Abstract
Mitochondria and other membranous organelles are frequently enriched in the nodes and paranodes of peripheral myelinated axons, particularly those of large caliber. The physiologic role(s) of this organelle enrichment and the rheologic factors that regulate it are not well understood. Previous studies suggest that axonal transport of organelles across the nodal/paranodal region is locally regulated. In this study, we have examined the ultrastructure of myelinated axons in the sciatic nerves of mice deficient in the contactin-associated protein (Caspr), an integral junctional component. These mice, which lack the normal septate-like junctions that promote attachment of the glial (paranodal) loops to the axon, contain aberrant mitochondria in their nodal/paranodal regions. These mitochondria are typically large and swollen and occupy prominent varicosities of the nodal axolemma. In contrast, mitochondria located outside the nodal/paranodal regions of the myelinated axons appear normal. These findings suggest that paranodal junctions regulate mitochondrial transport and function in the axoplasm of the nodal/paranodal region of myelinated axons of peripheral nerves. They further implicate the paranodal junctions in playing a role, either directly or indirectly, in the local regulation of energy metabolism in the nodal region.
Keywords: Contactin associated protein, paranode, myelin, sciatic nerve, axonal transport
Introduction
Myelin, generated by Schwann cells in the peripheral nervous system (PNS) and oligodendrocytes in the central nervous system (CNS), provides an insulating sheath around axons essential for proper axon function and integrity. Individual myelinated segments, comprised of the sheath and underlying axon, are organized into specialized, longitudinal domains (Poliak and Peles, 2003; Salzer, 2003). Among these domains are the paranodes which form between the ends of each segment and the axon. In the paranodal region, the myelin lamellae are not compacted, but rather terminate as a series of (paranodal) loops that contain cytoplasm and spiral around the axon. These loops form specialized paranodal junctions with the axon (Bhat, 2003). In longitudinal sections, these junctions appear as a series of transverse bands that span the intercellular space between the axolemma and the closely apposed glial loops; they have therefore been termed “septate-like” for their structural similarity to the septate junctions of invertebrates (Einheber et al., 1997; Bellen et al., 1998). Like septate-junctions, they are believed to provide a partial paracellular barrier that prevents diffusion of extracellular ions into the internodal space between the axolemma and inner glial membrane (Hirano and Llena, 1995).
Several major membrane protein components of the paranodal junctions have been identified. These include the axonal proteins contactin (Rios et al., 2000; Boyle et al., 2001) and the contactin-associated protein, Caspr (Einheber et al., 1997; Peles et al., 1997), which form a heteromeric complex at the paranodes (Rios et al., 2000). Caspr was independently identified and localized to this site as paranodin (Menegoz et al., 1997). It is a homologue of Drosophila Neurexin IV which has an orthologous function as a major component of invertebrate septate junctions; Caspr is therefore also referred to as NCP1 (for Neurexin, Caspr, Paranodin) (Bellen et al., 1998). Caspr and contactin, in turn, interact with components of the glial paranodal loops, including neurofascin (NF) 155, which is essential for junction formation (Tait et al., 2000; Sherman et al., 2005). The Caspr/contactin complex was originally proposed to bind to NF155 directly (Charles et al., 2002); recent evidence suggests NF155 may bind principally to contactin or via additional component (s) yet to be identified (Gollan et al., 2003).
The paranodes flank the nodes of Ranvier, small gaps that occur between adjacent myelin segments. At the node, the axolemma is relatively exposed to the surrounding environment. In the PNS, nodes are contacted by and form in association with the Schwann cell microvilli, which extend from the lateral borders of the myelin sheath to appose the nodal axolemma (Melendez-Vasquez et al., 2001; Scherer et al., 2001). Consistent with its role in impulse propagation, the nodal membrane is highly enriched in a protein complex containing voltage gated Na+ channels. Other components include the cell adhesion molecules NrCAM and NF186 (Davis et al., 1996), which interact in the PNS in trans with gliomedin, a membrane protein of the nodal microvilli (Eshed et al., 2005). Both gliomedin and neurofascin were recently demonstrated to be critical for node formation (Eshed et al., 2005; Sherman et al., 2005). The cytoplasmic domains of Na+ channels, NrCAM and NF186 are believed to be linked via their interactions with a subaxolemmal cytoskeleton composed of ankyrin G and beta IV spectrin (Berghs et al., 2000; Bennett and Chen, 2001).
Analyses of mice deficient in paranodal components have begun to elucidate the role of the paranodal junctions in the organization and integrity of the axon. Mice deficient in Caspr, contactin and NF155 each have substantially abnormal paranodal junctions (Bhat et al., 2001; Boyle et al., 2001; Sherman et al., 2005). Interestingly, mice defective in the synthesis of the myelin lipids, galactocerebroside (GalC) and sulfatide, also have aberrant paranodal junctions (Dupree et al., 1998), likely reflecting a role in trafficking of glial paranodal components such as NF155 (Schafer et al., 2004). In each of these mutant mice, transverse bands do not form and paranodal loops progressively detach from the axon indicating that the junctions are required for stable anchorage of the paranodal loops to the axon. Further, in the absence of normal paranodal interactions, nodal components diffuse along the axon and Na+ channel isoforms are misexpressed, particularly in the CNS (Rios et al., 2003). Finally, Shaker-type K+ channels, which normally are clustered in the juxtaparanode, are instead located in the paranodal region directly adjacent to Na+ channels at the node (Dupree et al., 1998; Bhat et al., 2001; Boyle et al., 2001). These disruptions in ion channel distribution and expression are accompanied by aberrant nerve conduction and likely contribute to the neurological abnormalities displayed by these mice, including mild tremor, hypomotility and aberrant gait (Bhat et al., 2001; Boyle et al., 2001).
These findings underscore the importance of the paranodal junctions in establishing and maintaining axonal domains. In this report, we have examined the role of these junctions in the organization of the nodal axoplasm, notably the distribution of organelles. We now report that a proportion of nodes in the sciatic nerve of Caspr-deficient mice exhibit abnormal accumulations of large and swollen mitochondria that cause bulging and distortion of the axolemma. These findings strongly suggest that the paranodal junctions regulate axonal transport of mitochondria across the nodal/paranodal region. In addition, as mitochondria are the major source of ATP in the cell, the findings raise the intriguing possibility that paranodal junctions may also contribute to the local regulation of energy metabolism in the nodal region.
Objective
The main goal of this study was to investigate the role of paranodal junctions in organizing the nodal axoplasm, particularly the distribution of membranous organelles. Accordingly, electron microscopy was used to compare the distribution and morphology of mitochondria in the nodal/paranodal axoplasm of myelinated axons in the sciatic nerves of Caspr-deficient mice, which exhibit disrupted paranodal junctions, to those of wild type mice.
Methods
Sciatic nerves from wild type (+/+), heterozygous (+/−) and homozygous Caspr-deficient mice (−/−) were processed for electron microscopy as described previously (Bhat et al., 2001) or with modifications (Einheber et al., 1996). In brief, mice were anesthetized with pentobarbital and perfused through the heart with either 4% paraformaldehyde/2.5% glutaraldehyde or 3.75% acrolein/2% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. The nerves were then postfixed in the same solution for another 18–24 hours at 4°C (paraformaldehyde/glutaraldehyde fixation) or 30 minutes at room temperature (acrolein/paraformaldehyde fixation) and embedded in EMbed (Electron Microscopy Sciences, Fort Washington, PA). Ultrathin sections (70 nm) of the embedded nerves from the mid-thigh region were cut, collected on copper grids and counterstained with 5% uranyl acetate and Reynold’s lead citrate and examined with a Philips CM10 electron microscope.
For these studies sciatic nerves from the following number of litter-matched mice of different postnatal (P) days of age were examined: P20 (two −/−, one +/+ and one +/−) and P44-45 (P44) (two −/− and two +/+). The sciatic nerves from a pair of 2 year old non litter-matched mice (one −/− and one +/+) were also studied.
Results
We compared the ultrastructure of the nodal/paranodal regions in the sciatic nerves of P44 wild type and Caspr −/− mice. By P44, myelinaton in the sciatic nerve in wild type mice is reported to be largely complete (Friede and Samorajski, 1968; Webster Hde, 1971). In agreement, the morphology of the myelinated axons including the segment between the two paranodal regions of adjacent myelin segments, referred to as the paranode/node/paranode (PNP) region, appears mature in wild type axons (Fig. 1). Paranodal loops were generally closely apposed to the axolemma and septate-like junctions were visible at many sites of contact (Fig. 1b). In addition, the diameter of many of the large myelinated axons in the PNP region was considerably reduced compared to that of the internodal region (Fig. 1). Mitochondria, small vesicles and other membranous organelles were numerous in the PNP axoplasm of many fibers. Mitochondria were located in both the central and peripheral (i.e. close to the axolemma) regions; their morphology was variable but typically elongate (Fig. 1). In some fibers, mitochondria and other membranous organelles appeared to be more enriched on one side of the node.
Figure 1. Mitochondria are distributed throughout the axoplasm of the PNP region in wild type mice.
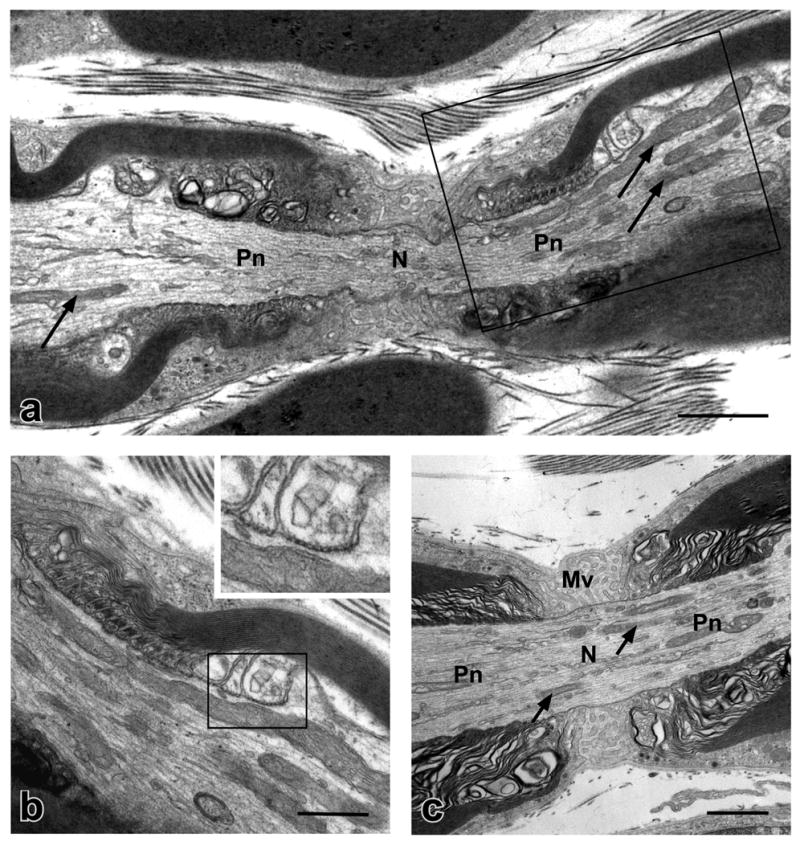
Electron micrographs of longitudinal sections through the PNP regions of myelinated axons in the sciatic nerves of P44 wild type mice are shown. (a) Note that the diameter of the axon in the PNP region is reduced considerably compared to that in the internode. Paranodal loops that make contact with the axolemma through septate-like junctions are visible on either side of the node (N). Mitochondria, many of which are elongated, are found throughout the axoplasm of the PNP region (arrows) and are particularly enriched in the paranodal (Pn) and juxtaparanodal regions on the right side. Small vesicles are also present in the PNP axoplasm. The paranodal loops surrounded by the box are shown at higher magnification in (b). (b) Septate-like junctions are clearly visible between some of the paranodal loops and the axolemma. Two such loops within the box are shown at higher magnification in the inset. (c) Thin elongated mitochondria (arrows) and small vesicles are distributed throughout the nodal and paranodal axoplasm of this myelinated axon. Microvilli (Mv) are visible between the paranodal loops at the node. Scale bars: a and c, 1 μm; b, 0.5 μm.
In agreement with our previous study (Bhat et al., 2001), septate-like junctions of myelinated axons in P44 Caspr −/− mice were aberrant whereas the internode and compact myelin sheath appeared normal. Thus, while paranodal loops were generally oriented towards the axolemma, they were not appropriately attached. In favorable planes of section small gaps between the paranodal loops and the axolemma were visible in the absence of the septate-like junctions (Fig. 2). Schwann cell microvilli, which are normally restricted to the nodal region, occasionally penetrated the space between the tips of the paranodal loops and axolemma as previously noted (Bhat et al., 2001). With the exception of these defects, the gross appearance of the majority of PNPs, including their axoplasm in the P44 Caspr −/− sciatic nerves, was comparable to that of the wild type mice.
