Abstract
PURPOSE
To investigate the feasibility of using femtosecond-pulse lasers to produce second-harmonic generated (SHG) signals to noninvasively assess corneal stromal collagen organization.
SETTING
The Eye Institute, University of California, Irvine, California, USA.
METHODS
Mouse, rabbit, and human corneas were examined by two-photon confocal microscopy using a variable-wavelength femtosecond lasers to produce SHG signals. Two types were detected: forward scattered and backward scattered. Wavelength dependence of the SHG signal was confirmed by spectral separation using the 510 Meta (Zeiss). To verify the spatial relation between SHG signals and corneal cells, staining of cytoskeletons and nuclei was performed.
RESULTS
Second-harmonic-generated signal intensity was strongest with an excitation wavelength of 800 nm for all 3 species. Second-harmonic-generated forward signals showed a distinct fibrillar pattern organized into bands suggesting lamellae, while backscattered SHG signals appeared more diffuse and indistinct. Reconstruction of SHG signals showed two patterns of lamellar organization: highly interwoven in the anterior stroma and orthogonally arranged in the posterior stroma. Unique to the human cornea was the presence of transverse, sutural lamellae that inserted into Bowman’s layer, suggesting an anchoring function.
CONCLUSIONS
Using two-photon confocal microscopy to generate SHG signals from the corneal collagen provides a powerful new approach to noninvasively study corneal structure. Human corneas had a unique organizational pattern with sutural lamellae to provide important biomechanical support that was not present in mouse or rabbit corneas.
The corneal stroma occupies the major part of the cornea and is composed predominantly of extracellular matrix containing types I and V collagen fibrils of 25 to 35 nm diameter organized in parallel bundles to form orthogonally arranged lamellae of variable width and thickness.1,2 Collagen lamellae organization in the anterior stroma is more undulated and interwoven, with branching of lamellae, than in the middle and posterior cornea, where lamellae run more parallel to the corneal surface.2,3 It is generally believed this collagen structure contributes to the cornea’s physical strength and shape.
Much information on the organization of collagen has been obtained from ultrastructural studies of very small regions of the cornea. Exceptions are reports that used high-voltage electron microscopy4 or attempted to serially section and reconstruct the stroma.5-7 However, ultrastructural analysis has limited ability to define the larger 3-dimensional (3-D) geometry of tissue and cell organization because of the need to mechanically section samples and the difficulty in aligning tissue sections to generate 3-D reconstructions.
Another approach to investigate collagen organization is X-ray scattering, which provides information on collagen fibril orientation and content. The collagen organization throughout the cornea and limbus was recently mapped using the synchrotron X-ray source, which has a highly focused beam, and was shown to have a preferred superior-inferior and nasal-temporal orientation in the central cornea.8,9 Scattering data also suggest the presence of anchoring lamellae that enter from the limbus predominantly at the major meridians and follow a curved trajectory through the peripheral cornea, exiting at the adjacent meridian. Although this approach provides new information on the bulk organization of collagen, it does not identify the organization of smaller populations of collagen lamellae that may define the micromechanical structure of the cornea.
Freund et al.10 showed intense laser light can produce second-harmonic generated (SHG) signals from tissues, particularly collagen. The SHG signals are created when two near-infrared photons interact with highly polarized and noncentrosymmetric materials such as collagen fibers to generate a single, visible photon with twice the energy and half the wavelength.11 With the recent advances in multiphoton confocal microscopy using femtosecond lasers with high energy pulses focused in very small volumes of the tissue, the application of second-harmonic imaging microscopy (SHIM) has been greatly expanded to include studies of actomyosin and tubulin.12
Hochheimer13 was the first to show SHG signals could be detected in the rabbit cornea as they were transmitted through the cornea (forward scattered) with respect to the excitation light. Particularly strong SHG signals can be obtained from the cornea14 and used to quantitatively establish collagen fibril orientation with the polarization dependence of the SHG signal.15 More recent studies use SHG signals to study 3-D collagen organization16,17 and the effects of laser photoablation.18
In our study, we used SHG signals to evaluate the organization of stromal collagen lamellae in three mammalian species. Our data showed distinct differences in the 3-D organization, in particular, the human cornea in which sutural collagen lamellae were detected that obliquely traverse the anterior cornea and insert into Bowman’s layer. We propose this unique organizational pattern may play an important role in defining the mechanical properties of the human cornea compared to corneas of other species.
MATERIALS AND METHODS
Preparation of Corneal Tissue
The corneas of mice, rabbits, and humans were examined in this study. Adult mouse eyes (n = 3) were fixed in paraformaldehyde 4%. Three corneas from 3 New Zealand White rabbits weighing 2 to 4 kg were obtained after anterior chamber perfusion with paraformaldehyde 2% in phosphate-buffered saline (PBS). Three human corneas were obtained from the San Diego Eye Bank, San Diego, California, USA. The donors were an 81-year-old white man, an 84-year-old Hispanic woman, and a 48-year-old woman of unknown race. All corneas appeared clear and normal viewed through a stereomicroscope. The human corneas were immediately fixed in paraformaldehyde 2% in PBS overnight. All animals and human specimens were treated according to the Association for Research in Vision and Ophthalmology and World Medical Association Declaration of Helsinki tenets.
