Abstract
It is generally thought that the early pre-tubular chick heart is formed by fusion of the anterior or cephalic limits of the paired cardiogenic fields. However, this study shows that the heart fields initially fuse at their midpoint to form a transitory “butterfly”-shaped, cardiogenic structure. Fusion then progresses bi-directionally along the longitudinal axis in both cranial and caudal directions. Using in vivo labeling, we demonstrate that cells along the ventral fusion line are highly motile, crossing future primitive segments. We found that mesoderm cells migrated cephalically from the unfused tips of the anterior/cephalic wings into the head mesenchyme in the region that has been called the secondary heart field. Perturbing the anterior/cranial fusion results in formation of a biconal heart. A theoretical role of the ventral fusion line acting as a “heart organizer” and its role in cardia bifida is discussed.
Keywords: chick embryo, heart development, cardia bifida, secondary heart forming field, cardiac fusion, anterior heart field, head mesoderm, cardiac patterning, ventral mesocardium, dorsal mesocardium, cardiogenic mesoderm, linear heart tube
INTRODUCTION
The mature heart is derived from several progenitor cell populations. The splanchnic mesoderm contributes the bulk of the atrial and ventricular myocardium as well as conduction tissue. Endocardial cells arise from an overlapping but more extensive area of splanchnic mesoderm, with potential contribution from cranial mesoderm (Rosenquist and DeHaan, 1966; Manasek, 1968; Noden, 1991). A variety of cells from outside these initial cardiogenic fields also contribute to the mature heart, including neural crest cells (Kirby et al., 1983; Noden et al., 1995) and proepicardial cells (Poelmann et al., 1993; Viragh et al., 1993). Recently, Kirby et al. (2003) have shown that the prechordal endoderm also contributes to both myocardial and endocardial cells.
Heart morphogenesis is a process that occurs over a long window of time. The generally recognized, paired heart-forming regions are situated anteriorly in the lateral plate mesoderm at Hamburger and Hamilton stage 5– 6 (Hamburger and Hamilton, 1951; Rawles, 1936). The mesoderm of these heart-forming regions is derived during gastrulation from embryonic cells that migrate through the primitive streak (Garcia-Martinez and Schoenwolf, 1993; Schoenwolf and Garcia-Martinez, 1995). Each plate of lateral mesoderm splits into somatic and splanchnic epithelial layers to generate the embryonic coelom (Linask, 1992; DeRuiter et al., 1993). The embryonic coelom extends from the aortic arches at the cranial border of the heart to somite 20 posteriorly by stage HH14 (Funayama et al., 1999). The well-known “cardiogenic crescent” is formed when the two sheets of lateral splanchnic mesoderm merge (or fuse) in the anterior portion of the coelom (i.e., the pericardial region of the coelom). Stalsberg and DeHaan (1969) concluded that this initial site of “first contact” occurred at the level of the future conus region (or proximal out-flow track) based on thymidine-labeled autologous implants in stage HH5 embryos. Over time, the cardiogenic crescent subsequently folds toward the ventral midline to form a primitive heart “trough” (or canal) (DeHaan, 1965; Manasek, 1968; van Mierop, 1979; DeRuiter et al., 1992). The dorsal margins of this trough fuse later to form the roof of the straight tube heart, which is suspended between the dorsal and ventral mesocardium. Both mesocardia eventually rupture to generate a heart tube at stage HH14 that is attached only at its anterior and posterior limits. The anterior mesocardium remains in close contact with the head mesenchyme (Noden, 1991; Shigetani et al., 2000).
While there is general acceptance that the paired heart fields fuse to form a tubular primitive heart, the exact mechanism by which this occurs is unclear. The results of fate mapping and extirpation experiments (Castro-Quezada et al., 1972; Argüello et al., 1975; de la Cruz et al, 1989; Kirby et al., 2003) and recent genetic analysis (Mjaatvedt et al., 2001; Waldo et al., 2001; Kelly et al., 2001) are not consistent with the historic view that fusion initially occurs at the level of the future conus. Instead, these studies collectively indicate that: (1) at stage HH10, the embryonic chick heart is composed of only two primitive cardiac segments, i.e., the future apical trabecular regions of the right and left ventricles, not four as originally suggested by Davies (1927), (2) looping of the heart tube (HH10 –HH12) accompanies the progressive addition of two new segments (AV inlet canal and primitive atrium), (3) a fifth segment (the proximal primitive outlet or conus) appears between stages HH11–HH12 from an anterior mesodermal source, and (4) a sixth segment, the distal outlet or truncus arteriosus appears at stage HH16, also from an anterior mesodermal source (Garcia-Pelaes and Arteaga, 1993; Markwald et al., 1998). Thus the current view is that the heart is built in a sequential and segmental manner by the continued recruitment/addition of cells to the anterior and posterior limits of the primitive trabeculated ventricles.
We present here multiple experimental approaches (i.e., morphological data, dye marking fate maps, extirpation experiments, and the localization pattern of sarcomeric MyHC) to re-examine the morphological events surrounding the initial fusion of the paired heart fields and its subsequent relationship to the formation of the primitive trabeculated ventricles and the addition of the proximal outlet segment (or conus). We also propose a possible mechanism by which the two populations of precursor cells might be integrated within the developing heart with the ventral fusion line acting as a pivotal locus for heart organization. These studies provide new insights into how early cardiac morphogenesis is linked to cardiac bifida and to congenital anterior/posterior segmental defect.
RESULTS AND DISCUSSION
Theoretically, there are several scenarios for fusion of the paired cardiogenic fields. Fusion could be initiated at the cephalic border (primitive outlet, Segment 5), at the caudal aspect (primitive atrial segment, Segment 4), or at some intermediate position (primitive right ventricle, Segment 1; primitive left ventricle, Segment 2; or primitive inlet, Segment 3). Stalsberg and DeHaan (1969) proposed that fusion commences at the cephalic level of the cardiogenic regions based on end-point (stage HH12) fate mapping experiments with thymidine-labeled stage HH5 tissue implants. Their mapping data have been largely verified by Redkar et al. (2001) and supported by the work of others (Colas et al., 2000; De Ruiter et al., 1992), but the point of fusion has not received significant attention. Much research has been based on an understanding that the cardiogenic regions fuse at the cephalic level, including its relationship to the recently described “anterior” or “secondary” heart fields (Mjaatvedt et al, 2001; Waldo et al., 2001; Kelly et al., 2001) as proposed by Abu-Issa et al. (2004). However, a cephalic level pattern of fusion is inconsistent with the fate mapping, extirpation experiments, and genetic analysis of others (Castro-Quezada et al., 1972; Argüello et al., 1975; de la Cruz et al., 1989, 2001; Kelly et al., 2001). If alternatively the precardiac mesodermal fields fused at an intermediate position rather than at the cephalic border, there would be good agreement with these experimental findings. This study was undertaken to specifically evaluate the point at which the cardiogenic fields fuse.
Figure 1 shows the spatial and temporal relationship between the heart-forming regions (HFRs) as drawn by Stalsberg and DeHaan (1969), sarcomeric myosin heavy chain expression (revealed by immunostaining with MF20), with the first somite as a common spatial reference point through the different developmental stages. At the 4-somite stage (HH8), sarcomeric myosin expression (red in Fig. 1) was restricted to bi-lateral streaks well within the heart-forming fields, representing but a fraction of the entire heart-forming area. At the 6-somite stage (HH9−), the sarcomeric myosin expression was located close to the midline of the embryo and in the lower extreme of the pericardial cavity as compared to the previous stage (see Fig. 1A, 4 to 6 s). At the 7-somite stage (HH9), sarcomeric myosin expression was more prominent in the cardiogenic fields as they approach one another and start to fuse. Additionally, expression was more broad and closer to the foregut. At the 9- to 10-somite stage (initiation of contraction), MF20 staining extends over the “primitive heart,” occupying about 3/4 of the length of the pericardial cavity. At the 13-somite stage (HH11; when the “conus” segment appears anterior to the primitive ventricle), MF20 staining extended over the entire pericardial cavity (see solid and open arrowheads in Fig. 1 and compare through different stages). Quantification of myosin expression (pixel area) for 4-, 6-, 7-, 10-, and 13-somite stage embryos showed that levels increased by 1, 1.3, 2.5, 4.5, and 10 times, respectively. The distance from the head to first somite (“e” in Fig. 1C) remained almost constant in 5- to 13-somite embryos. In contrast, the distance from the anterior intestinal portal to the first somite was found to decrease (“f” in Fig. 1C). It is important to note that the anterior end of the pericardial coelom (Fig. 1A, white arrowheads) remained at the same axial level from the 4-somite stage to the 13-somite stage (Fig. 1A,C). Conversely, the anterior intestinal portal (black arrowhead, Fig. 1A; see also Fig. 1C) was closer to the first somite in these same stages, which correlated with the elongation of the pericardial cavity. The pattern of sarcomeric myosin expression was reported previously by Han et al. (1992). However, they did not use a spatial reference point that would allow one to assess the position of mesodermal fusion. Nevertheless, while this sarcomeric myosin-positive cell population is generally taken to represent primary myocardium, its precise fate or role in cardiogenesis is unknown. Our data and those of Han et al. (1992) show that the fusion of the heart-forming fields starts at the 7-somite stage (HH9). This prompted us to investigate the developmental mechanism of cardiogenic mesoderm fusion with respect to sarcomeric myosin expression. We chose three important stages for this analysis: pre-fusion (5 somites), fusion (7-somites), and post-fusion (9 somites).
