Summary
Wnts are a family of secreted glycoproteins with diverse roles in development, including regulation of cell migration, however little is known about wnt signaling in mature T cells. We find that endothelial cell-derived wnts, acting through Frizzled (Fz) receptors, induce MMP2 and MMP9 expression in effector T cells. Blocking wnt signaling, or MMP activity, reduces T cell migration through basement membrane in vitro, and into inflamed skin in vivo. Wnt signaling stabilizes β-catenin protein in T cells and directly targets the MMP promoters through tandem LEF/TCF sites. Thus our data support a necessary, novel, and previously unexpected role for wnt signaling in T cell extravasation.
Introduction
The migration of T cells across the endothelial cell (EC) barrier that separates blood from tissue represents a critical event in inflammation, and the failure of this process, due to the lack of necessary recognition molecules, can result in recurrent infections and failure to clear pathogens (Madri and Graesser, 2000). The extravasation process involves complex morphological changes in both T cells and EC, as well as multiple interactions between cell-cell and cell-matrix adhesion molecules. Although the specifics of T cell recruitment are not well understood, the basic mechanism of extravasation employed by other leukocytes, in particular neutrophils and macrophages, has been described: selectins mediate the initial capture of the leukocyte and the rolling phase; chemokines expressed on the surface of EC then trigger affinity changes in leukocyte integrins resulting in arrest and firm adhesion. Less well understood are the molecular cues underlying the induction of morphological changes and the induction of new gene expression that are associated with migration across the EC monolayer, degradation of the underlying basement membrane (BM) and subsequent movement along chemokine gradients within the interstitial matrix. Progress has been made in our understanding of diapedesis, where the leukocyte squeezes between adjacent EC, through studies on monocyte migration where both PECAM-1 (CD31) and CD99 have been implicated (Muller, 2003). It is not known whether identical or similar processes regulate T lymphocyte transmigration.
The BM, composed largely of collagen type IV, laminin, nidogen, fibronectin and perlecan (a heparan sulfate proteoglycan) (Baluk et al., 2003), represents a major barrier to migrating T cells and necessitates the inducible expression by migrating cells of several proteases including matrix metallo-proteinase-2 (MMP2) and MMP9, also known as gelatinase A and B respectively (Goetzl et al., 1996; Madri and Graesser, 2000). These can be induced by T cell receptor triggering in vitro, however it is not known in vivo what the major inducing signal is, or when and where expression is induced. One possibility is that MMP expression is induced by interaction with the EC, and VCAM-VLA-4 interaction has been implicated in different settings (Madri et al., 1996; Romanic et al., 1997). Numerous studies have demonstrated the importance of MMP activity in T cell migration in vivo. For example, the MMP inhibitor alpha lipoic acid blocked T cell migration into spinal cord (Marracci et al., 2002), astilbin was suggested to block DTH responses in mice by inhibiting MMP activity and lymphocyte migration (Cai et al., 2003), and MMP2 and MMP9 were shown to be essential for T cell migration into lung in an allergen-induced airway inflammation model (Kumagai et al., 1999). T cell-associated MMP activity has also been implicated in the pathogenesis of EAE (Graesser et al., 1998; Madri and Graesser, 2000; Madri et al., 1996; Sixt et al., 2001). Notably, specific degradation of laminin10 has been correlated with efficient migration of encephalitogenic T cells into the brain parenchyma of mice in an EAE model (Sixt et al., 2001). Finally, a recent report demonstrated mobilization of matrix-bound chemokines by the MMP matrilysin (MMP7), indicating that MMPs may not only promote T cell migration by degrading matrix ahead of the cell, but may also influence the local free concentration of chemokines (Li et al., 2002). Interestingly, MMP7 is a wnt target in colon cancer (Crawford et al., 1999), and wnts have been implicated in cell migration and metastasis (Heisenberg et al., 2000; Qiang et al., 2005; Weeraratna et al., 2002). Wnt signaling is necessary for proper T cell development (see below) and we have previously shown that a wnt pathway regulates NFAT localization in mature T cells (Salazar Murphy and Hughes, 2002). Taken together, these findings raise the interesting possibility that wnts may regulate MMP expression in T cells.
Numerous developmental processes, as diverse as cell fate specification, induction of polarity, and cell migration are controlled by the wnt family of secreted glycoproteins (Gordon and Nusse, 2006; Logan and Nusse, 2004; Moon et al., 2004; Nusse, 2002; Wodarz and Nusse, 1998). The best understood wnt signaling pathway is dependent upon the transcriptional co-activator β-catenin (Nusse, 2002). As a result of wnt signaling, β-catenin is translocated to the nucleus where it binds TCF family proteins, converting them into transcriptional activators (Barker et al., 2000; Wodarz and Nusse, 1998). In the absence of β-catenin the LEF/TCF family members bind to DNA and act as negative regulators by recruiting the co-repressor groucho. In the absence of wnt, cytoplasmic β-catenin forms a complex with axin and APC where it becomes a target for phosphorylation by GSK-3β and degradation by the proteosome. Upon wnt ligation to the receptor Fz and the co-receptor LRP, the modular protein disheveled (Dsh in flies, Dvl in mammals) binds to the β-catenin-Axin-APC complex and reduces the activity of GSK-3β toward β-catenin. The accumulated β-catenin can then translocate to the nucleus and modulate LEF/TCF-dependent transcription (Moon, 2002). Examples of wnts that signal through this pathway include wnt1, wnt2B, wnt3A and wnt8B.
Studies on wnt signaling in T cells have, to date, largely focused on thymocyte development (Held et al., 2003; Ioannidis et al., 2001; Pongracz et al., 2006; Staal and Clevers, 2005; Staal et al., 2004). LEF-1 and TCF-1 are expressed in thymocytes where they drive expression of CD4 (Huang et al., 2006). TCF−/− mice have a block at the immature single positive (ISP) to double positive transition, due to a block in ISP proliferation (Schilham et al., 1998). Evidence that these effects reflect a direct role for wnts in thymocyte development comes from studies on wnt knockout mice. Although both wnt1 (McMahon and Bradley, 1990) and wnt4 (Stark et al., 1994) knockouts die within 24 hours of birth, thymocytes can be recovered. In the absence of either wnt1 or wnt4 there is a decrease of 20–30% in cell number, and a loss of over 70% in the double knockout (Mulroy et al., 2002).
We have previously shown that a non-β-catenin-dependent wnt pathway is active in T cells and mediates NFAT localization (Salazar Murphy and Hughes, 2002). Here we show that β-catenin-dependent wnt signaling is also active in mature, peripheral blood T cells and that EC-derived wnts induce MMP expression and augment T cell transmigration, both in vitro and in vivo. Thus, our data demonstrate a previously unrealized role for wnt signaling in the biology of mature T cells.
Results
Wnt signaling is active in mature T cells
Previous studies have failed to detect β-catenin protein in mature T cells, suggesting the absence of β-catenin-dependent wnt signaling (Chung et al., 2002). However, no attempt was made to stabilize β-catenin protein. In light of our previous studies showing a role for the Ca2+/PKC wnt pathway in regulating NFAT localization in mature T cells (Salazar Murphy and Hughes, 2002), we decided to re-address the issue of β-catenin-dependent signaling, which uses some of the same receptors, in these cells. By Western blot we found that both Li+ and wnt3A-containing conditioned medium (wnt3A-CM) led to the accumulation of β-catenin protein in effector T cells (Figure 1A) and also in resting T cells (data not shown). Li+ directly inhibits GSK-3β activity, whereas, wnt3A acts upstream through Frizzled receptors.
Figure 1. Endothelial cells induce β-catenin-dependent wnt signaling in T cells.
