Abstract
The sinoatrial node initiates the heartbeat and controls the rate and rhythm of contraction, thus serving as the pacemaker of the heart. Despite the crucial role of the sinoatrial node in heart function, the mechanisms that underlie its specification and formation are not known. Tbx3, a transcriptional repressor required for development of vertebrates, is expressed in the developing conduction system. Here we show that Tbx3 expression delineates the sinoatrial node region, which runs a gene expression program that is distinct from that of the bordering atrial cells. We found lineage segregation of Tbx3-negative atrial and Tbx3-positive sinoatrial node precursor cells as soon as cardiac cells turn on the atrial gene expression program. Tbx3 deficiency resulted in expansion of expression of the atrial gene program into the sinoatrial node domain, and partial loss of sinoatrial node-specific gene expression. Ectopic expression of Tbx3 in mice revealed that Tbx3 represses the atrial phenotype and imposes the pacemaker phenotype on the atria. The mice displayed arrhythmias and developed functional ectopic pacemakers. These data identify a Tbx3-dependent pathway for the specification and formation of the sinoatrial node, and show that Tbx3 regulates the pacemaker gene expression program and phenotype.
Keywords: Heart development, pacemaker, sinoatrial node, lineage, Tbx3, transgenic mice
The heart rhythm of higher vertebrates is driven by pacemaker cells. In mammals, these specialized heart muscle cells reside within the sinoatrial node (SAN), a structure located in the right atrium at the junction with the superior caval vein. The SAN has a distinct phenotype compared with atria, and runs a unique ion channel and gap junction gene expression program essential for normal pacemaker function (Boyett et al. 2000; Schram et al. 2002; Marionneau et al. 2005; Liu et al. 2006). Hcn4 encodes an isoform of the hyperpolarization-activated channels that is highly expressed in the SAN and is required for normal cardiac pacemaker activity (Baruscotti et al. 2005). Mutations in Hcn4 result in SAN dysfunction and bradycardia in humans and mice (Schulze-Bahr et al. 2003; Stieber et al. 2003; Milanesi et al. 2006). Genes expressed in the atrial working myocardium but normally inactive in the SAN include Nppa (ANF) and Smpx (Chisel), and gap junction genes Cx40 (Gja5) and Cx43 (Gja1), which are essential for fast propagation of the electrical impulse in the working myocardium (Moorman and Christoffels 2003; Gros et al. 2004; Bagwe et al. 2005). Pacemaker activity is essential for heart function and viability throughout the life span of an individual. Acquired heart disease, corrected congenital heart defects, pharmacological agents, or gene defects may cause sinus node dysfunction (sick sinus syndrome), a common disorder necessitating electronic pacemaker implantation.
A century after the discovery of the SAN structure in mammals by Keith and Flack (1907), its origin and genetic control are largely unknown. Although all cardiomyocytes of the early embryonic heart display pacemaker activity, this property is lost, or suppressed, when these cells mature to working myocardial cells. However, the mature SAN still has pacemaker activity and the slow intercellular conduction also found in the embryonic cardiomyocytes, suggesting that prevention of differentiation of working myocardium may be an essential element of SAN formation. Recently, we found that cardiac homeobox factor Nkx2-5, which is critical for cardiogenesis and working myocardium differentiation, is absent from the sinus venosus and SAN, providing a possible mechanism for the early confinement of the pacemaker phenotype to this region (Mommersteeg et al. 2007). However, the mechanism underlying the patterning and formation of the SAN domain and the regulation of its gene program have remained unclear. Tbx3 is a T-box transcription factor uniquely expressed in the developing conduction system (Hoogaars et al. 2004). Tbx3 is a transcriptional repressor involved in developmental patterning and the regulation of proliferation, senescence bypass, and apoptosis in a variety of tissues (He et al. 1999; Brummelkamp et al. 2002; Carlson et al. 2002; Davenport et al. 2003; Naiche et al. 2005). Mutations in TBX3 cause human ulnar-mammary syndrome, a disorder characterized by abnormal development of forelimbs, apocrine glands, teeth, and, occasionally, the heart (Bamshad et al. 1997; Meneghini et al. 2006). Because of its unique nodal expression pattern in the heart and functional equivalence to Tbx2, a potent repressor of markers for working myocardial differentiation including Nppa, Smpx, and Cx40 in the developing heart (Habets et al. 2002; Christoffels et al. 2004b; Harrelson et al. 2004), Tbx3 is an attractive candidate regulator for the SAN gene program. Here we investigated the role of Tbx3 in the regulation of SAN formation, gene expression, and function. We found lineage segregation of differentiating atrial and Tbx3-positive SAN cells, indicating that the SAN is formed by proliferation of Tbx3-positive precursor cells, and not by recruiting adjacent myocytes that have initiated the atrial gene program. Analysis of Tbx3-deficient embryos revealed that Tbx3 is required to induce and maintain the SAN gene program, while preventing the expansion of atrial gene expression into the SAN domain. Ectopic Tbx3 expression mouse models revealed that Tbx3 is sufficient to reprogram atrial cells into functional pacemaker cells that run a SAN gene program.
Results
Tbx3 defines the SAN domain
The SANs of late fetal and adult mice coexpress Tbx3 and Hcn4, a key SAN marker gene (Stieber et al. 2004; Baruscotti et al. 2005), whereas Cx40, Cx43, Smpx, and Nppa are selectively expressed in the atrial myocardium in a pattern strictly complementary to that of Tbx3 and Hcn4 (Supplementary Fig. 1; Hoogaars et al. 2004; Mommersteeg et al. 2007). Lbh, encoding a putative repressor of Nppa (Briegel et al. 2005), was found to be expressed in the SAN and not in the atria (Supplementary Fig. 1A). Furthermore, Hop, encoding a transcriptional cofactor required for Cx40 expression and function of the ventricular conduction system (Ismat et al. 2005), appeared to be expressed at relatively low levels in the SAN (Supplementary Fig. 1A). Thus, the SAN expresses Tbx3 and runs a gene program distinct from the atrial working myocardial cells that directly border the SAN.
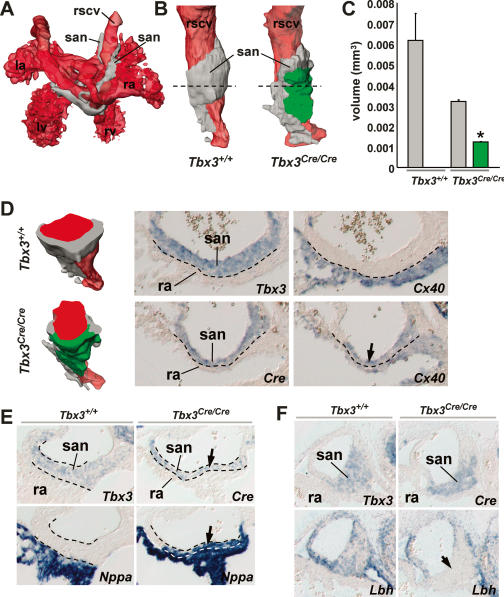
To assess the role of Tbx3 in the specification of SAN cells and in the regulation of gene expression, Tbx3-deficient mice were generated by replacing the first three codons in the first exon of Tbx3 with a Cre-pA cassette (Supplementary Fig. 2). Consistent with published results (Davenport et al. 2003), homozygous mutants (Tbx3Cre/Cre) died between embryonic day 11.5 (E11.5) and E14.5, displaying multiple malformations. These include limb malformations and failure to induce mammary glands, indicating that the Cre-pA insertion resulted in a functional null allele. Expression of Cre in Tbx3Cre/Cre embryos and endogenous Tbx3 in controls was highly similar, indicating that the Cre expression pattern mimics Tbx3 gene activity in mutants (Figs. 1A,D, 2D–F; Supplementary Fig. 2). To identify the primordial SAN region of interest, sections were probed for the expression of Tbx3 in wild type and for expression of Cre in mutants. Morphologically, a primordial SAN was formed in Tbx3 mutants, which expressed Hcn4 (Fig. 1A). Quantification of the volume of the SAN in wild-type and mutant littermates revealed that it was variable in size, but on average significantly smaller in mutants (Fig. 1C). Histological analysis of E12.5 and E14.5 SANs and three-dimensional (3D) reconstruction of an E14.5 SAN revealed that the wild-type and mutant SAN had comparable length along the right sinus horn, but differed in thickness (Fig. 2A,B,D,F). We next assessed the expression of Cx43, Cx40, Nppa, and Smpx, which in the embryonic heart mark differentiating atrial and ventricular working myocardium (Christoffels et al. 2004a). Throughout development, expression of these markers was excluded from Tbx3-positive myocardium of wild-type embryos. At E12.5, the expression domains of Cx43 and Smpx protruded into the Cre-positive primordial SAN of mutants (Fig. 1A,B,D). Cx40 and Nppa were not ectopically expressed in the SAN at this stage, probably because Nkx2-5, a crucial activator of these genes, is not yet expressed in the sinus venosus and SAN (Mommersteeg et al. 2007). However, from E13.5 onward, also Cx40 and Nppa were ectopically expressed in the SAN domain (Fig. 2B–E). 3D reconstruction and quantification and of Tbx3/Cre and Cx40 expression domains revealed that at E14.5, almost half of the mutant SAN coexpressed Cre and Cx40, whereas Cx40 was excluded from the SAN domain of wild-type littermates. In the region of Cre-Cx40 coexpression, Cx40 expression spanned the entire SAN domain from the atrium to the endothelial lining of the sinus horn (Fig. 2D). The cardiac sodium channel Nav1.5 (Scn5a), which in adults is expressed in working myocardium but at much lower levels in the SAN, is essential for impulse propagation (Papadatos et al. 2002; Lei et al. 2004). We observed that also at embryonic stages, Nav1.5 was almost undetectable in the developing SAN. However, a striking induction of expression was observed in the SAN domain of Tbx3 mutants (Fig. 1E). In Tbx3 mutants, Lbh expression was down-regulated in the SAN (Fig. 2F), indicating that Tbx3 not only suppresses atrial genes, but in some cases is also required for gene activity in the SAN. We did not observe abnormalities in gene expression in heterozygous Tbx3+/Cre embryos and adults. These observations demonstrate that a low dose of Tbx3 is required and sufficient to suppress atrial genes in the SAN domain and to activate Lbh.