Fig. 1.
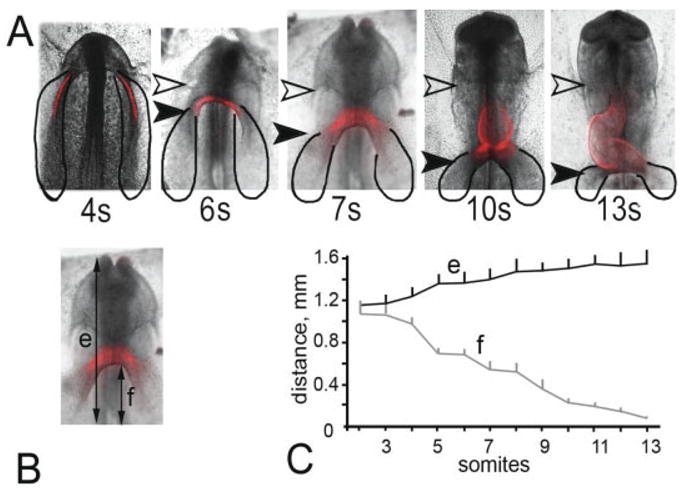
Spatial relationships between the heart-forming regions, sarcomeric myosin expression, and the location of the first somite. A: Embryos containing 4 –13 somites were aligned at the level of the first somite (bottom of each image). Six embryos per stage were immunostained to reveal sarcomeric myosin expression (red; MF20), relative to the cardiogenic region (black solid line) of Stalsberg and DeHaan (1969). The heart is situated in the intraembryonic coelom between the arrowheads. The posterior head fold (open arrowhead) remains at a constant distance from the first somite, while the margin of the foregut (solid arrowhead) progresses caudally. The MF20 staining in 4-somite embryos appears laterally, which by the 6-somite stage meets in the middle of the embryo. Later, the MF20 expands in both directions, cephalic and caudal (7 to 13 somites). B: This embryo (7 somites) shows the landmarks used to obtain measurements indicated in C. (e) is the distance (in mm) between the first somite to the anterior border of the head and (f) is refers to the posterior margin of the foregut. C: Head (e) and foregut (f) distance from the first somite (+SD) with respect to the number of somites. Note that the distance “e” remains nearly constant in 5- to 13-somite embryos. The margin of the foregut progresses down (solid arrowhead in A) and the heart is organized in that space between the arrows (pericardial region of coelom).
Fig. 4.
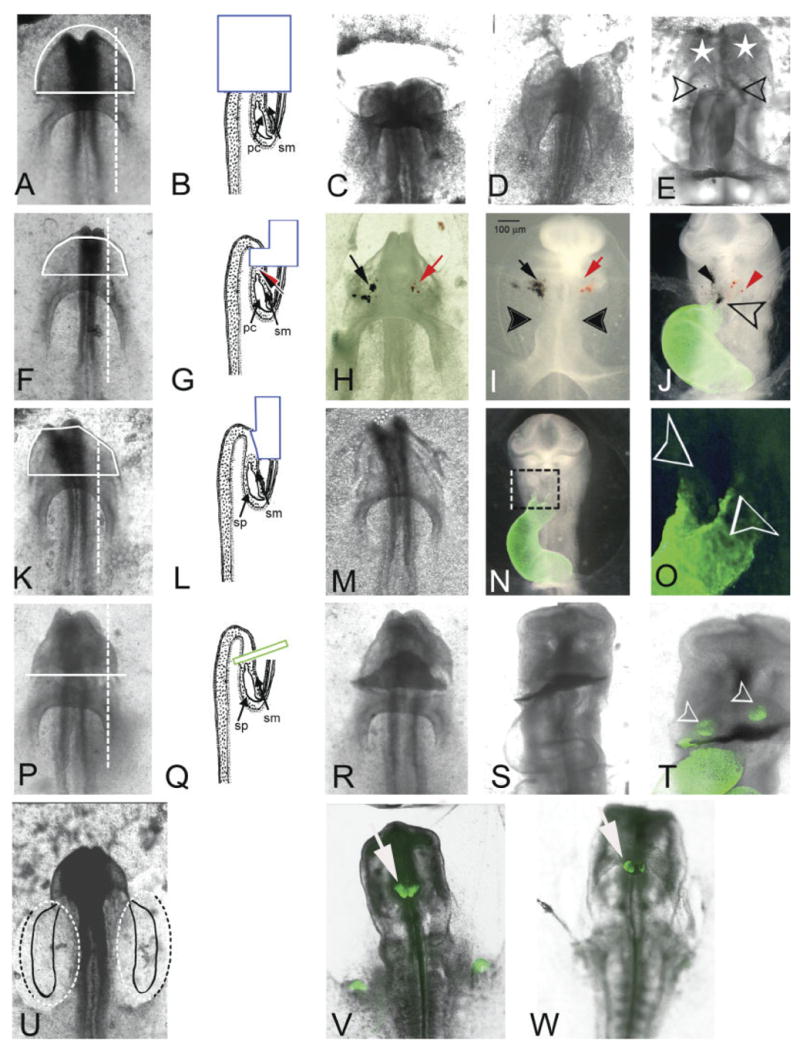
(Overleaf) Ablation mapping of cardiogenic potential of mesoderm from regions anterior to the pericardial region of the coelom in 6-somite stage embryos. Ventral views (A,F,K,P,U) of representative 6-somite embryos and cross-sectional diagrams (B,G,L,Q) of the corresponding surgical procedures performed. The positional relationship of the surgery with respect to the heart-forming regions is highlighted in U (see Fig. 1A). A–E: (n = 1) Although the entire head was removed (t = 0; C) the heart began to form within 3 hr (D). After 24 hr in culture (E), a conus-like structure with two-limb “biconal heart” (open arrows) was observed. The looping of the heart looks abnormal, and the head structures are missing. However, the head mesenchyme was partially restored (white stars). F–J: (n = 5) The ventral ectoderm, mesoderm, and endoderm of the anterior half of the head were removed (solid white line). H: After ablation the most anterior mesoderm tissue was labeled with black ferric-ferrous oxide and red ferric oxide (black and red arrows, respectively). The same embryo after 12 hr of incubation (I) and after 24 hr of incubation (J; MF20 immunostain overlay in green). Note that iron particle labels after 12 hr of culture (I) are outside the heart and far from the ventral middle line. Twenty-four hours later (J), the heart apparently is well developed with coincident distribution of both labels at the distal aspect of the ventral fusion line (open arrow), as well as dispersed individually (black and red arrows) in the lateral regions of head mesenchyme. K–O: (n = 15) Ectoderm alone was removed from the anterior half of the head (solid white line). M: The same embryo immediately following the ablation and (N) again after 24 hr in culture (16 somites; HH12). The organization of the head was not affected. O: Higher magnification of boxed area in N. Note that the outlet has two limbs (open arrows). P–T: (n = 3) An incision was made with a glass needle at the anterior border of the pericardial region of the coelom. Once ectoderm and endoderm were opened, we inserted a small piece of eggshell membrane (green box in Q) and the embryo was allowed to develop 24 hr in culture (S). T: Higher magnification of MF20 immunostained embryo in S. Note the heart was apparently well developed and the primitive outlet was below the eggshell membrane. Only two clusters of cells were MF20-positive above the eggshell membrane (open arrowheads). The growth of the head was not affected. U–W: Both mesoderm and endpderm were removed from posterior bilateral margins of the embryo (dotted line) that included the cardiogenic region as described by Stalsberg and DeHaan (1969). Two independent embryos that developed to the 16-somite stage are shown (V and W). The MF20 staining was present as two distinct masses on the foregut. pc, pericardial region of the coelom; sm, somatic mesoderm; sp, splanchnic mesoderm.
