Introduction
In 1999 Haissaguerre and his colleagues published a landmark article, showing that atrial fibrillation can be initiated by electrical activity in the pulmonary veins 1. Not only does it appear that electrical activity in the veins initiates fibrillation but it also may be responsible for perpetuating fibrillation. Subsequently similar evidence has suggested that other thoracic veins (vena cavae, coronary sinus, ligament of Marshall) may initiate and perpetuate atrial fibrillation 1.
How does electrical impulse initiation occur in the veins? The results of the numerous in vivo and in vitro studies on this subject have not conclusively defined a mechanism. Impulse initiation by automaticity and triggered activity as well as impulse initiation resulting from reentry have been suggested. The results of ablation procedures in preventing atrial fibrillation are consistent with both mechanisms. In this Chapter we focus only on those data suggesting the possibility that triggered activity may initiate and/or perpetuate atrial fibrillation. Our opinion from a review of the literature, is that both triggered activity and reentry are involved in the genesis of atrial fibrillation but that the relative importance of each cannot be determined at present.
Triggered Activity
Triggered activity is a term used to describe impulse initiation in cardiac fibers that is dependent on afterdepolarizations 2. Afterdepolarizations are oscillations in membrane potential that follow the upstroke of an action potential. Two kinds of afterdepolarizations may cause triggered activity. One occurs early, i.e., during phase 2 or 3 of repolarization of the action potential (early afterdepolarizations or EADs), and the other is delayed until repolarization is complete or nearly complete (delayed afterdepolarizations or DADs). When either kind of afterdepolarization is large enough to reach the threshold potential for activation of a regenerative inward current, action potentials result, and are referred to as “triggered.” Therefore, a key characteristic of triggered activity is that, to occur, at least one action potential must precede it (the trigger).
Afterdepolarizations and triggered activity have been demonstrated in isolated cardiac tissues and cells using transmembrane or patch clamp recordings of electrical activity. However, the demonstration that triggered activity is a cause of arrhythmias in vivo, such as atrial fibrillation, is a major problem that has not been completely solved. It has not been possible to reliably record transmembrane potentials demonstrating afterdepolarizations in vivo. While some studies show what has been interpreted to be afterdepolarizations in monophasic action potentials, the validity of such recordings has been questioned since motion artifact can produce deflections that resemble afterdepolarizations. One way often used to demonstrate that triggered activity may be a cause of an arrhythmia is to remove tissue from a region of the arrhythmic heart and then show that afterdepolarizations can be recorded from the cells of that tissue. However, the problem always exists that isolation and superfusion of cardiac tissues and cells may alter their properties. Thus, what is recorded in an isolated preparation may not always resemble what occurs in situ.
Because of these problems, it has been proposed that the mechanism of an arrhythmia in the in situ heart can be deduced from the response of the arrhythmia to cardiac pacing3. The following is just a brief summary of the stimulation protocols and the response of arrhythmias to stimulation that may identify triggered activity.
Delayed Afterdepolarizations (DADs)
The amplitude of DADs increases with a decrease in the cycle length at which action potentials occur until the afterdepolarization reaches threshold to cause triggered activity. Therefore, triggered arrhythmias caused by DADs in the in situ heart should be initiated by either overdrive pacing or programmed premature stimulation. Since automaticity is not initiated by pacing, automatic arrhythmias should be readily distinguished from triggered impulses arrhythmias (see Chapter 1 in 3). Reentrant arrhythmias also can be induced by the same stimulation. However, triggered activity caused by DADs is more easily induced by rapid pacing than by a single premature stimulus whereas reentry is more easily induced by premature stimulation.
During the initiation of DAD-dependent triggered rhythms, as the pacing cycle length (or coupling interval of premature impulses) decreases, the coupling interval from the last stimulated impulse to the first impulse of tachycardia should decrease (a direct relationship) since at short cycle lengths, the coupling interval of the afterdepolarizations to the proceeding action potential decreases. A direct relation like this is not expected during the initiation of reentrant arrhythmias where slowing of conduction causes the relationship to be inverse.
Both triggered rhythms and reentrant rhythms can be terminated by overdrive stimulation or single premature impulses 3. Automatic rhythms caused by normal automaticity show the phenomenon of overdrive suppression but are not terminated, while those caused by abnormal automaticity are little affected. Another feature of the response to electrical stimulation that differentiates DAD-induced triggered activity from reentry is that reentrant rhythms but not triggered rhythms can be entrained 3.
Early Afterdepolarizations (EADs)
Arrhythmias caused by EADs, that result from prolonged action potential duration, have been shown not to be inducible by overdrive or premature stimulation but can be initiated by slowing the basic heart rate. However, more recent studies have suggested that EAD dependent triggered activity under certain circumstances, can be induced by rapid pacing in pulmonary vein preparations (see below). Electrical stimulation (premature or overdrive) in general is not expected to terminate triggered rhythms caused by EADs. The response should be similar to that of abnormal automaticity that shows resetting but little overdrive suppression.
Triggered activity and atrial fibrillation
The response of initiators and perpetuators to electrical stimulation in vivo is mostly lacking and therefore, proof that triggered activity is related to onset and perpetuation of atrial fibrillation is mostly circumstantial. Most of the evidence for involvement of triggered activity in atrial fibrillation is from studies on isolated tissues and cells.