The corneas were further dissected into smaller tissue blocks (1.0 to 1.5 mm) taken from the central cornea, washed in PBS, mounted on glass coverslips with glycerol 50%/PBS, and then imaged. Selected samples were further stained overnight at 4°C with phalloidin (Alexa Fluor 488 phalloidin) and 5 μm Syto red (Syto 59, Molecular Probes) in triton dextran 50% buffer (0.5% dimethyl sulfoxide, 0.5% triton X, 2.5% dextran 40 in PBS, pH 7.4) to identify actin cytoskeleton and nuclei.
Conventional and Multiphoton Confocal Microscopy
Specimens were placed on an Axiovert 200 microscope (Zeiss) and imaged using ×20 (NA = 0.75) and ×40 (NA = 1.3) oil-immersion objective lenses (Zeiss). The fluorescent × signal detection from phalloidin and Syto 59 were obtained using the 488 nm and 633 nm laser lines of the argon and red helium-neon lasers, respectively. Emitted light was detected using a 500 to 550 nm and 650 long-pass filters for phalloidin and Syto 59, respectively. Two-photon SHG signals were generated using a mode-locked titanium:sapphire laser (Chameleon Coherent, Inc.). Laser light was circularly polarized using a one-quarter waveplate interposed between the laser and microscope. The SHG forward-scattered signals passing through the tissue were collected using a 0.8 NA condenser lens with a narrow band-pass filter (400/50) placed in front of the transmission light detector. Backward-scattered SHG signals were detected with the Meta detector on the Zeiss 510, which has 32 channel detectors capable of collecting different frequency ranges separated by 11 nm. Using the multitrack mode of the LSM 510 Meta, sequential SHG and single-photon fluorescent signals were obtained from the same optical slice. All samples were scanned using a 1 m or 2 μm z-axis step size to generate 3-D data sets extending from the corneal epithelial surface to the endothelium for the 20 objective or to a depth of 100 μm for the ×40 objective. All images were recorded as 12-bit, 512 × 512 images. Three-dimensional data sets were reconstructed using the LSM Image Examiner (Zeiss). For each corneal sample (3 human, 3 rabbit, and 3 mice), at least three 3-D data sets were collected from the central region of the cornea.
Spectral Detection of Second-Harmonic-Generated Signal
To determine the best wavelength to excite collagen using SHG signals, SHG emission spectra were detected using the lambda mode function on the LSM 510 Meta. For spectral analysis, the excitation wavelength was varied from 760 to 840 nm. Laser energy was held constant at 100 mW/cm2 for each wavelength tested by varying the power output of the laser.
RESULTS
Second-Harmonic-Generated Emission Spectra
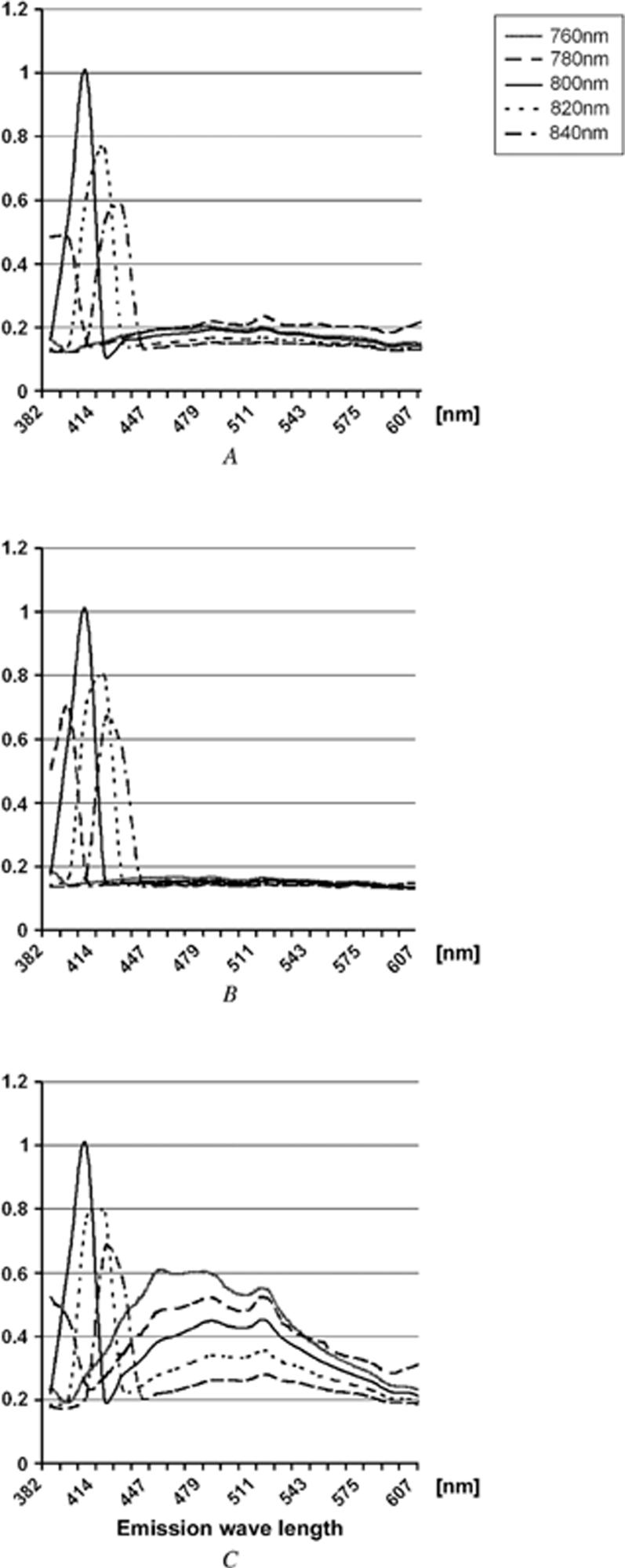
Figure 1 shows SHG signal emission spectra from the cornea. We assessed the emission spectra obtained from different infrared excitation wavelengths using the 510 Meta. For the mouse (Figure 1, A) and rabbit (Figure 1, B), excitation wavelength varying from 760 to 840 nm showed a single emission peak for each wavelength that was one half the excitation wavelength. Normalizing the detected signal showed that the maximum emission signal was obtained using an excitation wavelength of 800 nm for the mouse and rabbit; no other two-photon-excited signals were detected. The human cornea (Figure 1, C) showed a similar excitation/emission profile with peak emission intensity achieved with 800 nm infrared light. However, two-photon-excited fluorescent signals that peaked between 440 nm and 500 nm were also detected from the human cornea.
Figure 1.
Emission spectra from the mouse (A), rabbit (B), and human (C). The x-axis shows the emission wavelength in each species and y-axis, the normalized intensity of emission light.