Confocal microscopic images were taken of MF20-stained embryos before removing the endoderm and all the anterior ectoderm (head primordia) (Fig. 2A) to allow for scanning electron microscopy analysis of the exposed mesoderm (Fig. 2B,C). The pericardial region of the coelom was evident at the 5-somite stage (white arrow in Fig. 2B), with myosin expression correlating to the splanchnic mesoderm lateral and adjacent to the anterior intestinal portal (green in Fig. 2B). The region of the future “bucopharyngeal” orifice (i.e., “oral membrane”) was devoid of mesenchyme (white cross in Fig. 2C). However, mesenchymal cells were present in the “prechordal plate” (Fig. 2C, white star). The leading edge of the unfused mesoderm (black arrow in Fig. 2C) had migrated over the endoderm mesodermal face, relatively far from where the future ventral fusion line will eventually form.
Fig. 2.
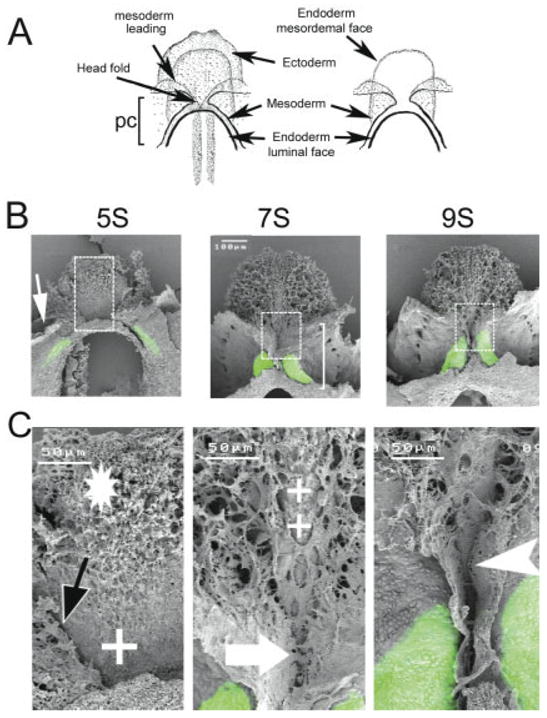
Fusion of the heart-forming regions. Scanning laser confocal micrographs were obtained from embryos (14) immunostained with MF20 to show sarcomeric myosin localization. The head ectoderm was removed from each embryo prior to being processed for scanning electron microscopy. A: Representation of an embryo before (left) and after (right) ectoderm removal (ventral view). The myosin expression pattern (green) was overlaid onto the sister SEM micrograph for each embryo. B: SEM/MF20 overlays from embryos at pre-fusion (5-somite stage), fusion (7-somite stage), and the post-fusion period (9-somite stage) are shown. C: Higher magnification of boxed region shown in B. At the pre-fusion stage (5 somites), the coelom is beginning to form (white arrow). Note that the MF20 localization is lateral with respect to the leading edge of the mesoderm layer (black arrow) at this stage of development. The endoderm layer (mesodermal face) has mesenchymal cells in the prechordal plate (*), and more caudally, the endoderm is without mesenchymal cells (+). At the 7-somite stage, sarcomeric myosin is expressed in the splanchnic mesoderm next to the region of the mesoderm fusion. The heart occupies only half of the pericardial coelom (white bracket). Note that the anterior portion of the pericardial coelom (above the large white arrow) has not fused. Some areas of the endoderm also lack mesenchymal cells (++). At the 9-somite stage, myosin expression extends over the length of the primitive heart canal and the ventral fusion line extends more cephalically (compare 7- to 9-somite images in C). In addition, the ventral fusion line extends caudally (white arrowhead). pc, pericardial coelom.
Sarcomeric myosin expression at the 7-somite stage was localized to the splanchnic mesoderm fields, which are approaching the mid-line to form the ventral fusion line (Fig. 2B,C). The myosin-positive area of the heart occupied approximately half of the pericardial coelom (“pc” in Fig. 2A; white bracket in Fig. 2B). Fusion of the splanchnic mesodermal heart fields was observed at the level of the mesodermal plate (large arrow in Fig. 2C), while the most anterior portions (“anterior wings”) that had not yet fused at this stage were MF20 negative. The ventral surface of the endoderm was almost fully covered by mesenchyme, except in the area of the prospective “oral plate” (++, Fig. 2C). It is important to mention that the sarcomeric myosin staining appeared at a lower level than observed at the previous stage (compare 5- and 7-somite stages in Fig. 2B). This movement might result from the folding of the cardiac mesoderm.
By the 9-somite stage, the ventral fusion line had extended both cephalically and caudally (compare 7- to 9-somite stage at the higher magnification Fig. 2B,C). Thus, the two first heart segments have been formed as a result of a bi-directional fusion, one at the caudal extension (i.e., future apical trabecular region of the left ventricle) and the other at the cephalic end (i.e., future apical trabecular region of the right ventricle). The persisting “cephalic mesoderm” that was initially derived from the mesenchyme of the prechordal plate and pre-cardiac field has, in turn, become organized into the “premandibular and mandibular” regions in the prospective head.
The data in Figures 1 and 2 were static images taken from different embryos fixed and evaluated at selected progressive stages of heart formation. In Figure 3, we show a more dynamic approach of imaging heart development over time within individual living embryos. The mesoderm of the anterior border of the pericardial region of the coelom was labeled with CFDA-SE at the 4-somite stage (green label in Fig. 3) and incubated for an additional 24 hr. Embryos were tattooed with dye in a line approximately 45° to the notochord (relative to the dotted line in Fig. 3A). By the 6-somite stage, the angle changed to 30° and the leading edge of the dye moved caudally toward the anterior intestinal portal (open arrow in Fig. 3A). This observation suggests that a caudal population of labeled cells arrived at the midline before those from a more cephalic region.
Fig. 3.
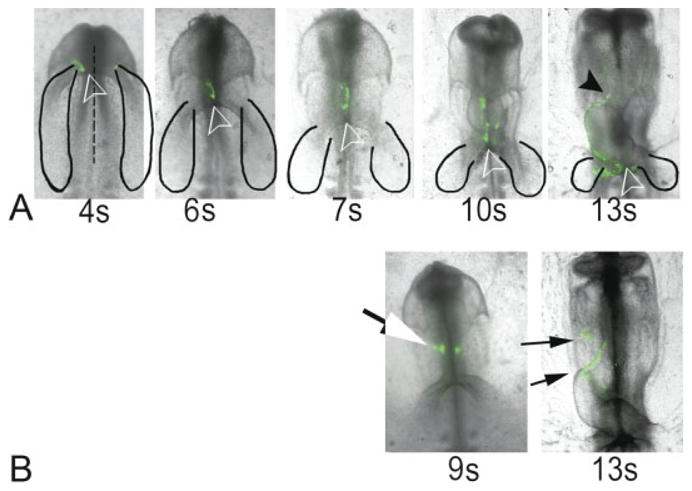
(Overleaf) Analysis of heart fusion by carboxyfluorescein dye marking. Time-lapse images are shown from representative embryos labeled at the 4-somite stage (A) and 9-somite (B) stage. Images were obtained up to the 13-somite stage and aligned relative to the first somite. The cardiogenic region (black solid line; as adapted from Stalsberg and DeHaan, 1969) is shown in each photograph. A: Label in this 4-s embryo was initially at a 45-degree angle relative to the central axis (broken line). This angle narrowed to 30 degrees at the 6-somite stage and 0 degrees at the 7-somite stage. The leading edge of the label (open arrowhead) remains next to the anterior foregut throughout all stages of development. Note that at the 13-somite stage, the label was distributed along the ventral fusion line of the heart from the conus (black arrowhead) to sinus venous (open arrowhead). B: Label placed slightly anterior to the fusion point in a 9-somite embryo (white) localizes to the conus region (small arrows) by the 13-somite stage. The image exposure time was different for successive stages of individual embryos in order to approximate the same intensity of the label in the initial image.
During subsequent development (10- and 13-somite stages, Fig. 3A), the labeled cells became positioned along the ventral fusion line of the heart and moved laterally to the right side of the embryo as the heart loops (Fig. 3A: compare 7s, 10s, 13s). Note that by the 13-somite stage, the entire length of the ventral fusion line of the heart was labeled (Fig. 3A: 13s) from the newly formed “primitive outlet/conus” (solid arrowhead, Fig. 3A) to the sinus venous area (open arrowhead on 13s, Fig. 3A). It is noteworthy that labeled cells were occasionally observed in the head mesenchyme at this time (data not shown). In other experiments, we labeled the anterior wing at the most cephalic part of the heart level, and next to the posterior subcephalic fold at the 9-somite stage. The embryo was allowed to develop to the 13-somite stage and all of the anterior fusion line was labeled (Fig. 3B).