Figure 1.
Tbx3 is required to suppress atrial genes in the SAN domain. (A) Ectopic expression of Cx43 in the Cre-positive SAN of a Tbx3-deficient (Tbx3Cre/Cre) embryo at E11.5 (black arrowheads). No difference in Hcn4 expression was observed between Tbx3+/+ and Tbx3Cre/Cre embryos. (B) Immunofluorescent double labeling of Cx43 (red) and Hcn4 (blue), and sytox green nuclear staining (green). In Tbx3Cre/Cre embryos, Cx43 showed overlap of expression (purple) with Hcn4 in the SAN region (white arrowheads). (C) Quantification of the volume of the SAN region (Tbx3 or Cre SAN expression domain) of individual E12.5 Tbx3+/+ and Tbx3Cre/Cre embryos (gray dots). The average SAN volume was significantly smaller in mutants; (*) P < 0.05. (D) Expression of Smpx in an E11.5 Tbx3+/+ embryo and Tbx3Cre/Cre littermate, showing ectopic expression in the developing SAN of Tbx3Cre/Cre embryos (black arrowheads). (E) Immunofluorescent labeling of Nav1.5 (red, top panel) and Hcn4 (blue, bottom panel) reveals ectopic expression of Nav1.5 in the Hcn4-positive SAN region of Tbx3Cre/Cre embryos (white arrowheads).
Figure 2.
3D reconstruction and expression analysis of the SAN region of Tbx3+/+ embryos and Tbx3Cre/Cre embryos. (A) Dorsal view of a 3D reconstructed E14.5 heart. The myocardium has been removed, exposing the blood-filled lumen (red). The Tbx3-expressing myocardial is shown in gray. The SAN region is embedded between the right atrium (ra) and right superior caval vein (rscv). (B) 3D reconstruction of serial sections of the SAN region in Tbx3+/+ embryos and Tbx3Cre/Cre embryos showing the Tbx3/Cre-positive SAN region (gray) and the lumen of the rscv (red). The atrium has been removed. Cx40 expression in the SAN domain (Cre+) is shown in green. The dashed line depicts cross-sections through the reconstructions shown in D. (C) Average of the volume of the SAN regions (gray) and Cx40-positive SAN regions (green) of E14.5 Tbx3+/+ embryos (n = 4) and Tbx3Cre/Cre embryos (n = 3). Error bars show the SD. Only in Tbx3Cre/Cre embryos was Cx40 expressed in the SAN region; (*) P < 0.05. (D) Left panels show cross-sections of 3D reconstructions shown in B (dashed lines). The right panels show the sections at that level. Dashed lines depict border between the SAN (Tbx3+/Cre+) and the atrium. Note that Cx40 is expressed throughout the SAN region in the Tbx3Cre/Cre embryo (black arrow). (E) Ectopic expression of Nppa in the SAN of an E14.5 Tbx3Cre/Cre embryo. Black arrow depicts Nppa expression in the Cre-positive SAN region. (F) Absence of Lbh expression in the Cre-positive SAN of an E13.5 Tbx3Cre/Cre embryo.
Differentiating atrial cells do not contribute to the Tbx3-positive SAN domain
Central conduction system components have been proposed to grow by continuous recruitment of cardiomyocytes into an initial framework of specified conduction system cells (Cheng et al. 1999; Pennisi et al. 2002). This implies that the Tbx3-positive SAN precursor pool is continuously supplemented with adjacent myocardial cells that adopt the SAN gene program. Alternatively, the SAN may form by proliferation of conduction system precursor cells specified early in development (Fig. 3A,B). From approximately E9.5 onward, the expression of Tbx3 allows the identification of the putative specified SAN precursors, whereas Nppa and Cx40 identify the bordering atrial cells differentiating from E9.5 onward (Christoffels et al. 2000; Hoogaars et al. 2004). To assess whether these atrial cells are being recruited into the Tbx3-positive SAN precursor pool after their differentiation, we deployed mice carrying an allele that expresses Cre under control of Nppa regulatory sequences (Cre3) (de Lange et al. 2003) selectively in the emerging atrial cells and not in the Tbx3-positive SAN domain (Fig. 3D). Cre3 mice were crossed with either R26R or Z/EG reporter mice to permanently label Cre-expressing cells and their daughters in double transgenic offspring (Fig. 3C). From their differentiation onward, atrial cells expressed Cre, resulting in β-galactosidase activity from the recombined lacZ gene from E10.5 onward (Fig. 3E). The expression domains of Cx40 and of Cre3-activated β-galactosidase activity colocalized, whereas β-galactosidase activity was not observed in the Tbx3-positive domain (Fig. 3F). We then investigated the expression borders in adults. Enhanced green fluorescent protein (EGFP) expression activated by Cre3 exactly matched Nppa expression, and Hcn4 expression was strictly complementary (Fig. 3G). We conclude that from approximately E10 on, the cells that differentiate to atrial myocardium (i.e., Cx40/Nppa-positive) and their daughters do not contribute to the SAN (i.e., Hcn4/Tbx3-positive), which therefore must increase in size by proliferation. Consistently, PCNA and phospho-histone H3 detection indicated that proliferation occurs at least until shortly before birth (Fig. 3H).
Figure 3.
Early specification of SAN precursor pool: Growth of the SAN does not occur by recruitment of atrial cells. (A,B) Schemes showing two mechanisms of SAN specification: growth of a pool of specified SAN precursors (A) or recruitment of myocardial cells into the SAN lineage (B). (C) Transgenes used to trace the fate of myocytes after they initiated the atrial gene expression program. A cross of Cre3 mice with R26R or Z/EG reporter mice results in activation of the lacZ or EGFP gene, respectively, in atrial myocardium. (D) Restricted expression of Cre in atrial working myocardium but not in Tbx3-positive SAN myocardium. (E) Cre is expressed in atrial myocardium from E10.5, as shown in whole-mount in situ hybridization and X-gal staining of Cre-R26R embryos. At E12.5, robust Cre-mediated recombination is observed in atrial myocardium. (F) Serial sections showing Cre-mediated lacZ activation in Cx40-positive atrial myocardium but not in the Tbx3-positive SAN region of E12.5 hearts. (G) Cross of Nppa-Cre mice with a Z/EG reporter mouse line resulting in expression of EGFP in atrial myocardium. Analysis of serial sections of adult Cre3-Z/EG mice revealed that EGFP expression is restricted to Nppa-positive atrial myocardium. The Hcn4-positive SAN region remained free of cells derived from EGFP-positive cells. (H) Presence of proliferating cells in the SANs of E16.5 and E17.5 hearts as demonstrated by the presence of PCNA and phosphorylated histone H3 (pHH3) in the Hcn4-positive SAN region. Black arrows show pHH3-posive cells in the SAN. (ina) Internodal artery.