Distinct from the SHG signals, the peak two-photon-excited fluorescent signals did not change with different excitation wavelength, although the intensity of the signals increased with decreasing excitation wavelength. The two-photon-excited fluorescent signals from the cornea may originate in part from collagen autofluorescence, which shows a peak emission around 500 nm. The 450 nm autofluorescence signal coincides with cellular fluorescence. Why autofluorescence was detected in the human cornea and not in the rabbit and mouse is not clear. The age of the tissues is a possible explanation because increased collagen cross-linking and deposition of lipofuscin pigment provided stronger autofluorescent signals in the older human samples. The contribution of the two-photon-excited fluorescent signals to the forward-scattered SHG signals detected from human corneas was minimized by using the 400/50 band-pass filter in front of the transmitted light detector.
Second-Harmonic-Generated Signal Detection of Stromal Collagen
Second-harmonic imaging microscopy of forward-directed SHG signals detected distinct fiber-like structures in the adult human cornea that were approximately 1 μm in diameter and varied in length and orientation depending on the depth in the cornea (Figure 2, A to C). In the anterior cornea, small bands of 5 or fewer parallel fibers appeared as short segments aligned in random orientations (Figure 2, A). Sequential images taken deeper in the cornea indicated that the short segments represented longer collagen fibers that composed narrow collagen lamellae running in and out of the plane of focus ( Video 1). This pattern suggested that the anterior collagen was organized into a highly interwoven lamellar structure with many lamellae running in a transverse, anterior-posterior direction and not parallel to the corneal surface. Deeper in the cornea, collagen bundles widened, containing from 5 to 10 fibers, but passed through multiple optical planes, suggesting that lamellae continued to be highly interwoven (Figure 2, B). In the posterior cornea, large numbers of fibers were grouped into orthogonally arranged lamellae that ran parallel to the corneal surface and showed markedly less interweaving than observed in the other regions. To ensure these differences were not the result of loss of signal deeper in the tissue or due to some cornea lens effect, selected tissue blocks were scanned epithelium to endothelium and then repositioned and scanned endothelium to epithelium. In both cases, the organization and size of the collagen lamellae were the same.
Video 1). This pattern suggested that the anterior collagen was organized into a highly interwoven lamellar structure with many lamellae running in a transverse, anterior-posterior direction and not parallel to the corneal surface. Deeper in the cornea, collagen bundles widened, containing from 5 to 10 fibers, but passed through multiple optical planes, suggesting that lamellae continued to be highly interwoven (Figure 2, B). In the posterior cornea, large numbers of fibers were grouped into orthogonally arranged lamellae that ran parallel to the corneal surface and showed markedly less interweaving than observed in the other regions. To ensure these differences were not the result of loss of signal deeper in the tissue or due to some cornea lens effect, selected tissue blocks were scanned epithelium to endothelium and then repositioned and scanned endothelium to epithelium. In both cases, the organization and size of the collagen lamellae were the same.
Figure 2.
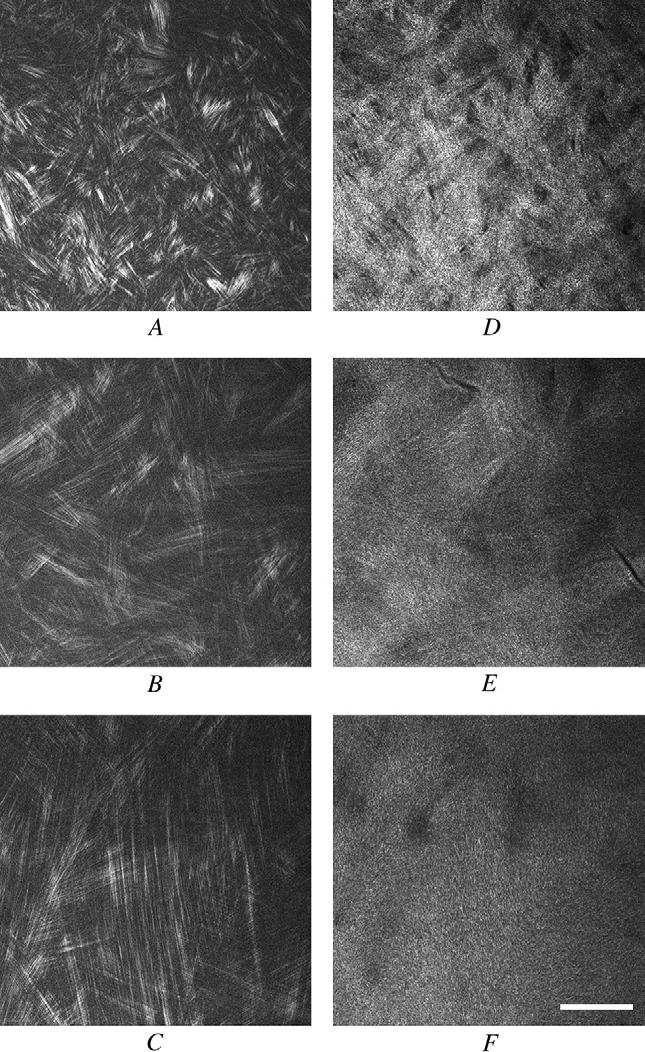
Forward-scattered (A to C) and back-scattered (D to F) SHG images of human corneal stroma taken in the anterior cornea (A and D), middle cornea (B and E), and posterior cornea (C and F) (bar = 50 μm).
Imaging of the backscattered signals did not fully resolve individual collagen fibers as clearly as detected in the forward-scattered images taken from the same optical plane (Figure 2, D to F), although the general outline of the lamellar organization could still be detected. As in the forward-scattered SHIM data, the anterior cornea showed a highly interwoven lamellar organization in which narrow lamellae running in random directions appeared to cross over and under multiple lamellae (Figure 2, D). Deeper within the cornea, broader lamellae that continued to exhibit an interwoven pattern (Figure 2 E) were detected. In posterior cornea, it was difficult to distinguish an organizational pattern, most likely because of the width of the lamellae and the orthogonal orientation.
Imaging of the forward scattered SHG signals in the mouse and rabbit cornea showed similar regions of interwoven lamellar and orthogonally arranged lamellae organization. For both species, the anterior cornea showed short lamellar bands arranged in groups of 5 or fewer collagen fibers that ran in random orientations (Figure 3, A and C; mouse and rabbit, respectively). Sequential images taken deeper in the cornea showed extension and interweaving of fiber bundles, indicating an interwoven lamellar organization. Deeper within the cornea, both the mouse cornea (Figure 3, B) and rabbit cornea (Figure 3, D) contained fibrils organized into much larger bands, forming orthogonally arranged lamellae that had less interweaving. This was more difficult to detect in the mouse cornea because of the steep corneal curvature (Figure 3, B).
Figure 3.
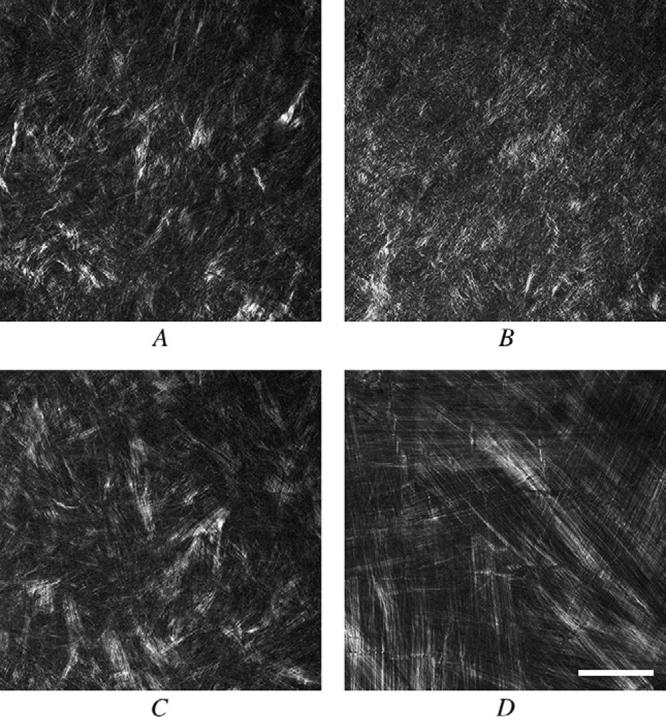
Forward-scattered SHG signals from the mouse (A and B) and rabbit (C and D) taken in the anterior stroma (A and C) and posterior stroma (B and D) (bar = 50 μm).
Spatial Organization of Corneal Collagen Lamellae
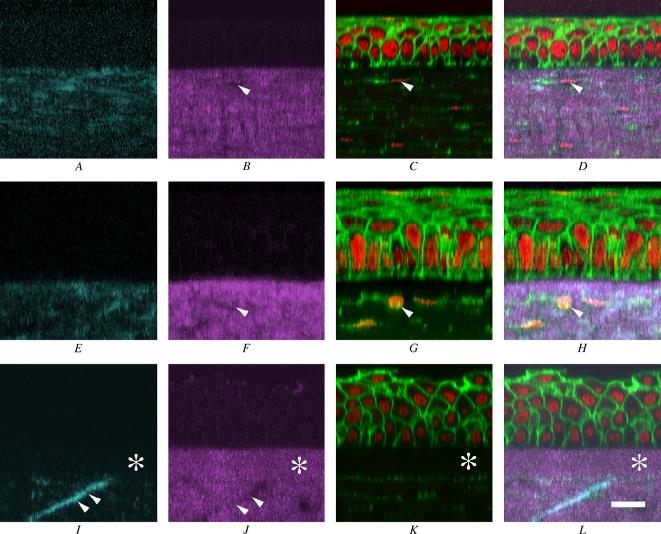
To more clearly identify the spatial organization of collagen lamellae, corneas were stained with phalloidin and Syto 59 to localize cells and collagen organization simultaneously. Serial optical sections were then taken through the entire mouse, rabbit, and human corneas, and the corneas were reconstructed in 3-D (Figure 4). Ten micron wide cross-sections through 3-dimensional data sets of the forward-scattered SHG signals (Figure 4, A, E, and I; cyan) and back-scattered SHG signals (Figure 4, B, F, and J; magenta) were compared to corneal epithelium and stromal cells stained for actin and DNA (Figure 4, C, G, and K; green and red, respectively) by merging the images (Figure 4, D, H, and L). In the mouse (Figure 4, A to D), collagen orientation detected with the forward-scattered SHG signal (A) and the back-scattered SHG signal (B) was predominantly parallel to the corneal surface and ran immediately underneath the corneal epithelium. Furthermore, corneal stromal cells (Figure 4, C and D; arrows) appeared to reside in regions devoid of SHG signals (Figure 4, B and D, arrows). The rabbit cornea showed a similar organizational pattern, with collagen fibrils oriented mostly parallel to the corneal surface and cells residing in defined regions devoid of SHG signals (Figure 4, F, G, and H; arrows). In the human, a distinctly different organizational pattern was detected (Figure 4, I to L). First, a defined region of the corneal stroma immediately below the corneal epithelium showed strong SHG back-scattering that did not show significant forward-scattered SHG signals (Figure 4, I to L; asterisk). This 10 μm thick region appeared to correspond to Bowman’s layer and was not detected in the mouse or rabbit cornea. Second, the forward-scattered SHG signal (Figure 4, I) identified collagen fibrils that appeared to run transversely through the corneal stroma (double arrowheads). These collagen lamellae also appeared to reside in regions of the stroma that did not show strong back-scattered signals (Figure 4, J; double arrowheads). Finally, transverse collagen lamellae also appeared to terminate at Bowman’s layer and gave the impression of inserting into Bowman’s layer.
Figure 4.
Reconstructed cross-sectional images of the mouse (A to D), rabbit (E to H), and human (I to J). A, E, and I: Cross-sectional images of SHG forward signal (cyan). B, F, and J: The SHG backward signal (magenta). C, G, and K: Double staining of actin (green)/nuclei (red). D, H, and L were merged images of an SHG forward signal, SHG backward signal, and double staining. The arrowheads in B to D and F to H indicate space in the stroma where cells reside. The double arrowheads in I and J indicate “anchoring” stromal lamellae, and the asterisk indicates the location of Bowman′s membrane (bar = 20 μm).
3-Dimensional Organization of Collagen Lamellae
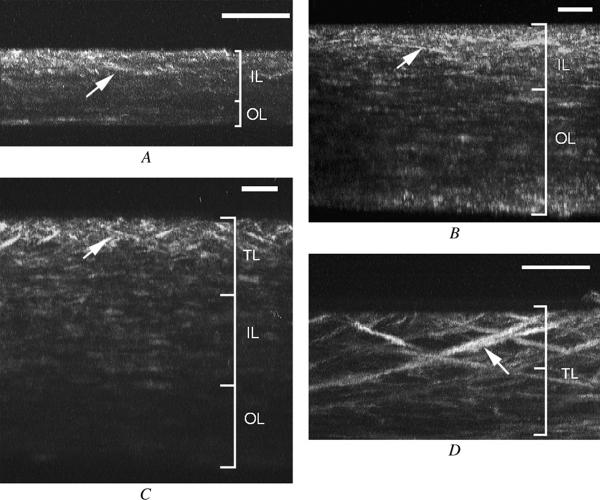
To evaluate the 3-D organization of collagen, maximum intensity projections of the 3-D forward-scattered data sets were generated along the z-x plane for 360 degrees ( Video 2). In the mouse, a few lamellae in the anterior stroma that appeared to have a transverse orientation (Figure 5, A; arrow) were identified; however, these lamellae did not reach the stromal surface, extended for a limited depth representing a few lamellar planes, and appeared to represent regions of more highly interwoven lamellae that were detected in the original 2-D data. Similarly, in the rabbit, transverse collagen lamellae were detected (Figure 5, B; arrow); however, almost all forward-scattering collagen lamellae appeared to run parallel to the corneal surface. Overall, there appeared to be two regions of lamellar organization, an anterior interwoven and a posterior orthogonal lamellar arrangement. The separation between these two regions was confirmed by viewing the original 2-D data set, after which an estimate of the relative thickness of the two stromal layers was then calculated. In general, the region of interwoven lamellar stroma comprised 66% and 34% of the mouse cornea (n = 3) and rabbit cornea (n = 3), respectively.
Video 2). In the mouse, a few lamellae in the anterior stroma that appeared to have a transverse orientation (Figure 5, A; arrow) were identified; however, these lamellae did not reach the stromal surface, extended for a limited depth representing a few lamellar planes, and appeared to represent regions of more highly interwoven lamellae that were detected in the original 2-D data. Similarly, in the rabbit, transverse collagen lamellae were detected (Figure 5, B; arrow); however, almost all forward-scattering collagen lamellae appeared to run parallel to the corneal surface. Overall, there appeared to be two regions of lamellar organization, an anterior interwoven and a posterior orthogonal lamellar arrangement. The separation between these two regions was confirmed by viewing the original 2-D data set, after which an estimate of the relative thickness of the two stromal layers was then calculated. In general, the region of interwoven lamellar stroma comprised 66% and 34% of the mouse cornea (n = 3) and rabbit cornea (n = 3), respectively.
Figure 5.
Three-dimensional reconstructions of an SHG forward-scattered signal derived from the mouse (A), rabbit (B), and human (C and D). Note the transverse-oriented collagen bundles (arrows). Organization of collagen lamellae are shown to the right (TL = transverse lamellae; IL = interwoven lamellae; OL = orthogonal lamellae; bar = 50 μm).
In contrast, the human cornea contained prominent transversely oriented collagen lamellae (Figure 5, C and D) that were much longer and extended over a much wider region of the anterior corneal stroma than observed in the mouse or rabbit. Many of the transverse lamellae appeared to insert into Bowman’s layer and extended deep into the corneal stroma, again suggesting an anchoring function similar to the sutural fibers identified in the shark. Because of the oblique angle of these lamellae, it is likely that many of the deeper transverse collagen lamellae detected in the lower magnification image (Figure 5 D) also inserted into Bowman’s layer in adjacent, out of view regions of the cornea. Overall, the zone of transverse lamellar was approximately 28% of the cornea (n = 3). Beneath the transverse lamellar zone, interwoven collagen lamellae, with relative short regions of transverse lamellae, could be identified. Taken together, the region of the cornea containing interwoven and transverse lamellae represented approximately 68% of the entire corneal stroma, with the remaining corneal lamellae organized in an orthogonal lamellar arrangement.
DISCUSSION
In our study, we showed that the generation of second-harmonic signals from corneal collagen using femtosecond pulsed infrared lasers can be used to assess the lamellar organization of the cornea. Because SHG images identify fibers with an approximate diameter of 1 μm, the SHG signal most likely represents groups of individual collagen fibrils that interact with pulsed laser light to generate second harmonic signals that can then be detected to determine the overall 3-D geometry of the lamellar organization. Using SHIM, three patterns of lamellar organization were detected in the human cornea: transverse, interwoven, and orthogonal. The detection of transverse lamellae supports observations by Komai and Ushiki,2 who report the presence of collagen lamellae that branch and insert into Bowman’s layer. However, because their study used transmission electron microscopy, the extension of these transverse lamellae deeper within the corneal stroma was not reported. The anterior cornea is known to have extensive anteroposterior, interweaving lamellae that obliquely pass from one lamellar layer to another, sometimes passing across several lamellae and occasionally insert to Bowman’s layer.21 These transverse lamellae may contribute to the anterior corneal mosaic that is visible in normal corneas as a polygonal pattern within the anterior stroma after instillation of fluorescein.21 Our findings indicate these transverse lamellae extend much deeper into the anterior cornea and are much more frequent than formerly appreciated.
Recent studies by Müller et al.6 of swollen human corneas also show that the anterior 100 to 120 μm of stroma is resistant to swelling and maintains the corneal curvature. Although it has been proposed that the interwoven nature of the anterior cornea is responsible for this mechanical property, this region also corresponds to the region containing transverse lamellae that extend to a depth of approximately 130 μm. Therefore, it is likely that the presence of transverse lamellae within the anterior cornea provides additional rigidity to the corneal stroma, much greater than that provided by lamellar interweaving, particularly if the lamellae insert into the corneal limbus, as Bron21 suggests.
Although transverse lamellae were only clearly identified in the anterior quarter of the cornea, it is likely these lamellae extend much deeper into the stroma. If so, the lamellae may represent a structural feature similar to that of “sutural fibers” identified in the dogfish.19,20 Sutural fibers are thought to provide rigidity to the dogfish cornea and play a role in the resistance to swelling following removal of the corneal endothelium, a similar function identified by Müller et al.6 for the anterior region of the human cornea. The importance of this lamellar organizational pattern to the mechanical strength of the cornea is also emphasized by Maurice and Monroe22 and Smolek and McCarey,23 who measured the interlamellar adhesive strength of rabbit and human corneas, respectively. Their studies showed the rabbit cornea is much less resistant than the human cornea to tearing in the lamellar plane. In our studies, lamellar interweaving in rabbit was limited to the anterior one third of the corneal stroma, while in the human, interweaving extended to the anterior two thirds. In addition, transverse, sutural lamellae were detected only in the human. This finding suggests that the transverse, sutural pattern and the more extensive interweaving of lamellae may be important in defining corneal rigidity and resistance to lateral sheer forces.
The distinct organizational pattern in the human cornea may also help explain differences in the risk for corneal ectasia after refractive surgical procedures. It is known that the posterior corneal stroma is mechanically weaker and that this weakness is generally thought to be caused by differences in the proteoglycan composition, collagen crosslinking, and keratocyte density.2,5,24 However, the regional differences in the 3-D collagen geometry, as noted by others and our report, support the hypothesis that the mechanical properties are defined by the microstructural organization and the anterior region of the stroma that contains transverse lamellae is much stiffer than the posterior stroma containing classically orthogonally arranged collagen. Surgical procedures that alter the relative contribution of the two organizational patterns might significantly alter the mechanical properties and susceptibility to ectasia. More important, variation in patient populations in the relative depth or thickness of these structures might also define these patients’ relative risk for ectasia. Although patient variation in corneal structural organization is not known, additional studies are needed to define differences in normal patients and in those with an increased risk for ectasia, such as patients with forme fruste keratoconus.25-27
Because SHG imaging microscopy is noninvasive, this new technology may provide an important approach to evaluating corneal structural organization in patients before refractive surgery. Transverse sutural lamellae were best detected with forward-scattered SHIM; however, outlines of the transverse lamellae were detected using back-scatter SHG signals that could potentially be used to estimate transverse lamellar density and depth in patients. Furthermore, varying the polarization or the angle of the excitation beam may provide better detection of the lamellae using the back-scattered signal detection.
Knowledge of the transverse, sutural lamellar density and how density changes in different patient populations may provide more accurate prediction of the final refractive surgical results as well as prediction of patients at risk for ectasia. Second-harmonic imaging microscopy is a promising in vivo imaging paradigm; however, additional study is needed before evaluation of patients is done.
Supplementary Material
Footnotes
No author has a proprietary or financial interest in any material or method mentioned.
Supported in part by National Institutes of Health grants EY07348 (Dr. Jester) and Infrastructure Grant EY016663, Lew Wasserman Merit Award and Support Grant from Research to Prevent Blindness, Inc., The Skirball Program in Molecular Ophthalmology, and the Japan Eye Bank Association.
REFERENCES
- 1.Hamada R, Giraud J-P, Graf B, Pouliquen Y. Étude analytique et statistique des lamelles, des keratocytes, des fibrilles de collagéne de la région centrale de la cornée humaine normale. (Microscopie optique et électronique) Arch Ophtalmol Rev Gen Ophtalmol. 1972;32:563–570. [PubMed] [Google Scholar]
- 2.Komai Y, Ushiki T. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Invest Ophthalmol Vis Sci. 1991;32:2244–2258. [PubMed] [Google Scholar]
- 3.Radner W, Zehetmayer M, Aufreiter R, Mallinger R. Interlacing and cross-angle distribution of collagen lamellae in the human cornea. Cornea. 1998;17:537–543. doi: 10.1097/00003226-199809000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Binder PS, Rock ME, Schmidt KC, Anderson JA. High-voltage electron microscopy of normal human cornea. Invest Ophthalmol Vis Sci. 1991;32:2234–2243. [PubMed] [Google Scholar]
- 5.Müller LJ, Pels L, Vrensen GFJM. Novel aspects of the ultrastructural organization of human corneal keratocytes. Invest Ophthalmol VisSci. 1995;36:2557–2567. [PubMed] [Google Scholar]
- 6.Müller LJ, Pels E, Vrensen GFJM. The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. Br J Ophthalmol. 2001;85:437–443. doi: 10.1136/bjo.85.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Müller LJ, Pels E, Schurmans LRHM, Vrensen GFJM. A new three-dimensional model of the organization of proteoglycans and collagen fibrils in the human corneal stroma. Exp Eye Res. 2004;78:493–501. doi: 10.1016/s0014-4835(03)00206-9. [DOI] [PubMed] [Google Scholar]
- 8.Aghamohammadzadeh H, Newton RH, Meek KM. X-Ray scattering used to map the preferred collagen orientation in the human cornea and limbus. Structure. 2004;12:249–256. doi: 10.1016/j.str.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Boote C, Dennis S, Huang Y, et al. Lamellar orientation in human cornea in relation to mechanical properties. J Struct Biol. 2005;149:1–6. doi: 10.1016/j.jsb.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Freund I, Deutsch M, Sprecher A. Connective tissue polarity; optical second-harmonic microscopy, crossed-beam summation, and small-angle scattering in rat-tail tendon. Biophys J. 1986;50:693–712. doi: 10.1016/S0006-3495(86)83510-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohler W, Millard AC, Campagnola PJ. Second harmonic generation imaging of endogenous structural proteins. Methods. 2003;29:97–109. doi: 10.1016/s1046-2023(02)00292-x. [DOI] [PubMed] [Google Scholar]
- 12.Campagnola PJ, Loew LM. Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nat Biotechnol. 2003;21:1356–1360. doi: 10.1038/nbt894. [DOI] [PubMed] [Google Scholar]
- 13.Hochheimer BF. Second harmonic light generation in the rabbit cornea. Appl Opt. 1982;21:1516–1518. doi: 10.1364/AO.21.001516. [DOI] [PubMed] [Google Scholar]
- 14.Williams RM, Zipfel WR, Webb WW. Multiphoton microscopy in biological research. Curr Opin Chem Biol. 2001;5:603–608. doi: 10.1016/s1367-5931(00)00241-6. [DOI] [PubMed] [Google Scholar]
- 15.Stoller P, Kim B-M, Rubenchik AM, et al. Polarization-dependent optical second-harmonic imaging of a rat-tail tendon. J Biomed Opt. 2002;7:205–214. doi: 10.1117/1.1431967. [DOI] [PubMed] [Google Scholar]
- 16.Yeh AT, Nassif N, Zoumi A, Tromberg BJ. Selective corneal imaging using combined second-harmonic generation and two-photon excited fluorescence. Optics Lett. 2002;27:2082–2084. doi: 10.1364/ol.27.002082. [DOI] [PubMed] [Google Scholar]
- 17.Zoumi A, Yeh A, Tromberg BJ. Imaging cells and extracellular matrix in vivo by using second-harmonic generation and two-photon excited fluorescence. PNAS. 2002;99:11014–11019. doi: 10.1073/pnas.172368799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han M, Zickler L, Giese G, et al. Second-harmonic imaging of cornea after intrastromal femtosecond laser ablation. J Biomed Opt. 2004;9:760–766. doi: 10.1117/1.1756919. [DOI] [PubMed] [Google Scholar]
- 19.Goldman JN, Benedek GB. The relationship between morphology and transparency in the nonswelling corneal stroma of the shark. Invest Ophthalmol. 1967;6:574–600. [PubMed] [Google Scholar]
- 20.Keller N, Pouliquen Y. Ultrastructural study of the posterior cornea of the dogfish “Scyliorhinus canicula L.”. Cornea. 198586;4:108–117. [PubMed] [Google Scholar]
- 21.Bron AJ. The architecture of the corneal stroma [editorial] Br J Ophthalmol. 2001;85:379–381. doi: 10.1136/bjo.85.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maurice DM, Monroe F. Cohesive strength of corneal lamellae. Exp Eye Res. 1990;50:59–63. doi: 10.1016/0014-4835(90)90011-i. [DOI] [PubMed] [Google Scholar]
- 23.Smolek MK, McCarey BE. Interlamellar adhesive strength in human eyebank corneas. Invest Ophthalmol Vis Sci. 1990;31:1087–1095. [PubMed] [Google Scholar]
- 24.Bettelheim FA. The hydration of proteoglycans of bovine cornea. Biochim Biophys Acta. 1975;381:203–214. doi: 10.1016/0304-4165(75)90202-0. [DOI] [PubMed] [Google Scholar]
- 25.Twa MD, Nichols JJ, Joslin CE, et al. Characteristics of corneal ectasia after LASIK for myopia. Cornea. 2004;23:447–457. doi: 10.1097/01.ico.0000122702.49054.12. [DOI] [PubMed] [Google Scholar]
- 26.Pallikaris IG, Kymionis GD, Astyrakakis NI. Corneal ectasia induced by laser in situ keratomileusis. J Cataract Refract Surg. 2001;27:1796–1802. doi: 10.1016/s0886-3350(01)01090-2. [DOI] [PubMed] [Google Scholar]
- 27.Randleman JB, Russell B, Ward MA, et al. Risk factors and prognosis for corneal ectasia after LASIK. Ophthalmology. 2003;110:267–275. doi: 10.1016/S0161-6420(02)01727-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.