Coronary Sinus
Although the pulmonary veins are the most important site for initiation of atrial fibrillation, we start with a description of triggered activity in the coronary sinus, because the musculature of the coronary sinus, in our opinion shows the most clear cut evidence of triggered activity. Atrial myocardium extends into the coronary sinus from its orifice. Some myocytes resembling the transitional cells of the sinus node are interspersed among working atrial myocytes and connected to them by scattered gap junctions. The structure of these cells in the coronary sinus resemble the structure of cells proposed to be the automatic cells in the sinus node (Albala A and Fenoglio JJ Jr unpublished observations).
Rapid atrial tachycardias have been shown to emanate from the coronary sinus by mapping techniques 4. Involvement of the coronary sinus in atrial fibrillation is evidenced by the demonstration that bursts of rapid activity in the coronary sinus, that were faster than in the atria, occurred in response to the rapid atrial pacing that initiated atrial fibrillation 5. In some patients with atrial fibrillation, rapid repetitive activity in musculature of the coronary sleeve may contribute to maintenance of the arrhythmia. Isolation of the coronary sinus from atrial myocardium has been shown to prevent atrial fibrillation in patients who had prior pulmonary vein ablation that did not prevent fibrillation. Other clinical studies have shown that the initiator of atrial fibrillation can sometimes be in the vicinity of the coronary sinus 6–8.
These clinical data however, do not address the mechanism for impulse initiation in any detail. Rapid coronary sinus activity and atrial fibrillation are initiated by atrial pacing but this does not eliminate the possibility of pacing-induced reentry. The other characteristics necessary to suggest DAD-induced triggered activity (for example, a direct relationship between pacing cycle length and the first cycle length of the induced activity) have not been obtained. That the coronary sinus in situ is capable of triggered activity has been shown in an experimental study on the canine heart, using the characteristics of response to electrical stimulation 9,10. Such studies need to be done in the human heart to relate triggered activity to atrial fibrillation initiation and maintenance.
There is a large body of information describing the cellular electrophysiology of coronary sinus musculature. Mapping of isolated preparations composed of coronary sinus and atrial musculature from the canine heart, showed that two different regions are capable of impulse initiation in the presence of norepinephrine; one just outside the orifice of the coronary sinus, and the other well within the walls of the coronary sinus11. The action potentials of musculature inside the coronary sinus resemble atrial action potentials but have a small plateau phase. However, the cells have a less negative resting potential that may result from a sodium leak current. In the absence of electrical stimulation this inward current causes a progressive loss of membrane potential that may result in a loss of excitability12. Norepinephrine causes delayed afterdepolarizations and triggered activity in musculature inside the coronary sinus (Figure 1), while causing spontaneous diastolic (pacemaker) depolarization in cells outside the coronary sinus orifice. Triggered activity in coronary sinus musculature is initiated either by a critically shortened stimulation cycle length (Figure 2) or critically timed premature impulse11. Delayed afterdepolarizations in coronary sinus are caused by a transient inward current similar to the transient inward current caused by cardiac glycosides in other tissues 13,14. It is likely to be related to calcium release from the sarcoplasmic reticulum15. Enhancing electrogenic sodium pump current during prolonged periods of triggered activity can terminate it 16.
Figure 1.
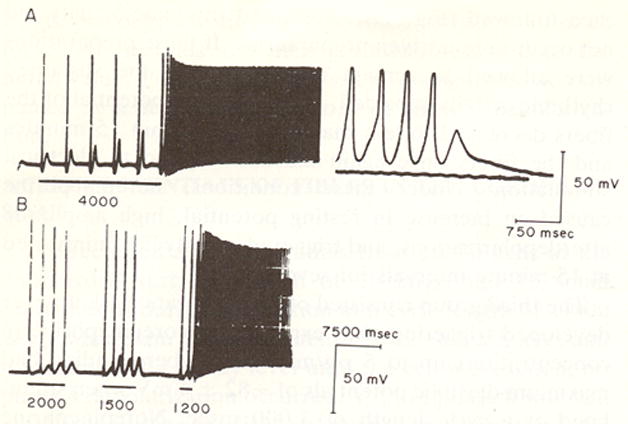
Transmembrane action potentials recorded from canine coronary sinus tissue in vitro. In A the preparation was stimulated at a cycle length of 4000 msec. DADs occur and get larger after each stimulated impulse until rapid triggered activity occurred. At the far right the time base was expanded and the shape of the triggered action potentials can be seen. Panel B shows effects of decreasing stimulation cycle length from 2000-1200 msec. DAD amplitude increases as stimulation cycle length is reduced until triggered activity occurred. (Reproduced from Wit and Cranefield Circ Res 1977 with permission)
Figure 2.