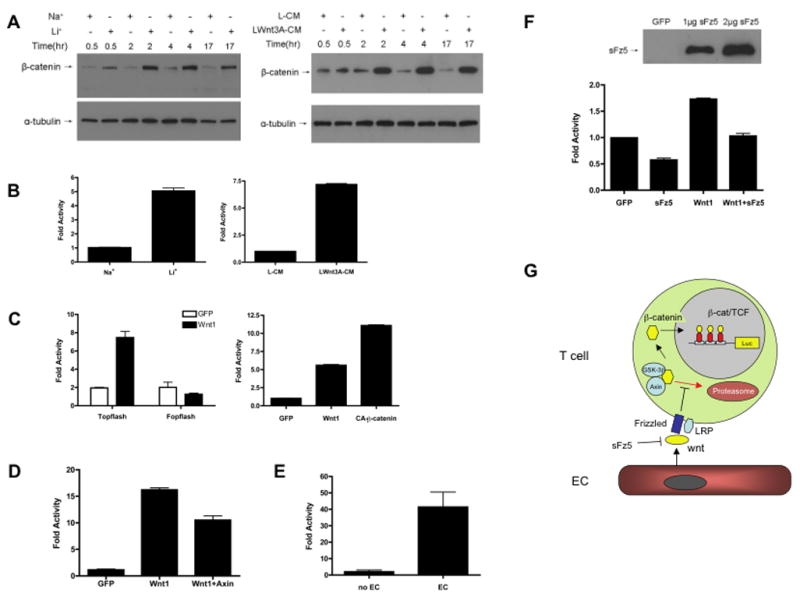
(A) Effector T cells were treated for the indicated times with either 10mM Li+ or Na+, or with control or wnt3A-conditioned medium and harvested for western blotting with an antibody against β-catenin. To confirm equal loading blots were stripped and re-probed for α-tubulin. One of at least three similar experiments. (B) Effector T cells were transfected with the TOPflash reporter vector and then incubated with either 10mM Li+ or Na+, or with control or wnt3A-conditioned medium. Luciferase activity was assayed after 24 hours. Mean and SD of triplicate wells, * – p<0.005 (t-test) for Na+ v Li+, p<0.005 (t-test) for L-CM v wnt3A-CM. One of three similar experiments. (C) (left) Effector T cells were transfected with either the TOPflash reporter or the control FOPflash, which contains mutated LEF/TCF binding sites, along with an expression plasmid for wnt1. (right) Effector T cells were transfected with the TOPflash reporter along with expression vectors for either wnt1 or constitutively active (CA) β-catenin (CA-β-catenin). Luciferase activity was assayed after 24 hours. Mean and SD of triplicate wells, * – p<0.005 (t-test) for GFP v wnt1 (TOPflash) and p<0.005 (t-test) for GFP v wnt1 or GFP v CA-β-catenin. One of three similar experiments. (D) Effector T cells were transfected with TOPflash and expression plasmids for wnt1 or axin as indicated. DNA load was balanced with control plasmid. Mean and SD of triplicate wells, * – p<0.005 (t-test) for wnt1 v wnt1 + axin. One of three similar experiments. (E) Effector T cells were transfected with TOPflash and incubated with EC for 24 hours before assay of luciferase activity. Mean and SD of triplicate wells, * – p<0.005 (t-test). One of three similar experiments. (F) Effector T cells were transfected with TOPflash and incubated with EC expressing sFz5 or wnt1 as indicated. DNA load was balanced with control plasmid. Luciferase activity was assayed after 24 hours. Mean and SD of triplicate wells, * – p<0.005 (t-test) for GFP v sFz5, GFP v wnt1, and wnt1 v wnt1+sFz5. Expression of tagged-sFz5 was confirmed by western blot using an anti-Flag antibody. One of at least three similar experiments. (G) EC-derived wnts signal through Frizzled/LRP receptors and block proteosome-mediated degradation of β-catenin. As β-catenin levels rise it enters the nucleus where it acts as a transcriptional co-factor with LEF/TCF to induce target genes, here the reporter TOPflash. sFz5 blocks wnt signaling.
To determine whether this stabilized β-catenin protein was active we employed the wnt reporter TOPflash, which consists of multimerized LEF/TCF binding motifs upstream of the luciferase gene (Korinek et al., 1997). The FOPflash control vector contains mutated binding sites and was used as a negative control. Consistent with the stabilization of β-catenin protein, both Li+ and wnt3A-CM strongly activated TOPflash in effector T cells (Figure 1B). TOPflash, but not FOPflash, activity was also increased in T cells expressing either wnt1 or a constitutively active form of β-catenin (S33Y) that has a targeted mutation of the serine phosphorylated by GSK-3β (Figure 1C). It is important to note that wnt1 and wnt3A are considered to be functionally redundant (see Discussion).
Axin, along with APC, performs a critical scaffolding function in the targeting of β-catenin for phosphorylation by GSK-3β, and overexpression of axin has previously been shown to disrupt wnt signaling (Nakamura et al., 1998). We confirmed this finding in T cells, where co-expression of axin reduced wnt1-induced TOPflash activity by almost 40% (Figure 1D). Taken as a whole, these results confirm that a functional β-catenin wnt pathway is present in both resting and effector T cells.
Mature T cells express multiple proximal components of the wnt signaling pathway
Wnt signals that act to stabilize β-catenin act through a receptor complex composed of a frizzled family member and either LRP5 or LRP6 (Tamai et al., 2000). This complex is then thought to trigger the translocation of dvl to the axin-APC-GSK-3β complex. We investigated the expression of Fz, LRP and dvl in mature T cells by semi-quantitative RT-PCR looking at both resting and effector T cells. Notably, all of the frizzleds present in resting T cells (Fz3, 4, 5, 6, 7) were strongly up regulated in effector T cells (Table 1). LRP5 was also up regulated, in contrast to LRP6 which showed significantly decreased expression. As antibodies specific for these molecules are currently unavailable we were not able to confirm these findings by western blot. All three dvl genes were expressed at roughly comparable levels between resting and effector T cells. The correlation between T cell activation and up regulation of wnt receptors suggested to us that wnt signaling may be particularly important in effector cells, and because of their obvious functional importance all subsequent experiments were performed with effector T cells.
Table 1.
T cells express components of the wnt signaling pathway1
| Resting | Effector | |
|---|---|---|
| Fz3 | + | +++ |
| Fz4 | + | +++ |
| Fz5 | + | +++ |
| Fz6 | + | +++ |
| Fz7 | + | +++ |
| LRP5 | + | +++ |
| LRP6 | +++ | + |
| Dvl1 | + | + |
| Dvl2 | + | + |
| Dvl3 | + | + |
Expression of Frizzled, LRP and Dvl family members was examined by semi-quantitative RT-PCR. Resting and effector T cells were prepared as described in Experimental Procedures. The relative expression level of each gene can be compared between resting and effector cells, however not between different genes.
Endothelial cells induce Wnt signaling in T cells
During circulation throughout the body, T cells continuously come into contact with the endothelial cells (EC) that line blood vessels. Naïve T cells re-circulate through the lymph nodes by crossing the high-endothelial venules, whereas memory/effector T cells follow a more complex path, homing both to lymph nodes and then, when re-activated, to sites of infection/inflammation. As previous reports have suggested that EC express various wnts (Cheng et al., 2003; Wright et al., 1999), we hypothesized that EC-derived wnts might activate signaling in T cells, and particularly in effector T cells, which express elevated levels of Fz receptors. As a first step to testing this hypothesis we examined wnt expression by cultured EC using semi-quantitative RT-PCR. Transcripts were detected for several wnts, including wnt1, wnt2B, wnt4, wnt5A and wnt8B (data not shown). Again, due to the lack of specific reagents we were not able to confirm these results at the protein level. However, to determine whether EC-derived wnts are functional we transfected effector T cells with the TOPflash wnt reporter and incubated them with EC. EC dramatically enhanced luciferase expression in T cells, increasing TOPflash activity more than 40-fold (Figure 1E). Thus, EC-derived wnts are fully capable of inducing β-catenin-dependent signaling in effector T cells.
The specificity of wnt-Fz interactions remains unclear. An individual wnt can bind to multiple Fzs and a single Fz may recognize multiple wnts (Wodarz and Nusse, 1998). Thus, to block wnt signaling, we generated an expression construct encoding the extracellular, cysteine-rich domain (CRD) of Fz5 (Salazar Murphy and Hughes, 2002) and confirmed expression by western blot (Figure 1F). We then demonstrated that this protein could act as a dominant negative to block EC-derived wnts from activating signaling in T cells (Figure 1F). Overexpression of soluble Fz5 (sFz5) in EC inhibited TOPflash activation in co-cultured effector T cells by 45%. sFz5 also reduced TOPflash activation induced by overexpressing wnt1 in EC to a similar degree (Figure 1F). Thus, EC-derived wnts can stimulate β-catenin-dependent wnt signaling in co-cultured effector T cells and this can be blocked by sFz5. A schematic, outlining EC-induced wnt signaling in T cells is shown in Figure 1G.
Wnt signaling regulates T cell transmigration
Based both on our finding that EC induce wnt signaling in effector T cells, and on the known role of wnts in cell migration and metastasis (Muller et al., 2002; Ouko et al., 2004; Weeraratna et al., 2002), we hypothesized that EC-derived wnts might regulate the extravasation of effector T cells – either by increasing T cell motility, or by facilitating egress across the EC monolayer and through the underlying extracellular matrix. To test this hypothesis, we established an in vitro transmigration assay (Figure 2A). Effector T cells migrate across an EC monolayer and through the collagen-coated Transwell into the lower well in response to the chemokine SDF1α. In the presence of control-transfected EC, approximately 70% of the T cells crossed into the lower well by 24 hours. In the presence of EC expressing sFz5, however, migration was reduced by 40–60% (Figure 2B). Similar results were obtained using sFz6 (data not shown). ICAM-1 is critically involved in T cell-EC interactions and an antibody against ICAM-1, used as a positive control, reduced migration by 80%. In addition to using sFz5 secreted by transfected EC, we also used a sFz5-Fc protein, purified over protein-G columns from supernatants of transfected HEK293, or purchased from R&D Systems. We confirmed purity of both proteins by Coomassie blue staining (Figure 2C). Compared to the control protein (BSA), purified sFz5-Fc significantly reduced T cell migration, by up to 80% (Figure 2D). Similar data were obtained using bacterially expressed sFz5 (data not shown). These findings are fully consistent with a necessary role for wnt signaling in T cell transmigration. The longer migration times in vitro compared to estimated transit times in vivo is likely due to the greater than 10-fold difference in distance the T cells must travel (membranes are 50μm thick without the collagen coating, and considerably more with, while venular wall/BM thickness is <5μm).
Figure 2. Blocking wnt signaling with sFz5 reduces T cell transmigration.