Figure 2. Large swollen nodal mitochondria in myelinated axons of Caspr-deficient mice.

Electron micrographs of longitudinal (a–d) and transverse (e and f) sections through the PNP regions of myelinated axons in the sciatic nerves of P44 Caspr-deficient (a–e) and wild type (f) mice are shown. Only the Caspr-deficient mice exhibit NMVs. (a) Profiles of swollen mitochondria with disrupted cristae (arrows) are located directly under the nodal axolemma, which bulges outward, on both sides of the node. Note the mostly normal appearance of the mitochondria throughout the rest of the axoplasm. (b) Higher magnification of the field in (a). Small vesicles are located between the larger mitochondrial profile and the nodal axolemma, which is indicated by arrowheads. Paranodal loops that are not closely apposed to the axolemma are also visible (arrows). (c) A NMV that contains two mitochondria on one side of the node and various membranous organelles on the other is shown. An elongated nodal mitochondrion that exhibits slight swelling is also present beneath the NMV (arrow). (d) Profiles of two swollen mitochondria (arrows) under a bulging nodal axolemma are visible. The paranodal loops indicated by the box are shown at higher magnification and inverted in the inset. Normal septate-like junctions are absent between the loops and the nodal axolemma. In the transverse sections swollen mitochondria that contain aberrant cristae are present in the node of the mutant (e, arrows) but not that of the wild type (f). Numerous Schwann cell microvilli (Mv) contact the nodal axolemma. Scale bars: a, e, f, 1.0 μm; b–d, 0.5 μm.
Of note, a subset of −/− myelinated fibers exhibited highly aberrant nodal regions characterized by the presence of one or more large, often swollen-appearing mitochondria located directly under the nodal axolemma. Since these mitochondria typically occupied bulges or outpockets of the nodal membrane, we refer to these outpockets as nodal mitochondria varicosities (NMVs). In most instances only a thin rim of cytoplasm separated the outer mitochondrial membrane from the nodal axolemma. However, in other cases small vesicles were situated between the outer mitochondrial membrane and the inner surface of the nodal axolemma. Multivesicular bodies were also occasionally present in the nodal outpocketings (data not shown). In some longitudinal sections of −/− myelinated axons (Fig. 2), mitochondrial profiles were present in bulges on both sides of the node. While these likely represent different mitochondria, we cannot exclude the possibility these profiles represent one mitochondrion wrapped around the node; serial reconstructions would be necessary to distinguish these possibilities. The matrix of the swollen mitochondria often appeared clear and the cristae misshapen (Fig. 2). Dense inclusions were also observed in some of the nodal mitochondria (data not shown). Although we did not directly assess whether there is a link between axon caliber and the presence of NMVs, it is our impression that larger caliber axons were more severely affected. To estimate the frequency of NMVs in the myelinated axons of the sciatic nerve of −/− mice, the total number of NMVs in thin sections of the sciatic nerves from one litter matched pair of −/− and +/+ mice at P44 were counted. From these counts it was found that one third of the nodes (4 of 12 nodes observed) in the sciatic nerve section of the −/− mouse exhibited NMVs compared to none in the section from the +/+ animal (0 of 16 nodes observed).
Whereas the vast majority of the swollen mitochondria in the PNP regions of the myelinated axons in the Caspr −/− mice were located directly adjacent to the nodal axolemma, in two instances mitochondria that appeared to be somewhat swollen with clear matrices and aberrant cristae were located closer to the central portion of the nodal axoplasm (Fig. 2c, arrow) or in the paranodal axoplasm (Fig. 3, asterisk). In both of these cases, swollen mitochondria also closely abutted the nodal axolemma to form NMVs in the axons. The swollen mitochondrion in the paranodal axoplasm of Fig. 3 is located within a cluster of other, more electron dense-appearing mitochondria.
Figure 3. Swollen mitochondria in the nodal and paranodal axoplasm of a Caspr-deficient mouse.
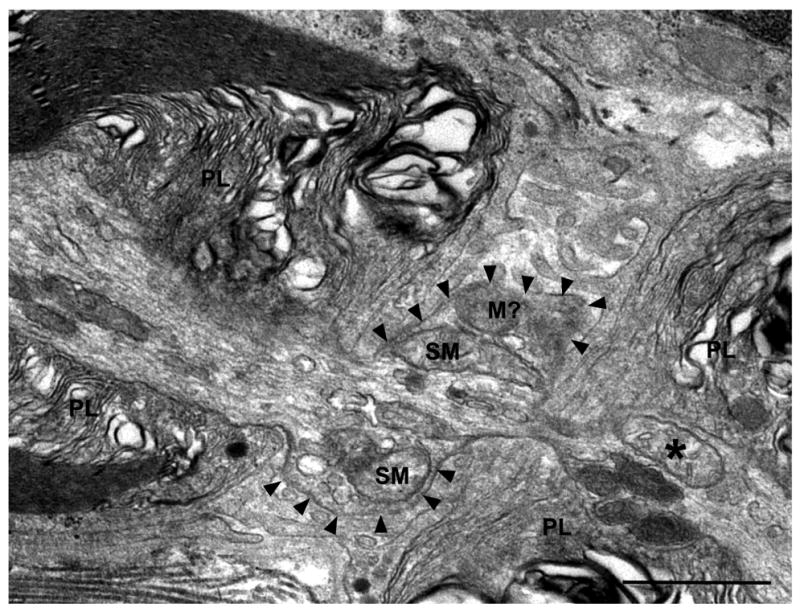
An electron micrograph of a longitudinal section through the PNP region of a myelinated axon in the sciatic nerve of a P44 Caspr-deficient mouse that exhibits a large NMV is shown. The contour of the nodal axolemma is indicated with arrowheads. Swollen mitochondria (SM) and other large membrane-bound organelles that may also be mitochondria (M?) but cannot be definitively identified, in addition to smaller vesicles, fill the axoplasm within the nodal varicosity. A swollen oval-shaped mitochondrion (indicated by an asterisk) is also present in the paranodal axoplasm in close proximity to four other more electron-dense mitochondria. Paranodal loops, PL. Scale bar: 1 μm.