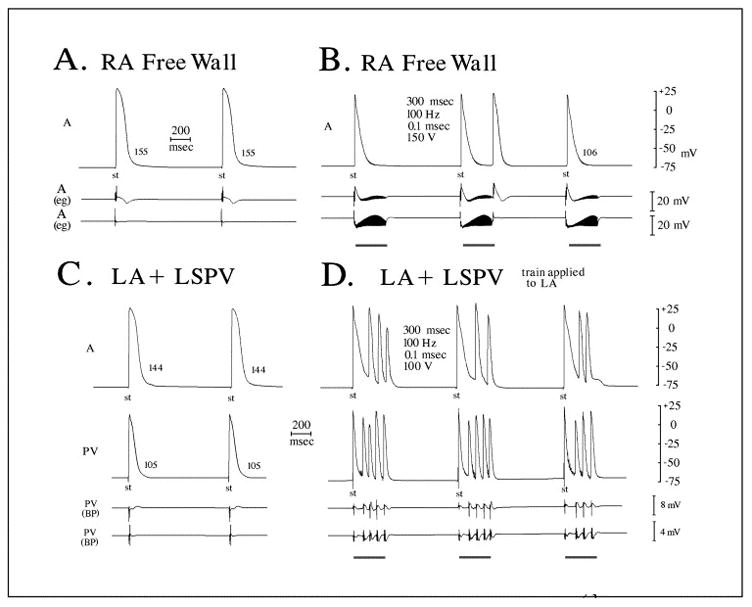
Autonomic nerve stimulation within right atrial free wall and within left atrium (LA) attached to pulmonary veins (PVs). A, B: Microelectrode and bipolar electrogram recordings from right atrial free wall (RA) before and during a maximal 150-V stimulus train. Although shortening is observed, only a break-shock beat is observed with stimulation. No arrhythmia was observed at less intense stimulus intensities. C, D: Microelectrode recordings from the left atrium (LA) and left superior pulmonary vein (LSPV) before and during a stimulus train introduced into LA myocardium, 3 mm from the PV os. Although shortening of the action potential is observed in both atrial and pulmonary vein recordings, triggered firing originates at a rapid rate within the PV sleeve 6 to 7 mm from the site where the stimulus train is introduced. The first beat appears earliest within the PV and precedes LA activation. (Reproduced from Patterson et al Heart Rhythm 2005;2:624 with permission))
Pulmonary Veins
Atrial muscle extends into the pulmonary veins. There is an extensive literature showing that electrical activity in this pulmonary vein musculature is related to the onset and perpetuation of atrial fibrillation 1,17. The ablation of pulmonary vein musculature can prevent atrial fibrillation. The mechanism for impulse origin in pulmonary veins is uncertain; automaticity, triggered activity, and reentry have all been proposed 18.
Specialized cardiac cells that are associated with pacemaking, resembling pale (P) cells and Purkinje cells have been described in rat 19 and human pulmonary vein 20 and in some 21 but not all 22,23 studies on canine pulmonary veins. The link suggesting triggered activity in pulmonary veins to atrial fibrillation is that rapid pacing of the atria can initiate pulmonary vein activity. However, no other evidence from in situ studies has shown the expected features of triggered activity in response to pacing protocols that we described at the beginning of this chapter. A majority of data suggesting a possible role of triggered activity has come from in vitro studies on tissues and cells. The results from studies on different species are somewhat varied, and add to the confusion as to whether pulmonary vein musculature is capable of triggered activity.
Automaticity, delayed afterdepolarizations and triggered activity do not readily occur in in vitro preparations of canine pulmonary vein where action potentials resemble atrial muscle and are characterized by rapid upstrokes 23,24,25 (see Figure 2, Panel C; trace labeled “PV”). Pulmonary vein muscle fibers have a less negative membrane potential than atrial muscle due to a smaller IK1, a slower phase-0 upstroke velocity (Vmax), likely caused by the reduced membrane potential, and shorter action potential duration (APD) associated with a larger IKr and IKs. Resting membrane potential and upstroke velocity are decreased more in the distal vein than proximal 23–25. The reduced upstrokes and structural anisotropy 23,26 along with differences in connexin expression 22 and heterogeneity of action potential duration, may cause reentry, a proposed mechanism for the rapid impulse initiation that can originate in the veins 27. Spontaneous activity arising just proximal to the venous ostium in the presence of isoproterenol, with an increased rate after rapid pacing(suggesting triggered activity) has been described in only one study on veins from normal dog hearts 27. Pacemaker potentials or afterdepolarizations were not evident, so a role for triggered activity is uncertain.
Canine pulmonary vein muscle can initiate rapid activity under special experimental conditions. One condition is the simultaneous activation of parasympathetic and sympathetic nerves in vitro (Figure 2). This rapid activity is caused by EADs during phase 3 of repolarization that induces triggered activity 28,29. Although the traditional mechanism for EAD induced triggered activity is dependent on action potential prolongation with reactivation of inward Ca2+ or Na+ current during the plateau phase, the proposed mechanism for EADs resulting from autonomic nerve activation in pulmonary veins is not dependent on action potential prolongation. The short duration of the atrial action potential in pulmonary vein muscle is associated with a peak Ca2+ transient (as deduced from force measurements) occurring during late repolarization, rather than during the plateau phase. Parasympathetic nerve activation increases this disparity by accelerating repolarization to make action potential duration even shorter. Presumably [Ca2+]i from the calcium transient remains elevated at a time when the membrane potential has mostly repolarized and is negative to the equilibrium potential for the Na/Ca exchanger current. Inward exchanger current is activated under these conditions. It is proposed that sympathetic activation augments the Ca2+ transient, enhances EADs and promotes triggering. Suppression of Na/Ca exchange suppresses the EAD induced triggered activity 28. Although the autonomic nervous system may sometimes be involved in the occurrence of atrial fibrillation in experimental animals 29 or in patients 30, it is uncertain how often its participation is obligatory. Late phase 3 EAD triggered activity caused by the above mechanisms may occur only under limited circumstances.