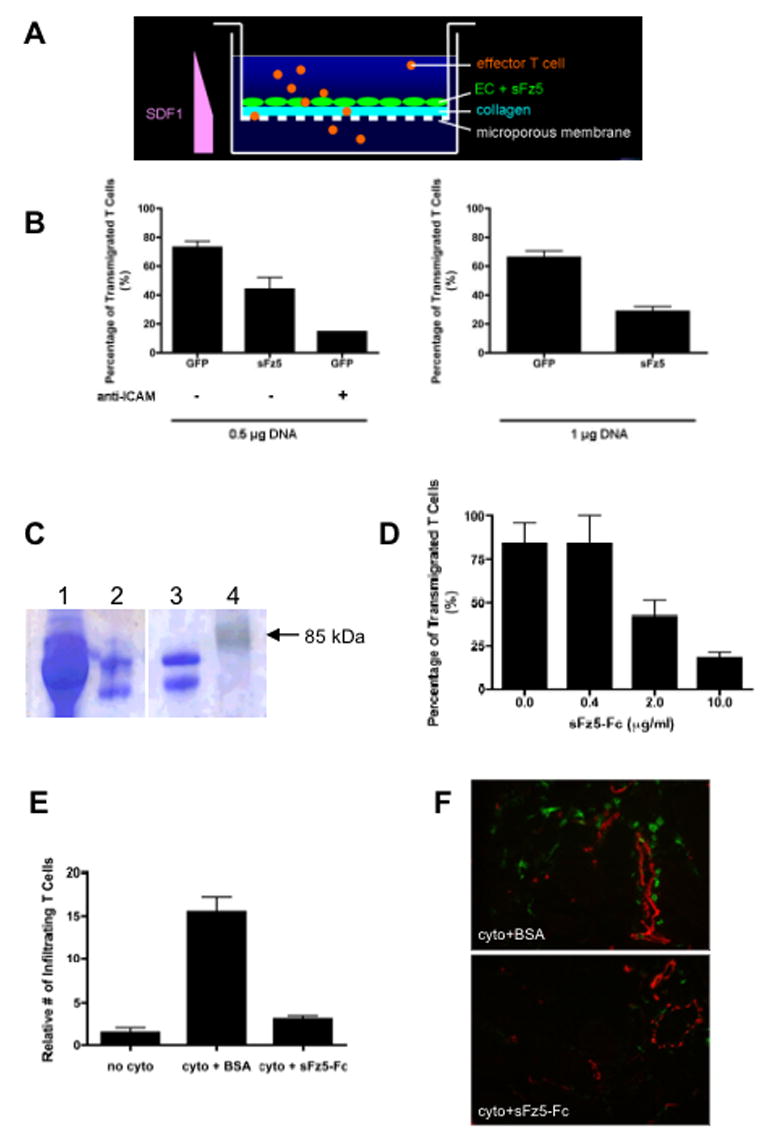
(A) Model system for T cell transmigration assays. (B) EC were transfected with 0.5 μg of GFP or sFz5 (left) or 1 μg of GFP or sFz5 (right), grown to confluence on collagen I-coated inserts, and then stimulated with TNF-α (10ng/ml) for 4hr to induce expression of adhesion molecules. Effector T cells were plated in the upper well in the presence of HB64 (−) or anti-ICAM-1 mAb (left). SDF1-α (100ng/ml) was added to the lower well. Cells that transmigrated into the lower well after 24 hours were counted. Results are representative of more than three independent experiments. Mean and SD of triplicate wells, * – p<0.05 (t-test) for GFP v sFz5, and p<0.005 (t-test) for control, v anti-ICAM-1 mAb. (C) Coomassie blue staining of an SDS-PAGE gel showing purified sFz5-Fc produced in HEK293 cells (lane 2), or purchased (lane 3). Supernatant from transfected HEK293 cells prior to purification is shown in lane 1. MW markers are in lane 4. (D) Effector T cells were added to untransfected EC monolayers in the presence of 10μg/ml BSA or sFz5-Fc protein. Transmigrated T cells were counted after 24 hours. Mean and SD of triplicate wells, * – p<0.005 (t-test) for control (0μg/ml sFz5-Fc, 10μg/ml BSA) v 10μg/ml sFz5-Fc. One of three similar experiments. (E) Mice were injected i.d. on the back with TNFα (10ng) and IFNγ (300U), or with TNF + IFNγ along with BSA or sFz5-Fc (1μg each). Controls received vehicle (PBS) alone, and all injections were in a total volume of 10μl. Skin was harvested after 22 hours and frozen. Sections were stained for CD3 (green) to show T cells and CD31 (red) to highlight blood vessels. Six fields from duplicate sites from each mouse were analyzed (blinded) for each condition. Mean and SD, * – p<0.01 (t-test) for BSA+cytokine v sFz5-Fc+cytokine. One of five similar experiments. (F) Representative sections stained for T cell CD3 (green) and EC CD31 (red). Conditions as indicated.
Soluble Fz5 inhibits T cell migration into inflamed tissues in vivo
In the in vitro migration assay, we found that sFz5 blocked T cell migration across a monolayer of EC and through a collagen matrix. To test whether wnt signaling has a similar role in vivo, we used a cytokine-induced inflammation model, which has been previously described (Kulidjian et al., 2002). Intradermal injection of TNFα and IFNγ reproducibly induces a T cell rich cutaneous inflammation. We injected sFz5-Fc protein or B□□ control protein together with the cytokines into mouse dorsal skin. After 22 hours, skin was collected and immunofluorescence staining was performed on frozen sections for T cells (CD3 – green) and blood vessels (CD31 – red). In the absence of cytokines, few infiltrating T cells were apparent, whereas cytokines induced a robust 8 to 9-fold increase (Figure 2E). Injection of cytokines in the presence of control protein led to a similarly pronounced T cell infiltration (Figure 2E, 2F). In sharp contrast, co-injection of sFz5-Fc dramatically decreased cytokine-induced T cell infiltration, by an average of 75% (Figure 2E, 2F). Similar results were obtained using purified, bacterially-expressed, sFz5 (data not shown). Thus wnt signaling is necessary for optimal T cell transmigration, both in vitro and in vivo.
To determine whether wnt signaling is sufficient to augment T cell migration we assayed migration in the presence of sub-confluent wnt1-expressing COS-7 cells. Consistent with a role for wnts in enhancing T cell migration, wnt1 increased the number of transmigrated T cells 2–3 fold (Figure 3A). Similarly, wnt3A-CM also dramatically augmented T cell migration through both interstitial collagen, type I, and basement membrane collagen, type IV (Figure 3B). Importantly, wnt3A-CM did not affect proliferation or survival of effector T cells over the course of 24 hours (data not shown). To determine specifically whether β-catenin-dependent wnt signaling is sufficient to enhance migration we used an N-terminal deletion of the LRP5 receptor (constitutively active (CA)-LRP5) and CA-β-catenin. Deletion of the extracellular, N-terminal domain of the LRPs has previously been shown to constitutively activate β-catenin-dependent signaling (Tamai et al., 2004). Overexpression of either of these constructs in effector T cells significantly increased migration through collagen gels (Figure 3C). Thus β-catenin-dependent signaling is sufficient to up-regulate T cell migration.
Figure 3. Wnt signaling is sufficient to augment T cell migration.
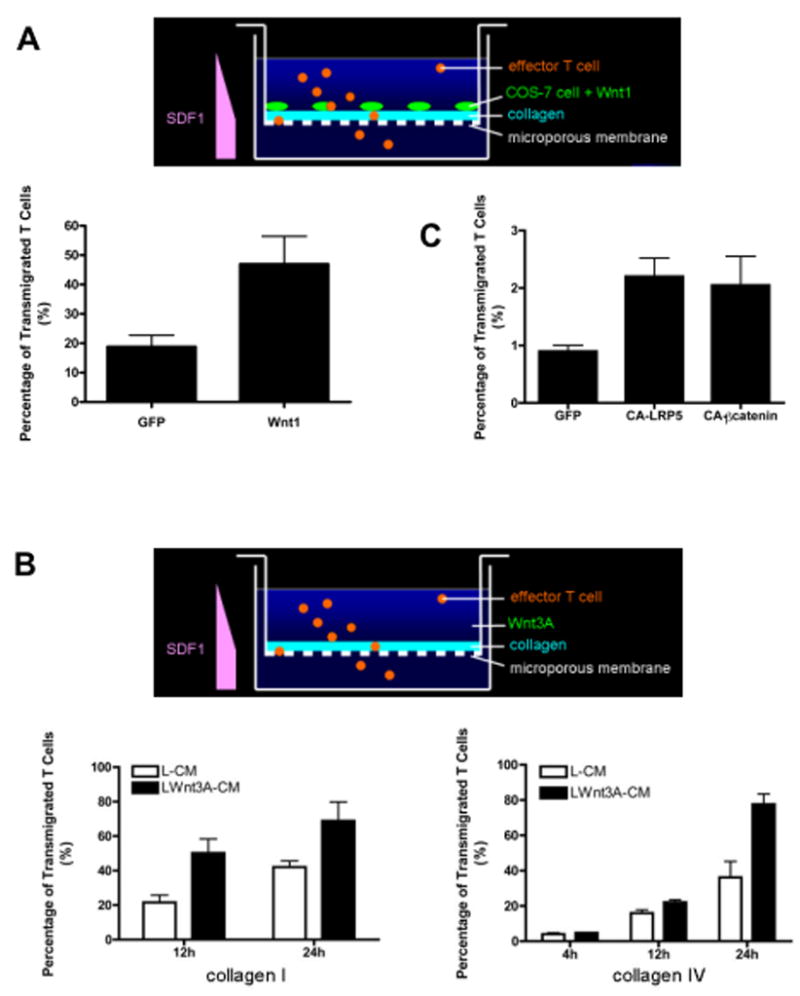
(A) Effector T cells were added to sub-confluent COS-7 cells expressing wnt1 and growing on collagen-coated wells. SDF1-α (100ng/ml) was added to the lower well, and cells that had transmigrated after 24 hours were counted. Mean and SD of triplicate wells, * – p<0.05 (t-test). One of three similar experiments. (B) Effector T cells were added to collagen-coated wells in the presence of control or wnt3A-conditioned medium. SDF1-α was added to the lower well, and cells that had transmigrated after the indicated times were counted. Mean and SD of triplicate wells, * – t-test: For collagen I: p<0.005 (12hr) and p<0.02 (24hr); for collagen IV: p<0.05 (12hr) and p<0.005 (24hr). One of three similar experiments. (C) Effector T cells were transfected with expression vectors for GFP, CA-LRP6 or CA-β-catenin and added to collagen IV-coated wells 24 hours post transfection. SDF1-α was added to the lower well, and cells that had transmigrated were counted. Mean and SD of triplicate wells, * – t-test: for GFP v CA-LRP6: p<0.005; for GFP v CA-β-catenin: p<0.05. One of three similar experiments. Transfected cells consistently migrate at a lower rate than non-transfected cells.