Atria of Cre-CT mice switch to the SAN gene program
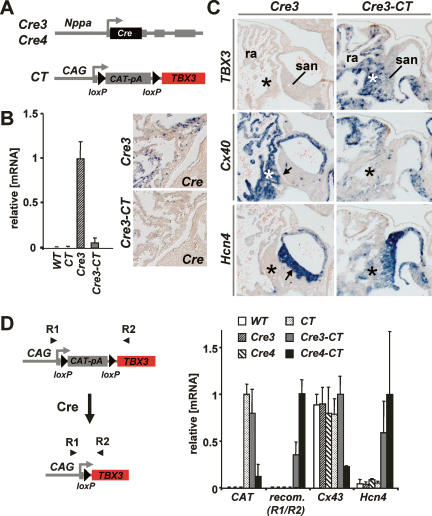
Because Tbx3 is partially redundant and mutants are embryonic lethal, Tbx3-deficient embryos do not allow detailed assessment of the function of Tbx3 in the regulation of the SAN phenotype. Therefore, to further explore the role of Tbx3 in regulating the SAN gene program and phenotype, we used a gain-of-function approach. Mice were generated that allow conditional activation of the human TBX3 cDNA by Cre recombinase (CT mice). In these mice, Cre-mediated excision of the CAT reporter gene activates TBX3 (Fig. 4A,D). CT mice were crossed with Cre3 mice in order to effectively expand the SAN Tbx3 expression domain into the atria. In double transgenic fetuses, TBX3 was induced in the atria, whereas Nppa promoter-driven Cre expression had disappeared (Fig. 4B–D; Supplementary Fig. 3A), showing that Tbx3 in vivo suppresses the Nppa promoter fragment that drives Cre expression. Furthermore, Cx40 and Nppa expression was down-regulated, whereas Hcn4 expression was strikingly induced in these atria (Fig. 4C; data not shown). These results indicate that Tbx3 is able to repress atrial genes and to induce a SAN gene in differentiated (Nppa-positive) atrial cardiomyocytes. Taken together, our data indicate that Tbx3 defines a domain where atrial working myocardial genes are repressed and expression of SAN-specific genes is permitted or induced, thereby establishing a phenotypic boundary between the SAN domain and the atrial working myocardium.
Figure 4.
Ectopic expression of TBX3 in atrial cardiomyocytes results in repression of Cx40 and in ectopic activation of pacemaker channel Hcn4. (A) Scheme showing the CT and Cre3/Cre4 alleles. (B) QRT–PCR and in situ hybridization analysis of Cre3 mice and Cre3-CT double transgenic mice. Cre expression under control of Nppa regulatory sequences is repressed in Cre3-CT mice, demonstrating that TBX3 is capable of suppressing Nppa promoter activity in vivo. (C) In situ hybridization on serial sections of prenatal (E17.5) Cre3 and Cre3-CT hearts showing TBX3 expression, down-regulation of Cx40, and ectopic induction of Hcn4 in the right atrium (ra) of Cre3-CT mice. Asterisks (*) depict comparable regions in the right atrium adjacent to the SAN. (D) QRT–PCR analysis of atria of Cre3-CT and Cre4-CT double transgenic mice compared with control mice. Expression of Cx43 is significantly down-regulated in Cre4-CT mice. Due to the partial, mosaic activation of TBX3 by Cre3, many atrial cells are not affected, and the repression of Cx43 is not observed in homogenates of Cre3-CT mice. Expression of Hcn4 is significantly up-regulated in both Cre3-CT and Cre4-CT mice. The product obtained with primer set R1/R2 reveals the activated TBX3-encoding transcripts.
We next crossed CT mice to Nppa-Cre4 (Cre4) mice, because Cre3, while efficiently recombining R26R or Z/EG alleles, appeared to recombine and activate the CT allele less efficiently and in a mosaic pattern (Fig. 4D; Supplementary Fig. 3). Hence, while on sections effective down-regulation of target genes could be assessed in a fraction of the atrial cells, this was not possible by quantitative RT–PCR (QRT–PCR) analysis due to the large fraction of cells in which TBX3 was not activated (Fig. 4D; Supplementary Fig. 3A,B). In contrast, the efficient activation of TBX3 by Cre4 allowed the assessment of gene repression by QRT-PCR (Fig. 4D). Three sets of genes were selected for expression analysis in the left atria of adult Cre4-CT mice. Set 1 genes represent cardiac transcription factors and structural and functional genes not enriched in either the SAN or atria (Schram et al. 2002; Marionneau et al. 2005). Set 2 genes are expressed at higher levels in the working myocardium of the atria, compared with the SAN, and are important for working myocardial function (Supplementary Fig. 1; Schram et al. 2002; Marionneau et al. 2005). This set includes Kir2.1 and Kir2.2, which encode inward rectifier potassium currents involved in the fast terminal repolarization and maintenance of the resting membrane potential of working cardiomyocytes (Miake et al. 2002). Set 3 genes are expressed at higher levels in the SAN compared with the atria, and are important for SAN function (Supplementary Fig. 1; Schram et al. 2002; Marionneau et al. 2005; Liu et al. 2006). This set includes the Hcn family of hyperpolarization-activated channels, which provide pacemaker activity (diastolic depolarization) to the myocardium (Baruscotti et al. 2005). Although Hcn4 is the dominant isoform in the SAN, Hcn1 and Hcn2 are also expressed in the SAN, at higher levels than in the atria (Marionneau et al. 2005). Cav3.1 encodes a T-type calcium channel that contributes to SAN pacemaker activity (Mangoni et al. 2006). Cx30.2 encodes a gap junction protein selectively expressed in the SAN and atrioventricular node of the conduction system that slows conduction velocity within the node (Kreuzberg et al. 2006). In situ hybridization of late fetal hearts, immunofluorescent labeling of adult hearts, and QRT–PCR analysis of adult atria showed that the genes required for regulation and function of cardiomyocytes in general, the set 1 genes, were not affected by TBX3 expression in the atria of Cre4-CT mice (Figs. 5B 6A). In contrast, all atrial tissue-enriched genes were significantly down-regulated, indicating that atrial myocytes adopt low levels of expression of these genes similar to the SAN (Figs. 5A,B, 6A). The SAN-enriched genes were up-regulated in the left atria of Cre4-CT mice (Figs. 5A,B, 6A), especially Hcn4 and Cx30.2. These data further indicate that Tbx3 converts the atrial gene program to the SAN gene program by coordinately activating and repressing key components of each program (Fig. 6C).
Figure 5.
TBX3 represses atrial working myocardial genes and induces SAN-specific genes. (A) In situ hybridization of serial sections of E17.5 hearts, showing down-regulation of Cx40 and Cx43 in the right atrium (ra) of Cre4-CT mice. Expression of Hcn4 and Lbh is up-regulated in atrial myocardium of Cre4-CT mice. (B) Immunofluorescent labeling of postnatal mouse heart sections showing that atrial myocytes of adult Cre4-CT mice ectopically expressed Hcn4, whereas Cx43 and Nav1.5 expression was reduced compared with control mice. (C) Immunofluorescent double staining of Cx43 (red) and Hcn4 (blue) in right atrial sections of Cre4 and Cre4-CT mice, respectively. In Cre4-CT mice, Cx43 is down-regulated, and the residual Cx43 expression is complementary to ectopic Hcn4 expression. Bar, 100 μm.
Figure 6.
TBX3 induces switch to the SAN gene expression program in the atria. (A, left panel) QRT–PCR analysis showed no difference in expression of set 1 (myocardial) genes in the left atria (LA) of Cre4-CT mice (black bars) as compared with control mice (white bars). (Right panel) Expression levels of set 2 (atrium-enriched) genes showed a significant down-regulation. (B) Expression levels of set 3 (SAN-enriched) genes were induced in the left atria of Cre4-CT mice. (C) Scheme indicating a central role for Tbx3 in the regulation of the SAN gene program and phenotype. Because Tbx3 is a repressor, it may activate genes indirectly (i.e., by repressing repressor R). Error bars in A and B represent SD (n = 4 per group). (*) P < 0.05.
To assess changes in target gene expression in response to a short period of Tbx3 exposure, αMhc-Cre mice were crossed to CT mice in order to activate TBX3 in the heart of embryos prior to and during chamber differentiation (Fig. 7A). Differentiation and expansion of the chamber myocardium were mildly to severely impaired (data not shown). Cx40 and other genes specific for the atrial and ventricular chambers were efficiently down-regulated in both mildly and severely affected hearts, indicative for a relative fast response to Tbx3 exposure (1–2 d) (Fig. 7A,B). In contrast, Hcn4, Cx30.2, and Lbh were not induced, which indicates that up-regulation of these genes, as observed in Cre3/4-CT mice, is indirect, requiring prolonged presence of Tbx3 (Fig. 7B).
Figure 7.