From these canine studies, triggered activity does not appear to be a normal intrinsic property of normal pulmonary vein myocardium, however, the properties of the vein musculature might be altered under conditions that favor the occurrence of atrial fibrillation. For example, stretch of the atria in a sheep model of stretch related AF, causes focal activity arising in the veins 31. In a canine model of pacing-induced heart failure, atrial tachycardia and fibrillation occur that may arise in the pulmonary veins 17,32,33. There is evidence that atrial tachycardia in this animal model is caused by DAD-induced triggered activity, some of which arises near or in pulmonary veins although atrial muscle may also be a source of impulse initiation. In superfused pulmonary vein preparations from a rapid-pacing induced heart failure model, both action potentials with spontaneous diastolic depolarization and automatic activity and those with phase 2 EADs have been recorded 34.
In contrast to the results of studies in tissues, both delayed and early afterdepolarizations and triggered activity have been found to be prevalent in single pulmonary vein myocytes isolated from normal canine pulmonary vein myocardium34 as well as myocytes from pulmonary vein obtained from dogs with pacing induced heart failure (Figure 3)35. Reasons why triggered activity is more prevalent in single myocytes are uncertain. Electrotonic inhibition of pacemaking cells by nonpacemaking cells may occur in tissues and not in isolated myocytes36. Additionally, isolation of single myocytes may result in abnormal calcium loading that can cause afterdepolarizations. The validity of results from isolated myocyte studies has been questioned and some results have been attributed to experimental artifacts24. In our opinion, studies on automaticity and triggered activity in isolated myocytes should be repeated by other laboratories.
Figure 3.
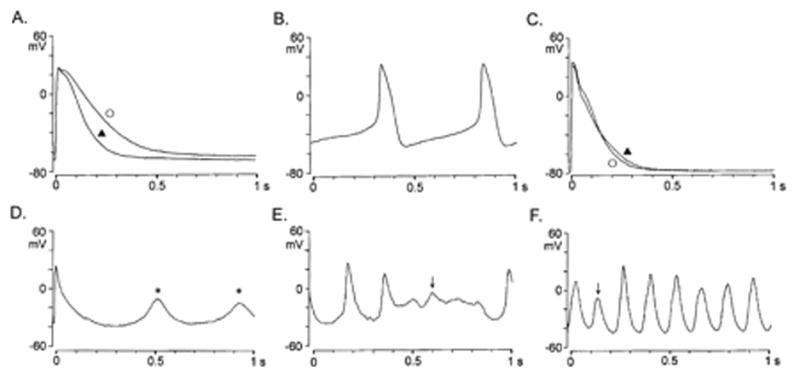
Action potential (APs) configurations and afterpotentials in control and right atrial paced (RAP) dog pulmonary vein myocytes (PVs). A and B, APs of control dog PV cardiomyocytes without and with pacemaker activity. C, APs of RAP dog PV cardiomyocytes without pacemaker activity; D, DAD in right atrial paced dog PV pacemaker cardiomyocytes during electrical stimulation at a rate of 0.1 Hz in normal Tyrode’s solution. E and F, EAD generated at depolarized levels during spontaneous beating. Electrical stimuli at 1 Hz (‘) and 0.1 Hz (_). Arrows indicate EAD; *, DAD. (Reproduced from Chen et al, Circulation. 2001;104:2849–2854 with permission.)
As in the dog, rabbit pulmonary vein tissue superfused in vitro shows typical atrial action potentials, is not spontaneously active and does not have afterdepolarizations or triggered activity37. Addition of ryanodine to the superfusate caused a depolarization of the resting potential, an increase in the plateau height, the development of pacemaker activity and rapid repetitive action potentials following pacing that were likely caused by delayed afterdepolarizations37(Figure 4). This behavior is consistent with the effects of ryanodine at low concentrations to lock the sarcoplasmic reticulum (SR) Ca2+ release channel, the ryanodine receptor (RyR), in a subconductance state, causing a Ca2+-independent Ca2+ release from the SR38. Ca2+ leakage during diastole causes traveling Ca2+ waves, increasing Ca2+ dependent ionic currents that may cause DADs 39,40. The inward current causing the DADs in this experimental model may be a Na/Ca exchanger current.
Figure 4.
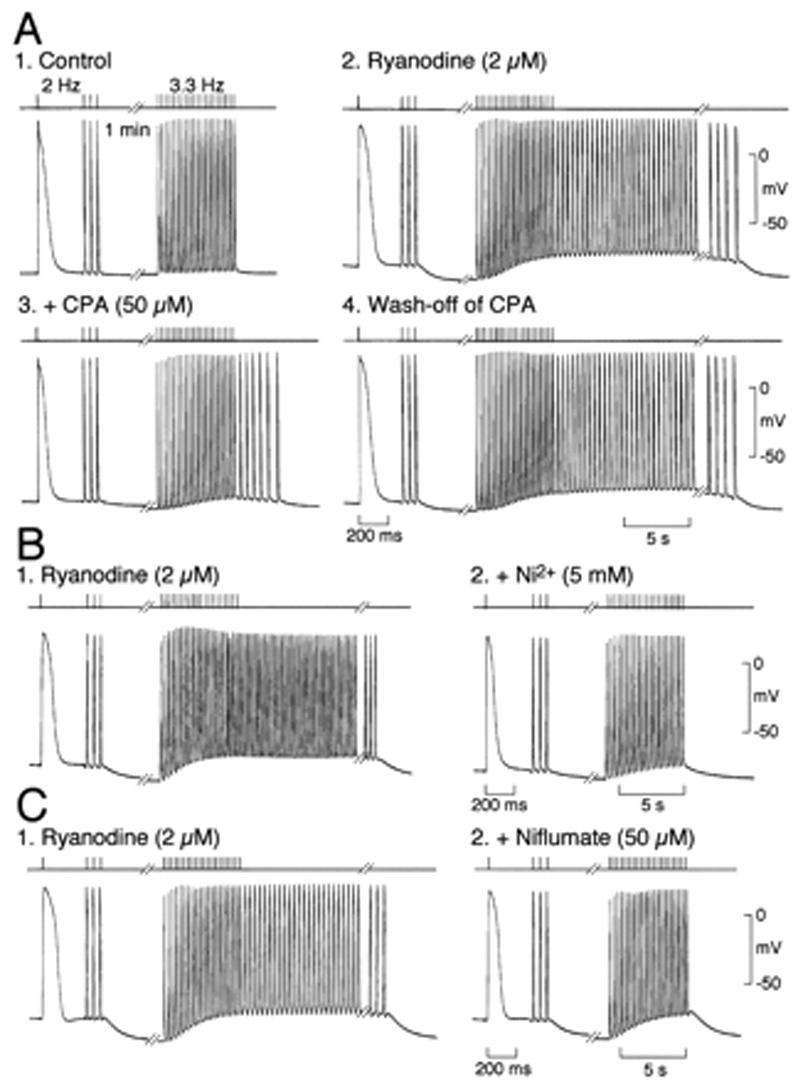
Effect of interventions that affect intracellular Ca2+ handling on pacing-induced spontaneous activity in PVMS from rabbit.. Stimulation in control superfusate, (A1) after treatment with 2 umol/L ryanodine (A2), in presence of 50 umol/L CPA after wash-off of ryanodine (A3), and after wash-off of cyclopiazonic acid (CPA) (A4). B, Same protocol after treatment with 2 umol/L ryanodine (B1) and in presence of 5 mmol/L Ni2 after wash-off of ryanodine (B2). C, Same protocol after treatment with 2 umol/L ryanodine (Ry) (C1) and in presence of 50 umol/L niflumate after wash-off of ryanodine (C2). Top, stimuli; bottom, membrane potential. (Reproduced from Honjo et al Circulation 107, 1937–1943 with permission)
Other sites of triggered activity
Other possible sites of triggered activity that may be related to the onset and perpetuation of atrial fibrillation include vena cavae, ligament of Marshall, atrial muscle and mitral valves. Ectopic activity has been recorded from the cardiac muscle that extends into the vena cavae 41 associated with the onset of atrial fibrillation42. Isoproterenol infusion and burst pacing, both of which can cause triggered activity, initiated atrial fibrillation with onset attributed to vena cava activity since the atrial fibrillation was prevented by ablation at the vein orifice. Isolated cardiomyocytes from the vena cavae have been shown to have pacemaker activity, DADs and triggered activity 43.
An electrically active muscle sleeve occurs in the ligament of Marshall, continuous with the muscle sleeve around the coronary sinus44. Rapid activity in this muscle sleeve has been shown to precede the onset of atrial fibrillation in some patients with ablation of the ligament preventing fibrillation 45,46. Action potentials recorded from the ligament in vitro resemble working atrial myocardial action potentials1. A role of triggered activity for the focal impulse initiation seen in situ has not been established.
Delayed afterdepolarizations and triggered activity in the presence of catecholamines readily occur in the atrial muscle that extends into the mitral valve47. Although a role for valve impulse initiation in atrial fibrillation has not been described, it is possible that there is a relationship. Under certain circumstance, triggered activity can also occur in working atrial muscle 48 particularly in the presence of underlying disease such as a cardiomyopathy49.
Conclusions
Although it is well accepted that electrical activity originating in the pulmonary and other thoracic veins, is sometimes intimately related to the onset and perpetuation of atrial fibrillation, the mechanism for impulse initiation is uncertain. Triggered activity does not appear to be a normal property of the atrial muscle that lines the pulmonary veins although it may be a normal property of coronary sinus muscle. Thus studies on normal canine hearts, while defining normal properties of this muscle lining the veins, do not indicate how pathology and/or age alter the electrophysiology. Experimental studies utilizing interventions that promote changes in intracellular calcium dynamics such as beta adrenergic stimulation, autonomic nerve stimulation, pacing induced atrial remodeling and induction of abnormal SR calcium channel function, have shown early and delayed afterdepolarizations in the thoracic veins, but the relationship of these interventions to pathologically-induced alterations in patients with atrial fibrillation remains to be determined. Nevertheless, the results of the studies that we have described warrant the conclusion that triggered activity participates in some as yet unspecified way in the occurrence of atrial fibrillation.
Footnotes
Supported by grants HL58860 and HL66140 from the National Heart Lung and Blood Institutes of Health, Bethesda, Maryland
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mechanisms and Treatment. Blackwell Futura: 2004. Thoracic Vein Arrhythmias. [Google Scholar]
- 2.Cranefield PF, Aronson RS. Cardiac Arrhythmias: The Role of Triggered Activity. 1. Mount Kisco: Futura Publishing Co. Inc; 1988. [Google Scholar]
- 3.Wit AL, Janse MJ. Electrophysiological Mechanisms. Mount Kisco, NY: Futura Publishing Co, Inc; 1993. The Ventricular Arrhythmias of Ischemia and Infarction. [Google Scholar]
- 4.Volkmer M, Antz M, Hebe J, Kuck KH. Focal atrial tachycardia originating from the musculature of the coronary sinus. J Cardiovasc Electr. 2002;13:68–71. doi: 10.1046/j.1540-8167.2002.00068.x. [DOI] [PubMed] [Google Scholar]
- 5.Oral H, Ozaydin M, Chugh A, Scharf C, Tada H, Hall B, Cheung P, Pelosi F, Knight BP, Morady F. Role of coronary sinus in maintenance of atrial fibrillation. J Cardiovasc Electr. 2003;14:1329–1336. doi: 10.1046/j.1540-8167.2003.03222.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen PS, Chou C. Coronary sinus as an arrhythmogenic structure. J Cardiovasc Electr. 2002;13:863–864. doi: 10.1046/j.1540-8167.2002.00863.x. [DOI] [PubMed] [Google Scholar]
- 7.Katritsis D, Ioannidis JPA, Giazitzoglou E, Korovesis S, Anagnostopoulos CE, Camm AJ. Conduction delay within the coronary sinus in Humans: Implications for atrial arrhythmias. J Cardiovasc Electr. 2002;13:859–862. doi: 10.1046/j.1540-8167.2002.00859.x. [DOI] [PubMed] [Google Scholar]
- 8.Sandres P, Jais P, Hocini M, Haissaguerre M. Electrical disconnection of the coronary sinus by radiofrequency catheter ablation to isolate a trigger of atrial fibrillation. J Cardiovasc Electr. 2004;15:364–368. doi: 10.1046/j.1540-8167.2004.03300.x. [DOI] [PubMed] [Google Scholar]
- 9.Johnson N, Rosen MR. Characteristics of initiation and termination of catecholamine-induced triggered activity in atrial fibers of the coronary sinus. Circ. 1986;74:1168–1179. doi: 10.1161/01.cir.74.5.1168. [DOI] [PubMed] [Google Scholar]
- 10.Malfatto G, Rosen TS, Rosen MR. The response to overdrive pacing of triggered atrial and ventricular arrhythmias in the canine heart. Circ. 1988;77:1139–1148. doi: 10.1161/01.cir.77.5.1139. [DOI] [PubMed] [Google Scholar]
- 11.Wit AL, Cranefield PF. Triggered and automatic activity in the canine coronary sinus. Circ Res. 1977;41(4):435–445. doi: 10.1161/01.res.41.4.434. [DOI] [PubMed] [Google Scholar]
- 12.Boyden PA, Cranefield PF, Gadsby DC, Wit AL. The basis for the membrane potential of quiescent cells of the canine coronary sinus. J Physiol. 1983;339:161–183. doi: 10.1113/jphysiol.1983.sp014710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tseng G-N, Wit AL. Characteristics of a transient inward current that causes delayed afterdepolarizations in atrial cells of the canine coronary sinus. JMCC. 1987;19:1105–1119. doi: 10.1016/s0022-2828(87)80354-1. [DOI] [PubMed] [Google Scholar]
- 14.Tseng GN, Wit AL. Effects of reducing [Na+]o on catecholamine-induced delayed afterdepolarizations in atrial cells. Am J Physiol Heart Circ Physiol. 1987;253:H115–H125. doi: 10.1152/ajpheart.1987.253.1.H115. [DOI] [PubMed] [Google Scholar]
- 15.Aronson RS, Cranefield PF. The effects of caffeine and ryanodine on the electrical activity of the canine coronary sinus. J Physiol. 1985;368:593–610. doi: 10.1113/jphysiol.1985.sp015878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wit AL, Cranefield PF, Gadsby DC. Electrogenic sodium extrusion can stop triggered activity in the canine coronary sinus. Circ Res. 1981;49:1029–1042. doi: 10.1161/01.res.49.4.1029. [DOI] [PubMed] [Google Scholar]
- 17.Okuyama Y, Miyauchi Y, Park AM, Hamabe A, Zhou S, Hayashi H, Miyauchi M, Omichi C, Pak HN, Brodsky LA. High resolution mapping of the pulmonary vein and the vein of marshall during induced atrial fibrillation and atrial tachycardia in a canine model of pacing-induced congestive heart failure. Journal of the American College of Cardiology. 2003;42:348–360. doi: 10.1016/s0735-1097(03)00586-2. [DOI] [PubMed] [Google Scholar]
- 18.Nattel S. Basic Electrophysiology of the Pulmonary Veins and Their Role in Atrial Fibrillation: Precipitators, Perpetuators, and Perplexers. Journal of Cardiovascular Electrophysiology. 2003;14:1372–1375. doi: 10.1046/j.1540-8167.2003.03445.x. [DOI] [PubMed] [Google Scholar]
- 19.Masani F. Node-like cells in the myocardial layer of the pulmonary vein of rats: an ultrastructural study. J Anat. 1986;145:133–142. [PMC free article] [PubMed] [Google Scholar]
- 20.Perez-Lugones A, McMahon JT, Ratliff NB, Saliba WI, Schweikert RA, Marrouche NF, Saad E, Navia JL, McCarthy PM, Tchou PJ, Gillinov AM, Natale A. Evidence of Specialized Conduction Cells in Human Pulmonary Veins of Patients with Atrial Fibrillation. J Cardiovasc Electr. 2003;14:803–809. doi: 10.1046/j.1540-8167.2003.03075.x. [DOI] [PubMed] [Google Scholar]
- 21.Chou C-C, Nihei M, Zhou S, Tan A, Kawasw A, Macias ES, Fishbein MC, Lin S-F, Chen P-S. Intracellular calcium dynamics and anisotropic reentry in isolated canine pulmonary veins and left atrium. Circulation. 2005;111:2889–2897. doi: 10.1161/CIRCULATIONAHA.104.498758. [DOI] [PubMed] [Google Scholar]
- 22.Verheule S, Wilson EE, Arora R, Engle SK, Scott LR, Olgin JE. Tissue structure and connexin expression of canine pulmonary veins. CARDIOVASCULAR RESEARCH. 2002;55:727–738. doi: 10.1016/s0008-6363(02)00490-x. [DOI] [PubMed] [Google Scholar]
- 23.Hocini M, Ho SY, Kawara T, Linnenbank AC, Potse M, Shah D, Jais P, Janse MJ, Haissaguerre M, de Bakker JMT. Electrical Conduction in Canine Pulmonary Veins: Electrophysiological and Anatomic Correlation. Circulation. 2002;105:2442–2448. doi: 10.1161/01.cir.0000016062.80020.11. [DOI] [PubMed] [Google Scholar]
- 24.Wang Tm, Chiang Ce, Sheu Jr, Tsou Ch, Chang Hm, Luk Hn. Homogenous distribution of fast response action potentials in canine pulmonary vein sleeves: a contradictory report. International Journal of Cardiology. 2003;89:187–195. doi: 10.1016/s0167-5273(02)00474-6. [DOI] [PubMed] [Google Scholar]
- 25.Ehrlich JR, Cha TJ, Zhang L, Chartier D, Melnyk P, Hohnloser SH, Nattel S. Cellular electrophysiology of canine pulmonary vein cardiomyocytes: action potential and ionic current properties. The Journal of Physiology. 2003;551:801–813. doi: 10.1113/jphysiol.2003.046417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamabe A, Okuyama Y, Miyauchi Y, Zhou S, Pak HN, Karagueuzian HS, Fishbein MC, Chen PS. Correlation Between Anatomy and Electrical Activation in Canine Pulmonary Veins. Circulation. 2003;107:1550–1555. doi: 10.1161/01.cir.0000056765.97013.5e. [DOI] [PubMed] [Google Scholar]
- 27.Arora R, Verheule S, Scott L, Navarrete A, Katari V, Wilson E, Vaz D, Olgin JE. Arrhythmogenic Substrate of the Pulmonary Veins Assessed by High-Resolution Optical Mapping. Circulation. 2003;107:1816–1821. doi: 10.1161/01.CIR.0000058461.86339.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patterson E, Lazzara R, Szabo B, Liu H, Tang D, Li YH, Scherlag BJ, Po SS. Sodium-Calcium Exchange Initiated by the Ca2+ Transient: An Arrhythmia Trigger Within Pulmonary Veins. Journal of the American College of Cardiology. 2006;47:1196–1206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 29.Schaueret P, Scherlag BJ, Patterson E. Focal atrial fibrillation:experimental evidence for a pathophysiologic role of the autonomic nervous system. J Cardiovasc Electr. 2001;12:592–599. doi: 10.1046/j.1540-8167.2001.00592.x. [DOI] [PubMed] [Google Scholar]
- 30.Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, Mazzone P, Tortoriello V, Landoni G, Zangrillo A, Lang C, Tomita T, Mesas C, Mastella E, Alfieri O. Pulmonary Vein Denervation Enhances Long-Term Benefit After Circumferential Ablation for Paroxysmal Atrial Fibrillation. Circulation. 2004;109:327–334. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- 31.Kalifa J, Jalife J, Zaitsev AV, Bagwe S, Warren M, Moreno J, Berenfeld O, Nattel S. Intra-Atrial Pressure Increases Rate and Organization of Waves Emanating From the Superior Pulmonary Veins During Atrial Fibrillation. Circulation. 2003;108:668–671. doi: 10.1161/01.CIR.0000086979.39843.7B. [DOI] [PubMed] [Google Scholar]
- 32.Stambler BS, Fenelon G, Shepard RK, Clemo HF, Guiraudon CM. Characterization of sustained atrial tachycardia in dogs with rapid ventricular pacing-induced heart failure. J Cardiovasc Electrophysiol. 2003;14:499–507. doi: 10.1046/j.1540-8167.2003.02519.x. [DOI] [PubMed] [Google Scholar]
- 33.Fenelon G, Shepard RK, Stambler BS. Focal origin of atrial tachycardia in dogs with rapid ventricular pacing-induced heart failure. J Cardiovasc Electr. 2003;14:1093–1102. doi: 10.1046/j.1540-8167.2003.03110.x. [DOI] [PubMed] [Google Scholar]
- 34.Chen YJ, Chen SA, Chang MS, Lin CI. Arrhythmogenic activity of cardiac muscle in pulmonary veins of the dog; implication for the genesis of atrial fibrillation. Cardiovascular Res. 2000;48:265–273. doi: 10.1016/s0008-6363(00)00179-6. [DOI] [PubMed] [Google Scholar]
- 35.Chen YJ, Chen SA, Chen YC, Yeh HI, Chan P, Chang MS, Lin CI. Effects of Rapid Atrial Pacing on the Arrhythmogenic Activity of Single Cardiomyocytes From Pulmonary Veins: Implication in Initiation of Atrial Fibrillation. Circulation. 2001;104:2849–2854. doi: 10.1161/hc4801.099736. [DOI] [PubMed] [Google Scholar]
- 36.Wilders R, Wagner MB, Golod DA, Kumar R, Wang Y-G, Goolsby WN, Joyner RW, Jongsma HJ. Effects of anisotropy on the development of cardiac arrhythmias associated with focal activity. Pflugers Arch. 2000;441:301–312. doi: 10.1007/s004240000413. [DOI] [PubMed] [Google Scholar]
- 37.Honjo H, Boyett MR, Niwa R, Inada S, Yamamoto M, Mitsui K, Horiuchi T, Shibata N, Kamiya K, Kodama I. Pacing-Induced Spontaneous Activity in Myocardial Sleeves of Pulmonary Veins After Treatment With Ryanodine. Circulation. 2003;107:1937–1943. doi: 10.1161/01.CIR.0000062645.38670.BD. [DOI] [PubMed] [Google Scholar]
- 38.Meissner G. Ryanodine activation and inhibtion of the Ca release channel of sarcoplasmic reticulum. J Biol Chem. 1986;261:6300–6306. [PubMed] [Google Scholar]
- 39.Schlotthauser K, Bers DM. Sarcoplasmic Reticulum Ca2+ Release Causes myocyte depolarization. Underlying Mechanism and threshold for Triggered Action potentials. Circ Res. 2000;87:774–780. doi: 10.1161/01.res.87.9.774. [DOI] [PubMed] [Google Scholar]
- 40.Ter Keurs HEDJ, Boyden PA. Calcium and Arrhythmogenesis. Physiol Rev. 2007 doi: 10.1152/physrev.00011.2006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hashizume H, Ushiki T, Abe K. A histological study of the cardiac muscle of the human superior and inferior venae cavae. Arch Histol Cytol. 1995;58:457–464. doi: 10.1679/aohc.58.457. [DOI] [PubMed] [Google Scholar]
- 42.Tsai CF, Tai CT, Hsieh MH, Lin WS, Yu WC, Ueng KC, Ding YA, Chang MS, Chen SA. Initiation of Atrial Fibrillation by Ectopic Beats Originating From the Superior Vena Cava : Electrophysiological Characteristics and Results of Radiofrequency Ablation. Circulation. 2000;102:67–74. doi: 10.1161/01.cir.102.1.67. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y-J, Chen Y-C, Yeh H-I, Lin C-I, Chen S-A. Electrophysiology and arrhythmogenic activity of single cardiac myocytes from canine superior vena cava. Circulation. 2002;105:2679–2685. doi: 10.1161/01.cir.0000016822.96362.26. [DOI] [PubMed] [Google Scholar]
- 44.Scherlag BJ, Yeh BK, Robinson MJ. Inferior interatrial pathway in the dog. Circ Res. 1972;31:18–35. doi: 10.1161/01.res.31.1.18. [DOI] [PubMed] [Google Scholar]
- 45.Hwang C, Karagueuzian HS, Chen P-S. Idiopathic paroxysmal atrial fibrillation induced by a focal discharge mechanism in the left superior pulmonary vein; possible roles of the ligament of Marshall. J Cardiovasc Electr. 1999;10:636–639. doi: 10.1111/j.1540-8167.1999.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 46.Hwang C, Wu TJ, Doshi RN, Peter CT, Chen PS. Vein of Marshall Cannulation for the Analysis of Electrical Activity in Patients With Focal Atrial Fibrillation. Circulation. 2000;101:1503–1505. doi: 10.1161/01.cir.101.13.1503. [DOI] [PubMed] [Google Scholar]
- 47.Wit AL, Cranefield PF. Triggered activity in cardiac muscle fibers of the simian mitral valve. Circ Res. 1976;38(2):85–98. doi: 10.1161/01.res.38.2.85. [DOI] [PubMed] [Google Scholar]
- 48.Saito T, Otoguro M, Matsubara T. Electrophysiological studies on the mechanism of electrically induced sustained rhythmic activity in the rabbit atrium. Circ Res. 1978;42:199–206. doi: 10.1161/01.res.42.2.199. [DOI] [PubMed] [Google Scholar]
- 49.Boyden PA, Tilley LP, Albala A, Liu SK, Fenoglio JJJr, Wit AL. Mechanisms for atrial arrhythmias associated with cardiomyopathy: a study of feline hearts with primary myocardial disease. Circulation. 1984;69:1036–1047. doi: 10.1161/01.cir.69.5.1036. [DOI] [PubMed] [Google Scholar]