Wnt signaling induces MMP expression in T cells
We considered two hypotheses to explain the effect of wnts on T cell migration: one, that wnts increase the inherent motility of effector T cells, which would be consistent with previous reports on the role of wnts in inducing cell motility in other systems (Muller et al., 2002; Ouko et al., 2004; Weeraratna et al., 2002); and two, that wnts induce a T cell phenotype that allows the cells to cross the EC and underlying collagen-rich basement membrane, an obvious candidate being induction of MMP expression. To test the first hypothesis we monitored in real-time the motility of effector T cells on a 2D collagen-IV matrix in the presence of control or wnt3A-CM. A plot of mean displacement versus the square root of time gave a roughly linear relationship for each group, indicating that individual T cells followed random tracks (Figure S1). Thirty cells were randomly selected for cell track and velocity measurements. We noted only a moderate, 18% increase in mean instantaneous velocity in the presence of wnt3A-CM (5.88 μm·min−1 in wnt3A-CM compared to 4.98 μm·min−1 in control medium. n=1200, p<0.01), suggesting that the effect of wnts on T cell transmigration cannot be accounted for solely by increases in inherent motility.
Importantly, we found that the effect of wnts on effector T cell migration is collagen-dependent as wnt3A did not increase migration of T cells across non-coated membranes (Figure S2). Thus, we favor the second explanation, that wnts induce a T cell phenotype conducive to migration through collagen. It has been suggested previously that T cell migration through collagen gels is independent of collagen degradation (Wolf et al., 2003). However, those studies used a very low concentration of collagen (1.67mg/ml), whereas, basement membrane and interstitial collagen are much denser (from 10–40mg/ml (Helary et al., 2006; Roeder et al., 2002)), and migration through them may well require MMP expression, consistent with previous findings of a role for MMPs in T cell extravasation in vivo (Cai et al., 2003; Graesser et al., 1998; Kumagai et al., 1999; Marracci et al., 2002; Sixt et al., 2001). As an initial test of the requirement for new protein synthesis in wnt-induced migration we assessed the ability of T cells treated with the protein synthesis inhibitor cycloheximide (CHX) to migrate through collagen gels in response to wnt3A stimulation. Even at low concentrations CHX dramatically inhibited effector T cell migration (Figure 4A), which reveals the necessity for protein synthesis, and which is consistent with a role for wnt-induced MMP expression.
Figure 4. Wnt signaling induces MMP expression.
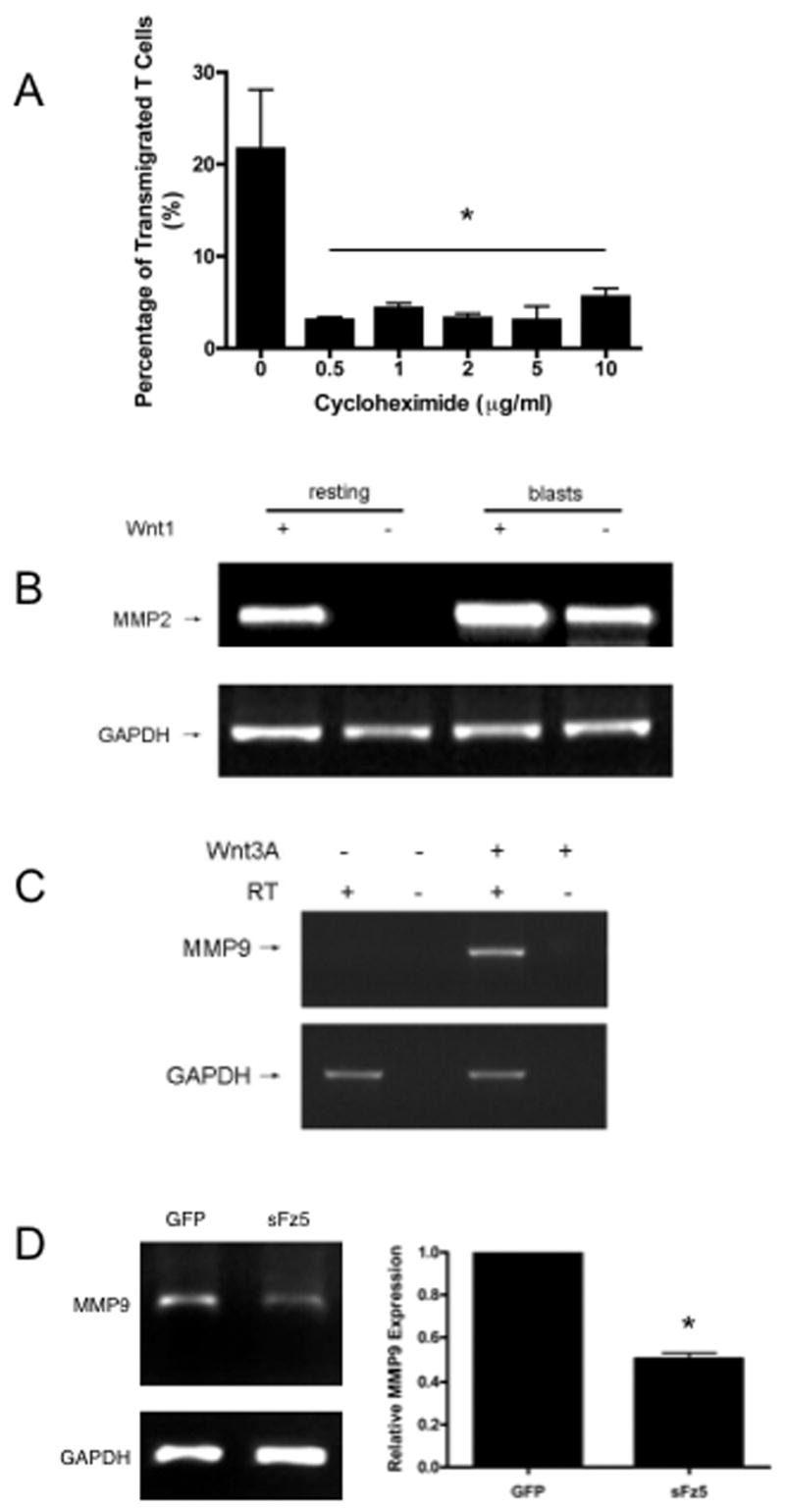
(A) Effector T cell migration is protein synthesis-dependent. Effector T cells were plated in collagen IV-coated wells in the presence of wnt3A-conditioned medium and allowed to migrate for 24 hours in response to SDF1-α (100ng/ml) in the lower well. Cycloheximide or vehicle was added at the indicated concentrations. Mean and SD of triplicate wells, * – p<0.05 (t-test) for all concentrations v control. One of two similar experiments. (B) Resting or effector T cells were transfected with expression plasmids for either GFP or wnt1 and harvested for semi-quantitative RT-PCR analysis 24 hours later. Results are shown for MMP2 and GAPDH. One of three similar experiments. (C) Effector T cells were allowed to migrate through collagen IV-coated wells in the presence of control or wnt3A-conditioned medium as described in Figure 3. Migrated T cells were harvested, and equal numbers were used for semi-quantitative RT-PCR. Results for MMP9 and GAPDH, in the presence or absence of reverse transcriptase (RT), are shown. One of three similar experiments. (D) Effector T cells were allowed to migrate through monolayers of EC expressing either GFP or sFz5, and were then analyzed for MMP9 expression by semi-quantitative RT-PCR. Mean and SD of triplicate RT-PCR samples are shown, * – p<0.005 (t-test) for GFP v sFz5. One of three similar experiments.