The fusion of the paired heart forming fields leads to the formation of a semi-tubular structure that progresses in both anterior and posterior directions from this initial point of contact/anastomosis to create a ventral fusion line. Closure of the dorsal aspect of this semi-tube structure forms the dorsal fusion-line (Romanoff, 1960). The rupture processes of dorsal and ventral mesocardium may involve a matrix metalloproteinase as suggested by Linask et al. (2005) for the dorsal mesocardium.
Based on these initial experiments (Figs. 1–3), we proposed a close relationship between the most anterior mesoderm of the pericardial region of the coelom (the anterior “wing” of the butterfly) and the development of the head mesoderm. Specifically, we hypothesized that mesoderm from the anterior regions of the pericardial coelom in the 5-somite stage embryo migrates into the head region before becoming incorporated into the tubular heart. In order to test this hypothesis, we conducted the following surgical experiments using embryos that had already begun to form anterior mesoderm in the head region (i.e., 6-somite stage): (1) removal of the entire head of the embryo (Fig. 4A–E), (2) removal of the endoderm, mesoderm, and ventral ectoderm in the head region (Fig. 4F–J), (3) removal of only the endoderm and mesoderm in the head region (Fig. 4K–O), (4) insertion of a physical barrier between the pericardial region of the coelom and the head (Fig. 4P–T), and, finally, (5) removal of the “traditional” (i.e., Stalsberg and DeHaan) bilateral cardiogenic regions (Fig. 4U–W). The goal in all these experimental manipulations was to prevent any mesoderm in the head region from being incorporated into the primitive heart at a later stage using progressively more selective interventions.
We deleted the entire head primordium at the level of the anterior border of the pericardial cavity of a cultured 6-somite stage (HH9−) embryo (Fig. 4A,B) followed by incubation for an additional 24 hr (Fig. 4E). This resulted in a heart-like structure with what resembled a branched conus (“bifed conus”) (open arrows in Fig. 4E), similar to that seen after removing the head ectoderm (Fig. 4O). Head structures were also missing, although some of the head mesenchyme above the anterior border of the pericardial cavity appeared to be partially restored (i.e., “regulated”) (white stars in Fig. 4E). It is important to mention that at this developmental stage the dorsal fusion line does not reach the conus area (Bellairs and Osmond, 1998), which will fuse at a later stage. In parallel in ovo experiments where this region of the head was removed and the embryos allowed to develop to stage HH23, the heart was relatively normal but it was misaligned (i.e., the “normal conus” was connected only with the right ventricular region and the AV canal was only connected with the left ventricular portion) (data not shown). The pharyngeal arches looked normal, but the head was severely truncated. How can this heart with two “partial conuses” at stage HH13 be regulated to form a single conus at a later stage? This may be the result of a late, secondary fusion that compensates for a malformed conus; however, this is only speculation.
Given the extreme nature of these “head ablation” experiments, we conducted progressively more selective removal of head tissue layers in order to evaluate their potential role in fusion of the anterior aspect of cardiac precursor mesoderm. We started by removing the frontal part of the anterior region of ectoderm, mesoderm, and endoderm of cultured embryos at the 5- to 7-somite stage (Fig. 4F,G). The most anterior end of the persisting mesoderm just below the ablation was labeled with red and black iron oxide particles (arrows in Fig. 4G,H) and the embryos were allowed to develop in culture. At the 10-somite stage (HH10), the label was located lateral to the midline of the embryo and about 100 μm from the future ventral fusion line and about 100 μm from the heart (Fig. 4I, solid arrowheads). However, after additional incubation (19-somite stage; HH13) coincident red and black labels were found at the midline and adjacent to the distal aspect of the heart (Fig. 4J, open arrow). The future pharyngeal area was also labeled with the iron particles (red and black arrowheads in Fig. 4J).
When we removed only the ectoderm that overlaid the cephalic region of the 6-somite stage embryos (Fig. 4K,L) that were then allowed to develop in ovo into 19-somite stage (HH13) embryos (Fig. 4N,O), we found that 40% of the experimental embryos had hearts with two conus segments (“bifed conus” or “double conuses”) (Fig. 4N,O). Both conal regions were positive for sarcomeric myosin staining (open arrows in Fig. 4O). Other regions of the embryo did not appear to be affected by the ablation or experimental conditions.
As a complement to the ablation experiments, we used egg shell membrane to physically block both the reintroduction of mesoderm that had previously migrated anteriorly into the head region back into the heart, as well as prevent any further contribution to the anterior mesoderm from more posterior mesoderm. When an eggshell barrier membrane (Fig. 4P,Q) was inserted between the head mesenchyme and the pericardial region of the coelom of cultured embryos at the 6-somite stage (Fig. 4P,Q), and the embryo incubated to the 19-somite stage (HH13), we observed a fully “normal heart” (for that stage of development) on the caudal side of the eggshell membrane (Fig. 4S and T). In addition, two clusters of sarcomeric myosin-positive cells were located above (cranial) the membrane (open arrows in Fig. 4T), which further supports the cardiogenic potential of mesoderm from the anterior region of the pericardial coelom. No abnormalities were observed in the head region of any of the manipulated embryos.
In order to confirm the effectiveness of the eggshell membrane in blocking continued (post-6-somite stage) posteriorly-derived contributions to the conus, we removed the posterior bilateral regions of endoderm and mesoderm that contained the traditional heart-forming fields (Fig. 4U) as previously published (Mjaatvedt et al., 2001). Care was taken to not perturb the most anterior part of the pericardial region of the coelom and the foregut. These ablated embryos developed two separate sarcomeric myosin-positive domains at the margin of the foregut (white arrow in Fig. 4V and W) suggesting that fusion of the cranial precursors of the heart are dependent upon fusion of more caudal aspects. The source of this cranial MF20-positive tissue is uncertain, although the data in Figures 2 and 3 indicate that it came from the most anterior part of the pericardial region of the coelom or would represent a derived mesoderm that migrates into the head prior to fusion of the traditional heart fields. Previous studies support this notion based on the late incorporation of the conus/outlet to the heart (Mjaatvedt et al., 2001). The study of Mjaatvedt et al. (2001) and others postulate that the progenitor cells for the outlet come from a secondary heart field in the pharyngeal region (Castro-Quezada et al., 1972; Argüello et al., 1975; de la Cruz et al., 1989).
The sum of the data presented here was used to propose a model for fusion of the heart-forming regions (HFRs) (Fig. 5). This process is divided into three steps. The first involves the formation of the intraembryonic coelom (Fig. 5A). This space has an anterior-posterior extension and starts to form at the 1-somite stage (Linask, 1992; DeRuiter et al., 1993). During the second step of the fusion process, the most external bilateral splanchnic mesoderm, or prospective ventral fusion line (see Fig. 2 and blue line in Fig. 5A,A’), starts to fold internally (folding arrows in Fig. 5A’) aligning with the future dorsal fusion line, (purple in Fig. 5B,B’). This step is completed when the ventral fusion line complex is stabilized at the point of contact (Fig. 5C,C’). By examining the expression patterns of the transcription factor, Islet-1, as well as atrial myosin light chain 2 (MLC2a) on mouse embryos at equivalent developmental stages, Cai et al. (2003) were similarly able to show MLC2a+ tissue crossing from the external area to the fusion area (fig. 2 in Cai et al., 2003). From our data, we can see that the point of initiation does not occur at the cephalic point of the intraembryonic coelom, but rather initiation occurs at an intermediate position, resulting in a “butterfly”-shaped pre-tubular heart field. Note that the anterior wings of the “butterfly” are MF20 negative, while the posterior wings are MF20 positive (Fig. 2). A pattern similar to the “butterfly”-shaped, pretubular heart described here has also been seen in chick embryos with respect to mRNA expression patterns of alpha smooth muscle actin (Colas et al., 2000) and cardiac troponin T (Antin et al., 2002). A similar pattern was also described in zebrafish based on the expression of Nkx2.5 and cardiac light chain 2 (Yelon et al., 1999). It is also important to note that the ventral mesoderm at the initially point of HFR’s fusion corresponds to cells that contribute to the primitive interventricular septum as described several years ago (de la Cruz et al. 1997).
Fig. 5.