Activation of TBX3 in embryonic hearts leads to decreased expression of atrial genes but not up-regulation of SAN genes. (A) Whole-mount in situ hybridization of E10.5 αMhc-Cre-CT double transgenic embryos, showing ectopic expression of TBX3 in the whole heart (black arrow). Expression of Cx40 is decreased specifically in the hearts of αMhc-Cre-CT embryos (black arrowheads). Yellow arrow depicts staining in endothelial cells of the dorsal aorta, which is comparable in αMhc-Cre-CT and αMhc-Cre control embryos. (B) QRT–PCR analysis showed a significant down-regulation of atrium-enriched genes Nppa, Smpx, and Cx40, but not of SAN-enriched genes Hcn4, Lbh, and Cx30.2. (C) Chromatin was isolated from H10 rat cardiomyocyte cells stably expressing Flag-tagged Tbx3. PCR analysis of input DNA using primers surround the Cx43 TBEs at position −450 pb is shown in lane 1. Lane 2 and 3 show TBE target amplification using Flag-immunoprecipitated chromatin from cells expressing Flag-Tbx3 or a control not expressing Flag-Tbx3, respectively. Amplification of a region ∼0.6 kb upstream of the TBE can only be achieved using the input DNA (lane 4), whereas amplification using Flag-Tbx3-derived chromatin fails to give an amplification product (lane 5).
Both promoters of Nppa and Cx43 contain a T-box-binding element (TBE) required for their suppression in the atrioventricular canal and limb in vivo, respectively (Habets et al. 2002; Chen et al. 2004), indicating that Tbx3 directly represses these genes. To assess whether Tbx3 interacts with the Cx43 gene in vivo, chromatin was isolated from H10 rat cardiomyocyte cells (Jahn et al. 1996) stably expressing Flag-tagged Tbx3. Figure 7C shows the immunoprecipitation of chromatin using anti-Flag Sepharose with subsequent PCR amplification for the Cx43 promoter TBE (Chen et al. 2004). Only in the presence of Tbx3 can the TBE be amplified. These results indicate that Cx43 is directly repressed by Tbx3.
Tbx3 induces the formation of ectopic pacemaker sites in the atria
Next, we investigated whether the switch to the SAN gene program in atria of double transgenic mice was sufficient to generate functional pacemaker activity. Cre4-CT mice developed dilatation and fibrosis of the atria several weeks after birth, possibly resulting from electrical and structural remodeling (Supplementary Fig. 4A,B). Therefore, we used Cre3 mice that provide an incomplete, mosaic TBX3 activation pattern of the CT allele in the atria (Fig. 4; Supplementary Fig. 3). As expected, the response of Tbx3 target genes in the atria of Cre3-CT was comparable with that in atria of Cre4-CT mice, but less pronounced (Supplementary Fig. 4C,D). Whole-mount and histological examination of adult atria of Cre3-CT mice and controls revealed no abnormalities in size or tissue structure (Supplementary Fig. 4A,B). Electrocardiography revealed normal RR, PQ, QRS, and QT intervals and individual waveforms in the double transgenic mice (Fig. 8A,B). However, close examination of the RR intervals of double transgenic mice showed periods of normal activity ranging from seconds to minutes followed by short periods of one to three irregular, premature beats (Fig. 8A,C).
Figure 8.
Ectopic pacemaker activity in Cre3-CT mice. (A) Three-minute segment of RR intervals, displaying heart rate instability. Inspection of an ECG segment in an expanded time scale revealed a premature beat in the Cre3-CT group. (B,C) ECG analysis uncovered an increase in premature beats in the Cre3-CT mice group compared with the three control groups, without changes in ECG parameters. (D) Only Cre3-CT mice showed spontaneous electrical activity in both isolated right and left atria. (E) Activation map of isolated right atria of Cre3-CT mice, showing the presence of stable ectopic nodal impulse formation originating from the site marked by an asterisk. The numbers on isochrones indicate activation time in milliseconds. (F) Typical example of pacemaker formation (left) and hyperpolarization-activated current (right) in a single right atrial Cre4-CT cardiomyocyte. (*) P < 0.05.
The arrhythmia in Cre3-CT mice could result from dysfunction of the SAN, due to an increase in atrial premature complexes, or ectopic pacemaker activity. To discriminate between these possibilities, intact left and right atria of Cre3-CT mice were isolated free from SAN and internodal tract myocytes (Fig. 8D; Yamamoto et al. 2006). Ninety percent of right and 40% of left atria, respectively, showed ectopic spontaneous electrical activity, while this was absent in control mice (Fig. 8D). Mapping of the activation pattern of isolated atria in double transgenic mice revealed at least one stable ectopic pacemaker. Nodal-like action potentials could be recorded at the site of the earliest activation in the ectopic pacemaker region (Fig. 8E). To exclude cell-to-cell coupling-induced modifications, pacemaker activity was studied in isolated right atrial myocytes. Pacemaker formation was observed in seven out of 15 single myocytes (47%) of Cre4-CT mice, while it was absent from single myocytes (n = 11) of control mice (P = 0.01) (Fig. 8F). The spontaneously active Cre4-CT myocytes had nodal-like action potentials with typical low action potential upstrokes, maximal diastolic depolarization potential around −60 mV, and diastolic depolarization resulting in pacemaker activity with an intrinsic cycle length of 243 ± 61 msec. The myocytes of Cre4-CT mice exhibiting pacemaker activity had a clear hyperpolarization activated current (−8.4 ± 1.9 pA/pF [n = 7] at −110 mV), while such current was virtually absent in the quiescent myocytes of Cre4-CT and control mice. Our experimental evidence thus showed that the ectopic expression of TBX3 in the atria induces the formation of pacemaker activity resulting in functional ectopic nodes in the atria in vivo.
Discussion
The present study uses loss- and gain-of-function strategies and genetic lineage analysis to define the potential role of Tbx3 in the regulation of SAN formation, and identifies Tbx3 as a central regulatory factor that controls the SAN gene program and phenotype in the heart. Tbx3 appears to be sufficient to reprogram atrial cells into functional pacemaker cells. During embryonic development, Tbx3 defines the SAN region by shielding the cells within its own expression domain from atrial gene expression and differentiation, while at the same time inducing SAN gene expression. The precursor cells within this domain then expand to form the SAN, without receiving contributions from the bordering atrial myocytes, which were found to segregate soon after their differentiation. As such, these results identify a new paradigm for the specification and formation of the SAN, and provide an important aspect of the underlying molecular mechanism. Tbx3 expression in the developing nodal components of the conduction system is conserved among higher vertebrates (Hoogaars et al. 2004), indicating that Tbx3 is an evolutionarily conserved control of SAN formation and phenotype.
Tbx3 and the formation of the SAN
Compared with the working myocardium, the cells of the SAN are small and pale and have poorly developed sarcomeres and sarcoplasmic reticulum. These nodal cells are automatic and express few gap junctions, and their intercellular coupling is poor (Bleeker et al. 1980; Canale et al. 1986). Interestingly, these properties and gene expression patterns of the SAN resemble those of the early embryonic myocytes (for review, see Moorman and Christoffels 2003). This suggests that while embryonic cardiac muscle cells differentiate and mature into working myocytes, the SAN retains properties of embryonic myocardium. We now show that Tbx3 is expressed in the developing and mature SAN and is required to suppress the expression of atrial differentiation marker genes. These findings indicate that Tbx3 specifies the SAN domain by suppressing differentiation to working myocardium, thereby allowing the cells within its expression domain to retain the properties required for their pacemaker function in the mature heart. However, during development, the phenotype of the SAN is not fixed, but also matures. The finding that Tbx3 not only suppresses working myocardial genes, but also is required for the activity of SAN genes such as Lbh, and is able to induce important SAN genes including Hcn-family members and Cx30.2, indicates that Tbx3 plays an active role in the establishment of the mature SAN phenotype.
Analysis of E12.5 Tbx3Cre/Cre embryos revealed that a primordial SAN was formed that was variable in size. This indicates that Tbx3 may be involved in, but is not strictly required for, the induction of the primordial SAN. Furthermore, the primordial SAN expressed Cre, indicating that Tbx3 is not required for its own expression. At E12.5 we found ectopic expression of Cx43 and Smpx in the Cre expression domain of the primordial SAN. However, only at E13.5–E14.5 were Cx40 and Nppa ectopically induced in the primordial SAN of Tbx3Cre/Cre embryos, suggesting redundancy of repressive Tbx3 function or the absence of an activating factor of these genes at earlier stages. The cardiac homeobox transcription factor Nkx2-5, an essential activator of Cx40 and Nppa (Harvey 2002; Dupays et al. 2005), is not expressed in the SAN region until approximately E13–E14 (Mommersteeg et al. 2007), explaining why the requirement of Tbx3 to suppress Cx40 and Nppa in Tbx3-deficient embryos only becomes apparent at this stage. Therefore, the expression of Nkx2-5-regulated genes may initially not be altered in the primordial SAN of Tbx3-deficient embryos.