MMP7 and MMP26 are known target genes of the β-catenin-dependent wnt pathway in other cells (Crawford et al., 1999; Marchenko et al., 2002), however they are not expressed by T cells. Instead, T cells express primarily MMP2 and MMP9 (Madri et al., 1996). To test for wnt induction of MMP2 we transfected either resting or effector T cells with a wnt1 expression plasmid or a control plasmid and harvested RNA for analysis by semi-quantitative RT-PCR. MMP2 was not expressed in control resting T cells but was strongly induced by wnt1 (Figure 4B). Effector T cells expressed MMP2 and this was up regulated by wnt1, confirming that MMP2 is indeed a wnt target gene in T cells. These results were confirmed by qRT-PCR, which showed a six-fold induction of MMP2 by wnt3A (Figure S3). It is also important to know how migration through the collagen gel affects wnt-induced MMP expression, as interactions with matrix molecules are known to modulate gene expression triggered by other pathways (Hood and Cheresh, 2002; Jolivet et al., 2005; Schwartz and Ginsberg, 2002). To examine this we collected effector T cells that had migrated through a collagen-IV-coated Transwell in the presence of either control or wnt3A-CM and examined MMP9 expression by semi-quantitative RT-PCR. In the control-treated cells no transcripts for MMP9 were detectable (Figure 4C), however, a robust signal was detected in cells that had migrated in the presence of wnt3A, suggesting that in effector T cells MMP9 is also a wnt target. To confirm that induction of MMP expression by EC is dependent on wnt signaling we collected T cells that had migrated through GFP- or sFz5-expressing EC and examined MMP expression by RT- and qRT-PCR. As before, sFz5 reduced T cell migration by over 30% (data not shown). The suppression of wnt signaling by sFz5 also significantly reduced MMP9 expression, by close to 50% (Figure 4D). Analysis by qRT-PCR confirmed this result, showing a decrease of 52%. Addition of purified sFz5 also strongly blocked wnt induced MMP2 and MMP9 expression (data not shown). These findings confirm, therefore, that T cell MMP expression is under the control of EC-derived wnts.
MMPs are transcriptional targets of wnt
We next asked whether MMP2 and MMP9 are transcriptional targets of wnt signaling in T cells. We cloned a 1.9kb fragment of the human MMP2 proximal promoter and a 2.1kb fragment of the MMP9 proximal promoter into the pGL3-Luc reporter vector and examined their activity in effector T cells. Both promoters responded strongly to activation by wnt1 (Figure 5A and 5B). To determine whether the promoters are responsive to β-catenin-dependent signals we co-transfected T cells with the MMP9 reporter and expression constructs for either constitutively active (CA)-LRP5 (LRP5-ΔN) or CA-LRP6 (LRP6-ΔN). These constructs strongly activated the MMP9 reporter (Figure 5B), as well as the MMP2 reporter (Figure S4), confirming that both genes are transcriptional targets of the wnt-β-catenin pathway in T cells.
Figure 5. Wnt signaling regulates MMP expression at the transcriptional level.
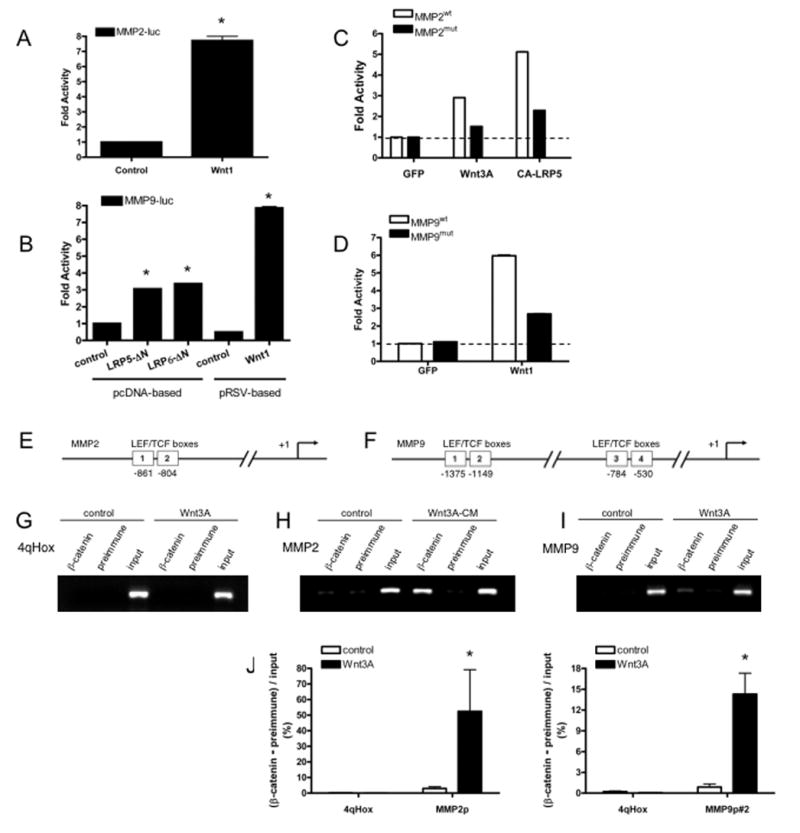
(A) Effector T cells were transfected with MMP2-Luc along with either a control or wnt1 expression plasmid and harvested 24 hours later for analysis of luciferase expression. Mean and SD for triplicate wells, * – p<0.005 (t-test). One of more than three similar experiments. (B) Effector T cells were transfected with MMP9-Luc along with either a control vector, or expression vectors for wnt1, LRP5-ΔN or LRP6-ΔN. Cells were harvested 24 hours later for analysis of luciferase expression. Mean and SD for triplicate wells, * – p<0.005 (t-test) for control v LRP5-ΔN or LRP6-ΔN, or control v wnt1. One of three similar experiments. (C) Effector T cells were transfected with expression vectors for GFP, wnt3A or CA-LRP5, along with either the wild-type (WT) or mutant MMP2 promoter luciferase reporters. The promoter was mutated at both the upstream and downstream putative LEF/TCF sites. Cells were assayed for luciferase activity at 17 hours. Reduced activity of the mutant promoter is relative to the GFP control (dotted line). One of four similar experiments. (D) Effector T cells were transfected with expression vectors for GFP or wnt1, along with either the wild-type (WT) or mutant MMP9 promoter luciferase reporters. The promoter was mutated at both of the proximal putative LEF/TCF sites in the truncated (800bp) promoter. Cells were assayed for luciferase activity at 17 hours. Reduced activity of the mutant promoter is relative to the GFP control (dotted line). One of three similar experiments. (E) Schematic of the MMP2 promoter showing the two putative LEF/TCF sites. (F) Schematic of the MMP9 promoter showing the four putative LEF/TCF sites, one upstream pair and one downstream pair. The downstream pair are within the 800bp truncated promoter. (G, H, I) ChIP analysis was performed by immunoprecipitating DNA/protein complexes from effector T cells with an anti-β-catenin antibody. DNA was amplified using primers specific for an irrelevant sequence, 4qHox, the MMP2 promoter, and the MMP9 promoter (2 sets of primers, only one shown). Controls were immunoprecipitated with pre-immune serum. Input DNA (before immunoprecipitation) was amplified as a positive control for the PCR. Band intensities for each of three PCR reactions were measured and immune minus pre-immune intensities are plotted (J, K). One of three similar experiments.
Further, in silico, analysis of the promoters revealed two putative LEF/TCF binding sites in the MMP2 proximal promoter, and 4 in the MMP9 proximal promoter (Figure 5E and 5F). To determine the requirement for these sites we generated mutations in these sites and tested the resultant promoters for responsiveness to wnt signals. Both sites in the MMP2 promoter were mutated, either alone or in combination, so as to destroy the core CTTTG motif. The MMP9 promoter was truncated between the two pairs of sites and the remaining two (proximal) LEF/TCF sites were mutated as described for MMP2. Mutation of these sites in either promoter strongly reduced responsiveness to wnt or to CA-LRP5 (Figure 5C, D). Induction of MMP2 was blocked 65–75% relative to GFP control, while MMP9 was blocked by 65% relative to GFP. These results confirm that the LEF/TCF sites are necessary for promoter responsiveness to wnt signaling, however they leave open the possibility that other sites are also important.
To test whether the promoters are direct targets of the wnt pathway we performed chromatin immunoprecipitation (ChIP) assays. We used an anti-β-catenin antibody to immunoprecipitate DNA capable of binding β-catenin in vivo and amplified this using primers specific for: a fragment of the MMP2 promoter containing the two putative LEF/TCF binding sites; two separate fragments of the MMP9 promoter, containing the two pairs of sites; and a fragment of chromosome four, which acted as a negative control. As shown in Fig. 5G neither the specific nor pre-immune serum precipitated the negative control DNA. In contrast, the β-catenin antibody immunoprecipitated both the MMP2 and MMP9 promoters (Figure 5H, I and data not shown), although the signal for MMP9 was weaker. Wnt induced an 18-fold increase in signal for MMP2 (Figure 5J) and an 18–20-fold increase in signal for MMP9 (proximal sites, Figure 5K). A similar signal was obtained for the second set of MMP9 primers (data not shown). Further ChIP experiments (not shown) also confirm the presence of LEF/TCF bound to the MMP2 and MMP9 promoters. We conclude, therefore, that both the MMP2 and MMP9 proximal promoters bind a β-catenin/LEF/TCF transcription factor complex and that these two genes are thus direct targets of the wnt signaling pathway.
MMP activity is necessary for Wnt-enhanced T cell transmigration
Finally, to test explicitly whether MMP activity is necessary for Wnt-induced T cell migration through the collagen matrix, we employed two MMP inhibitors. OA-Hy has a broad specificity with a Ki for MMP2 of 1.7 μM, whereas inhibitor IV has higher specificity for MMP2 and MMP9 (MMP2 Ki = 13.9nM; MMP9 Ki = 600nM). Both inhibitors effectively blocked T cell migration augmented by Wnt3A (Figure. 6A and 6B), demonstrating that MMP activity is necessary for the wnt-induced effects on T cell migration.
Figure 6. Wnt3A-enhanced migration can be blocked by MMP-specific inhibitors.
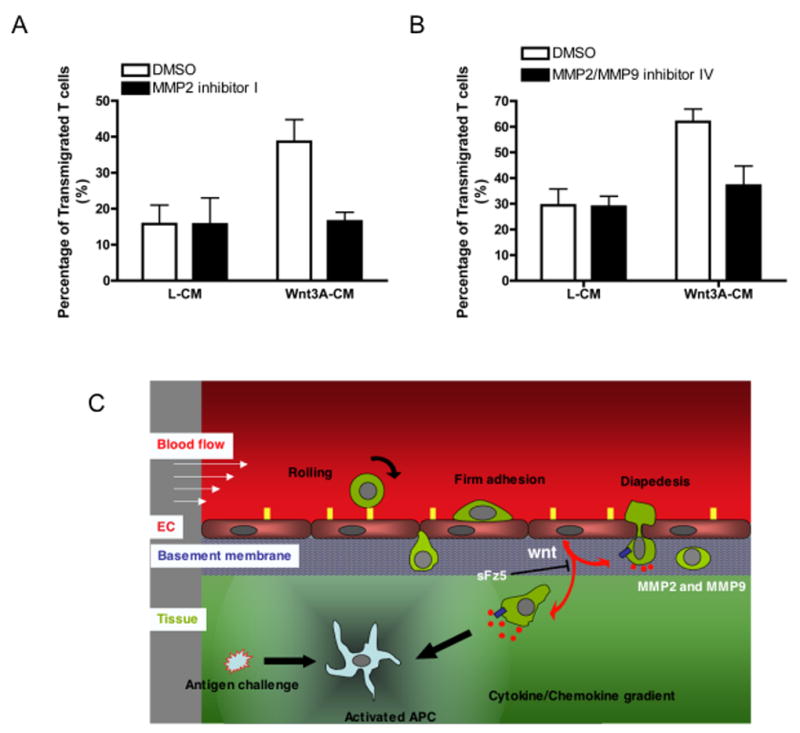
(A) Effector T cells were stimulated to migrate across collagen IV gels as described above, in the presence of control or wnt3A-CM, with DMSO or 10μM MMP2 inhibitor OA-HY. Migrated T cells were harvested and counted. Mean and SD for triplicate wells, * – p<0.05 (t-test) for DMSO(Wnt3A) v inhibitor(Wnt3A). One of three similar experiments. (B) Similar to (A) except MMP inhibitor IV (10nM) was used. Mean and SD for triplicate wells, * – p<0.01 (t-test) for DMSO(Wnt3A) v inhibitor(Wnt3A). One of three similar experiments. (C) Model for wnt regulation of T cell migration. Antigen challenge activates local APC to express cytokines/chemokines that induce adhesion molecules (yellow rectangles) on EC. As recruited T cells cross the EC monolayer lining blood vessels they receive a wnt signal through Fz receptors (blue rectangle). Induction of MMP expression (red dots) allows the T cells to cross the BM and migrate along chemokine gradients through interstitial collagen toward the inflammatory source.
Discussion
We have identified a novel mechanism for regulation of T cell migration through collagen. EC-derived wnts trigger up-regulation of MMP2 and MMP9 in effector T cells, enhancing their ability to cross the sub-endothelial basement membrane and migrate through the underlying interstitial collagen. A soluble form of the wnt receptor Fz5 (sFz5-Fc) blocks wnt-dependant MMP induction and inhibits T cell transmigration in vitro and T cell extravasation in vivo. A schematic of this process is shown in Figure 6C.
The early stages of leukocyte extravasation have been described (Alon and Feigelson, 2002; Steeber and Tedder, 2000), however it is still unclear whether the mechanisms used by neutrophils and monocytes are also employed by lymphocytes. Less is known about the subsequent steps of diapedesis and migration of leukocytes through the basement membrane. While elegant studies have established clear roles for PECAM (CD31) and CD99 in monocyte diapedesis (Muller et al., 1993; Schenkel et al., 2002), we do not know whether these are necessary for transmigration of lymphocytes. The mechanisms underlying induction of MMP expression in T cells are even less clear – indeed, even the requirement for MMP activity in this process has been questioned (Wolf et al., 2003). Several studies have suggested an important role for VCAM-VLA-4 interactions in MMP induction during T cell trafficking into the brain of EAE mice (Graesser et al., 1998), however these findings have yet to be generalized to other vascular beds. Our results clearly demonstrate that wnt signaling can play an important role in up regulating MMP expression in T cells migrating into peripheral inflammatory sites. We propose that wnt signaling fits well into the sequential nature of T cell extravasation: subsequent to chemokine-induced firm adhesion T cells become polarized and move toward EC junctions. Either before or during diapedesis T cells receive wnt signals from the EC and up regulate MMP expression. Although the early stages of T cell extravasation are known to occur over a time course of minutes, transit through the basement membrane and into the interstitial collagen is slower, and indeed, perivascular cuffing of T cells is a commonly-observed phenomenon in inflamed tissues (Tanaka et al., 1975).
This delay in T cell migration likely relates to the density of the extracellular matrix (Roeder et al., 2002) and the necessary induction of, and subsequent matrix degrading activity of MMPs. When MMP induction is reduced, here by sFz5, the onward movement of the T cells through the collagen basement membrane is also reduced and T cells likely re-cross the EC and re-enter the circulation. Consistent with this, we have observed by time-lapse video microscopy similar crossing and re-crossing of an EC monolayer in vitro (unpublished observations). An alternative explanation for the failure to see T cell migration and/or cuffing would be that T cells are absent in the circulation, however we can easily rule this out as BSA-injected and sFz5-injected tissue is harvested from opposite flanks of the same mice. Thus, although we cannot prove this point in vivo, the most parsimonious explanation for our data is that T cells re-enter the circulation when further movement through the basement membrane is blocked.
There appears to be considerable redundancy in wnt signaling, as evidenced by the ability of sFz5 to block signaling induced by the multiple wnts expressed by EC. Gene knockout studies in mice have shown that wnt1 and wnt3A are likely functionally redundant (McMahon and Bradley, 1990; Takada et al., 1994), and we have treated them as such in our studies. Wnt signaling has been shown to regulate cell movements in a number of contexts. Non-canonical, β-catenin-independent, wnt signaling through wnt5a induces actin reorganization and increased cell adhesion in metastatic melanoma cells (Weeraratna et al., 2002), while wnt11 induces cell polarity and regulates convergent extension movements in zebrafish (Heisenberg et al., 2000). Interestingly, wnt signaling has been implicated in tumor cell metastasis – a process not unlike T cell extravasation (Kim et al., 2002; Muller et al., 2002). Of particular relevance to the current study, it was recently shown that invasion of myeloma plasma cells is promoted by wnt signaling, in this instance through a pathway involving rhoA and members of the PKC family (Qiang et al., 2005). We have previously demonstrated the presence of a PKC-dependent wnt pathway in T cells that controls NFAT export from the nucleus, and we have not ruled out a role for β-catenin independent wnt signaling, possibly involving rac, rho and PKC, in T cell extravasation. Such a pathway would complement the wnt-β-catenin-MMP pathway we have described here. Indeed, a reasonable hypothesis would be that β-catenin-independent wnt signal might be involved in the polarization of newly adherent T cells on the luminal surface of the EC, while β-catenin-dependent wnt signaling would regulate MMP expression. Certainly chemokines have been implicated in the transition of T cells from a non-polarized, non-migratory phenotype to a phenotype specialized for directional migration, however a role for wnts has not been excluded.
At least two MMPs have been identified as wnt targets in non-T cells: MMP7 is downstream of β-catenin in a human colon cancer (Crawford et al., 1999), while MMP26 is a target of TCF4 in cancer cells of epithelial origin (Marchenko et al., 2004). Here we show that both MMP2 and MMP9 are downstream of wnt and β-catenin in T cells and are direct transcriptional targets of wnt signaling through tandem LEF/TCF binding sites. Consistent with our findings, functional LEF/TCF sites have been previously identified in the MMP7 (Matrilysin) promoter (Crawford et al., 1999).
Several of the wnts, frizzleds and wnt pathway genes have been knocked-out in mice and nearly all result in embryonic or perinatal lethality, precluding analysis of lymphocyte trafficking. Recently, a T cell-specific deletion of β-catenin was reported by two groups (Cobas et al., 2004; Xu et al., 2003). Curiously, their findings were quite different. In one case (Cobas et al., 2004) no defect was noted in T cell development, proliferation or activation, whereas in the other (Xu et al., 2003), significant defects were seen in early T cell development, especially at the β-selection checkpoint. In neither case was T cell trafficking studied and no explanation for these widely discrepant findings has yet emerged. When it does, these mice will likely prove useful in further characterizing the role of wnts in T cell trafficking.