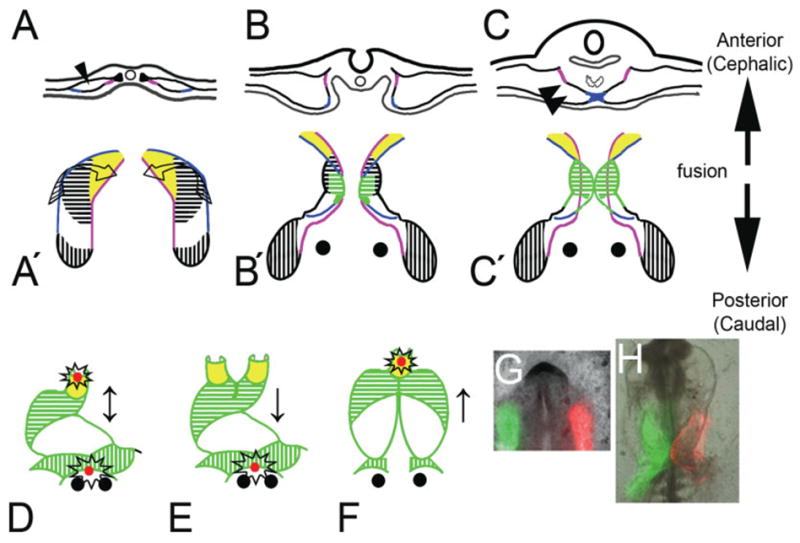
(Overleaf) The heart-forming fusion model with respect to different types of cardia bifida. A–C: A transversal section through an embryo at the level of fusion of the heart fields. A′–C′: A ventral view (mesoderm only) of how the zipper complex is formed and fuses in a bi-directional fashion in corresponding embryos (anterior/cephalic vs. posterior/caudal; arrows). A,A′: One- to foursomite stage. B,B′: Five- to six-somite stage. C,C′: Seven-to tensomite stage. A: The mesoderm separates to form the coelom (arrowhead). Later, the mesoderm folds (twisted arrows in A′), and the previously outer margins (blue) meet to form the ventral fusion line, which originates at the level of the prospective right ventricle (area shaded with green horizontal lines in C’), at the same time the pericardial coelom is formed too (twoarrowheads). Fusion proceeds in both directions (↕), which allows for the caudal incorporation of the apical trabecular portion of the left ventricle (white area) and the cephalic addition of the prospective outlet (yellow area). D: Remnants of the ventral fusion line are located at the caudal and cranial aspects in a normal 16-somite embryo (open stars). If the complex fusion does not form, then a complete cardia bifida occurs (not shown). E: Failure in cranial extension (↓) would produce a biconal heart. F: Failure in the caudal extension (↑) would predict a posterior cardiac bifida. G: An embryo painted with green and red mitotracker showed defective fusion in both cranial and caudal directions and produce anterior and posterior bifide heart (H). This embryo did not develop beyond the 10 somite stage. Prospective outlet tissue (yellow), apical trabecular region of the right (horizontal hatch), left ventricle and AV canal (white), sinoatrial tissue (perpendicular hatch), and ventral fusion line (blue). The green represents the MF20 staining during several developmental stages (B′, C′, D, E, and F).
In the third or last step, the fusion complex is established. A normal heart is formed if the fusion occurs in a bi-directional manner (Fig. 5D; ↕). Blocking of the bi-directional fusion process of the heart-forming regions could potentially result in several types of cardia bifida. A total absence of HFR fusion could result in complete or true cardia bifida (not represented in Fig. 5). There is evidence that fusion of the heart-forming regions is not necessary in order to obtain chamber specification (Shanru et al., 2004). A failure in the progressive fusion in both the anterior and caudal directions could be predicted to result in inlet and outlet bifida (Fig. 5G and H). If fusion is perturbed in the anterior direction alone, a biconal heart or anterior cardia bifida would result (Fig. 4A–E and K–O; Fig. 5E ↓). Osmond et al. (1991) described such a double “coni” or biconal heart (branched conus or “bifed conus”) as two hearts sharing a common posterior appendage. In contrast, if fusion in the caudal direction was perturbed, a posterior cardiac bifida would result (Fig. 5F; ↑). Osmond et al. (1991) and Linask and Lash (1988) described this as a “cranially stunted” heart. This type of cardia bifida can be produced experimentally by a midline dissection of the foregut (DeHaan, 1959; Nadal-Ginard and Garcia, 1972). These data support our proposal for a bi-directional fusion model for the paired, heart-forming fields.
Labeling of the anterior wings of the “butterfly” indicates that they give rise to precursor cells for the outlet limb of the heart, specifically, the future right ventricle and proximal half of the outflow track or the conus region (Fig. 3B). Cells within the anterior wing moved further cephalically into the aortic sac and its associated aortic arches (Fig. 4F–J). Label in the posterior wings (between stages HH9/10 and HH14) ended up in the future left ventricle, the primitive inlet (atrioventricular canal), and, lastly, the sinoatrial elements. Thus, as initially suggested by Stalsberg and DeHaan (1969), and more recently by Redkar et al. (2001), the splanchnic mesoderm known as the “heart-forming fields/regions” contains precursors for most of the future primitive cardiac segments. We interpret our labeling studies to indicate that the different primitive segments are added to both ends of the developing cardiac tube as a progressive bidirectional fusion (“zipping”) of the paired HFRs from a 7- to 14-somite stage embryo.
It is noteworthy that when the anterior extreme of the Stalsberg and DeHaan’s heart-forming regions was labeled prior to their contact (i.e., 4-somite stage), the label was distributed along the entire length of the ventral fusion line including the contiguous atrial, ventricular and conal segments (Fig. 3A). One explanation for this widespread distribution (elongation) of label is that there is a population of MF20-negative mesodermal cells in this area of the HFRs that are highly motile, crossing over segmental boundaries in the process before differentiating into the cardiac tissue of each associated segment. While this specific interpretation/hypothesis has not been previously suggested, data on the differential migration of cells in the cardiogenic mesoderm are present in the literature. Stalsberg and De-Haan (1969) were the first to demonstrate that presumptive cardiogenic cells, initially labeled with iron particles in a rostral position, eventually ended up in a caudal position, but they never commented on these data. The bi-directional fusion of the HFRs to form the transitional “butterfly” generally agrees with the description made by Argüello et al (1975) in the chick, who described the incorporation and differentiation of mesodermal cells to the anterior end of the heart. More recently, Redkar et al. (2001) presented labeling data indicating that the same region from the cardiogenic area at stage HH4 can contribute to the atrium, ventricle, bulbus cordis (primitive right ventricle and conus), and head mesenchyme, but the authors did not comment as to how this occurred.
The “zipper” hypothesis proposed in the current study is similar to what Glickman et al. (2004) showed for zebrafish, where fusion of the cardiogenic regions begins in a caudal location and then proceeds in an anterior direction. It is uncertain whether there is a similar bi-directional fusion process in other species. However, Kelly et al. (2001) used a transgene enhancer trap approach in mice to suggest that undifferentiated mesoderm associated with the aortic sac contributes to the myocardium of the distal outflow track after looping and torsion of the heart. More recently Zaffran et al. (2004) used a combined culture and DI labeling approach to show that pharyngeal mesoderm is incorporated into the cephalic end of the primitive heart of the mouse. Results from cultures of myosin light chain promoter transgenic mice showed that cells from this region contributed extensively to the primitive right ventricular segment and the conus. However, without knowing the level at which the cardiogenic fields fuse in the mouse, it is unclear if these results directly correlate with events in chick heart morphogenesis. Interestingly, Viragh and Challice (1973) have shown that sarcomere formation in the myocardium of the mouse proceeds in a bi-directionally fashion, i.e., in both a caudal and cephalic direction beginning at the ventricular level. Thus, further investigation may show this phenomenon to be common across a wide range of species, with the potential for the ventral and dorsal “zippers” acting as “heart organizers.”
Because cardia bifida can also be produced by environmental (e.g., retinoic acid, Dickman and Smith, 1996) or genetic perturbations (e.g., Mesp1, Saga et al., 1999), we suggest that the bi-directional fusion of cardiogenic primordia is a developmentally regulated process. Besides Mesp1, other candidates are bon (bonnie and clyde), cas (casanova; Dickmeis et al., 2001), GATA 5 (faust, fau), and an EGF-CFC-related gene, oep (one eyed pinhead) (Griffin and Kimelman, 2002, 2003; David and Rosa, 2001), all of which produce cardia bifida when mutated. Cardiac bifida resulting from zygotic oep mutations (Reiter et al., 2001) indicates that nkx2.5, a transcription factor critical for cardiogenesis, may be an important downstream regulator of ventral and dorsal fusion line formation. These findings are consistent with the observation that inhibitors of BMP’s, known regulators of nkx2.5 expression (Harvey 1996, Lien et al., 1999) disrupt fusion and produce cardia bifida in Xenopus embryos (Walters et al., 2001).
