Abstract
Chemotactic migration of macrophages is critical for recruitment of leukocytes to inflamed tissues. Macrophages utilize a specialized adhesive structure called podosome to migrate. Podosome formation requires the Wiskott-Aldrich syndrome protein (WASP) which is a product of the gene defective in an Xid disorder, Wiskott-Aldrich Syndrome (WAS). Macrophages from WASP-deficient WAS patients lack podosomes, resulting in defective chemotactic migration. However molecular basis for podosome formation is not fully understood. I have shown that WASP interacting protein (WIP), a binding partner of WASP plays an important role in podosome formation in macrophages. I showed that WASP bound WIP to form a complex at podosomes and that knockdown of WIP impairs podosome formation. When WASP binding to WIP was blocked, podosome formation was also impaired. When WASP expression was reduced by siRNA transfection, the amount of a complex of WASP with WIP decreased, resulting in reduced podosome formation. Podosomes were restored by reconstitution of the WASP-WIP complex in WASP knockdown cells. These results indicate that the WASP-WIP complex is required for podosome formation in macrophages. When podosome formation was reduced by blocking WASP binding to WIP, transendothelial migration of macrophages, the most crucial process in macrophage trafficking was impaired. These results suggest that a complex of WASP with WIP plays a critical role in podosome formation, thereby mediating efficient transendothelial migration of macrophages.
Keywords: Monocytes/Macrophages, Immunodeficiency Disorder, Cell Trafficking, Wiskott-Aldrich syndrome protein (WASP), WASP interacting protein (WIP)
The Wiskott-Aldrich Syndrome (WAS)3 is an X-linked inherited immunodeficiency disorder. WAS is characterized by thrombocytopenia, eczema, recurrent infections, autoimmune diseases and an increased risk of lymphoreticular malignancy (1-3). The causative gene underlying WAS encodes Wiskott-Aldrich syndrome protein (WASP) (4). WASP contains several domains that regulate its activity and subcellular localization (5). N-WASP, the more widely expressed homolog has the same domain organization as WASP (6). The WASP C-terminal VCA (verprolin/cofilin/acdic) domain stimulates actin polymerization by interacting with an actin-related protein complex, the Arp2/3 (actin related protein) complex (7, 8).
In contrast to the well-characterized WASP C-terminal functions, the WASP N-terminal functions are still unclear. Three mammalian verprolins have been identified as proteins binding to the WASP N-terminal region (residues 1-170), WASP interacting protein (WIP) (9); CR16 (10); and WIP and CR16 homologous protein (WICH) (11) or WIP-related protein (WIRE) (12). Two independent groups identified WICH/WIRE simultaneously. Verprolin was originally identified as a yeast protein essential for cell polarization (13). These three mammalian verprolins share the same domain organization that consists of the verprolin homologous (VH) domain, a proline-rich (Pro-rich) domain and a WASP binding (WB) domain (9-12, 14). WIP and WICH/WIRE are expressed in hematopoietic cells such as T cells and monocytes/macrophages. WIP is crucial for localizing WASP activity both in a vaccinia-based actin motility system and to the immune synapse after TCR ligation (15). In addition, WIP synergizes with N-WASP to induce filopodia when overexpressed in fibroblast (16). WICH/WIRE has actin-bundling activity and plays a critical role in platelet-derived growth factor receptors endocytosis (17, 18).
Chemotactic migration of monocytes and macrophages is critical for recruitment of leukocytes to inflamed tissues. Macrophage chemotaxis requires regulated cell-cell and cell-extracellular matrix interactions. A specialized adhesive structure called podosome is thought to play an important role in macrophage chemotactic migration (19). Podosomes are highly dynamic, actin-rich adhesion structures found in monocyte-derived cells such as macrophages, osteoclasts and dendritic cells (19-23). They also have been found in certain transformed fibroblast and carcinoma cell lines (24-27). It has been previously reported that WASP is a critical component of podosomes in macrophages (28), but the role of WIP, WASP binding partner in podosome formation is unknown. Here, I investigate the role of the WIP in podosome formation in macrophages.
Materials and Methods
Reagents
Recombinant human M-CSF-1 (macrophage-colony stimulating factor-1) and TNF-α (tumor necrosis factor-α) were purchased from R&D systems. Anti-WASP mAb and anti-WIP polyclonal antibody were purchased from Santa Cruz Biotechnology. PMSF, leupeptin, pepstatin A, aprotinin, anti-mouse IgG agarose, Protein A sepharose, and PMA were obtained from Sigma-Aldrich. RPMI1640 and other tissue culture reagents were obtained from Invitrogen, except for the reagents for endothelial cell culture.
Cells and transfection
Human monocyte cell line, THP-1 was obtained from American Type Culture Collection and cultured in RPMI1640 containing 10% fetal calf serum (FCS), 100 units/ml penicillin and 0.1 mg/ml streptomycin. THP-1 cells were transfected with the WIP or WASP constructs using Cell Line Nucleofector Kit V and amaxa Nucleofector (amaxa Inc.) according to the manufacturer’s instructions. After transfection, cells were cultured for 3 days in RPMI1640 containing 10% FCS supplemented with 12.5 ng/ml of PMA. For human primary monocyte isolation, 20-40 ml of peripheral blood was drawn from healthy volunteer, after informed consent was obtained. Monocytes were isolated from blood samples using Monocyte Isolation Kit II (Miltenyi Biotech). Cells were cultured in RPMI1640 containing 10% FCS supplemented with 20 ng/ml of recombinant human M-CSF-1. Monocytes cultured for 6 days in this medium attained morphology characteristic of macrophages, and their differentiated state was confirmed by FACS analysis for CD14+ status. Monocytes cultured for 3 days were harvested and transfected with the WIP or WASP construct using Human Monocyte Nucleofector Kit and amaxa Nucleofector (amaxa Inc.) according to the manufacturer’s instructions. After transfection, cells were cultured for 3 days additionally. Cells were cotransfected with GFP expressing plasmid, pmaxGFP (amaxa Inc.) as a transfection marker. The efficiency of transfection measured using pmaxGFP was 40-60% for THP-1 cells or monocytes. Transfection of short interfering RNA (siRNA) was performed using DharmaFECT 2 (Dharmacon Inc.). The following sequences were chosen to generate siRNA: for WASP, 5′-GCCGAGACCTCTAAACTTA-3′ (sense), 5′-CGGCCAGATCTCAATATCAT-3′ (scrambled); for WIP, 5′-GATCCACATCTGCGAAACC -3′ (sense), 5′-AACCTCGGAGCCTCAACTA-3′ (scrambled); for WICH/WIRE, 5′-GAGAACCTAGCTGGTAAGC-3′ (sense), 5′-CACCAGCATTGGACATGGA-3′ (scrambled). The efficiency of siRNA transfection measured using FITC-conjugated control siRNA, BLOCK-IT (Invitrogen), was 40-60%. The human umbilical vein endothelial cells (HUVEC) were obtained from Cell Applications Inc. Cells were cultured using Endothelial Cell Growth Medium (Cell Applications Inc.) at 37°C in a 5% CO2 atmosphere. The Internal Review Board of Burnham Institute for Medical Research approved these experiments.
Immunofluorescence microscopy
THP-1 cells seeded on coverslips were stimulated with 12.5 ng/ml PMA for 3 days. Human primary monocytes were stimulated with 20 ng/ml M-CSF-1 for 6 days. Stimulated THP-1 cells and monocytes obtain macrophage-like phenotypes (28-30). Cells were fixed with 4% paraformaldehyde (Fluka) and permeabilized with 0.1% saponin. Cells were stained with Alexa 568-labeled phalloidin (Invitrogen), and anti-WIP or anti-WASP antibodies (Santa Cruz Biotechnology). Secondary antibodies were Alexa 488-labeled anti-rabbit or mouse IgG (Invitrogen). Cell staining was examined under a fluorescence microscope (Zeiss Exoplan AR) or MRC 1024 SP Bio-Rad Laser Point Scanning Confocal Microscope (Bio-Rad).
Immunoprecipitation
For immunoprecipitation of WASP or WIP, cells (2-5 × 107 cells) were lysed in buffer A (50 mM Tris-HCl, pH7.5, 150 mM NaCl, 1% IGEPAL CA-630 (Sigma-Aldrich), 1 mM PMSF, 1 mM sodium orthovanadate, 1 μg/ml leupeptin, 1 μg/ml pepstatin A, 1 μg/ml aprotinin). Lysates were centrifuged at 10,000 × g at 4°C for 15 min. The supernatant was incubated with 2 μg/ml anti-WASP mAb or anti-WIP antibody (Santa Cruz Biotechnology) at 4°C for 2 h, and then incubated with anti-mouse IgG agarose or Protein A sepharose (Sigma-Aldrich). The resin binding the immune complex was washed with 0.5 ml of buffer A three times and the complex was eluted with 1 x Laemmli’s SDS-PAGE sample buffer. Eluted proteins were subjected to SDS-PAGE and analyzed by immunoblotting using anti-WASP antibody, anti-WIP polyclonal antibody (Santa Cruz Biotechnology) and anti-WICH/WIRE polyclonal antibody (31).
Transendothelial migration assay
A confluent monolayer of HUVEC cells was generated by plating 3-5 × 104 cells on Attachment Factor Solution (Cell Applications Inc.)-coated 13-mm diameter coverslips in 24-well plates overnight. HUVEC cells were activated to induce maximal expression of cell adhesion molecules by incubation with 25 ng/ml TNF-α (R&D systems) for 6 hours. PMA-differentiated THP-1 cells or human primary macrophages were transfected with the WIP construct and the pmaxGFP plasmid. Transfected cells (5 × 104 cells) were seeded per well in 0.5 ml RPMI. After 2 hours, cocultures of THP-1 cells or macrophages on the HUVEC monolayer were washed once with PBS and fixed with 4% paraformaldehyde in PBS. Three sequential confocal optical sections were taken at the top, center and bottom of the HUVEC monolayer of randomly chosen fields. I scored the percentage of GFP-positive cells per coverslip found on either the surface of the monolayer, spanning the monolayer, or having fully crossed the monolayer for 25 cells per coverslip and three coverslips per experiment.
Statistical analysis
The significance of differences between groups was calculated by the Student’s t-test. Confidence (95%) was set a priori as the desired level of statistical significance.
Results
WASP binds WIP to form a complex at podosomes
A complex of WASP with WIP functions in important cellular processes in T cells, fibroblasts or monocytes (15, 16, 31, 32). It has been shown that WASP localizes at podosomes and plays a critical role in podosome formation (28, 33). I therefore hypothesized that WIP might play a role in podosome formation as a binding partner of WASP in macrophages. I first examined whether WASP bound WIP to form a complex in human macrophages. Monocytes isolated from peripheral blood were cultured in the medium containing M-CSF-1 and differentiated into macrophages. WASP was immunoprecipitated with anti-WASP antibody from the lysates of human primary macrophages (Fig. 1A, lanes 1 and 2). Fig. 1A shows that WIP coimmunoprecipitated with WASP (Fig. 1A, lane 4), indicating that WASP binds WIP in macrophages (Fig. 1A). I previously showed that WASP binds WIP in THP-1 cells (31).
FIGURE 1.
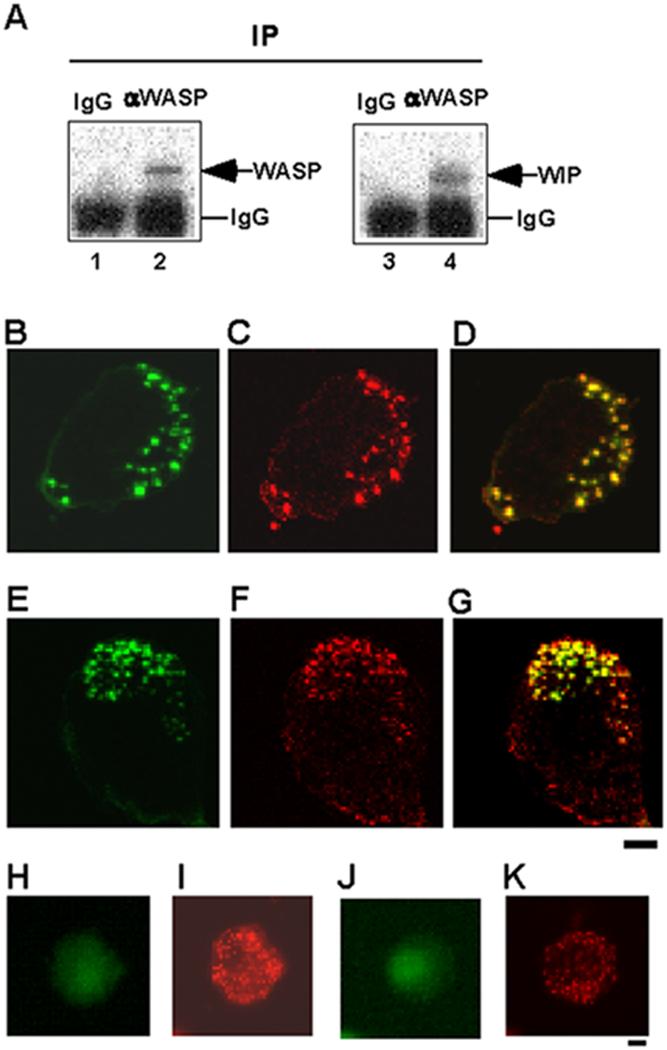
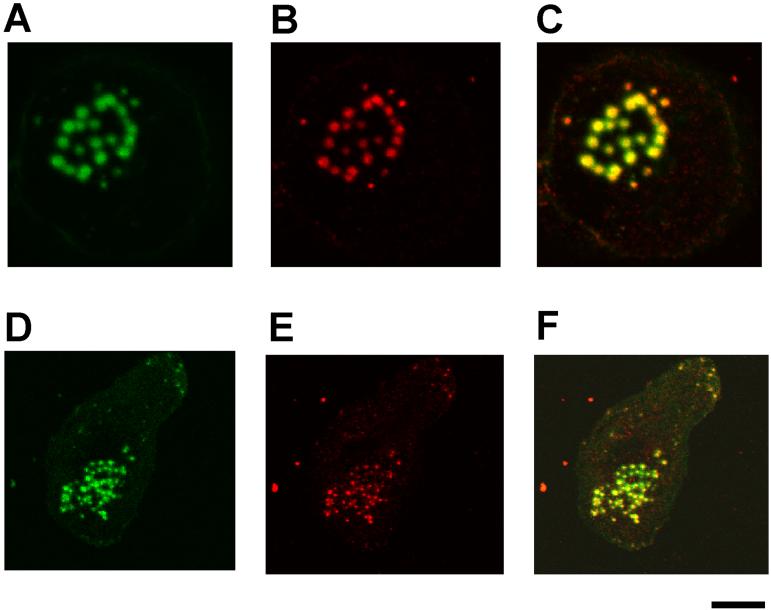
WASP binds WIP to form a complex at podosomes. A, Coimmunoprecipitation of WIP with WASP. WASP was immunoprecipitated with anti-WASP mAb or a control IgG from the lysates of human primary macrophages followed by immunoblotting for WASP (lanes 1 and 2) and WIP (lanes 3 and 4). B-G, Confocal laser scanning micrographs of PMA-differentiated THP-1 cells (B-D) and human primary macrophages (E-G). WIP staining (B and E), actin staining (C and F), and overlay of WIP and actin staining (D and G). Yellow color indicates colocalization of green (WIP) and red (actin). THP-1 cells (H and I) and macrophages (J and K) were stained with a control antibody (rabbit IgG) and phalloidin. Bars, 10 μm.
To determine if WIP localizes at podosomes, I performed immunofluorescence experiments. THP-1 cells were differentiated by stimulation with PMA to obtain macrophage-like phenotypes, which closely resemble human monocyte-derived macrophages, as previously reported (29, 30). PMA-differentiated THP-1 cells were stained with anti-WIP antibody and phalloidin. Phalloidin staining gives a typical punctate pattern of F-actin in core of podosomes (Fig. 1C). Double staining revealed colocalization of F-actin and WIP to the core of podosomes in PMA-differentiated THP-1 cells (Fig. 1B-D). I also showed that WIP colocalizes at F-actin at core of podosomes in human primary macrophages by double staining (Fig. 1E-G). PMA-differentiated THP-1 cells (Fig. 1H and I) and human primary macrophages (Fig. 1J and K) were stained with a control antibody (rabbit IgG) and phalloidin. Phalloidin staining gives a typical pattern of F-actin in core of podosomes (Fig. 1I and K),but control antibody staining gives no positive staining for podosomes (Fig. 1H and J). These results indicate that WASP binds WIP to form a complex at core of podosomes in macrophages (Fig. 1).
The role of WIP in podosome formation in macrophages
I then asked whether WIP plays a role in podosome formation in macrophages. To address this question, I examined whether knockdown of WIP and WICH/WIRE, mammalian verprolins predominantly expressed in macrophages (31), affects podosome formation. To do this, expression of both verprolins, WIP and WICH/WIRE was knocked down in THP-1 cells by transfection of siRNA, and then podosome formation of THP-1 cells was assayed, since both verprolins are expressed at the same level and are complexed with WASP in THP-1 cells (31).
Transfection of siRNAs for WIP and WICH/WIRE decreased the amount of WIP and WICH/WIRE in PMA-differentiated THP-1 cells compared with transfection of scrambled control siRNAs (Fig. 2A, lanes 1-4). Transfection of siRNAs for control, WIP and WICH/WIRE barely affected the expression of WASP and β-actin (Fig. 2A, lanes 5 and 6), suggesting that gene silencing by siRNAs for WIP and WICH/WIRE is specific for WIP and WICH/WIRE. I then examined podosome formation of THP-1 cells and macrophages. To quantify podosome formation, I scored the percentage of cells with podosomes per siRNA-transfected cells (FITC-control siRNA-positive cells). When expression of both WIP and WICH/WIRE was knocked down, podosome formation of THP-1 cells (Fig. 2B) or human primary macrophages (Fig. 2C) was reduced. A representative cell of each experiment was shown in Fig. 2 D-G. Podosome formation was not affected in THP-1 cells or macrophages by transfection of control siRNAs (Fig. 2D and F), but impaired by transfection of siRNAs for WIP and WICH/WIRE (Fig. 2E and G). Introduction of siRNAs itself barely affected podosome formation (Fig. 2B and C). Figs. 1 and 2 indicate that WIP is a component of podosomes and that WIP plays an important role in podosome formation in macrophages.
FIGURE 2.
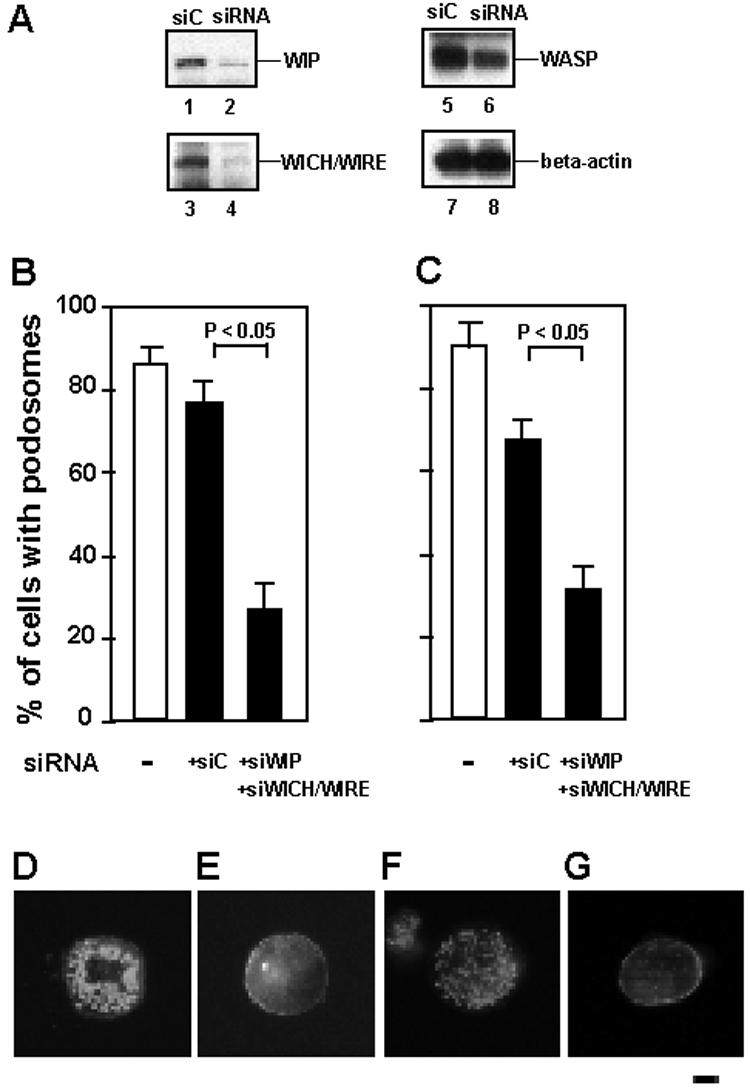
The role of WIP in podosome formation. A, Expression of WIP and WICH/WIRE was reduced by transfection of siRNA. THP-1 cells were transfected with siRNAs for WIP (siWIP) and WICH/WIRE (siWICH/WIRE) (lanes 2, 4, 6, and 8), or their scrambled control siRNAs (siC) (lanes 1, 3, 5, and 7). Cells were cotranfected with FITC-conjugated control siRNA (Invitrogen). FITC-positive cells were sorted by FACS. Total lysates of sorted cells were analyzed by immunoblotting for WIP (lanes 1 and 2), WICH/WIRE (lanes 3 and 4), WASP (lanes 5 and 6), and β-actin (lanes 7 and 8). B and C, Quantification of podosome formation. The percentage of cells with podosomes per siRNA-transfected cells (FITC-conjugated control siRNA-positive cells) was scored. □, podosome formation of untransfected cells. ■, podosome formation of siRNA-transfected cells. siWIP and WICH/WIRE indicate siRNA for WIP and WICH/WIRE, respectively. siC indicates the mixture of scrambled control siRNAs of siWIP and siWICH/WIRE. Data represent the mean ± SD of triplicate measurements. D-G, Immunofluorescence micrographs of a representative cell of each experiment. Cells were stained for F-actin with Alexa 568-phalloidin. PMA-differentiated THP-1 cells transfected with the scrambled controls (D), or siRNAs for WIP and WICH/WIRE (E); Human primary macrophages transfected with the scrambled controls (F), or siRNAs for WIP and WICH/WIRE (G). Bar is 10 μm.
The role of WASP-WIP complex in podosome formation
I next asked whether formation of a complex of WASP with WIP is critical for podosome formation. To address this question, I blocked binding of WASP to WIP in macrophages and examining those cells for podosome formation.
To block WASP binding to WIP in cells, FLAG-tagged WIP C-terminal fragment containing the WASP-binding domain of WIP (residues 321-503) (F-WB) was overexpressed in cells by transient transfection. As a negative control, FLAG-tagged PDZ-guanine nucleotide exchange factor (PDZ-GEF) C-terminal fragment (residues 1146-1429) (F-C) (34) was expressed in cells, because this fragment is stable in cytosol as well as WASP or WIP and its molecular weight (25 kDa) is similar to that of F-WB (21 kDa) (Fig. 3A, lanes 7 and 8). I confirmed that binding of this control protein to WASP or WIP was undetectable (data not shown). Cells were cotransfected with GFP expressing plasmid as a trasnfection marker, and then GFP-positive cells were sorted by FACS. WASP was immunoprecipitated from lysates of sorted cells expressing the F-C or F-WB fragment (Fig. 3). When the F-C fragment was expressed in THP-1 cells (Fig. 3A, lanes 1, 3, 5, 7, 9, 11, and 13), both WIP and WICH/WIRE coimmunoprecipitated with WASP (Fig. 3A, lanes 11 and 13), indicating that both WIP and WICH/WIRE bound to WASP in the THP-1 cells. When the F-WB fragment was expressed in THP-1 cells (Fig. 3A, lanes 2, 4, 6, 8, 10, 12, and 14), the amounts of coimmunoprecipitated WIP and WICH/WIRE with WASP were much lower than when the F-C fragment was expressed (Fig. 3A, lanes 11-14). These results indicate that overexpression of the F-WB fragment blocks WASP binding to WIP and WICH/WIRE in THP-1 cells by a dominant-negative effect.
FIGURE 3.
The role of WASP-WIP complex in podosome formation. A, Binding of WASP to WIP and WICH/WIRE was blocked by the FLAG-tagged WB fragment. THP-1 cells were transfected with the FLAG-tagged PDZ-GEF (F-C) as a negative control (lanes 1, 3, 5, 7, 9, 11, and 13), or the FLAG-tagged WASP binding site of WIP (residues 321-503) (F-WB) (lanes 2, 4, 6, 8, 10, 12, and 14). Cells were cotransfected with GFP expressing plasmid (pmaxGFP). GFP-positive cells were sorted by FACS. Total lysates of sorted cells were analyzed by immunoblotting for WASP (lanes 1 and 2), WIP (lanes 3 and 4), WICH/WIRE (lanes 5 and 6), and FLAG-tagged proteins (lanes 7 and 8). WASP was immunoprecipitated from the lysates of sorted cells followed by immunoblotting for WASP (lanes 9 and 10), WIP (lanes 11 and 12), and WICH/WIRE (lanes 13 and 14). B and C, Quantification of podosome formation. The percentage of cells with podosomes per transfected cells (GFP-positive cells) was scored. □, podosome formation of cells transfected with FLAG-tagged PDZ-GEF (F-C) or Myc-tagged PDZ-GEF (M-C) as a negative control. ■, podosome formation of cells transfected with FLAG-tagged WASP binding site of WIP (F-WB) or Myc-tagged WIP binding site of the WASP N-terminus (M-WN) to block WASP binding to WIP. Podosome formation of PMA-differentiated THP-1 cells (B) or human primary macrophages (C) was shown. Data represent the mean ± SD of triplicate measurements. D-K, Immunofluorescence micrographs of a representative cell of each experiment. Cells were stained for F-actin with Alexa 568-phalloidin. PMA-differentiated THP-1 cells transfected with F-C (D), F-WB (E), M-C (F), or M-WN (G). Human primary macrophages transfected with F-C (H), F-WB (I), M-C (J), or M-WN (K). Bar is 10 μm. L, THP-1 cells were cotransfected with FLAG-tagged constructs, Myc-tagged constructs and GFP expressing plasmid. M-42 is the Myc-tagged constitutively active form of Cdc42 (V12Cdc42). The total lysates were immunoblotted by anti-FLAG (lanes 1-3) or anti-Myc monoclonal antibodies (lanes 4-6) to detect expression of each protein. M and N, Cells were stained with phalloidin, and podosome and filopodia formation of transfected cells (GFP-positive cells) were examined. The percentages of cells with podosomes (M) and filopodia (N) per transfected cells were scored, respectively. Podosome and filopodia formation of cells transfected with F-C as a negative control (□). Podosome and filopodia formation of cells transfected with F-WB to block WASP binding to WIP (■) (M and N). O-Q, Immunofluorescence micrographs of a representative cell of each experiment. Cells were cotransfected with F-C and M-C (O), F-WB and M-C (P) and F-WB and M-42 (Q), and then stained with Alexa 568-phalloidin. Bar is 10 μm. R, THP-1 cells were cotransfected with FLAG-tagged constructs, Myc-tagged WASP murant constructs and GFP expressing plasmid. dW is a WASP deletion mutant lacking the WIP binding site (residues 171-502). dW Y291E is the deletion mutant with Y291E mutation. dW Y291F is the deletion mutant with Y291F mutation. The total lysates were immunoblotted by anti-FLAG (lanes 1-4) or anti-Myc monoclonal antibodies (lanes 5-8) to detect expression of each protein. S and T, Cells were stained with phalloidin, and podosome and filopodia formation of transfected cells (GFP-positive cells) were examined. The percentages of cells with podosomes (S) and filopodia (T) were scored, respectively. Podosome and filopodia formation of cells transfected with F-C as a negative control (□). Podosome and filopodia formation of cells transfected with F-WB to block WASP binding to WIP (■) (S and T). U-X, Immunofluorescence micrographs of a representative cell of each experiment. Cells were cotransfected with F-C and dW (U), F-WB and dW (V), F-WB and dW Y291E (W) and F-WB and dW Y291F (X). Bar is 10 μm. Data represent the mean ± SD of triplicate measurements.
When the F-WB fragment was overexpressed in PMA-differentiated THP-1 cells and cells were stained with Alexa 568-phalloidin for F-actin, the percentage of cells with podosomes significantly decreased, compared with when the F-C fragment was overexpressed (Fig. 3B). A representative cell of each experiment was shown in Fig. 3 (F-C, D; F-WB, E). When the F-WB fragment was overexpressed in human primary macrophages and cells were stained with phalloidin, the percentage of cells with podosomes also significantly decreased (Fig. 3C). A representative cell of each experiment was shown in Fig. 3 (F-C, H; F-WB, I). These results indicate that podosome formation was significantly impaired when WASP binding to WIP was blocked in macrophages. To confirm these results, WASP binding to WIP was blocked by overexpression of Myc-tagged WASP N-terminal fragment containing the WIP binding domain of WASP (M-WN) in PMA-differentiated THP-1 cells and human primary macrophages. Podosome formation was also impaired by overexpression of the M-WN fragment in THP-1 cells (Fig. 3B, F, and G) and macrophages (Fig. 3C, J, and K). These results suggest that a complex of WASP with WIP plays a critical role in podosome formation in macrophages.
The WASP C-terminal VCA domain interacts with Arp2/3 complex and stimulates actin polymerization, thereby resulting in formation of filopodia (7, 8). This WASP C-terminal activity is mainly regulated by Cdc42. In free WASP molecule, the VCA domain is bound in intramolecular fashion to the GTPase binding domain (GBD, residues 231-310) of WASP, resulting in autoinhibition toward Arp2/3 complex (7). Binding of GTP-bound form of Cdc42 to the GBD causes destabilization of autoinhibited GBD-VCA domain, leading to release of the VCA, facilitating its activation of Arp2/3 complex. Overexpression of constitutively active form of Cdc42 (V12Cdc42) in dendritic cells highly activates the WASP C-terminus and causes a dramatic increase in cell spreading, resulting in formation of numerous filopodia (51). To examine if overexpression of V12Cdc42 causes podosome formation when WASP binding to WIP was blocked by the F-WB fragment, PMA-differentiated THP-1 cells were cotransfected with the F-WB fragment and Myc-tagged V12Cdc42 (M-42) (Fig. 3L, lanes 3 and 6), and then assayed for podosome formation and filopodia formation (Fig. 3M-Q). Expression of F-C, F-WB, M-C (Myc-tagged PDZ-GEF as a control) and M-42 was confirmed by immunoblotting using anti-FLAG and Myc antibodies (Fig. 3L). Expression of the F-WB fragment blocked WASP binding to WIP, reducing podosome formation (Fig. 3M, O and P). Overexpression of V12Cdc42 increased cell spreading and filopodia formation (Fig. 3N), but did not increase podosome formation in cells expressing the F-WB fragment and few podosomes were observed in spread cells and cells with filopodia (Fig. 3M, N and Q). These results indicate that overexpression of V12Cdc42 did not restore podosomes, when WASP binding to WIP was blocked (Fig. 3L-Q).
The WASP C-terminal activity is also regulated by Src family tyrosine kinases. Binding of Cdc42 to the GBD of WASP increases the accessibility of tyrosine at residue 291 (Y291) of WASP to Src kinases. In addition to Cdc42 binding to the GBD, phosphorylation at Y291 by the kinases promotes destabilization of the GBD-VCA domain interaction, increasing the basal activity of WASP toward Arp2/3 complex (48-50). Expression of the WASP mutant mimicking tyrosine-phosphorylated form (Y291E) induces more filopodia, compared with wild-type WASP (48). Expression of non-phosphorylated form of WASP (Y291F) induces less filopodia, compared with wild-type WASP (48). Thus, the activity of the WASP C-terminus is also regulated by tyrosine phosphorylation at residue 291 of WASP. To examine if such altered phosphorylation patterns of WASP cause podosome formation when WASP binding to WIP was blocked, PMA-differentiated THP-1 cells were cotransfected with the F-WB fragment and the Myc-tagged WASP mutant constructs (Fig. 3R), and then assayed for podosome formation and filopodia formation. I used a WASP deletion mutant lacking the WIP binding site (dW, residues 171-502). Although this WASP deletion mutant does not bind to WIP, its C-terminal activity is still regulated by Cdc42 and tyrosine phosphorylation, since it contains the GBD and Y291. I also used the WASP deletion mutant with Y291E mutation (dW Y291E, residues 171-502) and the WASP deletion mutant with Y291F mutation (dW Y291F, residues 171-502). Expression of F-C, F-WB, dW, dW Y291E and dW Y291F was confirmed by immunoblotting using anti-FLAG and Myc antibodies (Fig. 3R). Expression of the F-WB fragment blocked WASP binding to WIP, reducing podosome formation in cells expressing the WASP deletion mutant, dW (Fig. 3S, U and V). Expression of the dW Y291E increases the basal activity toward Arp2/3 complex, thereby inducing more filopodia than wild-type WASP (Fig. 3T), but podosome formation was not significantly increased in cells expressing dW Y291E compared with cells expressing dW and few podosomes were observed in cells with filopodia (Fig. 3S, T and W). Expression of Y291F decreases the basal activity toward Arp2/3 complex, thereby inducing less filopodia than wild-type WASP (Fig. 3T). Podosome formation was not significantly increased by expression of dW Y291F (Fig. 3T and X). These results indicate that altered phosphorylation patterns did not restore podosomes, when WASP binding to WIP was blocked (Fig. 3R-X).
These results also indicate that in addition to actin polymerization, WASP binding to WIP is required for efficient podosome formation, suggesting that WIP plays a critical role in localizing the WASP activity to podosomes by forming a complex with WASP.
Complex formation of WASP with WIP causes podosome formation
These results from the experiments taking advantage of a dominant-negative effect showed that inhibition of formation of a complex of WASP with WIP reduced podosome formation in macrophages (Fig. 3). I then asked whether formation of the WASP-WIP complex causes podosome formation. To address this question, podosome formation was reduced by decreasing the amount of the WASP-WIP complex in cells, and then I tested if reduced podosome formation would be reversed by reconstitution of the WASP-WIP complex. To do this, I took advantage of the fact that only 15 of 19 bases of siRNA for human WASP are conserved in the mouse WASP cDNA. When cells were cotransfected with human WASP siRNA and mouse WASP cDNA, human WASP expression was reduced (Fig. 4A,lanes 1-3), but mouse WASP expression was barely affected by human WASP siRNA, because of the difference in the gene sequence between human and mouse WASP (Fig. 4A, lanes 3 and 4-6). Transfection of siRNA for human WASP barely affect expression of WIP and β-actin (Fig. 4A, lanes 7-12). This experiment was designed based on a previous report by Seals et al. (27). To examine the role of an adaptor protein, Tks5/Fish in podosome formation in NIH3T3 cells, they reduced mouse Tks5/Fish expression by siRNA transfection and then transfected those cells with human Tks5/Fish cDNA. Human Tks5/Fish was successfully expressed in mouse Tks5/Fish knockdown cells. They took advantage of the fact that only 15 of 19 bases of siRNA for mouse Tks5/Fish are conserved in human Tks5/Fish cDNA.
FIGURE 4.

Complex formation of WASP with WIP causes podosome formation. A, Knockdown of human WASP and expression of mouse WASP. THP-1 cells were transfected with siRNA for human WASP (siW) or its scrambled control (siC) and FLAG-tagged mouse WASP (F-mW). Cells were cotranfected with FITC-conjugated control siRNA. FITC-positive cells were sorted by FACS. Total lysates of sorted cells were analyzed by immunoblotting with anti-WASP (lanes 1-3), anti-FLAG (lanes 4-6), anti-WIP (lanes 7-9), and anti-β-actin (lanes 10-12). B, Decrease in the amount of the human WASP-WIP complex and reconstitution of the mouse WASP-WIP complex. WIP was immunoprecipitated from the lysates of sorted cells followed by immunoblotting with anti-WIP (lanes 1-3), anti-WASP (lanes 4-6), and anti-FLAG (lanes 7-9). C and D, Quanitfication of podosome formation. The percentage of cells with podosomes per transfected cells (FITC-conjugated control siRNA-positive cells) was scored. □, podosome formation of cells transfected with the scrambled control siRNA for WASP (siC). ■, podosome formation of cells transfected with siRNA for WASP (siW). F-C and F-mW indicate FLAG-tagged PDZ-GEF (a negative control) and FLAG-tagged mouse WASP, respectively. Podosome formation of PMA-differentiated THP-1 cells (C) or human primary macrophages (D) was shown. Data represent the mean ± SD of triplicate measurements. E and F, Immunofluorescence micrographs of a representative cell of each experiment. Cells were stained for F-actin with Alexa 568-phalloidin. PMA-differentiated THP-1 cells transfected with human WASP siRNA (siW) and FLAG-tagged mouse WASP cDNA (F-mW) (E). Human primary macrophages transfected with human WASP siRNA (siW) and FLAG-tagged mouse WASP cDNA (F-mW) (F). Bar is 10 μm.
Transfection of siRNA for human WASP into PMA-differentiated THP-1 cells reduced expression of WASP (Fig. 4A, lane 2). The amount of coimmunoprecipitated WASP with WIP significantly decreased in cells transfected with human WASP siRNA (Fig. 4B, lanes 1-5), indicating that the amount of WASP-WIP complex decreased in cells. Podosome formation was reduced in such human WASP knockdown cells (Fig. 4C, +siW). N-WASP expression is not high enough to compensate for reduced WASP expression, since N-WASP expression in THP-1 cells is lower than WASP (less than 5% of WASP) based on RT-PCR results (31). This result is consistent with the observation that podosomes are absent in macrophages from WASP-deficient WAS patients (28). FLAG-tagged mouse WASP was expressed in human WASP knockdown cells (Fig. 4A, lanes 3 and 6). FLAG-tagged mouse WASP coimmunoprecipitated with WIP, indicating that mouse WASP bound WIP to form a complex in human WASP knockdown cells (Fig. 4B, lanes 6 and 9). When THP-1 cells were transfected with human WASP siRNA and FLAG-tagged mouse WASP cDNA (F-mW), transfected cells showed significant increase in podosome formation compared with when transfected with human WASP siRNA and FLAG-tagged control cDNA (F-C, FLAG-tagged PDZ-GEF) (Fig. 4C). Transfected human primary macrophages also showed significant increase in podosome formation, when cells were transfected with human WASP siRNA and FLAG-tagged mouse WASP cDNA (F-mW) (Fig. 4D). A representative THP-1 cell (Fig. 4E) and macrophage (Fig. 4F) with podosomes formed by transfection of mouse WASP were shown. These results indicate that podosomes are restored by reconstitution of the WASP-WIP complex in human WASP knockdown cells, suggesting that formation of a complex of WASP with WIP causes podosome formation in macrophages.
To confirm that the exogenous mouse WASP localizes at podosomes restored in human WASP knockdown cells, transfected cells were stained with anti-FLAG mAb and phalloidin. Double-staining revealed localization of exogenous mouse WASP at the core of podosomes in PMA-differentiated THP-1 cells (Fig. 5A-C) or human primary macrophages (Fig. 5D-F), as previously reported WASP localization (28).
FIGURE 5.
Exogenous mouse WASP localizes at restored podosomes. Confocal laser scanning micrographs of cells transfected with human WASP siRNA and FLAG-tagged mouse WASP cDNA. PMA-differentiated THP-1 cells (A-C) and human primary macrophages (D-F). Cells were stained with anti-FLAG mAb for FLAG-tagged mouse WASP (A and D), actin staining (B and E), and overlay of mouse WASP and actin staining. Yellow color indicates colocalization of green (mouse WASP) and red (actin), Bar is 10 μm.
WASP-WIP complex plays a role in macrophage transendothelial migration
Macrophage migration across the endothelium (transendothelial migration) is a critical process for recruitment of macrophages to inflamed tissues (35). Since podosomes are thought to have an important function in not only cell adhesion but also migration (19, 36), I tested if a complex of WASP with WIP plays a role in transendothelial migration.
PMA-differentiated THP-1 cells were plated and cocultured on a monolayer of HUVEC endothelial cells for 2 hours, and then transendothelial migration was measured by scoring the percentage of transmigrated cells. Seventy four % of THP-1 cells transfected with control plasmid (F-C) fully crossed the monolayer (Fig. 6A). When WASP binding to WIP was blocked by FLAG-tagged WB construct (F-WB) in THP-1 cells, only 22% of cells crossed the monolayer (Fig. 6A). When WASP binding to WIP was blocked in human primary macrophages, the percentage of cells fully crossed the monolayer also decreased (Fig. 6B). Blocking WASP binding to WIP resulted in impaired transendothelial migration of macrophages across the monolayer of HUVEC endothelial cells (Fig. 6), indicating that the WASP-WIP complex plays an important role in transendothelial migration of macrophages. I demonstrated that the WASP-WIP complex is required for podosome formation (Figs. 1-5). These results taken together suggest that a complex of WASP with WIP causes podosome formation, thereby mediating efficient transendothelial migration of macrophages.
FIGURE 6.
The role of the WASP-WIP complex in transendothelial migration of macrophages. PMA-differentiated THP-1 cells (A) or human primary macrophages (B) were transfected with FLAG-tagged PDZ-GEF as a negative control (F-C, □), or FLAG-tagged WB (F-WB, ■) to block WASP binding to WIP. Cells were cotransfected with GFP expressing plasmid (pmaxGFP). Cells were cocultured on a monolayer of HUVEC cells for 2 hours. The percentage of transfected cells (GFP-positive cells) transmigrated (Basal), transmigrating (Transmigrating), or retained on the apical surface of the monolayer (Apical) was scored. Data represent the mean ± SD of triplicate measurements.
Discussion
It has been previously reported that 95% of WASP is complexed with WIP in lymphocytes (14, 15) and that WASP plays a critical role in podosome formation in macrophages (28), but the role of WIP in podosome formation is unknown. In the present study, I demonstrate that WASP binds WIP at podosomes and that a complex of WASP with WIP is required for podosome formation in macrophages. Moreau et al. suggested that binding of N-WASP to WIP is involved in the formation of podosome-like structures in aortic endothelial cells (37). The porcine aortic endothelial cells used in their study are overexpressing the constitutively active mutant of Cdc42 (V12Cdc42). I demonstrated the importance of the WASP-WIP complex in podosome formation in a physiologically relevant system using primary macrophages.
Crossing monolayer of endothelial cells is the most crucial process in recruitment of macrophages to inflamed tissues (19, 35). Podosomes are thought to play an important role in this process. Transendotheilal migration assay is a very good method to examine this process in vitro (38). I showed that blocking WASP binding to WIP reduced podosome formation (Fig. 3) and transendothelial migration in PMA-differentiated THP-1 cells or human primary macrophages (Fig. 6). These results suggest that podosomes formed by a complex of WASP with WIP mediate efficient transendothelial migration of macrophages.
In T cells, WIP plays a crucial role in localizing WASP activity both in a vaccinia-based actin motility system and to the immune synapse after TCR ligation (15, 16). Considering that podosomes are also actin-rich and actin-based structures as well as the immune synapse, it is very likely that WIP plays an important role in localizing WASP activity to podosomes by forming a complex with WASP.
In WASP-deficient mice, podosome-like clusters of F-actin dots were observed in macrophages (36). The reason for this is thought to be that some other proteins from the WASP family, most likely N-WASP can at least partially compensate for the WASP deficiency in the mouse system, since N-WASP can be recruited to podosomes (26, 36).
Patients from X-linked thrombocytopenia (XLT), a milder form of WAS, have missense mutations in the WASP N-terminus (residues 1-137), which is required for binding to WIP or WICH/WIRE, mammalian verprolins predominantly expressed in monocytes/macrophages. XLT patients express the mutant WASPs at a lower concentration than normal subjects, and the defects are observed in only platelets, but not in other hematopoietic cells (39-43). Recently, Linder et al. reported that XLT macrophages, previously thought to be unaffected in this disorder, are compromised in podosome formation (44). Complex formation of mutant WASPs with WIP is impaired in XLT patients, because XLT mutations reduced WASP binding to WIP (45). These studies suggest that inefficient formation of a complex of WASP with WIP causes reduced podosome formation, consistent with these results (Figs. 2 and 3). Reduced podosome formation causes the failure of chemotaxis in WAS macrophages, contributing to recurrent infections in WAS patients (33, 46).
In conclusion, I have shown that WIP is one of the components of podosomes and that a complex of WASP with WIP is required for podosome formation in macrophages. Furthermore, the WASP-WIP complex is required for transendothelial migration of macrophages. These findings suggest that WASP and WIP function as a unit in podosome formation and that the WASP-WIP complex plays a critical role in recruitment of macrophages to inflamed tissues. In WASP-deficient WAS patients, the deficiency of the WASP-WIP complex causes loss of podosomes. Consequently, loss of podosomes would impair transendotheilal migration of macrophages. Impaired transendothelial migration of macrophages most likely contributes to recurrent infections in WAS patients. These findings thus provide an important information on a potential disease mechanism underlying recurrent infections in WAS patients.
Acknowledgements
I thank Ms. Jennifer Meerloo for expert assistance with the confocal microscope.
Footnotes
This work was supported by a grant from the National Institute of Health (R01HD42752).
- WAS
- Wiskott-Aldrich Syndrome
- WASP
- Wiskott-Aldrich Syndrome protein
- WIP
- WASP interacting protein
- WICH
- WIP and CR16 homologous protein
- WIRE
- WIP-related protein
- siRNA
- small interfering RNA
Disclosures
The author has no financial conflict of interest.
References
- 1.Wiskott A. Familiarer, angeborener Morbus Welhofii? Monatsschr Kinderheilkd. 1937;68:212–216. [Google Scholar]
- 2.Aldrich RA, Steinberg AG, Campbell DC. Pedigree demonstrating a sex-linked recessive condition characterized by draining ears, eczematoid dermatitis and bloody diarrhea. Pediatrics. 1954;13:133–139. [PubMed] [Google Scholar]
- 3.Ochs HD, Thrasher AJ. The Wiskott-Aldrich syndrome.[see comment] Journal of Allergy & Clinical Immunology. 2006;117:725–738. doi: 10.1016/j.jaci.2006.02.005. quiz 739. [DOI] [PubMed] [Google Scholar]
- 4.Derry JM, Ochs HD, Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. [Published erratum appears in 1994 Cell 79: following 922.] Cell. 1994;78:635–644. [PubMed] [Google Scholar]
- 5.Burns S, Cory GO, Vainchenker W, Thrasher AJ. Mechanisms of WASp-mediated hematologic and immunologic disease. Blood. 2004;104:3454–3462. doi: 10.1182/blood-2004-04-1678. [DOI] [PubMed] [Google Scholar]
- 6.Miki H, Miura K, Takenawa T. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J. 1996;15:5326–5335. [PMC free article] [PubMed] [Google Scholar]
- 7.Kim AS, Kakalis LT, Abdul-Manan N, Liu GA, Rosen MK. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature. 2000;404:151–158. doi: 10.1038/35004513. [DOI] [PubMed] [Google Scholar]
- 8.Prehoda KE, Scott JA, Mullins RD, Lim WA. Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science. 2000;290:801–806. doi: 10.1126/science.290.5492.801. [DOI] [PubMed] [Google Scholar]
- 9.Ramesh N, Anton IM, Hartwig JH, Geha RS. WIP, a protein associated with wiskott-aldrich syndrome protein, induces actin polymerization and redistribution in lymphoid cells. Proc. Natl. Acad. Sci. U S A. 1997;94:14671–14676. doi: 10.1073/pnas.94.26.14671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho HY, Rohatgi R, Ma L, Kirschner MW. CR16 forms a complex with N-WASP in brain and is a novel member of a conserved proline-rich actin-binding protein family. Proc. Natl. Acad. Sci. U S A. 2001;98:11306–11311. doi: 10.1073/pnas.211420498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato M, Miki H, Kurita S, Endo T, Nakagawa H, Miyamoto S, Takenawa T. WICH, a novel verprolin homology domain-containing protein that functions cooperatively with N-WASP in actin-microspike formation. Biochem. Biophys. Res. Commun. 2002;291:41–47. doi: 10.1006/bbrc.2002.6406. [DOI] [PubMed] [Google Scholar]
- 12.Aspenstrom P. The WASP-binding protein WIRE has a role in the regulation of the actin filament system downstream of the platelet-derived growth factor receptor. Exp. Cell Res. 2002;279:21–33. doi: 10.1006/excr.2002.5576. [DOI] [PubMed] [Google Scholar]
- 13.Vaduva G, Martin NC, Hopper AK. Actin-binding verprolin is a polarity development protein required for the morphogenesis and function of the yeast actin cytoskeleton. J. Cell Biol. 1997;139:1821–1833. doi: 10.1083/jcb.139.7.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anton IM, Jones GE. WIP: A multifunctional protein involved in actin sytoskeleton regulation. European Journal of Cell Biology. 2006;85:295–304. doi: 10.1016/j.ejcb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Sasahara Y, Rachid R, Byrne MJ, de la Fuente MA, Abraham RT, Ramesh N, Geha RS. Mechanism of recruitment of WASP to the immunological synapse and of its activation following TCR ligation. Mol. Cell. 2002;10:1269–1281. doi: 10.1016/s1097-2765(02)00728-1. [DOI] [PubMed] [Google Scholar]
- 16.Moreau V, Frischknecht F, Reckmann I, Vincentelli R, Rabut G, Stewart D, Way M. A complex of N-WASP and WIP integrates signalling cascades that lead to actin polymerization. Nat. Cell Biol. 2000;2:441–448. doi: 10.1038/35017080. [DOI] [PubMed] [Google Scholar]
- 17.Kato M, Takenawa T. WICH, a member of WASP-interacting protein family, cross-links actin filaments. Biochem. Biophys. Res. Commun. 2005;328:1058–1066. doi: 10.1016/j.bbrc.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 18.Aspenstrom P. The mammalian verprolin homologue WIRE participates in receptor-mediated endocytosis and regulation of the actin filament system by distinct mechanisms. Exp. Cell Res. 2004;298:485–498. doi: 10.1016/j.yexcr.2004.04.050. [DOI] [PubMed] [Google Scholar]
- 19.Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–385. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 20.Lehto VP, Hovi T, Vartio T, Badley RA, Virtanen I. Reorganization of cytoskeletal and contractile elements during transition of human monocytes into adherent macrophages. Laboratory Investigation. 1982;47:391–399. [PubMed] [Google Scholar]
- 21.Amato PA, Unanue ER, Taylor DL. Distribution of actin in spreading macrophages: a comparative study on living and fixed cells. J. Cell Biol. 1983;96:750–761. doi: 10.1083/jcb.96.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchisio PC, Cirillo D, Naldini L, Primavera MV, Teti A, Zambonin- Zallone A. Cell-substratum interaction of cultured avian osteoclasts is mediated by specific adhesion structures. J. Cell Biol. 1984;99:1696–1705. doi: 10.1083/jcb.99.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burns S, Thrasher AJ, Blundell MP, Machesky L, Jones GE. Configuration of human dendritic cell cytoskeleton by Rho GTPases, the WAS protein, and differentiation. Blood. 2001;98:1142–1149. doi: 10.1182/blood.v98.4.1142. [DOI] [PubMed] [Google Scholar]
- 24.Tarone G, Cirillo D, Giancotti FG, Comoglio PM, Marchisio PC. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp. Cell Res. 1985;159:141–157. doi: 10.1016/s0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- 25.Marchisio PC, Cirillo D, Teti A, Zambonin-Zallone A, Tarone G. Rous sarcoma virus-transformed fibroblasts and cells of monocytic origin display a peculiar dot-like organization of cytoskeletal proteins involved in microfilament-membrane interactions. Exp. Cell Res. 1987;169:202–214. doi: 10.1016/0014-4827(87)90238-2. [DOI] [PubMed] [Google Scholar]
- 26.Mizutani K, Miki H, He H, Maruta H, Takenawa T. Essential role of neural Wiskott-Aldrich syndrome protein in podosome formation and degradation of extracellular matrix in src-transformed fibroblasts. Cancer Research. 2002;62:669–674. [PubMed] [Google Scholar]
- 27.Seals DF, Azucena EF, Jr., Pass I, Tesfay L, Gordon R, Woodrow M, Resau JH, Courtneidge SA. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell. 2005;7:155–165. doi: 10.1016/j.ccr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Linder S, Nelson D, Weiss M, Aepfelbacher M. Wiskott-Aldrich syndrome protein regulates podosomes in primary human macrophages. Proc. Natl. Acad. Sci. U S A. 1999;96:9648–9653. doi: 10.1073/pnas.96.17.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Auwerx J. The human leukemia cell line, THP-1: a multifacetted model for the study of monocyte-macrophage differentiation. Experientia. 1991;47:22–31. doi: 10.1007/BF02041244. [DOI] [PubMed] [Google Scholar]
- 30.Dreskin SC, Thomas GW, Dale SN, Heasley LE. Isoforms of Jun kinase are differentially expressed and activated in human monocyte/macrophage (THP-1) cells. J. Immunol. 2001;166:5646–5653. doi: 10.4049/jimmunol.166.9.5646. [DOI] [PubMed] [Google Scholar]
- 31.Tsuboi S. A complex of Wiskott-Aldrich syndrome protein with mammalian verprolins plays an important role in monocyte chemotaxis. J. Immunol. 2006;176:6576–6585. doi: 10.4049/jimmunol.176.11.6576. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Quiles N, Rohatgi R, Anton IM, Medina M, Saville SP, Miki H, Yamaguchi H, Takenawa T, Hartwig JH, Geha RS, Ramesh N. WIP regulates N-WASP-mediated actin polymerization and filopodium formation. Nat. Cell Biol. 2001;3:484–491. doi: 10.1038/35074551. [DOI] [PubMed] [Google Scholar]
- 33.Jones GE, Zicha D, Dunn GA, Blundell M, Thrasher A. Restoration of podosomes and chemotaxis in Wiskott-Aldrich syndrome macrophages following induced expression of WASp. Int. J. Biochem. Cell Biol. 2002;34:806–815. doi: 10.1016/s1357-2725(01)00162-5. [DOI] [PubMed] [Google Scholar]
- 34.Rebhun JF, Castro AF, Quilliam LA. Identification of guanine nucleotide exchange factors (GEFs) for the Rap1 GTPase. Regulation of MR-GEF by M- Ras-GTP interaction. J. Biol. Chem. 2000;275:34901–34908. doi: 10.1074/jbc.M005327200. [DOI] [PubMed] [Google Scholar]
- 35.Worthylake RA, Burridge K. Leukocyte transendothelial migration: orchestrating the underlying molecular machinery. Cur. Opin. in Cell Biol. 2001;13:569–577. doi: 10.1016/s0955-0674(00)00253-2. [DOI] [PubMed] [Google Scholar]
- 36.Calle Y, Chou HC, Thrasher AJ, Jones GE. Wiskott-Aldrich syndrome protein and the cytoskeletal dynamics of dendritic cells. J. Pathol. 2004;204:460–469. doi: 10.1002/path.1651. [DOI] [PubMed] [Google Scholar]
- 37.Moreau V, Tatin F, Varon C, Genot E. Actin can reorganize into podosomes in aortic endothelial cells, a process controlled by Cdc42 and RhoA. Molecular & Cellular Biology. 2003;23:6809–6822. doi: 10.1128/MCB.23.19.6809-6822.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calle Y, Charranger NO, Thrasher A, Jones G. Inhibition of calpain stabilities podosomes and impairs dendritic cell motility. J. Cell Sci. 2006;119:2375–2385. doi: 10.1242/jcs.02939. [DOI] [PubMed] [Google Scholar]
- 39.Olivier A, Jeanson-Leh L, Bouma G, Compagno D, Blondeau J, Seye K, Charrier S, Burns S, Thrasher AJ, Danos O, Vainchenker W, Galy A. A partial down- regulation of WASP is sufficient to inhibit podosome formation in dendritic cells. Molecular Therapy: the Journal of the American Society of Gene Therapy. 2006;13:729–737. doi: 10.1016/j.ymthe.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Zhu Q, Watanabe C, Liu T, Hollenbaugh D, Blaese RM, Kanner SB, Aruffo A, Ochs HD. Wiskott-Aldrich syndrome/X-linked thrombocytopenia: WASP gene mutations, protein expression, and phenotype. Blood. 1997;90:2680–2689. [PubMed] [Google Scholar]
- 41.Nonoyama S, Ochs HD. Wiskott-Aldrich syndrome. Curr. Allergy Asthma Rep. 2001;1:430–437. doi: 10.1007/s11882-001-0028-0. [DOI] [PubMed] [Google Scholar]
- 42.Thrasher AJ. WASp in immune-system organization and function. Nat. Rev. Immunol. 2002;2:635–646. doi: 10.1038/nri884. [DOI] [PubMed] [Google Scholar]
- 43.Imai K, Nonoyama S, Ochs HD. WASP (Wiskott-Aldrich syndrome protein) gene mutations and phenotype. Curr. Opin. Allergy Clin. Immunol. 2003;3:427–436. doi: 10.1097/00130832-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Imai K, Morio T, Zhu Y, Jin Y, Itoh S, Kajiwara M, Yata J, Mizutani S, Ochs HD, Nonoyama S. Clinical course of patients with WASP gene mutations. Blood. 2004;103:456–464. doi: 10.1182/blood-2003-05-1480. [DOI] [PubMed] [Google Scholar]
- 45.Linder S, Wintergerst U, Bender-Gotze C, Schwarz K, Pannicke U, Aepfelbacher M. Macrophages of patients with X-linked thrombocytopenia display an attenuated Wiskott-Aldrich syndrome phenotype. Immunol. Cell Biol. 2003;81:130–136. doi: 10.1046/j.0818-9641.2002.01147.x. [DOI] [PubMed] [Google Scholar]
- 46.Stewart DM, Tian L, Nelson DL. Mutations that cause the Wiskott- Aldrich syndrome impair the interaction of Wiskott-Aldrich syndrome protein (WASP) with WASP interacting protein. J. Immunol. 1999;162:5019–5024. [PubMed] [Google Scholar]
- 47.Zicha D, Allen WE, Brickell PM, Kinnon C, Dunn GA, Jones GE, Thrasher AJ. Chemotaxis of macrophages is abolished in the Wiskott-Aldrich syndrome. Br. J. Haematol. 1998;101:659–665. doi: 10.1046/j.1365-2141.1998.00767.x. [DOI] [PubMed] [Google Scholar]
- 48.Cory GO, Garg R, Cramer R, Ridley AJ. Phosphorylation of tyrosine 291 enhances the ability of WASp to stimulate actin polymerization and filopodium formation. Wiskott-Aldrich Syndrome protein. J. Biol. Chem. 2002;277:45115–45121. doi: 10.1074/jbc.M203346200. [DOI] [PubMed] [Google Scholar]
- 49.Torres E, Rosen MK. Contingent phosphorylation/dephosphorylation provides a mechanism of molecular memory in WASP. Mol. Cell. 2003;11:1215–1227. doi: 10.1016/s1097-2765(03)00139-4. [DOI] [PubMed] [Google Scholar]
- 50.Torres E, Rosen MK. Protein-tyrosine kinase and GTPase signals cooperate to phosphorylate and activate Wiskott-Aldrich syndrome protein (WASP)/neuronal WASP. J. Biol. Chem. 2006;281:3513–3520. doi: 10.1074/jbc.M509416200. [DOI] [PubMed] [Google Scholar]
- 51.Swetman CA, Leverrier Y, Garg R, Gan CH, Ridley AJ, Katz DR, Chain BM. Extension, retraction and contraction in the formation of a dendritic cell dendrite: distinct roles for Rho GTPases. Eur. J. of Immunol. 2002;32:2074–2083. doi: 10.1002/1521-4141(200207)32:7<2074::AID-IMMU2074>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]