In conclusion, we have identified a novel role for wnt signaling in regulating T cell extravasation. In the absence of a robust wnt signal T cells fail to up regulate MMP expression and are consequently prevented from crossing the basement membrane and entering inflammatory tissues. Manipulation of T cell wnt signaling may therefore be useful for controlling immune inflammation.
Experimental Procedures
Reagents
Cell culture and transfections
Wnt3A-producing L cells and control L cells were purchased from ATCC (Rockville, MD, USA). Conditioned media were obtained after 7-day culture. HUVEC were isolated from human umbilical veins and cultured as described previously (Hughes et al., 1990). Resting T cells were purified from human blood using the EasySepTM T cell enrichment kit (StemCell Technologies, Vancouver, BC Canada). This procedure yielded 98% CD3+ T cells. Effector T cells were obtained from PBMC by stimulation with PHA (3μg/ml) and IL-2 (25U/ml) for 3 days, followed by growth in the presence of IL-2 (25U/ml) for 2 days. Effector cells were typically >98% CD3+, ca. 55% CD4+, ca. 40% CD8+, >90% CD25+, ca. 20% CD69+, and <2% CD14+ or CD19+. HUVEC (p2–4) or COS-7 cells were transiently transfected with 1μg of DNA using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Resting T cells were transiently transfected with 5μg of DNA by Nucleofection (Amaxa, GmbH, Koln, Germany). Effector T cells were transiently transfected with 10μg of DNA using a Gene Pulser (Bio-Rad, Beverly, MA, USA) at 250V, 960μFD. All transfected cells were used for experiments 24hr later. Cotransfection with a GFP-expressing vector showed typical efficiencies of 70–90% for HUVEC and COS-7 cells, 50%–60% for resting T cells and 30%–40% for effector T cells (see Supplementary Methods).
Plasmids
TOPflash and FOPflash luciferase reporters were gifts from H. Clevers (Center of Biomedical Genetics, Utrecht, Netherlands). The wnt3A expression plasmid was from Karl Willert (Stanford University). Truncated LRP5 and LRP6 constructs were kindly provided by A. Brown (Weill Medical College of Cornell University, New York, NY, USA). S33Y β-catenin was a gift from A. Ben-Ze’ev (Weizmann Institute of Science, Rehovot, Israel). Axin expression vector was made by cloning of mouse Axin form 2 (a gift from F. Costantini, Columbia University, New York, NY, USA) into pcDNA3.1(+) (Invitrogen). pcDNA-sFz5-Flag was obtained as described previously (Salazar Murphy and Hughes, 2002). The extracellular domain of Fz5 was cloned into the Gateway pEmpty4 vector (Invitrogen) and then recombined into a vector that adds the mouse IgG2a-Fc (pDEST-Ig, created in our lab). MMP2 and MMP9 partial promoters were cloned from human genomic DNA by PCR and inserted into the pGl3e luciferase reporter vector (Promega). The putative LEF/TCF sites in the MMP2 promoter were mutated by PCR from CTTTGC to CCCTGC (upstream), and from CTTTGT to CCCTGG (downstream). The two LEF/TCF sites in a truncated (800bp) MMP9 proximal promoter were mutated from TCAAAG to GCGCAG (both upstream and downstream). The site-directed mutagenesis was performed using the QuickChange XL from Stratagene.
RT-PCR
Protein purification
In vitro T cell transmigration assay
For trans-endothelial migration, HUVEC were plated in triplicate on the upper wells of 24-well collagen-coated 3μm Costar transwells (Fisher, Tustin, CA, USA) and grown to confluence. Cells were then treated with TNFα (10ng/ml) for 4hr to up regulate expression of adhesion molecules. Human SDF1α was added to the lower wells at 100ng/ml and effector T cells were added to the upper wells. After 24hr, T cells that had transmigrated into the lower wells were collected and counted. For some experiments, effector T cells were added to the upper wells of 24-well collagen I-coated or collagen IV-coated transwells in the absence of EC. Wnt3A-CM or control medium was then added to the upper wells.
T cell infiltration into inflamed tissue in vivo
Female BALB/c mice were obtained from Jackson Laboratories. Mice were used at 6–10 weeks of age. All mouse experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee of UCI. Mice were anesthetized with ketamine and xylazine. The backs were shaved and each of 5 mice was injected i.d. in eight patches on the back with 10μl, in duplicate, of diluent alone (1xHBSS), cytokines (10ng TNFα + 300U IFNγ), cytokines with sFz5-Fc protein (1μg) or cytokines with BSA control protein (1μg). After 22hr, skin was harvested and frozen. Frozen sections at 5μm thick were fixed with acetone and stained with hamster anti-mouse CD3 (10μg/ml), followed by Alexa-488 conjugated goat anti-hamster IgG, and biotin-anti-CD31 (5μg/ml) together with Texas-red-Streptavidin (1:200) and DAPI. The number of infiltrating T cells was analyzed using NIH Image J software (Bethesda, MD, USA).
ChIP assay
ChIP analysis was performed according to the manufacturer’s protocol (Upstate Biotechnology). Effector T cells (3x106) were harvested and DNA was precipitated with 20μg of polyclonal anti-β-catenin antibody (Santa Cruz), or a preimmune rabbit IgG antibody as a negative control. Precipitated DNA was amplified using the following primer sets: for the MMP2 promoter, 5′-GAGGTCGCTTTCTTTGCCATCT-3′ (upper) and 5′-AGCGACTCCATCTTGAACAGG-3′ (lower); for the MMP9 promoter #1, 5′-AAGTTAATTATCTCCATCTC ACAGTCTCAT-3′ (upper) and 5′-CGGCATCGGGCAGGGTCT-3′ (lower); and for the MMP9 promoter #2, 5′-CACTGTATCCTTGACCTTCTTTCTGG-3′ (upper) and 5′-GCTTCCTCTCCCTGCTTCATCTG-3′ (lower). PCR signal intensity was analyzed using the Quantity One program from Bio-Rad.
Statistical analysis
Data are represented as mean ± SD. Student’s t-test (Microsoft Excel) was used to analyze differences between experimental groups as indicated in the figure legends. A p value of less than 0.05 was considered significant. Unless indicated otherwise all experiments were performed at least three times with similar results.
Supplementary Material
Acknowledgments
This work was supported by RO1 AI 40710. SPC is supported by an NIH Training Grant, T32 AI 60573. We thank Dr. Marian Waterman for critical reading of the manuscript and for the gift or reagents. We also thank Dr. Kyoko Yokomori and Weihua Zeng for help with the ChIP assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alon R, Feigelson S. From rolling to arrest on blood vessels: leukocyte tap dancing on endothelial integrin ligands and chemokines at sub-second contacts. Semin Immunol. 2002;14:93–104. doi: 10.1006/smim.2001.0346. [DOI] [PubMed] [Google Scholar]
- Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2003;163:1801–1815. doi: 10.1016/S0002-9440(10)63540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Morin PJ, Clevers H. The Yin-Yang of TCF/beta-catenin signaling. Adv Cancer Res. 2000;77:1–24. doi: 10.1016/s0065-230x(08)60783-6. [DOI] [PubMed] [Google Scholar]
- Cai Y, Chen T, Xu Q. Astilbin suppresses delayed-type hypersensitivity by inhibiting lymphocyte migration. J Pharm Pharmacol. 2003;55:691–696. doi: 10.1211/002235703765344612. [DOI] [PubMed] [Google Scholar]
- Cheng CW, Smith SK, Charnock-Jones DS. Wnt-1 signaling inhibits human umbilical vein endothelial cell proliferation and alters cell morphology. Exp Cell Res. 2003;291:415–425. doi: 10.1016/j.yexcr.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Chung EJ, Hwang SG, Nguyen P, Lee S, Kim JS, Kim JW, Henkart PA, Bottaro DP, Soon L, Bonvini P, et al. Regulation of leukemic cell adhesion, proliferation, and survival by beta-catenin. Blood. 2002;100:982–990. doi: 10.1182/blood.v100.3.982. [DOI] [PubMed] [Google Scholar]
- Cobas M, Wilson A, Ernst B, Mancini SJ, MacDonald HR, Kemler R, Radtke F. Beta-catenin is dispensable for hematopoiesis and lymphopoiesis. J Exp Med. 2004;199:221–229. doi: 10.1084/jem.20031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford HC, Fingleton BM, Rudolph-Owen LA, Goss KJ, Rubinfeld B, Polakis P, Matrisian LM. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene. 1999;18:2883–2891. doi: 10.1038/sj.onc.1202627. [DOI] [PubMed] [Google Scholar]
- Goetzl EJ, Banda MJ, Leppert D. Matrix metalloproteinases in immunity. J Immunol. 1996;156:1–4. [PubMed] [Google Scholar]
- Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- Graesser D, Mahooti S, Haas T, Davis S, Clark RB, Madri JA. The interrelationship of alpha4 integrin and matrix metalloproteinase-2 in the pathogenesis of experimental autoimmune encephalomyelitis. Lab Invest. 1998;78:1445–1458. [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Helary C, Ovtracht L, Coulomb B, Godeau G, Giraud-Guille MM. Dense fibrillar collagen matrices: a model to study myofibroblast behaviour during wound healing. Biomaterials. 2006;27:4443–4452. doi: 10.1016/j.biomaterials.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Held W, Clevers H, Grosschedl R. Redundant functions of TCF-1 and LEF-1 during T and NK cell development, but unique role of TCF-1 for Ly49 NK cell receptor acquisition. Eur J Immunol. 2003;33:1393–1398. doi: 10.1002/eji.200323840. [DOI] [PubMed] [Google Scholar]
- Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- Huang Z, Xie H, Ioannidis V, Held W, Clevers H, Sadim MS, Sun Z. Transcriptional regulation of CD4 gene expression by T cell factor-1/beta-catenin pathway. J Immunol. 2006;176:4880–4887. doi: 10.4049/jimmunol.176.8.4880. [DOI] [PubMed] [Google Scholar]
- Hughes CC, Savage CO, Pober JS. Endothelial cells augment T cell interleukin 2 production by a contact-dependent mechanism involving CD2/LFA-3 interaction. J Exp Med. 1990;171:1453–1467. doi: 10.1084/jem.171.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis V, Beermann F, Clevers H, Held W. The beta-catenin--TCF-1 pathway ensures CD4(+)CD8(+) thymocyte survival. Nat Immunol. 2001;2:691–697. doi: 10.1038/90623. [DOI] [PubMed] [Google Scholar]
- Jolivet G, Pantano T, Houdebine LM. Regulation by the extracellular matrix (ECM) of prolactin-induced alpha s1-casein gene expression in rabbit primary mammary cells: role of STAT5, C/EBP, and chromatin structure. J Cell Biochem. 2005;95:313–327. doi: 10.1002/jcb.20397. [DOI] [PubMed] [Google Scholar]
- Kim K, Lu Z, Hay ED. Direct evidence for a role of beta-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol Int. 2002;26:463–476. doi: 10.1006/cbir.2002.0901. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Kulidjian AA, Issekutz AC, Issekutz TB. Differential role of E-selectin and P-selectin in T lymphocyte migration to cutaneous inflammatory reactions induced by cytokines. Int Immunol. 2002;14:751–760. doi: 10.1093/intimm/dxf045. [DOI] [PubMed] [Google Scholar]
- Kumagai K, Ohno I, Okada S, Ohkawara Y, Suzuki K, Shinya T, Nagase H, Iwata K, Shirato K. Inhibition of matrix metalloproteinases prevents allergen-induced airway inflammation in a murine model of asthma. J Immunol. 1999;162:4212–4219. [PubMed] [Google Scholar]
- Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Madri JA, Graesser D. Cell migration in the immune system: the evolving inter-related roles of adhesion molecules and proteinases. Dev Immunol. 2000;7:103–116. doi: 10.1155/2000/79045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madri JA, Graesser D, Haas T. The roles of adhesion molecules and proteinases in lymphocyte transendothelial migration. Biochem Cell Biol. 1996;74:749–757. doi: 10.1139/o96-082. [DOI] [PubMed] [Google Scholar]
- Marchenko GN, Marchenko ND, Leng J, Strongin AY. Promoter characterization of the novel human matrix metalloproteinase-26 gene: regulation by the T-cell factor-4 implies specific expression of the gene in cancer cells of epithelial origin. Biochem J. 2002;363:253–262. doi: 10.1042/0264-6021:3630253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko ND, Marchenko GN, Weinreb RN, Lindsey JD, Kyshtoobayeva A, Crawford HC, Strongin AY. Beta-catenin regulates the gene of MMP-26, a novel metalloproteinase expressed both in carcinomas and normal epithelial cells. Int J Biochem Cell Biol. 2004;36:942–956. doi: 10.1016/j.biocel.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Marracci GH, Jones RE, McKeon GP, Bourdette DN. Alpha lipoic acid inhibits T cell migration into the spinal cord and suppresses and treats experimental autoimmune encephalomyelitis. J Neuroimmunol. 2002;131:104–114. doi: 10.1016/s0165-5728(02)00269-2. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62:1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- Moon RT. Connections Map Pathway: Wnt/beta-catenin. 2002. [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- Muller T, Bain G, Wang X, Papkoff J. Regulation of epithelial cell migration and tumor formation by beta-catenin signaling. Exp Cell Res. 2002;280:119–133. doi: 10.1006/excr.2002.5630. [DOI] [PubMed] [Google Scholar]
- Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulroy T, McMahon JA, Burakoff SJ, McMahon AP, Sen J. Wnt-1 and Wnt-4 regulate thymic cellularity. Eur J Immunol. 2002;32:967–971. doi: 10.1002/1521-4141(200204)32:4<967::AID-IMMU967>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Hamada F, Ishidate T, Anai K, Kawahara K, Toyoshima K, Akiyama T. Axin, an inhibitor of the Wnt signalling pathway, interacts with beta-catenin, GSK-3beta and APC and reduces the beta-catenin level. Genes Cells. 1998;3:395–403. doi: 10.1046/j.1365-2443.1998.00198.x. [DOI] [PubMed] [Google Scholar]
- Nusse R. The wnt homepage. 2002. [Google Scholar]
- Ouko L, Ziegler TR, Gu LH, Eisenberg LM, Yang VW. Wnt11 signaling promotes proliferation, transformation, and migration of IEC6 intestinal epithelial cells. J Biol Chem. 2004;279:26707–26715. doi: 10.1074/jbc.M402877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongracz JE, Parnell SM, Jones T, Anderson G, Jenkinson EJ. Overexpression of ICAT highlights a role for catenin-mediated canonical Wnt signalling in early T cell development. Eur J Immunol. 2006;36:2376–2383. doi: 10.1002/eji.200535721. [DOI] [PubMed] [Google Scholar]
- Qiang YW, Walsh K, Yao L, Kedei N, Blumberg PM, Rubin JS, Shaughnessy J, Jr, Rudikoff S. Wnts induce migration and invasion of myeloma plasma cells. Blood. 2005;106:1786–1793. doi: 10.1182/blood-2005-01-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder BA, Kokini K, Sturgis JE, Robinson JP, Voytik-Harbin SL. Tensile mechanical properties of three-dimensional type I collagen extracellular matrices with varied microstructure. J Biomech Eng. 2002;124:214–222. doi: 10.1115/1.1449904. [DOI] [PubMed] [Google Scholar]
- Romanic AM, Graesser D, Baron JL, Visintin I, Janeway CA, Jr, Madri JA. T cell adhesion to endothelial cells and extracellular matrix is modulated upon transendothelial cell migration. Lab Invest. 1997;76:11–23. [PubMed] [Google Scholar]
- Salazar Murphy LL, Hughes CC. Endothelial cells stimulate T cell NFAT nuclear translocation in the presence of cyclosporin A: involvement of the wnt/glycogen synthase kinase-3 beta pathway. J Immunol. 2002;169:3717–3725. doi: 10.4049/jimmunol.169.7.3717. [DOI] [PubMed] [Google Scholar]
- Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol. 2002;3:143–150. doi: 10.1038/ni749. [DOI] [PubMed] [Google Scholar]
- Schilham MW, Wilson A, Moerer P, Benaissa-Trouw BJ, Cumano A, Clevers HC. Critical involvement of Tcf-1 in expansion of thymocytes. J Immunol. 1998;161:3984–3991. [PubMed] [Google Scholar]
- Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nat Cell Biol. 2002;4:E65–68. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- Sixt M, Engelhardt B, Pausch F, Hallmann R, Wendler O, Sorokin LM. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J Cell Biol. 2001;153:933–946. doi: 10.1083/jcb.153.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal FJ, Clevers HC. WNT signalling and haematopoiesis: a WNT-WNT situation. Nat Rev Immunol. 2005;5:21–30. doi: 10.1038/nri1529. [DOI] [PubMed] [Google Scholar]
- Staal FJ, Weerkamp F, Baert MR, van den Burg CM, van Noort M, de Haas EF, van Dongen JJ. Wnt target genes identified by DNA microarrays in immature CD34+ thymocytes regulate proliferation and cell adhesion. J Immunol. 2004;172:1099–1108. doi: 10.4049/jimmunol.172.2.1099. [DOI] [PubMed] [Google Scholar]
- Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- Steeber DA, Tedder TF. Adhesion molecule cascades direct lymphocyte recirculation and leukocyte migration during inflammation. Immunol Res. 2000;22:299–317. doi: 10.1385/IR:22:2-3:299. [DOI] [PubMed] [Google Scholar]
- Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, He X. A mechanism for Wnt coreceptor activation. Mol Cell. 2004;13:149–156. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- Tanaka R, Iwasaki Y, Koprowski H. Ultrastructural studies of perivascular cuffing cells in multiple sclerosis brain. Am J Pathol. 1975;81:467–478. [PMC free article] [PubMed] [Google Scholar]
- Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, Trent JM. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1:279–288. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Wolf K, Muller R, Borgmann S, Brocker EB, Friedl P. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood. 2003;102:3262–3269. doi: 10.1182/blood-2002-12-3791. [DOI] [PubMed] [Google Scholar]
- Wright M, Aikawa M, Szeto W, Papkoff J. Identification of a Wnt-responsive signal transduction pathway in primary endothelial cells. Biochem Biophys Res Commun. 1999;263:384–388. doi: 10.1006/bbrc.1999.1344. [DOI] [PubMed] [Google Scholar]
- Xu Y, Banerjee D, Huelsken J, Birchmeier W, Sen JM. Deletion of beta-catenin impairs T cell development. Nat Immunol. 2003;4:1177–1182. doi: 10.1038/ni1008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