The data presented in this report shed new light on whether cardiogenic splanchnic mesoderm contributes to the head mesenchyme as suggested by the pioneering studies of Stalsberg and DeHaan (1969), and more recently by Bredman et al. (1991). As shown by labeling the cells of the anterior wings of the butterfly, over time, some of the labeled cells could be tracked into the head mesenchyme. The cardiogenic potential of these cells was revealed by combining myosin staining with barrier and extirpation experiments that prevented “reincorporation” of these cells into the heart. These results are entirely consistent with our previous studies using older chick embryos (Mjaatvedt et al., 2001), which demonstrated that head mesenchymal cells (those associated with branchial arches and the aortic sac) constitute an “anterior heart-forming field,” and, specifically, that cells from this anterior heart field could be recruited into the growing distal end of the heart tube as the truncus or distal outflow track. The work of Waldo et al. (2001) and Kirby et al. (2003) also support a cranial or “secondary” heart-forming field that is restricted to the remnants of the dorsal mesocardium associated with the foregut in stage HH14 embryos. The existence of a putative “patch” of cardiogenic mesoderm within the head mesenchyme is supported by gene expression studies (Marvin et al., 2001; Tzahor and Lassar, 2001; Shigetani et al., 2000) that show nkx2.5, which is essential for differentiation of mesoderm into myocardial cells, is also required for the formation of head mesenchyme (Tanaka et al., 2001). Yutzey and Kirby (2002) describe cells within the secondary heart-forming field (likely to correspond to our “anterior wing”) that have markers characteristic of precardiac mesoderm. In addition, there are data that suggest that the head ectoderm can block the differentiation of mesoderm into myocardium through wnt signaling pathways in both chick and zebra fish (Marvin et al., 2001; Tzahor and Lazar, 2001; Goldstein and Fishman, 1998). These data suggest that cells located anterior to the straight tube heart have the ability to either become definitive heart or contribute to head mesenchyme. Bredman et al. (1991) have also suggested a similar embryologic origin for both head mesenchyme and cardiac mesenchyme.
The gene, molecules, cellular plus extracellular components, and the forces involved in the process of bidirectional fusion and “zipper complex” formation are unclear and are the focus of future work. The “Zipper model” of bi-directional fusion and its importance to formation of the heart “one piece at a time” creates new experimental opportunities for understanding the role of the anterior and posterior heart-forming regions, as well as elucidating the contributions of head mesoderm and anterior and posterior proepicardial tissue.
EXPERIMENTAL PROCEDURES
Chicken Embryos
Fertilized chicken eggs (Hubbard ISA Hatchery, Statesville, NC) were incubated at 37°C in a moist atmosphere with hourly rotation. After the appropriate incubation times, embryos were isolated and staged according to Hamburger and Hamilton (1951).
Immunofluorescence
Embryos were lifted from the surface of the egg with a filter paper ring (Satin et al., 1988), then fixed at room temperature for 20 min with freshly prepared 3.7% formaldehyde in phosphate buffered saline (PBS; Sigma, St. Louis, MO), pH 7.4, followed by treatment with cold (−20°C) absolute ethanol for 2 hr to permeabilize the tissue. Fixed embryos were rehydrated into PBS through a graded series of ethanol, rinsed two times with PBS containing 0.1% Tween-20, then incubated overnight at 4°C in PBS containing 1% bovine serum albumin (Sigma; initial fraction) and 0.75% normal goat serum to block non-specific binding sites. Sarcomeric myosin heavy chain (MyHC) expression was assessed in intact embryos using the monoclonal antibody MF20 (supernatant fluid from Developmental Studies Hybridoma Bank, Iowa City, IA, overnight at 4°C). Embryos were rinsed extensively with PBS/Tween-20 followed by overnight incubation (4°C) with rhodamine- or fluorescein-conjugated goat anti-mouse IgG (Cappel; 1:100 in PBS containing 0.2% normal goat serum). Stained embryos were washed for 1 hr in PBS then PBS/Tween-20 (20 × 1 min each) and cover slipped with 50% glycerin/50% PBS containing 10% (w:v) DAPCO (Sigma) as an anti-quenching agent. Antibody staining was observed using an epi-fluorescent Leica DMLB microscope equipped with a Hamamatsu model C5818 color chilled 3CCD digital camera. The bright field picture was merged to the corresponding fluorescence image using Adobe Photoshop 6 for Windows.
Morphometric Analysis (Fig. 1)
Digital images were obtained from embryos containing from 2 to 12 somites using an Olympus DP11 camera attached to a Nikon dissection scope (SMZ-2T). Embryos (6 to 12 per stage) were measured from the middle of the first somite to: (1) the anterior tip of the head and (2) to the anterior intestinal portal.
Scanning Electron Microscopy (Fig. 2)
Fixed embryos that had been stained previously with MF20 were dissected with glass needles to remove only the head ectoderm, exposing the tissue below. Briefly, for stage HH8− (5 somites) embryos, the entire head ectodermal cap above the posterior head fold was removed along with dorsal ectoderm overlying the primitive streak anterior to the first somite (Fig. 2A). For stage HH9 (7 somites) and HH10− (9 somites) embryos, the pericardial cavity was opened at the level of posterior head fold margin and all anterior ectoderm removed, exposing the underlying endoderm and head mesoderm. The resected embryos were post-fixed overnight with 2% paraformaldehyde and 3% glutaraldehyde solution in 0.1 M sodium cacodylate, pH 7.2, and then dehydrated through a graded alcohol series. Each embryo was attached to a strip of masking tape between two glass slides to keep them stationary during dehydration and critical-point drying (Jeol EMS850 using liquid CO2). Specimens were sputter-coated with gold (Polaron SC7640) and examined using a Jeol JSM5410LV scanning electron microscope. The digital scanning images were overlaid with those from the MF20 immunohistochemistry using Photoshop (adjusted for minor differences in size due to critical point drying).
Dye-Marking Studies (Fig. 3)
The mesoderm underlying the anterior margin of the pericardial region of the coelom was labeled with 5-(and-6)-carboxy-2′,7′-dichlorofluorescein diacetate succinimidyl ester (CFDA-SE; Molecular Probes, Eugene, OR). These amine-reactive reagents can passively diffuse into cells and are colorless until their acetate groups are cleaved by intracellular esterases to yield a highly fluorescent product. Upon reaction with amine-containing residues of intracellular proteins, these probes form dye-protein adducts that are retained inside cells as they move and divide during embryonic development (Griffith and Hay, 1992). A 10-mM stock solution of CFDA-SE in dimethyl sulfoxide was diluted 1:500 in Earle’s balanced salt solution (with Ca/Mg, and without bicarbonate and phenol red, EBSS; Gibco) and pre-warmed to 37°C prior to use. Ten embryos were microinjected (0.1– 0.3 μl) into the mesoderm layer though a slit in the pericardial region of the coelom using a screw-controlled syringe (Gilmont Instrument). Digital images were acquired using a Hamamatsu color chilled 3CCD C5818 camera system attached to a Leica DMLB epifluorescence microscope. The fluorescence image was masked onto the bright field image using Adobe Photoshop 6.
Surgical Procedures (Fig. 4)
Eggs were cleaned with 70% ethanol, allowed to air-dry, then broken into a 100 × 15 mm Petri dish. The embryos were removed with a paper ring, washed ventral side up in EBSS, then dissected as follows using sterile glass needles. For head ablation, the head was removed at the level of the anterior end of the pericardial region of the coelom from 10 embryos (5–7 somites) (Fig. 4A,B). For anterior ectoderm-mesoderm-endoderm ablation, the anterior ectoderm, mesoderm, endoderm from the rostral region of 10 embryos (5–7 somites) were dissected (Fig. 4F,G) and the persisting anterior mesoderm labeled with ferric-ferrous oxide black (Fe3O4, Fisher Science) and ferric oxide red (Fe2O3 Fisher Science). For ectoderm ablation, the ectoderm was removed from the anterior half of 5 embryos (5–7 somites) with boundaries similar to the anterior endoderm-mesoderm ablation (Fig. 4K,L) (only the proamnios was removed in control embryos). In the eggshell membrane experiment, an incision was made with a glass needle at the anterior border of the pericardial region of the coelom in 4 embryos (5–7 somites). Once the ectoderm, mesoderm, and endoderm were opened, a small piece of eggshell membrane was inserted (Fig. 4P,Q). In the heart-forming fields extirpation experiment, the heart-forming regions of Stalsberg and DeHaan (1969) were removed from 10 embryos (5–7 somites) as previously reported (Mjaatvedt et al., 2001) (Fig. 4U). After the different surgical procedures, embryos were transferred to a center-well organ culture dish (Falcon), containing fresh egg albumen (1:3) in EBSS. The embryos were cultured in an incubator at 37.5°C, and 100% humidity, until the appropriate stages were reached. Images were recorded digitally during development. All the digital image processing was done using Adobe Photoshop 6 for Windows.