To address whether NMVs were also present in mice of other ages, we examined the sciatic nerves at two distinct ages: P20 and two years old. P20 corresponds to the end of the most active period of myelination during development in mouse sciatic nerve. Whereas no NMVs were noted in the myelinated axons of the P20 wild type or heterozygotes, they were observed in those of the P20 −/− mice suggesting that NMVs were present during active myelination (Fig. 4). Interestingly, in a single sciatic nerve of a 2yr old Caspr −/− mouse that was examined, no NMVs were observed (data not shown); these results suggest that either the defect does not persist to this age or that fibers with this defect degenerated. Most Caspr −/− mice die at about P21 and very few survive beyond 1 yr (Bhat et al., 2001). For this reason, only a single 2 yr old Caspr mutant was examined.
Figure 4. NMVs are present in myelinated axons of P20 Caspr-deficient mice.
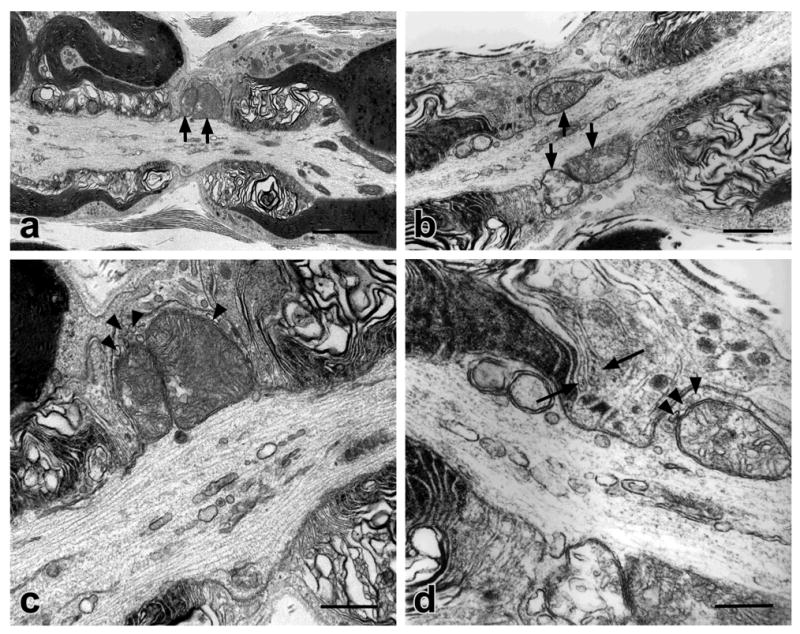
ppPairs of electron micrographs (a and c, low and high magnification images of the same field, respectively) and (b and d, low and high magnification images of the same field, respectively) demonstrate NMVs in the sciatic nerves of P20 Caspr-deficient mice. In (a) and (c) a bulging nodal axolemma surrounds two large and tightly packed mitochondria (indicated by arrows in a). Small vesicles are located between the mitochondria and nodal axolemma (indicated by arrowheads in c). (b) Profiles of large swollen mitochondria (arrows) are evident under the bulging nodal axolemma. (d) At higher magnification, small vesicles are visible between a mitochondrion and the nodal axolemma (arrowheads). Some paranodal loops are distant from the axolemma (arrows). Scale bars: aqp, 2.0 μm; b and c, 0.5 μm; d, 0.25 μm.
Conclusions
A proportion of the nodal regions of myelinated axons in the sciatic nerves of Caspr-deficient mice contain mitochondria with aberrant morphology that are not found in wild type mice.
The aberrant mitochondria are typically large and swollen and occupy prominent varicosities of the nodal axolemma.
Mitochondria located outside the nodal/paranodal regions of the myelinated axons in the Caspr-deficient mice appear normal.
Aberrant mitochondria occur in myelinated axons in three week old (during the period of active myelination) and six week old Caspr mutants.
Discussion
We report here that a subset of the PNP regions of myelinated axons in the sciatic nerves of Caspr-deficient mice exhibit large, often swollen mitochondria; these were most prominent directly under the nodal axolemma where they form large nodal varicosities. These results provide evidence that paranodal junctions modulate mitochondrial transport and function in the PNP region of myelinated axons in the sciatic nerve. They further suggest that the paranodal junctions may play a role in the local regulation of energy metabolism in the nodal region. These results are considered further below.
Accumulation of swollen mitochondria at nodes of Caspr-deficient mice
Mitochondria and other membranous organelles are normally enriched in the PNP region of peripheral myelinated axons, particularly those of large caliber axons (Fabricius et al., 1993). The physiologic role of this accumulation and the rheologic factors that regulate it are not understood. Mitochondria are normally targeted to regions of cells where energy demand, i.e. ATP consumption, is high (Hollenbeck, 1996; Ames, 2000). In axons, these regions are thought to include growth cones, synaptic terminals and sites of action potential initiation and regeneration, i.e. the distal part of the initial segment and nodes of Ranvier (Ruthel and Hollenbeck, 2003; Li et al., 2004a). In synaptic terminals mitochondria produce ATP needed to mobilize reserve pools of vesicles for neurotransmission in a process that involves activation of protein kinase A and phosphorylation of myosin light chain by myosin light chain kinase (Verstreken et al., 2005). In nodes of Ranvier, mitochondria have been suggested to play an important role in impulse conduction by producing the ATP that is necessary to maintain the activity of energy-demanding ion pumps such as the Na+/K+ -ATPase (Aiello and Bach-y-Rita, 2000). Consistent with this notion, approximately five times more mitochondria occur in the PNP axoplasm of large caliber peripheral axons than in the corresponding internodal regions of these fibers (Berthold, 1978).
The normal enrichment of mitochondria in the PNP region may be established by factors unrelated to ATP consumption, notably PNP-induced alterations in the rate of axonal transport. The rate of axonal transport is reduced in the nodal region (Armstrong et al., 1987) and likely accounts for the accumulation of membranous organelles at this site (Cooper and Smith, 1974; Armstrong et al., 1987; Fabricius et al., 1993; Zimmermann, 1996). This reduction in transport rates may result from the constriction of the axonal diameter in the nodal region. This constriction, which may be 50–70% compared to that of the internode in large caliber axons, may create a “bottleneck” in axoplasmic flow and enhance the accumulation of membranous organelles in the PNP axoplasm (Sward et al., 1995). In addition, changes in the organization of the nodal cytoskeleton including increased packing of microtubules and intermediate filaments may also play a role (Reles and Friede, 1991).
However, the accumulation of mitochondria at the nodes of Caspr-deficient mice is strikingly abnormal as evidenced by their displacement to the periphery of the axon and swollen morphology. Mitochondria swell in response to various experimental conditions such as anoxia (Webster and Ames, 1965; Waxman et al., 1992; Waxman et al., 1994), Ca2+ influx (Bernardi et al., 1999; Halestrap et al., 2002) and insufficient fixation (Li and Zochodne, 2003). We consider it unlikely that the NMVs observed in the peripheral myelinated axons of Caspr −/− mice represent an artifact of sample preparation for several reasons. First, enlarged nodal mitochondria were only observed in the −/− animals, not in the +/+ or +/− animals. Second, both −/− and +/+ litter-matched pairs of animals were perfusion fixed and their isolated sciatic nerves post-fixed under identical conditions and in parallel. Third, enlarged mitochondria were only observed in the PNP axoplasm and not elsewhere along the internode. Finally, in a number of cases, two or more large mitochondria were tightly packed into the nodal outpocketings, occupying most of the space directly under the nodal axolemma. It seems likely that such dense packing of organelles in the NMVs would only have occurred in vivo and not as the result of inadequate fixation. Nodal evaginations or outpocketings of variable size containing mitochondria (sometimes swollen), multivesicular bodies, and other membranous organelles have also been observed in peripheral nerves of other mutant strains and occasionally even in normal animals (Uhrik and Stampfli, 1981; Berthold and Rydmark, 1983; Saito et al., 2003; Yang et al., 2004). However, the NMVs observed in the myelinated axons of the Caspr −/− mice are distinguished by highly exaggerated nodal evaginations and the selective enrichment of tightly packed and frequently swollen mitochondria.
Potential mechanisms of NMV formation
The mechanism(s) accounting for these defects are not yet known. Aberrant transport and accumulation of organelles in the PNP region is a frequent accompaniment of myelin protein mutations (Salzer, 2003), being reported, for example, in mice with mutations of PLP or CNPase (Lappe-Siefke et al., 2003; Edgar et al., 2004). However, a striking finding in the Caspr mutant mice is that mitochondria accumulate in the nodal region out of proportion to other membranous organelles. These findings suggest a more specific disruption of mechanisms responsible for the transport and localization of mitochondria.
Mitochondrial transport involves both actin- and microtubule-dependent mechanisms (Hollenbeck and Saxton, 2005). Microtubule motors regulate transport, i.e. kinesin 1 and possibly kinesin 3 in the anterograde direction and, dyneins in the retrograde direction (Hollenbeck and Saxton, 2005). Potentially, alterations in the axonal cytoskeleton of the Caspr-deficient mice, including loss of actin cytoskeleton in the paranodes (Garcia-Fresco et al., 2006), may contribute to these defects. As mitochondria associate with actin filaments in addition to microtubules during axonal transport (Ligon and Steward, 2000), disruption of the actin cytoskeleton in the paranodal region may perturb mitochondrial transport through the PNP region. Alteration or weakening of the subplasmalemal cytoskeleton may also alter axonal constriction or packing of actin filaments or microtubules in the PNP region or permit evagination of the nodal membrane and promote trapping of the mitochondria in the outpocketings that form as observed in the beta lV spectrin deficient-mice (Lacas-Gervais et al., 2004; Yang et al., 2004). However, it should be noted that the major components of the nodal cytoskeleton remain intact in the Caspr mutant mice (Rios et al., 2003) and that the prevalence and sizes of the nodal protrusions is far greater in the CNS of the beta lV spectrin mutant mouse compared to the PNS than observed here (data not shown) indicating that these mutations do not phenocopy each other.
Redistribution of axolemmal components that result from disrupted paranodal junctions (Bhat, 2003; Salzer, 2003) may also contribute to nodal abnormalities. In the absence of paranodal junctions, voltage gated Na+ channels are more diffusely expressed at the node and K+ channels are displaced into the paranodes (Dupree et al., 1999; Bhat et al., 2001; Rios et al., 2003). Alterations in the distribution of another axolemmal component, Na+/K+ -ATPase, could also contribute to NMV formation in the Caspr mutant. This possibility is suggested by its proposed role in the pathophysiology of anoxic injury in myelinated axons (Stys et al., 1992; LoPachin and Lehning, 1997; Stys, 2004). During anoxia depletion of ATP levels in myelinated axons results in reduced Na+/K+ -ATPase activity. Such decreases in Na+/K+ -ATPase activity may cause disruption of Na+ and K+ gradients across the axolemma and lead to reverse operation of the Na+ -Ca2+ exchanger located in the nodal membrane. In this model, reversal of the Na+ -Ca2+ exchanger produces an influx of toxic extracellular Ca2+ (Stys et al., 1992; LoPachin and Lehning, 1997; Stys, 2004). By analogy, dispersion of Na+/K+ -ATPase in the nodal membrane of the Caspr mutant could, by reducing the effective enzyme activity over a wider expanse of membrane area, potentially contribute to metabolic stress and reverse operation of the Na+ - Ca2+ exchanger. The targeting of mitochondria to the nodes of the mutant might thus reflect compensatory mechanisms by the neuron to increase local ATP concentrations to fuel the dispersed Na+/K+ -ATPase or buffer the influx of intracellular Ca2+ (Simpson and Russell, 1998). An influx of Ca2+ at the node could account for the mitochondrial swelling observed in the Caspr mutant (Bernardi et al., 1999; Halestrap et al., 2002). Moreover, as elevated Ca2+ levels are associated with cessation of mitochondrial movement in neurons, an increase in nodal Ca2+ levels might also play a role in the focal accumulation or positioning of mitochondria at the nodes of the mutants (Li et al., 2004b; Glater et al., 2006). In support of this hypothesis, optic nerves subjected to anoxic insult exhibit swollen mitochondria in the nodal region and detachment of terminal paranodal loops (Waxman et al., 1992; Waxman et al., 1994). Taken together, these studies suggest a possible correlation between disruption of the paranodal junctions and the presence of swollen mitochondria in the nodal region under some conditions.
It should be noted that Na+/K+ -ATPase is a component of septate junctions in Drosophila and is required for their function (Genova and Fehon, 2003; Paul et al., 2003) and that Na+/K+ -ATPase subunits have recently been detected by mass spectroscopy in paranodal membrane preparations (Ogawa et al., 2006). However, it is not yet known whether Na+/K+ -ATPase plays a role in the function of paranodal junctions.
Interestingly, in the present study NMVs were only observed in myelinated axons of the PNS, and not in the CNS (Einheber, S. and Salzer, J., unpublished observations). While we have not observed significant defects in the nodal regions of myelinated axons in several areas of the CNS, accumulation of SER, mitochondria and other organelles were recently reported in the axons of Purkinje cells, leading to neuronal degeneration (Garcia-Fresco et al., 2006). Thus defects of axonal transport appear to be a common sequella of Caspr-deficiency although there are regional differences. As defects are also observed in CGT-deficient mice (Garcia-Fresco et al., 2006), these changes may reflect a common role of the paranodal junctions in regulating axonal transport.
In summary, we report aberrant accumulation of swollen mitochondria in the nodal region of Caspr-deficient mice. The mechanism(s) involved may reflect abnormalities in the components of the paranodal region resulting in local alterations of axonal transport, ion homeostasis or energy metabolism. In the future, direct measurements of mitochondrial transport rates and additional analysis of other paranodal mutants, will be useful to further clarify the mechanisms involved. As mitochondrial disorders have been associated with neurodegeneration (Beal, 2005), including in demyelinating disorders such as multiple sclerosis (Andrews et al., 2005; Dutta et al., 2006), it will also be important to determine whether these abnormalities are associated with axonal loss in the PNS as they are in the CNS (Garcia-Fresco et al., 2006).
Acknowledgments
This study was supported by National Institute of Health Grants NS43474 (J.L.S.) and GM63074 (M.A.B.) and by National Multiple Sclerosis Society Grant RG2311-C-6 to J.L.S. We thank Dr. Teresa Milner for valuable assistance with the electron microscopy and Dr. Aurea Sousa for comments on the manuscript.
References
- Aiello GL, Bach-y-Rita P. The cost of an action potential. J Neuroscience Methods. 2000;103:145–149. doi: 10.1016/s0165-0270(00)00308-3. [DOI] [PubMed] [Google Scholar]
- Ames A., 3rd CNS energy metabolism as related to function. Brain Research Reviews. 2000;34:42–68. doi: 10.1016/s0165-0173(00)00038-2. [DOI] [PubMed] [Google Scholar]
- Andrews HE, Nichols PP, Bates D, Turnbull DM. Mitochondrial dysfunction plays a key role in progressive axonal loss in Multiple Sclerosis. Medical Hypotheses. 2005;64:669–677. doi: 10.1016/j.mehy.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Armstrong R, Toews AD, Morell P. Axonal transport through nodes of Ranvier. Brain Research. 1987;412:196–199. doi: 10.1016/0006-8993(87)91461-2. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondria take center stage in aging and neurodegeneration. Annals of Neurologyl. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- Bellen HJ, Lu Y, Beckstead R, Bhat MA. Neurexin IV, caspr and paranodin--novel members of the neurexin family: encounters of axons and glia. Trends in Neurosciences. 1998;21:444–449. doi: 10.1016/s0166-2236(98)01267-3. [DOI] [PubMed] [Google Scholar]
- Bennett V, Chen L. Ankyrins and cellular targeting of diverse membrane proteins to physiological sites. Current Opinion in Cell Biology. 2001;13:61–67. doi: 10.1016/s0955-0674(00)00175-7. [DOI] [PubMed] [Google Scholar]
- Berghs S, Aggujaro D, Dirkx R, Jr, Maksimova E, Stabach P, Hermel JM, Zhang JP, Philbrick W, Slepnev V, Ort T, Solimena M. betaIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. Journal of Cell Biology. 2000;151:985–1002. doi: 10.1083/jcb.151.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi P, Scorrano L, Colonna R, Petronilli V, Di Lisa F. Mitochondria and cell death. Mechanistic aspects and methodological issues. European Journal of Biochemistry. 1999;264:687–701. doi: 10.1046/j.1432-1327.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- Berthold C. Morphology of Normal Peripheral Axons. In: Waxman S, editor. Physiology and Pathobiology of Axons. Vol. 3. Raven Press; 1978. p. 63. [Google Scholar]
- Berthold CH, Rydmark M. Electron microscopic serial section analysis of nodes of Ranvier in lumbosacral spinal roots of the cat: ultrastructural organization of nodal compartments in fibres of different sizes. Journal of Neurocytology. 1983;12:475–505. doi: 10.1007/BF01159386. [DOI] [PubMed] [Google Scholar]
- Bhat MA. Molecular organization of axo-glial junctions. Current Opinion in Neurobiology. 2003;13:552–559. doi: 10.1016/j.conb.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–383. doi: 10.1016/s0896-6273(01)00294-x. [DOI] [PubMed] [Google Scholar]
- Boyle ME, Berglund EO, Murai KK, Weber L, Peles E, Ranscht B. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 2001;30:385–397. doi: 10.1016/s0896-6273(01)00296-3. [DOI] [PubMed] [Google Scholar]
- Charles P, Tait S, Faivre-Sarrailh C, Barbin G, Gunn-Moore F, Denisenko-Nehrbass N, Guennoc AM, Girault JA, Brophy PJ, Lubetzki C. Neurofascin is a glial receptor for the paranodin/Caspr-contactin axonal complex at the axoglial junction. Current Biology. 2002;12:217–220. doi: 10.1016/s0960-9822(01)00680-7. [DOI] [PubMed] [Google Scholar]
- Cooper PD, Smith RS. The movement of optically detectable organelles in myelinated axons of Xenopus laevis. Journal of Physiology. 1974;242:77–97. doi: 10.1113/jphysiol.1974.sp010695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JQ, Lambert S, Bennett V. Molecular composition of the node of Ranvier: identification of ankyrin-binding cell adhesion molecules neurofascin (mucin+/third FNIII domain-) and NrCAM at nodal axon segments. Journal of Cell Biology. 1996;135:1355–1367. doi: 10.1083/jcb.135.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupree JL, Coetzee T, Blight A, Suzuki K, Popko B. Myelin galactolipids are essential for proper node of Ranvier formation in the CNS. Journal of Neuroscience. 1998;18:1642–1649. doi: 10.1523/JNEUROSCI.18-05-01642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupree JL, Girault JA, Popko B. Axo-glial interactions regulate the localization of axonal paranodal proteins. Journal of Cell Biology. 1999;147:1145–1152. doi: 10.1083/jcb.147.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R, McDonough J, Yin X, Peterson J, Chang A, Torres T, Gudz T, Macklin WB, Lewis DA, Fox RJ, Rudick R, Mirnics K, Trapp BD. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Annals of Neurology. 2006;59:478–489. doi: 10.1002/ana.20736. [DOI] [PubMed] [Google Scholar]
- Edgar JM, McLaughlin M, Yool D, Zhang SC, Fowler JH, Montague P, Barrie JA, McCulloch MC, Duncan ID, Garbern J, Nave KA, Griffiths IR. Oligodendroglial modulation of fast axonal transport in a mouse model of hereditary spastic paraplegia. Journal of Cell Biology. 2004;166:121–131. doi: 10.1083/jcb.200312012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einheber S, Schnapp LM, Salzer JL, Cappiello ZB, Milner TA. Regional and ultrastructural distribution of the alpha 8 integrin subunit in developing and adult rat brain suggests a role in synaptic function. Journal of Comparative Neurology. 1996;370:105–134. doi: 10.1002/(SICI)1096-9861(19960617)370:1<105::AID-CNE10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Einheber S, Zanazzi G, Ching W, Scherer S, Milner TA, Peles E, Salzer JL. The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. Journal of Cell Biology. 1997;139:1495–1506. doi: 10.1083/jcb.139.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshed Y, Feinberg K, Poliak S, Sabanay H, Sarig-Nadir O, Spiegel I, Bermingham JR, Jr, Peles E. Gliomedin mediates Schwann cell-axon interaction and the molecular assembly of the nodes of Ranvier. Neuron. 2005;47:215–229. doi: 10.1016/j.neuron.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Fabricius C, Berthold CH, Rydmark M. Axoplasmic organelles at nodes of Ranvier. II. Occurrence and distribution in large myelinated spinal cord axons of the adult cat. Journal of Neurocytology. 1993;22:941–954. doi: 10.1007/BF01218352. [DOI] [PubMed] [Google Scholar]
- Friede RL, Samorajski T. Myelin formation in the sciatic nerve of the rat. A quantitative electron microscopic, histochemical and radioautographic study. Journal of Neuropathology and Experimental Neurology. 1968;27:546–570. [PubMed] [Google Scholar]
- Garcia-Fresco GP, Sousa AD, Pillai AM, Moy SS, Crawley JN, Tessarollo L, Dupree JL, Bhat MA. Disruption of axo-glial junctions causes cytoskeletal disorganization and degeneration of Purkinje neuron axons. Proceedings of the National Academy of Sciences, U S A. 2006;103:5137–5142. doi: 10.1073/pnas.0601082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genova JL, Fehon RG. Neuroglian, Gliotactin, and the Na+/K+ ATPase are essential for septate junction function in Drosophila. Journal of Cell Biology. 2003;161:979–989. doi: 10.1083/jcb.200212054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. Journal of Cell Biology. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan L, Salomon D, Salzer JL, Peles E. Caspr regulates the processing of contactin and inhibits its binding to neurofascin. Journal of Cell Biology. 2003;163:1213–1218. doi: 10.1083/jcb.200309147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, McStay GP, Clarke SJ. The permeability transition pore complex: another view. Biochimie. 2002;84:153–166. doi: 10.1016/s0300-9084(02)01375-5. [DOI] [PubMed] [Google Scholar]
- Hirano A, Llena JF. Morphology of central nervous system axons. In: Waxman S, Kocsis J, Stys P, editors. The Axon. Oxford University Press; 1995. pp. 49–67. [Google Scholar]
- Hollenbeck PJ. The pattern and mechanism of mitochondrial transport in axons. Frontiers in Bioscience. 1996;1:d91–102. doi: 10.2741/a118. [DOI] [PubMed] [Google Scholar]
- Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. Journal of Cell Science. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacas-Gervais S, Guo J, Strenzke N, Scarfone E, Kolpe M, Jahkel M, De Camilli P, Moser T, Rasband MN, Solimena M. BetaIVSigma1 spectrin stabilizes the nodes of Ranvier and axon initial segments. Journal of Cell Biology. 2004;166:983–990. doi: 10.1083/jcb.200408007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nature Genetics. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- Li XQ, Zochodne DW. Microvacuolar neuronopathy: a postmortem artifact of sensory neurons. Journal of Neurocytology. 2003;32:393–398. doi: 10.1023/B:NEUR.0000011333.06638.b6. [DOI] [PubMed] [Google Scholar]
- Li YC, Zhai XY, Ohsato K, Futamata H, Shimada O, Atsumi S. Mitochondrial accumulation in the distal part of the initial segment of chicken spinal motoneurons. Brain Research. 2004a;1026:235–243. doi: 10.1016/j.brainres.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004b;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Ligon LA, Steward O. Role of microtubules and actin filaments in the movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. Journal of Comparative Neurology. 2000;427:351–361. doi: 10.1002/1096-9861(20001120)427:3<351::aid-cne3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- LoPachin RM, Lehning EJ. Mechanism of calcium entry during axon injury and degeneration. Toxicology and Applied Pharmacology. 1997;143:233–244. doi: 10.1006/taap.1997.8106. [DOI] [PubMed] [Google Scholar]
- Melendez-Vasquez CV, Rios JC, Zanazzi G, Lambert S, Bretscher A, Salzer JL. Nodes of Ranvier form in association with ezrin-radixin-moesin (ERM)-positive Schwann cell processes. Proceedings of the National Academy of Sciences, U S A. 2001;98:1235–1240. doi: 10.1073/pnas.98.3.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegoz M, Gaspar P, Le Bert M, Galvez T, Burgaya F, Palfrey C, Ezan P, Arnos F, Girault JA. Paranodin, a glycoprotein of neuronal paranodal membranes. Neuron. 1997;19:319–331. doi: 10.1016/s0896-6273(00)80942-3. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Schafer DP, Horresh I, Bar V, Hales K, Yang Y, Susuki K, Peles E, Stankewich MC, Rasband MN. Spectrins and ankyrinB constitute a specialized paranodal cytoskeleton. Journal of Neuroscience. 2006;26:5230–5239. doi: 10.1523/JNEUROSCI.0425-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SM, Ternet M, Salvaterra PM, Beitel GJ. The Na+/K+ ATPase is required for septate junction function and epithelial tube-size control in the Drosophila tracheal system. Development. 2003;130:4963–4974. doi: 10.1242/dev.00691. [DOI] [PubMed] [Google Scholar]
- Peles E, Nativ M, Lustig M, Grumet M, Schilling J, Martinez R, Plowman GD, Schlessinger J. Identification of a novel contactin-associated transmembrane receptor with multiple domains implicated in protein-protein interactions. Embo Journal. 1997;16:978–988. doi: 10.1093/emboj/16.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nature Reviews Neuroscience. 2003;4:968–980. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- Reles A, Friede RL. Axonal cytoskeleton at the nodes of Ranvier. Journal of Neurocytology. 1991;20:450–458. doi: 10.1007/BF01252273. [DOI] [PubMed] [Google Scholar]
- Rios JC, Melendez-Vasquez CV, Einheber S, Lustig M, Grumet M, Hemperly J, Peles E, Salzer JL. Contactin-associated protein (Caspr) and contactin form a complex that is targeted to the paranodal junctions during myelination. Journal of Neuroscience. 2000;20:8354–8364. doi: 10.1523/JNEUROSCI.20-22-08354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios JC, Rubin M, St Martin M, Downey RT, Einheber S, Rosenbluth J, Levinson SR, Bhat M, Salzer JL. Paranodal interactions regulate expression of sodium channel subtypes and provide a diffusion barrier for the node of Ranvier. Journal of Neuroscience. 2003;23:7001–7011. doi: 10.1523/JNEUROSCI.23-18-07001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthel G, Hollenbeck PJ. Response of mitochondrial traffic to axon determination and differential branch growth. Journal of Neuroscience. 2003;23:8618–8624. doi: 10.1523/JNEUROSCI.23-24-08618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito F, Moore SA, Barresi R, Henry MD, Messing A, Ross-Barta SE, Cohn RD, Williamson RA, Sluka KA, Sherman DL, Brophy PJ, Schmelzer JD, Low PA, Wrabetz L, Feltri ML, Campbell KP. Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron. 2003;38:747–758. doi: 10.1016/s0896-6273(03)00301-5. [DOI] [PubMed] [Google Scholar]
- Salzer JL. Polarized domains of myelinated axons. Neuron. 2003;40:297–318. doi: 10.1016/s0896-6273(03)00628-7. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Bansal R, Hedstrom KL, Pfeiffer SE, Rasband MN. Does paranode formation and maintenance require partitioning of neurofascin 155 into lipid rafts? Journal of Neuroscience. 2004;24:3176–3185. doi: 10.1523/JNEUROSCI.5427-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer SS, Xu T, Crino P, Arroyo EJ, Gutmann DH. Ezrin, radixin, and moesin are components of Schwann cell microvilli. Journal of Neuroscience Research. 2001;65:150–164. doi: 10.1002/jnr.1138. [DOI] [PubMed] [Google Scholar]
- Sherman DL, Tait S, Melrose S, Johnson R, Zonta B, Court FA, Macklin WB, Meek S, Smith AJ, Cottrell DF, Brophy PJ. Neurofascins are required to establish axonal domains for saltatory conduction. Neuron. 2005;48:737–742. doi: 10.1016/j.neuron.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Simpson PB, Russell JT. Role of mitochondrial Ca2+ regulation in neuronal and glial cell signalling. Brain Research Reviews. 1998;26:72–81. doi: 10.1016/s0165-0173(97)00056-8. [DOI] [PubMed] [Google Scholar]
- Stys PK. White matter injury mechanisms. Current Molecular Medicine. 2004;4:113–130. doi: 10.2174/1566524043479220. [DOI] [PubMed] [Google Scholar]
- Stys PK, Waxman SG, Ransom BR. Ionic mechanisms of anoxic injury in mammalian CNS white matter: role of Na+ channels and Na(+)-Ca2+ exchanger. Journal of Neuroscience. 1992;12:430–439. doi: 10.1523/JNEUROSCI.12-02-00430.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sward C, Berthold CH, Nilsson-Remahl I, Rydmark M. Axonal constriction at Ranvier's node increases during development. Neuroscience Letters. 1995;190:159–162. doi: 10.1016/0304-3940(95)11528-5. [DOI] [PubMed] [Google Scholar]
- Tait S, Gunn-Moore F, Collinson JM, Huang J, Lubetzki C, Pedraza L, Sherman DL, Colman DR, Brophy PJ. An oligodendrocyte cell adhesion molecule at the site of assembly of the paranodal axo-glial junction. Journal of Cell Biology. 2000;150:657–666. doi: 10.1083/jcb.150.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhrik B, Stampfli R. Ultrastructural observations on nodes of Ranvier from isolated single frog peripheral nerve fibres. Brain Research. 1981;215:93–101. doi: 10.1016/0006-8993(81)90493-5. [DOI] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Black JA, Ransom BR, Stys PK. Anoxic injury of rat optic nerve: ultrastructural evidence for coupling between Na+ influx and Ca(2+)-mediated injury in myelinated CNS axons. Brain Research. 1994;644:197–204. doi: 10.1016/0006-8993(94)91680-2. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Black JA, Stys PK, Ransom BR. Ultrastructural concomitants of anoxic injury and early post-anoxic recovery in rat optic nerve. Brain Research. 1992;574:105–119. doi: 10.1016/0006-8993(92)90806-k. [DOI] [PubMed] [Google Scholar]
- Webster Hde F, Ames A., 3rd Reversible and irreversible changes in the fine structure of nervous tissue during oxygen and glucose deprivation. Journal of Cell Biology. 1965;26:885–909. doi: 10.1083/jcb.26.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster Hde F. The geometry of peripheral myelin sheaths during their formation and growth in rat sciatic nerves. Journal of Cell Biology. 1971;48:348–367. doi: 10.1083/jcb.48.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Lacas-Gervais S, Morest DK, Solimena M, Rasband MN. BetaIV spectrins are essential for membrane stability and the molecular organization of nodes of Ranvier. Journal of Neuroscience. 2004;24:7230–7240. doi: 10.1523/JNEUROSCI.2125-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H. Accumulation of synaptic vesicle proteins and cytoskeletal specializations at the peripheral node of Ranvier. Microscopy Research and Technique. 1996;34:462–473. doi: 10.1002/(SICI)1097-0029(19960801)34:5<462::AID-JEMT6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]