Tbx3 is a potent transcriptional repressor (He et al. 1999). Nppa was shown to contain a TBE critical for the suppression of Nppa promoter activity in the Tbx3-positive atrioventricular canal in vivo, to which Tbx3 binds in vitro (Habets et al. 2002; Hoogaars et al. 2004). Also promoter fragments of Cx40 and Cx43 contain TBEs that are involved in T-box factor-mediated gene regulation in cell culture, and in limb in vivo (Bruneau et al. 2001; Chen et al. 2004). It is therefore likely that Tbx3 directly suppresses the activity of atrial genes in vivo. Indeed, chromatin immunoprecipitation analysis revealed that Tbx3 specifically interacts with the TBE-containing DNA region of the Cx43 gene in HL-10 cells in vivo, providing strong indication that Tbx3 directly suppresses Cx43. In addition, Tbx3 was found to efficiently activate Lbh, previously shown to suppress the Nppa promoter (Briegel et al. 2005), and to suppress Hop, required for Cx40 expression (Ismat et al. 2005). Therefore, we speculate that Tbx3 may suppress atrial genes both directly and indirectly through modulating the expression of suppressors and activators of atrial genes.
The repressor function of Tbx3 implies that the induction of SAN genes in the atria is indirect. Consistently, when Tbx3 was ectopically expressed in the entire heart of embryos before or during chamber differentiation using the αMhc-Cre mouse line, SAN genes were not significantly induced (Fig. 7A,B), indicating that a much longer period of time is required to activate these genes, as was observed in Cre3/4-CT mice. Furthermore, although Lbh expression was lost from the SAN in Tbx3-deficient embryos, we did not observe loss of Hcn4 expression, and the induction of SAN-specific genes in the atria of Cre4-CT mice was mosaic, again with the exception of Lbh, which was homogeneously induced in these atria. These observations indicate that Tbx3 does not directly induce Hcn4 and other SAN genes, but imposes a phenotype on cells that allow those genes to be activated. To identify the underlying activation mechanism, future work will focus on the analysis of Hcn4 and Lbh regulation in vivo.
Heterozygous mutations in TBX3 causes ulnar-mammary syndrome in human, but mice appear to be less sensitive to a lower Tbx3 dose (Naiche et al. 2005). Consistently, we did not observe alterations in gene expression in the SAN of heterozygous Tbx3+/− embryos and adults. Affected conduction system morphology or function has not been reported in ulnar-mammary syndrome patients, suggesting that the reduced TBX3 dose is sufficient for SAN regulation in humans. However, a subset of ulnar-mammary syndrome patients has structural congenital heart defects, indicating that reduction of Tbx3 dose in the context of certain genetic backgrounds or additional mutations becomes important for heart development. It is therefore possible that a closer examination of a large number of ulnar-mammary syndrome patients may reveal conduction system anomalies.
Formation of the SAN: recruitment versus proliferation of specified precursors
The central conduction system components have been proposed to form by continuous recruitment throughout development of myocytes into an initial framework of specified conduction system cells. This proposal was based on elegant retrospective clonal analyses in which rare single-cell-derived clones found in the central conduction system always extended into the adjacent working myocardium (Cheng et al. 1999; Pennisi et al. 2002). The phenotype of the infected founder cell could not be assessed. However, during development, the cells of the central conduction system, including those of the SAN (Erokhina and Rumyantsev 1986), proliferate much less compared with the adjacent myocytes forming the working myocardium, which indicated that the latter enter the conduction system lineage, rather than the other way around (Cheng et al. 1999). The spatio-temporal expression patterns we observed indicate specification of the sinoatrial junction in Tbx3/Hcn4-positive nodal precursors and adjacent Cx40/Nppa-positive atrial precursors as early as E10. Therefore, SAN specification and growth can be envisioned to follow either of two modes as presented in Figure 3, A and B. Using the Cre-loxP system, the emerging atrial lineage bordering the Tbx3-positive domain was irreversibly labeled, allowing assessment of recruitment of the atrial cells into the SAN lineage. We found that the pattern of β-galactosidase or EGFP, irreversibly activated by the Nppa-Cre allele from E10 on, exactly matched the patterns of Cx40 and Nppa expression and was strictly complementary to those of Tbx3 and Hcn4 expression. These data indicate that from their differentiation onward, atrial myocytes are not recruited into the SAN lineage. Our assay did not rule out the possibility that initially Tbx3-positive cells differentiate to atrial working myocardium later in development. Tbx3 expression was observed to be associated with the SAN during development. In addition, Tbx3 was found to be able to suppress the atrial genes, including the Nppa-Cre cassette used to label atrial myocytes, and appeared to be required to suppress atrial genes in the primordial SAN. From the combined data, we conclude that the mature SAN is formed from the Tbx3-positive embryonic precursor population.
Tbx3 imposes pacemaker activity on atrial myocytes
Many time-dependent currents contribute to SAN function. These currents are encoded by several gene families, each comprising several family members (Boyett et al. 2000; Schram et al. 2002; Marionneau et al. 2005). Other important properties include intercellular coupling, which is largely defined by the number and type of gap junctions present in and around the SAN. The difference in function between atrial working myocytes and SAN cells is at least in part defined by genes differentially expressed between the SAN and atria. To date, only a limited number of differentially expressed genes have been identified, but their contribution to either SAN-specific or atrial working myocardial-specific function is substantial. Some of these genes were found to be essential for pacemaker function or for impulse propagation, whereas others are sufficient to generate ectopic pacemaker activity (Miake et al. 2002; Papadatos et al. 2002; Schram et al. 2002; Rosen et al. 2004; Stieber et al. 2004; Bucchi et al. 2006; Tse et al. 2006). In the present study, we found the remarkable ability of Tbx3 to selectively regulate the expression of this differentially expressed and functionally important set of genes, indicating that it acts upstream in a pathway that controls pacemaker phenotype. Not only does it repress genes required for working myocardial function, it also stimulates genes required for pacemaker function, including pacemaker channels (Hcn), calcium channel (Cav3.1), and slow gap junction Cx30.2, thereby efficiently reprogramming atrial myocytes into functional pacemaker cells. In contrast, genes not implicated in the functional or structural distinction between atria and SAN were not affected by Tbx3. These results indicate that Tbx3 is an efficient tool to identify genes that define the distinction between working myocardium and pacemaker cells, and to gain further insight into the molecular genetic underpinnings of pacemaker formation and function.
Currently, attempts to generate bio-artificial pacemakers with normal entopic electrophysiological characteristics using virally delivered channel genes are ongoing (Miake et al. 2002; Rosen et al. 2004; Bucchi et al. 2006; Tse et al. 2006). These pacemakers provide a novel inroad into treatment of common arrhythmias. It will be of great clinical interest to examine whether Tbx3 could be applied in the generation of bio-artificial pacemakers. Although Tbx3 maintains proliferation and stimulates senescence bypass in specific cell types (Brummelkamp et al. 2002; Naiche et al. 2005), it is unlikely to have this role in developing or mature cardiac myocytes, possibly rendering it a useful tool to reprogram working myocytes ex vivo into pacemaker cells displaying normal electrophysiological characteristics.
Materials and methods
Mice and gene targeting
Z/EG (Novak et al. 2000) and R26R (Soriano 1999) reporter mice have been described previously. The CAG–CAT–TBX3 (CT) transgene construct harbors a human TBX3 cDNA encoding a full-length human TBX3 protein (Brummelkamp et al. 2002) with an in-frame fusion to a hemagglutinin (HA) tag in a modified backbone from pCAG–CAT–Z (Araki et al. 1995). CT transgenic mice were bred with Nppa-Cre3, Nppa-Cre4 (de Lange et al. 2003), or αMhc-Cre mice (Agah et al. 1997) to generate double-transgenic mice conditionally expressing TBX3 in the atria or the whole heart, respectively. The transgenic mice were identified by PCR analysis using primers specific for CAT and Cre genes.
A cosmid with Tbx3, isolated from the 129/Ola cosmid genomic library obtained from the Resourcenzentrum (RZPD) in Berlin, was kindly provided by Dr. Andreas Kispert (Institut fur Molekularbiologie, Medizinische Hochschule Hannover, Hannover, Germany). Homologous DNA sequences (6.1 kb of upstream sequence and 1.9 kb of downstream sequence) were ligated to a Cre-polyA-Frt-flanked PGK-neo cassette derived from pKOII (Bardeesy et al. 2002) to generate a Tbx3-targeting construct (Supplementary Fig. 2) in which the first three codons of the Tbx3-coding region were replaced by the Cre-pA cassette. The linearized targeting construct was electroporated into E141B10 embryonic stem (ES) cells to generate targeted cell lines. A diphtheria toxin A cassette was used to positively select for homologous recombinants. Chimeras were generated by injection of targeted ES cells into C57Bl6 host blastocysts. Germline transmission of the targeted allele was obtained by mating with FVB females. Subsequently, Tbx3CreNEO mice were crossed with FlpE mice (Rodriguez et al. 2000) to remove the PGK-neo cassette. Progeny was screened by PCR for the presence of the Tbx3Cre allele using the following primers: fw1 (AGCGGAGCCAAGCCAGCA), rv1 (CCTTGGCCTCCAG GTGCAC), and rv2 (GCTAGAGCCTGTTTTGCACGTTCA). The Tbx3Cre allele has been maintained on a FVB background. Animal care was in accordance with national and institutional guidelines.
Chromatin immunoprecipitation
Chromatin was isolated from the rat neonatal cardiomyocyte cell line, H10 (Jahn et al. 1996), stably expressing Flag-tagged Tbx3. A kit (Active Motif) and Flag M2 Sepharose (Sigma) were used to capture the Flag-tagged protein according to the manufacturer’s instructions. DNA fragments were analyzed by PCR with primers specific for the rat Cx43 TBEs ∼450 base pairs (bp) upstream of the transcription start site (5′-CCGTGTTTAAGA GGAGGAGAATTAGG-3′ and 5′-GGGACAAGGTCAACTCG TGCAGAC-3′) or for the control region ∼1 kb upstream (5′-AG GAGCTGCCCACCCTTAGGAATG-3′ and 5′-GAGTTTCCA GATACATTATGTTAGC-3′).
In situ hybridization, immunohistochemistry, and histology
Nonradioactive in situ hybridization, 3D reconstruction, and quantification of expression domains were performed as described previously (Moorman et al. 2001; Hoogaars et al. 2004). The probes used have been described previously (Hoogaars et al. 2004). Probes for Hcn4 (Santoro et al. 2000; Garcia-Frigola et al. 2003) and Hop (Chen et al. 2002) were generously provided by B. Santoro (Center for Neurobiology and Behavior, Columbia University, New York, NY) and J. Epstein (Department of Cell and Developmental Biology, University of Pennsylvania School of Medicine, Philadelphia, PA), respectively. For immunohistochemistry, adult hearts were isolated in PBS, rapidly frozen in liquid nitrogen, and cut into 10-μm sections. The primary antibodies used were HCN4 rabbit polyclonal (1:250; Chemicon), Cx40 rabbit polyclonal (1:250; Chemicon), Cx43 mouse monoclonal (1:250; BD Transduction Laboratory), cTnI rabbit polyclonal (1:1000; Hitest Ltd.), and Nav1.5 rabbit polyclonal (1:100; Alomone Laboratories). The secondary antibodies used were Alexa 647 anti-rabbit, Alexa 568 anti-rabbit, and Alexa 568 anti-mouse antibodies (1:100; Molecular Probes). Nuclei were stained using Sytox Green nucleic acid stain (1:30,000; Molecular Probes). Fibrosis was determined by Pico Sirius red staining.
Quantitative expression analysis
Total RNA was isolated from atrial appendices of adult mice (2–4 mo) using the RNeasy Mini Kit according to the manufacturer’s protocol (Qiagen). cDNA was reverse-transcribed from 300 ng of total RNA using the SuperScript II system (Invitrogen). Expression of different genes was assayed with quantitative real-time PCR using the MyiQ Single-Color RT–PCR Detection System (Bio-Rad). The relative start concentration [N(0)] was calculated using the following equation: N(0) = 10[log(threshold)−Ct(mean Eff)]. Values were normalized to Gapdh expression levels.
ECGs
Lead I ECG configuration recordings were obtained in anesthetized (isofluraan 1.5%) mice. Plots of successive RR intervals during 15-min epochs were inspected for periods of irregularity in heart rate. Premature beats were defined as a rhythm during which P waves occurred prior to the expected P wave.
Electrophysiology
Electrophysiological mapping of the atrial region was accomplished at 37.0 ± 0.3°C as recently described (Verheijck et al. 2001). Transmembrane potentials were recorded at 5 kHz by conventional glass microelectrodes filled with 2.7 M KCl and 2 mM K-citrate (resistance 15–30 MΩ). For the construction of activation maps, impalements were made 0.1 mm apart and in the ectopic pacemaker area down to 0.05 mm apart. Ectopic pacemakers showed diastolic depolarization and low maximal upstroke velocity <10 V/sec. A bipolar silver wire electrode was placed on the edge of the preparation. This electrode provided a surface electrogram of which the first deflection served as a time reference for the determination of the ectopic conduction time and construction of the activation map. Single right atrial cardiomyocytes were isolated by enzymatic dissociation. Action potentials and hyperpolarizing-activated currents were recorded using the perforated patch-clamp technique (Axopatch 200B Clamp amplifier; Axon Instruments, Inc.). Signals were low-pass-filtered (cut-off frequency 5 kHz) and digitized at 5 kHz. Series resistance was compensated ≥80%, and potentials were corrected for liquid-junction potential. For voltage control, data acquisition, and analysis, custom-made software was used. The superfusion solution (37°C) contained 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5.5 mM glucose, and 5 mM HEPES [pH 7.4 (NaOH)]. The pipette solution contained 125 mM K-gluc, 20 mM KCl, 5 mM NaCl, 2.2 mM amphotericin-B, and 10 mM HEPES [pH 7.2 (KOH)]. Hyperpolarizing-activated current was measured by 500-msec hyperpolarizing steps from −40 mV.
Statistics
Results are expressed as mean ± SEM. Data are considered different if P < 0.05. The Mann-Whitney U-test, unpaired t-test, or Fisher’s exact test was used if appropriate.
Acknowledgments
We thank Tilly Mommersteeg, Kees Jan Boogerd, Jan Ruijter, André Linnenbank, Corrie de Gier-de Vries, Alexandre Soufan, Ephie Kraneveld, and Toon van Veen for their contributions and advice; the GGM facility for generating mice; Susan Dymecki for providing FlpE deleter mice; Michael Schneider for providing αMhc-Cre mice; and Andreas Kispert, Bina Santoro, Ronald DePinho, Jonathan Epstein, and Jun-ichi Miyazaki for reagents. This work was supported by NWO 912-03-043 to E.E.V. and V.M.C.; by NHS grant 1996M002 to A.F.M. and V.M.C.; by NWO 864-05-006 to V.M.C.; and by NHS grant 2005B076 to V.M.C.
Footnotes
Supplemental material is available at http://www.genesdev.org.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.416007
References
- Agah R., Frenkel P.A., French B.A., Michael L.A., Overbeek P.A., Schneider M.D., Frenkel P.A., French B.A., Michael L.A., Overbeek P.A., Schneider M.D., French B.A., Michael L.A., Overbeek P.A., Schneider M.D., Michael L.A., Overbeek P.A., Schneider M.D., Overbeek P.A., Schneider M.D., Schneider M.D. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J. Clin. Invest. 1997;100:169–179. doi: 10.1172/JCI119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K., Araki M., Miyazaki J., Vassalli P., Araki M., Miyazaki J., Vassalli P., Miyazaki J., Vassalli P., Vassalli P. Site-specific recombination of a transgene in fertilized eggs by transient expression of Cre recombinase. Proc. Natl. Acad. Sci. 1995;92:160–164. doi: 10.1073/pnas.92.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagwe S., Berenfeld O., Vaidya D., Morley G.E., Jalife J., Berenfeld O., Vaidya D., Morley G.E., Jalife J., Vaidya D., Morley G.E., Jalife J., Morley G.E., Jalife J., Jalife J. Altered right atrial excitation and propagation in connexin40 knockout mice. Circulation. 2005;112:2245–2253. doi: 10.1161/CIRCULATIONAHA.104.527325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamshad M., Lin R.C., Law D.J., Watkins W.C., Krakowiak P.A., Moore M.E., Franceschini P., Lala R., Holmes L.B., Gebuhr T.C., Lin R.C., Law D.J., Watkins W.C., Krakowiak P.A., Moore M.E., Franceschini P., Lala R., Holmes L.B., Gebuhr T.C., Law D.J., Watkins W.C., Krakowiak P.A., Moore M.E., Franceschini P., Lala R., Holmes L.B., Gebuhr T.C., Watkins W.C., Krakowiak P.A., Moore M.E., Franceschini P., Lala R., Holmes L.B., Gebuhr T.C., Krakowiak P.A., Moore M.E., Franceschini P., Lala R., Holmes L.B., Gebuhr T.C., Moore M.E., Franceschini P., Lala R., Holmes L.B., Gebuhr T.C., Franceschini P., Lala R., Holmes L.B., Gebuhr T.C., Lala R., Holmes L.B., Gebuhr T.C., Holmes L.B., Gebuhr T.C., Gebuhr T.C., et al. Mutations in human TBX3 alter limb, apocrine and genital development in ulnar-mammary syndrome. Nat. Genet. 1997;16:311–315. doi: 10.1038/ng0797-311. [DOI] [PubMed] [Google Scholar]
- Bardeesy N., Sinha M., Hezel A.F., Signoretti S., Hathaway N.A., Sharpless N.E., Loda M., Carrasco D.R., DePinho R.A., Sinha M., Hezel A.F., Signoretti S., Hathaway N.A., Sharpless N.E., Loda M., Carrasco D.R., DePinho R.A., Hezel A.F., Signoretti S., Hathaway N.A., Sharpless N.E., Loda M., Carrasco D.R., DePinho R.A., Signoretti S., Hathaway N.A., Sharpless N.E., Loda M., Carrasco D.R., DePinho R.A., Hathaway N.A., Sharpless N.E., Loda M., Carrasco D.R., DePinho R.A., Sharpless N.E., Loda M., Carrasco D.R., DePinho R.A., Loda M., Carrasco D.R., DePinho R.A., Carrasco D.R., DePinho R.A., DePinho R.A. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature. 2002;419:162–167. doi: 10.1038/nature01045. [DOI] [PubMed] [Google Scholar]
- Baruscotti M., Bucchi A., DiFrancesco D., Bucchi A., DiFrancesco D., DiFrancesco D. Physiology and pharmacology of the cardiac pacemaker (‘funny’) current. Pharmacol. Ther. 2005;107:59–79. doi: 10.1016/j.pharmthera.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Bleeker W.K., Mackaay A.J.C., Masson-Pevet M., Bouman L.N., Becker A.E., Mackaay A.J.C., Masson-Pevet M., Bouman L.N., Becker A.E., Masson-Pevet M., Bouman L.N., Becker A.E., Bouman L.N., Becker A.E., Becker A.E. Functional and morphological organization of the rabbit sinus node. Circ. Res. 1980;46:11–22. doi: 10.1161/01.res.46.1.11. [DOI] [PubMed] [Google Scholar]
- Boyett M.R., Honjo H., Kodama I., Honjo H., Kodama I., Kodama I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc. Res. 2000;47:658–687. doi: 10.1016/s0008-6363(00)00135-8. [DOI] [PubMed] [Google Scholar]
- Briegel K.J., Baldwin H.S., Epstein J.A., Joyner A.L., Baldwin H.S., Epstein J.A., Joyner A.L., Epstein J.A., Joyner A.L., Joyner A.L. Congenital heart disease reminiscent of partial trisomy 2p syndrome in mice transgenic for the transcription factor Lbh. Development. 2005;132:3305–3316. doi: 10.1242/dev.01887. [DOI] [PubMed] [Google Scholar]
- Brummelkamp T.R., Kortlever R.M., Lingbeek M., Trettel F., MacDonald M.E., van Lohuizen M., Bernards R., Kortlever R.M., Lingbeek M., Trettel F., MacDonald M.E., van Lohuizen M., Bernards R., Lingbeek M., Trettel F., MacDonald M.E., van Lohuizen M., Bernards R., Trettel F., MacDonald M.E., van Lohuizen M., Bernards R., MacDonald M.E., van Lohuizen M., Bernards R., van Lohuizen M., Bernards R., Bernards R. TBX-3, the gene mutated in Ulnar-Mammary Syndrome, is a negative regulator of p19ARF and inhibits senescence. J. Biol. Chem. 2002;277:6567–6572. doi: 10.1074/jbc.M110492200. [DOI] [PubMed] [Google Scholar]
- Bruneau B.G., Nemer G., Schmitt J.P., Charron F., Robitaille L., Caron S., Conner D.A., Gessler M., Nemer M., Seidman C.E., Nemer G., Schmitt J.P., Charron F., Robitaille L., Caron S., Conner D.A., Gessler M., Nemer M., Seidman C.E., Schmitt J.P., Charron F., Robitaille L., Caron S., Conner D.A., Gessler M., Nemer M., Seidman C.E., Charron F., Robitaille L., Caron S., Conner D.A., Gessler M., Nemer M., Seidman C.E., Robitaille L., Caron S., Conner D.A., Gessler M., Nemer M., Seidman C.E., Caron S., Conner D.A., Gessler M., Nemer M., Seidman C.E., Conner D.A., Gessler M., Nemer M., Seidman C.E., Gessler M., Nemer M., Seidman C.E., Nemer M., Seidman C.E., Seidman C.E., et al. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell. 2001;106:709–721. doi: 10.1016/s0092-8674(01)00493-7. [DOI] [PubMed] [Google Scholar]
- Bucchi A., Plotnikov A.N., Shlapakova I., Danilo P., Jr., Kryukova Y., Qu J., Lu Z., Liu H., Pan Z., Potapova I., Plotnikov A.N., Shlapakova I., Danilo P., Jr., Kryukova Y., Qu J., Lu Z., Liu H., Pan Z., Potapova I., Shlapakova I., Danilo P., Jr., Kryukova Y., Qu J., Lu Z., Liu H., Pan Z., Potapova I., Danilo P., Jr., Kryukova Y., Qu J., Lu Z., Liu H., Pan Z., Potapova I., Kryukova Y., Qu J., Lu Z., Liu H., Pan Z., Potapova I., Qu J., Lu Z., Liu H., Pan Z., Potapova I., Lu Z., Liu H., Pan Z., Potapova I., Liu H., Pan Z., Potapova I., Pan Z., Potapova I., Potapova I., et al. Wild-type and mutant HCN channels in a tandem biological-electronic cardiac pacemaker. Circulation. 2006;114:992–999. doi: 10.1161/CIRCULATIONAHA.106.617613. [DOI] [PubMed] [Google Scholar]
- Canale E.D., Campbell G.R., Smolich J.J., Campbell J.H., Campbell G.R., Smolich J.J., Campbell J.H., Smolich J.J., Campbell J.H., Campbell J.H. Cardiac muscle. Springer Verlag; Berlin: 1986. [Google Scholar]
- Carlson H., Ota S., Song Y., Chen Y., Hurlin P.J., Ota S., Song Y., Chen Y., Hurlin P.J., Song Y., Chen Y., Hurlin P.J., Chen Y., Hurlin P.J., Hurlin P.J. Tbx3 impinges on the p53 pathway to suppress apoptosis, facilitate cell transformation and block myogenic differentiation. Oncogene. 2002;21:3827–3835. doi: 10.1038/sj.onc.1205476. [DOI] [PubMed] [Google Scholar]
- Chen F., Kook H., Milewski R., Gitler A.D., Lu M.M., Li J., Nazarian R., Schnepp R., Jen K., Biben C., Kook H., Milewski R., Gitler A.D., Lu M.M., Li J., Nazarian R., Schnepp R., Jen K., Biben C., Milewski R., Gitler A.D., Lu M.M., Li J., Nazarian R., Schnepp R., Jen K., Biben C., Gitler A.D., Lu M.M., Li J., Nazarian R., Schnepp R., Jen K., Biben C., Lu M.M., Li J., Nazarian R., Schnepp R., Jen K., Biben C., Li J., Nazarian R., Schnepp R., Jen K., Biben C., Nazarian R., Schnepp R., Jen K., Biben C., Schnepp R., Jen K., Biben C., Jen K., Biben C., Biben C., et al. Hop is an unusual homeobox gene that modulates cardiac development. Cell. 2002;110:713–723. doi: 10.1016/s0092-8674(02)00932-7. [DOI] [PubMed] [Google Scholar]
- Chen J.R., Chatterjee B., Meyer R., Yu J.C., Borke J.L., Isales C.M., Kirby M.L., Lo C.W., Bollag R.J., Chatterjee B., Meyer R., Yu J.C., Borke J.L., Isales C.M., Kirby M.L., Lo C.W., Bollag R.J., Meyer R., Yu J.C., Borke J.L., Isales C.M., Kirby M.L., Lo C.W., Bollag R.J., Yu J.C., Borke J.L., Isales C.M., Kirby M.L., Lo C.W., Bollag R.J., Borke J.L., Isales C.M., Kirby M.L., Lo C.W., Bollag R.J., Isales C.M., Kirby M.L., Lo C.W., Bollag R.J., Kirby M.L., Lo C.W., Bollag R.J., Lo C.W., Bollag R.J., Bollag R.J. Tbx2 represses expression of connexin43 in osteoblastic-like cells. Calcif. Tissue Int. 2004;74:561–573. doi: 10.1007/s00223-003-0106-5. [DOI] [PubMed] [Google Scholar]
- Cheng G., Litchenberg W.H., Cole G.J., Mikawa T., Thompson R.P., Gourdie R.G., Litchenberg W.H., Cole G.J., Mikawa T., Thompson R.P., Gourdie R.G., Cole G.J., Mikawa T., Thompson R.P., Gourdie R.G., Mikawa T., Thompson R.P., Gourdie R.G., Thompson R.P., Gourdie R.G., Gourdie R.G. Development of the cardiac conduction system involves recruitment within a multipotent cardiomyogenic lineage. Development. 1999;126:5041–5049. doi: 10.1242/dev.126.22.5041. [DOI] [PubMed] [Google Scholar]
- Christoffels V.M., Habets P.E.M.H., Franco D., Campione M., de Jong F., Lamers W.H., Bao Z.Z., Palmer S., Biben C., Harvey R.P., Habets P.E.M.H., Franco D., Campione M., de Jong F., Lamers W.H., Bao Z.Z., Palmer S., Biben C., Harvey R.P., Franco D., Campione M., de Jong F., Lamers W.H., Bao Z.Z., Palmer S., Biben C., Harvey R.P., Campione M., de Jong F., Lamers W.H., Bao Z.Z., Palmer S., Biben C., Harvey R.P., de Jong F., Lamers W.H., Bao Z.Z., Palmer S., Biben C., Harvey R.P., Lamers W.H., Bao Z.Z., Palmer S., Biben C., Harvey R.P., Bao Z.Z., Palmer S., Biben C., Harvey R.P., Palmer S., Biben C., Harvey R.P., Biben C., Harvey R.P., Harvey R.P., et al. Chamber formation and morphogenesis in the developing mammalian heart. Dev. Biol. 2000;223:266–278. doi: 10.1006/dbio.2000.9753. [DOI] [PubMed] [Google Scholar]
- Christoffels V.M., Burch J.B.E., Moorman A.F.M., Burch J.B.E., Moorman A.F.M., Moorman A.F.M. Architectural plan for the heart: Early patterning and delineation of the chambers and the nodes. Trends Cardiovasc. Med. 2004a;14:301–307. doi: 10.1016/j.tcm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Christoffels V.M., Hoogaars W.M.H., Tessari A., Clout D.E.W., Moorman A.F.M., Campione M., Hoogaars W.M.H., Tessari A., Clout D.E.W., Moorman A.F.M., Campione M., Tessari A., Clout D.E.W., Moorman A.F.M., Campione M., Clout D.E.W., Moorman A.F.M., Campione M., Moorman A.F.M., Campione M., Campione M. T-box transcription factor Tbx2 represses differentiation and formation of the cardiac chambers. Dev. Dyn. 2004b;229:763–770. doi: 10.1002/dvdy.10487. [DOI] [PubMed] [Google Scholar]
- Davenport T.G., Jerome-Majewska L.A., Papaioannou V.E., Jerome-Majewska L.A., Papaioannou V.E., Papaioannou V.E. Mammary gland, limb and yolk sac defects in mice lacking Tbx3, the gene mutated in human ulnar mammary syndrome. Development. 2003;130:2263–2273. doi: 10.1242/dev.00431. [DOI] [PubMed] [Google Scholar]
- de Lange F.J., Moorman A.F.M., Christoffels V.M., Moorman A.F.M., Christoffels V.M., Christoffels V.M. Atrial cardiomyocyte-specific expression of Cre recombinase driven by an Nppa gene fragment. Genesis. 2003;37:1–4. doi: 10.1002/gene.10220. [DOI] [PubMed] [Google Scholar]
- Dupays L., Jarry-Guichard T., Mazurais D., Calmels T., Izumo S., Gros D., Theveniau-Ruissy M., Jarry-Guichard T., Mazurais D., Calmels T., Izumo S., Gros D., Theveniau-Ruissy M., Mazurais D., Calmels T., Izumo S., Gros D., Theveniau-Ruissy M., Calmels T., Izumo S., Gros D., Theveniau-Ruissy M., Izumo S., Gros D., Theveniau-Ruissy M., Gros D., Theveniau-Ruissy M., Theveniau-Ruissy M. Dysregulation of connexins and inactivation of NFATc1 in the cardiovascular system of Nkx2-5 null mutants. J. Mol. Cell. Cardiol. 2005;38:787–798. doi: 10.1016/j.yjmcc.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Erokhina I.L., Rumyantsev P.P., Rumyantsev P.P. Ultrastructure of DNA-synthesizing and mitotically dividing myocytes in sinoatrial node of mouse embryonal heart. J. Mol. Cell. Cardiol. 1986;18:1219–1231. doi: 10.1016/s0022-2828(86)80426-6. [DOI] [PubMed] [Google Scholar]
- Garcia-Frigola C., Shi Y., Evans S.M., Shi Y., Evans S.M., Evans S.M. Expression of the hyperpolarization-activated cyclic nucleotide-gated cation channel HCN4 during mouse heart development. Brain Res. Gene Expr. Patterns. 2003;3:777–783. doi: 10.1016/s1567-133x(03)00125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros D., Dupays L., Alcolea S., Meysen S., Miquerol L., Theveniau-Ruissy M., Dupays L., Alcolea S., Meysen S., Miquerol L., Theveniau-Ruissy M., Alcolea S., Meysen S., Miquerol L., Theveniau-Ruissy M., Meysen S., Miquerol L., Theveniau-Ruissy M., Miquerol L., Theveniau-Ruissy M., Theveniau-Ruissy M. Genetically modified mice: Tools to decode the functions of connexins in the heart—New models for cardiovascular research. Cardiovasc. Res. 2004;62:299–308. doi: 10.1016/j.cardiores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Habets P.E.M.H., Moorman A.F.M., Clout D.E.W., van Roon M.A., Lingbeek M., Lohuizen M., Christoffels V.M., Moorman A.F.M., Clout D.E.W., van Roon M.A., Lingbeek M., Lohuizen M., Christoffels V.M., Clout D.E.W., van Roon M.A., Lingbeek M., Lohuizen M., Christoffels V.M., van Roon M.A., Lingbeek M., Lohuizen M., Christoffels V.M., Lingbeek M., Lohuizen M., Christoffels V.M., Lohuizen M., Christoffels V.M., Christoffels V.M. Cooperative action of Tbx2 and Nkx2.5 inhibits ANF expression in the atrioventricular canal: Implications for cardiac chamber formation. Genes & Dev. 2002;16:1234–1246. doi: 10.1101/gad.222902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrelson Z., Kelly R.G., Goldin S.N., Gibson-Brown J.J., Bollag R.J., Silver L.M., Papaioannou V.E., Kelly R.G., Goldin S.N., Gibson-Brown J.J., Bollag R.J., Silver L.M., Papaioannou V.E., Goldin S.N., Gibson-Brown J.J., Bollag R.J., Silver L.M., Papaioannou V.E., Gibson-Brown J.J., Bollag R.J., Silver L.M., Papaioannou V.E., Bollag R.J., Silver L.M., Papaioannou V.E., Silver L.M., Papaioannou V.E., Papaioannou V.E. Tbx2 is essential for patterning the atrioventricular canal and for morphogenesis of the outflow tract during heart development. Development. 2004;131:5041–5052. doi: 10.1242/dev.01378. [DOI] [PubMed] [Google Scholar]
- Harvey R.P. Patterning the vertebrate heart. Nat. Rev. Genet. 2002;3:544–556. doi: 10.1038/nrg843. [DOI] [PubMed] [Google Scholar]
- He M., Wen L., Campbell C.E., Wu J.Y., Rao Y., Wen L., Campbell C.E., Wu J.Y., Rao Y., Campbell C.E., Wu J.Y., Rao Y., Wu J.Y., Rao Y., Rao Y. Transcription repression by Xenopus ET and its human ortholog TBX3, a gene involved in ulnar-mammary syndrome. Proc. Natl. Acad. Sci. 1999;96:10212–10217. doi: 10.1073/pnas.96.18.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogaars W.M.H., Tessari A., Moorman A.F.M., de Boer P.A.J., Hagoort J., Soufan A.T., Campione M., Christoffels V.M., Tessari A., Moorman A.F.M., de Boer P.A.J., Hagoort J., Soufan A.T., Campione M., Christoffels V.M., Moorman A.F.M., de Boer P.A.J., Hagoort J., Soufan A.T., Campione M., Christoffels V.M., de Boer P.A.J., Hagoort J., Soufan A.T., Campione M., Christoffels V.M., Hagoort J., Soufan A.T., Campione M., Christoffels V.M., Soufan A.T., Campione M., Christoffels V.M., Campione M., Christoffels V.M., Christoffels V.M. The transcriptional repressor Tbx3 delineates the developing central conduction system of the heart. Cardiovasc. Res. 2004;62:489–499. doi: 10.1016/j.cardiores.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Ismat F.A., Zhang M., Kook H., Huang B., Zhou R., Ferrari V.A., Epstein J.A., Patel V.V., Zhang M., Kook H., Huang B., Zhou R., Ferrari V.A., Epstein J.A., Patel V.V., Kook H., Huang B., Zhou R., Ferrari V.A., Epstein J.A., Patel V.V., Huang B., Zhou R., Ferrari V.A., Epstein J.A., