Several models of attention explain how a priori knowledge about stimulus characteristics results in modulations of neural activity at the time of stimulus presentation and, in turn, enhanced perception of the stimulus (e.g., Desimone & Duncan, 1995; Tsotsos et al., 1995). There has been little focus, however, on how neural signals preceding stimulus presentation encode this a priori information and how these signals affect perception. We argue that a significant portion of behavioral variability on psychophysical tasks may be explained by variability in the neural signals preceding, rather than following, the sensory target. Modelers should consider this important source of behavioral variability.
Currently, variability in the observer 's percept of a visual stimulus is thought to reflect variability in the neural signals evoked by that stimulus – due to either noise in the stimulus itself (‘external noise’) or noise inherent to the evoked neural signals (‘internal noise’). Many single unit studies have addressed the statistical nature of the internal noise affecting visually evoked responses and how it affects the clarity of the internal representation of the stimulus (e.g., Britten, Shadlen, Newsome, & Movshon, 1992; Newsome, Britten, & Movshon, 1989). Because of the Poisson-like distribution of spike counts over repeated presentations of the same stimulus, the information present in neural signals is proportional to the mean neural signal. In Figure 1A, we re-plot data from Heuer and Britten (2004) illustrating the relation between average modulations and information content of stimulus-triggered spike trains. The blue line represents average spike rates in a single MST neuron following presentation of a motion stimulus, while the red line portrays the ability of an ideal observer to discriminate the direction of motion at each time point from the activity of the MST neuron. Clearly, the timecourse of signals carrying sensory information follows the mean signal under conditions in which internal noise limits perception. It has been suggested that attention can reduce the effects of internal noise by increasing the gain of the mean neural response (Hillyard, Vogel, & Luck, 1998; Reynolds, Pasternak, & Desimone, 2000).
Figure 1.
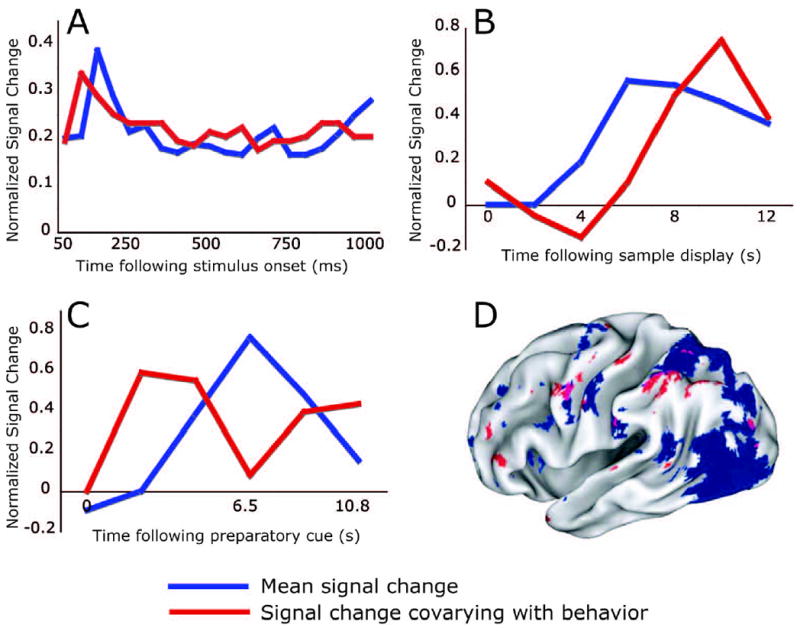
Timecourses of mean activity and the component of mean activity that varies with behavior (A-C). In each panel, blue lines represent average activity modulation over all trials (A) or over all correct trials (B,C) in the epoch of interest. Red lines indicate the component of this signal that varied with performance, as measured by the ability of the signal at each time point to predict trial accuracy. All data were normalized to allow mean and predictive timecourses to be drawn on the same plots. (A) Single-unit recordings from MST following presentation of a motion stimulus (Heuer & Britten, 2004). Note that in this stimulus-evoked activity, the predictive component closely follows the mean signal. (B) BOLD activity in the right intraparietal sulcus following presentation of a visual stimulus to be held in working memory (Pessoa et al., 2002). (C) BOLD activity in MT following a spatial cue indicating the probable location of an upcoming motion stimulus (Sapir et al., 2005). Note that in these preparatory signals (B,C) the predictive component of the signal differs from the mean signal. Panel D displays a map highlighting brain regions with consistent preparatory signals averaged over trials (blue) and regions whose preparatory activity was most predictive of performance (red) (Sapir et al., 2005). Purple indicates overlap. Note the spatial segregation between these signals.
Figure 1A modified, with permission, from figures 5A and 9 from Heuer and Britten (2005). Figure 1B modified, with permission, from figures 3B and 4 from Pessoa et al. (2002). Figure 1C modified, with permission, from figure 5A from Sapir et al. (2005). Figure 1D modified, with permission, from figure 2C from Sapir et al. (2005).
Another source of behavioral variability, less commonly considered, may arise from preparatory processes preceding target presentation. Under conditions of low stimulus visibility, for example, human subjects generate preparatory signals in visual cortex, and variability in these preparatory signals at the time of target presentation predicts accuracy of stimulus detection (Ress, Backus, & Heeger, 2000)1. Preparatory signals also seem to be modulated by spatial attention (Corbetta, Kincade, & Shulman, 2002; Jack, Shulman, Snyder, McAvoy, & Corbetta, 2006; Kastner, Pinsk, De Weerd, Desimone, & Ungerleider, 1999; Serences, Yantis, Culberson, & Awh, 2004). Preliminary evidence suggests that one function of preparatory signals may be to reduce external noise (Serences et al., 2004). These observations raise the intriguing possibility that variability in preparatory processes may be related to variability in stimulus-evoked signals.
To understand how preparatory signals influence perception, we highlight specific results from recent neuroimaging studies (Pessoa, Gutierrez, Bandettini, & Ungerleider, 2002; Sapir, d'Avossa, McAvoy, Shulman, & Corbetta, 2005; Weissman, Roberts, Visscher, & Woldorff, 2006). In each case, the timecourse of preparatory signals affecting behavioral performance did not follow the mean preparatory response, suggesting that performance related variability in preparatory signals does not resemble the variability of stimulus-evoked responses. In Figure 1, we re-plot data from two neuroimaging studies during epochs between the presentation of a sample stimulus or an attentional cue and visual targets. Each panel displays the pre-stimulus mean BOLD response timecourse (blue lines), and the timecourse that is predictive of behavioral performance (red lines). Pessoa et al. (2002) (Figure 1B) showed that variability in preparatory activity in a right intraparietal sulcus region predicted, trial-by-trial, whether subjects would correctly match a memorized sample to an upcoming test display. Sapir et al. (2005) (Figure 1C) showed that preparatory activity in MT following a spatial foveal cue predicted on a trial-by-trial basis whether subjects would correctly identify the direction of motion of a test stimulus at an attended or unattended location. Finally, Weissman et al. (2006) showed that variability in pre-trial neural signals in the anterior cingulate cortex, predicted subjects’ reaction time on a letter discrimination task.
These studies suggest that variations in endogenous signals (memory, attention) affect trial-by-trial perceptual performance. From where is this variability arising? Since the timecourses of endogenous signals predictive of behavior and mean signals are not identical, the possibility that variability is a by-product of noisy neural transmission of information seems unlikely. We offer three possible explanations. First, given its low spatial resolution, the BOLD signal may reflect several processes (neural populations), of which only a subset is relevant to behavior. Hence, the predictive components of the BOLD signal would reflect only behaviorally relevant processes (neural signals), whereas the mean signal would represent all task processes. Interestingly, Sapir et al. (2005) found that predictive and mean BOLD signals tend to segregate spatially across the brain (Figure 1D), buttressing the notion of independence between mean and predictive signals.
Second, variability may result from patterned ongoing neural signals unrelated to the specific task periods, which nevertheless affect task behavior. Ongoing intrinsic activity in visual cortex has been shown to re-play patterns of activity evoked by previously seen stimuli (Kenet, Bibitchkov, Tsodyks, Grinvald, & Arieli, 2003); it is plausible that the state of this activity immediately preceding stimulus presentation could affect behavior. Recently, coherent intrinsic BOLD activity across brain regions has been found to recapitulate patterns of task-evoked activation (Biswal, Yetkin, Haughton, & Hyde, 1995; Fox et al., 2005). Others have speculated that this intrinsic synchrony may be partly responsible for the behavioral and neural variability observed during active tasks (Fox, Snyder, Zacks, & Raichle, 2006).
A third possibility is that this variability reflects the result of subjects changing strategy, trial-by-trial, to optimize performance. Sapir et al. (2005), in fact, argued that preparatory signals related to performance reflected the variable use of a probabilistic cue. It should be noted that ideal performance in tasks whose demands are constant would be achieved by using a strategy that does not change from trial to trial. The presence of variable signals would suggest that humans, under some circumstances, use sub-optimal strategies. Variable preparatory signals may accordingly provide insights into how observers update internal representations of a priori information.
In conclusion, we reviewed recent evidence that variability in preparatory neural signals may significantly influence trial-by-trial behavioral performance on visual psychophysical tasks. The evidence suggests a departure from the standard view that perception is limited exclusively by variability in neural signals following stimulus presentation. Rather processes preceding stimulus presentation may make a substantial contribution to trial-to-trial variability and therefore accuracy of perception. Models of human visual attention should consider the reasons why preparatory signals are variable.
Footnotes
In this experiment preparatory signals were isolated presenting targets only some trials. However, as preparatory and target-related signals were not temporally separated, it is still possible that the observed neural variability reflects accumulation of sensory information at the time of target detection.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Biswal B, Yetkin F, Haughton V, Hyde J. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: A comparison of neuronal and psychophysical performance. Journal of Neuroscience. 1992;12:4745–4765. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationship with working memory. Journal of Cognitive Neuroscience. 2002;14(3):508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci. 2006;9(1):23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- Heuer HW, Britten KH. Optic flow signals in extrastriate area MST: comparison of perceptual and neuronal sensitivity. J Neurophysiol. 2004;91(3):1314–1326. doi: 10.1152/jn.00637.2003. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Vogel EK, Luck SJ. Sensory gain control (amplification) as a mechanism of selective attention: electrophysiological and neuroimaging evidence. Philosophical Transactions of the Royal Society of London B. 1998;353:1257–1270. doi: 10.1098/rstb.1998.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack AI, Shulman GL, Snyder AZ, McAvoy M, Corbetta M. Separate modulations of human V1 associated with spatial attention and task structure. Neuron. 2006;51(1):135–147. doi: 10.1016/j.neuron.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kenet T, Bibitchkov D, Tsodyks M, Grinvald A, Arieli A. Spontaneously emerging cortical representations of visual attributes. Nature. 2003;425(6961):954–956. doi: 10.1038/nature02078. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Britten KH, Movshon JA. Neuronal correlates of a perceptual decision. Nature. 1989;341:52–54. doi: 10.1038/341052a0. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Gutierrez E, Bandettini P, Ungerleider L. Neural correlates of visual working memory: fMRI amplitude predicts task performance. Neuron. 2002;35(5):975–987. doi: 10.1016/s0896-6273(02)00817-6. [DOI] [PubMed] [Google Scholar]
- Ress D, Backus BT, Heeger DJ. Activity in primary visual cortex predicts performance in a visual detection task. Nat Neurosci. 2000;3(9):940–945. doi: 10.1038/78856. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Pasternak T, Desimone R. Attention increases sensitivity of V4 neurons. Neuron. 2000;26(3):703–714. doi: 10.1016/s0896-6273(00)81206-4. [DOI] [PubMed] [Google Scholar]
- Sapir A, d'Avossa G, McAvoy M, Shulman GL, Corbetta M. Brain signals for spatial attention predict performance in a motion discrimination task. Proc Natl Acad Sci U S A. 2005;102(49):17810–17815. doi: 10.1073/pnas.0504678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Yantis S, Culberson A, Awh E. Preparatory activity in visual cortex indexes distractor suppression during covert spatial orienting. J Neurophysiol. 2004;92(6):3538–3545. doi: 10.1152/jn.00435.2004. [DOI] [PubMed] [Google Scholar]
- Tsotsos JK, Culhane S, Wai W, Lai Y, Davis N, Nuflo F. Modeling visual attention via selective tuning. Artificial Intelligence. 1995;78:507–547. [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nature Neuroscience. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]