Acknowledgments
We thank Dr. Tom Trusk, Sue Tjepkema-Burrows, and Josh Spruill for their valuable technical contributions in preparing this manuscript. Special recognition goes to Victor Fresco, MS, for his valuable help in the editing of this manuscript.
Footnotes
Grant sponsor: NIH; Grant numbers: HL66231, HL33756, HL52813, HL56596, HD39946, C06-RR018823 (from the Extramural Research Facilities Program of the National Center for Research Resources), and FOGARTY-NIH PA-95-011.
References
- Abu-Issa R, Waldo K, Kirby ML. Heart fields: one, two or more? Dev Biol. 2004;272:281–285. doi: 10.1016/j.ydbio.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Antin PB, Bates MA, Zhang W, Garriock RJ, Yatskievych TA. Precocious expression of cardiac troponin T in early chick embryos is independent of bone morphogenetic protein signaling. Dev Dyn. 2002;225:135–141. doi: 10.1002/dvdy.10148. [DOI] [PubMed] [Google Scholar]
- Argüello C, De la Cruz MV, Sanchez-Gomez C. Experimental study of the formation of the heart tube in the chick embryo. J Embryol Exp Morph. 1975;33:1–11. [PubMed] [Google Scholar]
- Bellairs R, Osmond M. The atlas of chick development. New York: Academic Press; 1998. Plate 15a,b. [Google Scholar]
- Bredman JJ, Wessels A, Weijs WA, Korfage JA, Soffers CA, Moorman AF. Demonstration of “cardiac-specific” myosin heavy chain in masticatory muscles of human and rabbit. Histochem J. 1991;23:160 –170. doi: 10.1007/BF01046587. [DOI] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Quezada A, Nadal-Ginard B, De la Cruz MV. Experimental study of the formation of the bulboventricular loop in the chick. J Embryol Exp Morphol. 1972;27:623–637. [PubMed] [Google Scholar]
- Colas J, Lawson A, Schoenwolf G. Evidence that translation of smooth muscle alpha-actin mRNA is delayed in the chick promyocardium until fusion of the bilateral heart-forming regions. Dev Dyn. 2000;218:316 –330. doi: 10.1002/(SICI)1097-0177(200006)218:2<316::AID-DVDY6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- David NB, Rosa FM. Cell autonomous commitment to an endodermal fate and behaviour by activation of Nodal signalling. Development. 2001;128:3937–3947. doi: 10.1242/dev.128.20.3937. [DOI] [PubMed] [Google Scholar]
- Davis CL. Development of the human heart from its first appearance to the stage found in embryos of twenty paired somites. Carnegie Contrib Embryol. 1927;19:245–284. [Google Scholar]
- De la Cruz MV, Sánchez-Gómez C, Palomino MA. The primitive cardiac regions in the straight tube heart (Stage 9-) and their anatomical expression in the mature heart: an experimental study in the chick embryo. J Anat. 1989;165:121–131. [PMC free article] [PubMed] [Google Scholar]
- De La Cruz MV, Castillo MM, Villavicencio L, Valencia AM, Moreno-Rodriguez RA. The primitive interventricular septum, its primordium, and its contribution in the definitive interventricular septum. “in vivo” labelling study in the chick embryo heart. Anat Rec. 1997;247:512–520. doi: 10.1002/(SICI)1097-0185(199704)247:4<512::AID-AR10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- De la Cruz MV, Markwald RR, Krug EL, Rumenoff L, Sanchez Gomez C, Sadowinski S, Galicia TD, Gomez F, Salazar GM, Villavicencio GL, Reyes AL, Moreno-Rodriguez RA. Living morphogenesis of the ventricles and congenital pathology of their component parts. Cardiol Young. 2001;11:588 –600. doi: 10.1017/s1047951101000932. [DOI] [PubMed] [Google Scholar]
- De Ruiter MC, Poelman RE, deVries VP, Mentink MMT, Gittenberger-de Goot AC. The development of the myocardium and endocardium in mouse embryos. Fusion of two heart tubes? Anat Embryol. 1992;185:461–473. doi: 10.1007/BF00174084. [DOI] [PubMed] [Google Scholar]
- De Ruiter MC, Poelman RE, Mentink MMT, Vaniperen L, Gittenberger-de Goot AC. Early formation of the vascular system in quail embryos. Anat Rec. 1993;235:261–274. doi: 10.1002/ar.1092350210. [DOI] [PubMed] [Google Scholar]
- DeHaan RL. Cardia bifida and the development of pacemaker function in the early chick heart. Dev Biol. 1959;1:586 –602. [Google Scholar]
- DeHaan RL. Morphogenesis of the vertebrate heart. In: DeHaan RL, Ur-sprung H, editors. Organogenesis. New York: Holt, Rinehart, and Winston; 1965. pp. 377–419. [Google Scholar]
- Dickman ED, Smith SM. Selective regulation of cardiomyocyte gene expression and cardiac morphogenesis by retinoic acid. Dev Dyn. 1996;206:39 –48. doi: 10.1002/(SICI)1097-0177(199605)206:1<39::AID-AJA4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Dickmeis T, Mourrain P, Saint-Etienne L, Fischer N, Aanstad P, Clark M, Strahle U, Rosa F. A crucial component of the endoderm formation pathway, CASANOVA, is encoded by a novel sox-related gene. Genes Dev. 2001;15:1487–1492. doi: 10.1101/gad.196901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama N, Sato Y, Matsumoto K, Ogura T, Takahashi Y. Coelom formation: binary decision of the lateral plate mesoderm is controlled by the ectoderm. Development. 1999;126:4129 –4138. doi: 10.1242/dev.126.18.4129. [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez V, Schoenwolf GC. Primitive streak origin of the cardiovascular system in avian embryos. Dev Biol. 1993;159:706 –719. doi: 10.1006/dbio.1993.1276. [DOI] [PubMed] [Google Scholar]
- Garcia-Pelaez I, Arteaga M. Experimental study of the development of the truncus arteriosus of the chick embryo heart. I. Time of appearance. Anat Rec. 1993;237:378 –384. doi: 10.1002/ar.1092370311. [DOI] [PubMed] [Google Scholar]
- Glickman N, Tsai HJ, Yelon D. Differentially directed cell movements drive heart assembly in zebrafish. Weinstein Conference A19, Leiden, The Netherlands, May. 2004;24:2004. [Google Scholar]
- Goldstein AM, Fishman MC. Notochord regulates cardiac lineage in zebrafish embryos. Dev Biol. 1998;201:247–252. doi: 10.1006/dbio.1998.8976. [DOI] [PubMed] [Google Scholar]
- Griffin KJ, Kimelman D. One-Eyed Pinhead and Spadetail are essential for heart and somite formation. Nature Cell Biol. 2002;4:821–825. doi: 10.1038/ncb862. [DOI] [PubMed] [Google Scholar]
- Griffin KJ, Kimelman D. Interplay between FGF, one-eyed pinhead, and T-box transcription factors during zebrafish posterior development. Dev Biol. 2003;264:456 –466. doi: 10.1016/j.ydbio.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Griffith CM, Hay ED. Epithelial-mesenchymal transformation during palatal fusion: carboxyfluorescein traces cells at light and electron microscopic levels. Development. 1992;116:1087–1099. doi: 10.1242/dev.116.4.1087. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49 –92. [PubMed] [Google Scholar]
- Han Y, Dennis JE, Cohen-Gould L, Bader DM, Fischman DA. Expression of sarcomeric myosin in the presumptive myocardium of chicken embryos occurs within six hours of myocyte commitment. Dev Dyn. 1992;193:257–265. doi: 10.1002/aja.1001930306. [DOI] [PubMed] [Google Scholar]
- Harvey RP. NK-2 homeobox genes and heart development. Dev Biol. 1996;178:203–216. doi: 10.1006/dbio.1996.0212. [DOI] [PubMed] [Google Scholar]
- Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1:435–440. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Gale TF, Stewart DE. Neural crest cells contribute to normal aorticopulmonary septation. Science. 1983;220:1059 –1061. doi: 10.1126/science.6844926. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Lawson A, Stadt HA, Kumiski DH, Wallis KT, McCraney E, Waldo KL, Li YX, Schoenwolf GC. Hensen’s node gives rise to the ventral midline of the foregut: implications for organizing head and heart development. Dev Biol. 2003;253:175–188. doi: 10.1016/s0012-1606(02)00024-6. [DOI] [PubMed] [Google Scholar]
- Lien CL, Wu C, Mercer B, Webb R, Richardson JA, Olson EN. Control of early cardiac-specific transcription of Nkx2–5by a GATA-dependent enhancer. Development. 1999;126:75–84. doi: 10.1242/dev.126.1.75. [DOI] [PubMed] [Google Scholar]
- Linask KK. N-cadherin localization in early heart development and polar expression of Na+, K(+)-ATPase, and integrin during pericardial coelom formation and epithelialization of the differentiating myocardium. Dev Biol. 1992;151:213–224. doi: 10.1016/0012-1606(92)90228-9. [DOI] [PubMed] [Google Scholar]
- Linask KK, Lash JW. A role for fibronectin in the migration of avian pre-cardiac cells. II. Rotation of the heart-forming region during different stages and its effects. Dev Biol. 1988;129:324 –329. doi: 10.1016/0012-1606(88)90379-x. [DOI] [PubMed] [Google Scholar]
- Linask KK, Han M, Cai DH, Brauer PR, Maisastry SM. Cardiac morphogenesis: Matrix metalloproteinase coordination of cellular mechanisms underlying heart tube formation and directionality of looping. Dev Dyn. 2005;233:739 –753. doi: 10.1002/dvdy.20377. [DOI] [PubMed] [Google Scholar]
- Manasek FJ. Embryonic development of the heart. I. A light and electron microscope study of myocardial development in the early chick embryo. J Morphol. 1968;125:329 –366. doi: 10.1002/jmor.1051250306. [DOI] [PubMed] [Google Scholar]
- Markwald RR, Trusk T, Moreno-Rodriguez RA. Formation and septation of the tubular heart: integrating the dynamics of morphology with emerging molecular concepts. In: De la Cruz MV, Markwald, editors. Living morphogenesis of the heart. Boston: Birkhäuser; 1998. pp. 43–84. [Google Scholar]
- Marvin MJ, DiRocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316 –327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjaatvedt CH, Nakaoka T, Moreno-Rodriguez RA, Norris C, Kern MJ, Eisenberg CA, Turner D, Markwald RR. The outflow tract of the heart is recruited from a novel heart-forming field. Dev Biol. 2001;238:97–109. doi: 10.1006/dbio.2001.0409. [DOI] [PubMed] [Google Scholar]
- Nadal-Guinard B, Garcia MP. The morphologic expression of each cardiac primordium in the chick embryo. J Embryol Exp Morph. 1972;28:141–152. [PubMed] [Google Scholar]
- Noden DM. Origins and patterning of avian outflow tract endocardium, Development. 1991;111:857–861. doi: 10.1242/dev.111.4.867. [DOI] [PubMed] [Google Scholar]
- Noden DM, Poelmann RE, Gittenberger-de Groot A. Cell origins and tissue boundaries during outflow tract development. Trends Cardiovasc Med. 1995;5:69 –75. doi: 10.1016/S1050-1738(99)80002-4. [DOI] [PubMed] [Google Scholar]
- Osmond MK, Buttler AJ, Voon FCT, Bellairs R. The effets of retinoic acid on heart formation in the early chick embryo. Development. 1991;113:1405–1417. doi: 10.1242/dev.113.4.1405. [DOI] [PubMed] [Google Scholar]
- Poelmann RE, Gittenberger-de Groot AC, Mentink MMT, Bökenkamp R, Hogers B. Development of the cardiac coronary vascular endothelium, studied with antiendothelial antibodies, in chicken-quail chimeras. Circ Res. 1993;73:559 –568. doi: 10.1161/01.res.73.3.559. [DOI] [PubMed] [Google Scholar]
- Rawles M. A study in the localization of organ forming areas of the chick blastoderm of the head process stage. J Exp Zool. 1936;72:271–315. [Google Scholar]
- Redkar A, Montgomery M, Litvin J. Fate map of early avian cardiac progenitor cells. Development. 2001;128:2269 –2279. doi: 10.1242/dev.128.12.2269. [DOI] [PubMed] [Google Scholar]
- Reiter JF, Verkade H, Stainier DY. Bmp2b and Oep promote early myocardial differentiation through their regulation of gata5. Dev Biol. 2001;234:330 –338. doi: 10.1006/dbio.2001.0259. [DOI] [PubMed] [Google Scholar]
- Romanoff AL. The avian embryo. New York: Macmillan Company; 1960. pp. 684–687. [Google Scholar]
- Rosenquist GC, DeHaan RL. Migration of precardiac cells in the chick embryo: a radioautographic study. Carnegie Inst Wash Contrib Embryol. 1966;38:111–121. [Google Scholar]
- Saga Y, Miyagawa-Tomita S, Takagi A, Kitajima S, Miyazaki J, Inoue T. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development. 1999;126:3437–3447. doi: 10.1242/dev.126.15.3437. [DOI] [PubMed] [Google Scholar]
- Satin J, Fujii S, DeHaan RL. Development of cardiac beat rate in early chick embryos is regulated by regional cues. Dev Biol. 1988;129:103–113. doi: 10.1016/0012-1606(88)90165-0. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Garcia-Martinez V. Primitive-streak origin and state of commitment of cells in the cardiovascular system in avian and mammalian embryos. Cell Mol Biol Res. 1995;41:233–240. [PubMed] [Google Scholar]
- Shanru Li, Zhou D, Lu MM, Morrisey EE. Advanced cardiac morphogenesis does not require heart tube fusion. Science. 2004;305:1619 –1622. doi: 10.1126/science.1098674. [DOI] [PubMed] [Google Scholar]
- Shigetani Y, Nobusada Y, Kuratani S. Ectodermally derived FGF8 defines the maxillomandibular region in the early chick embryo: Epithelial-mesenchymal interactions in the specification of the craniofacial ectomesenchyme. Dev Biol. 2000;228:73–85. doi: 10.1006/dbio.2000.9932. [DOI] [PubMed] [Google Scholar]
- Stalsberg H, DeHaan RL. The pre-cardiac areas and formation of the tubular heart in the chick embryo. Dev Biol. 1969;19:128 –159. doi: 10.1016/0012-1606(69)90052-9. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Schinke M, Liao HS, Yamasaki N, Izumo S. Nkx2.5 and Nkx2.6, homologs of Drosophila tinman, are required for development of the pharynx. Mol Cell Biol. 2001;21:4391– 4398. doi: 10.1128/MCB.21.13.4391-4398.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzahor E, Lassar AB. Wnt signals from the neural tube block ectopic cardiogenesis. Genes Dev. 2001;15:255–260. doi: 10.1101/gad.871501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Mierop LHS. Morphological development of the heart. Handbook of physiology: the cardiovascular system. In: Berne RM, editor. The heart. I. Bethesda, MD: Ann Physiol Society; 1979. pp. 1–28. [Google Scholar]
- Viragh S, Challice CE. Origin and differentiation of cardiac muscle cells in the mouse. J Ultrastruct Res. 1973;42:1–24. doi: 10.1016/s0022-5320(73)80002-4. [DOI] [PubMed] [Google Scholar]
- Viragh S, Gittenberger-de Groot AC, Poelmann RE, Kalman F. Early development of quail heart epicardium and associated vascular and glandular structures. Anat Embryol. 1993;188:381–393. doi: 10.1007/BF00185947. [DOI] [PubMed] [Google Scholar]
- Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, Kirby ML. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–3188. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- Walters M, Wayman G, Christian J. Bone morphogenetic protein function is required for terminal differentiation of the heart but not for early expression of cardiac marker genes. Mech Dev. 2001;100:263–273. doi: 10.1016/s0925-4773(00)00535-9. [DOI] [PubMed] [Google Scholar]
- Yelon D, Horne SA, Stainier DY. Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev Biol. 1999;214:23–37. doi: 10.1006/dbio.1999.9406. [DOI] [PubMed] [Google Scholar]
- Yutzey KE, Kirby ML. Wherefore heart thou? Embryonic origins of cardiogenic mesoderm. Dev Dyn. 2002;223:307–320. doi: 10.1002/dvdy.10068. [DOI] [PubMed] [Google Scholar]
- Zaffran S, Kelly RG, Meilhac SM, Buckingham ME, Brown NA. Right ventricular myocardium derives from the anterior heart field. Circ Res. 2004;95:261–268. doi: 10.1161/01.RES.0000136815.73623.BE. [DOI] [PubMed] [Google Scholar]


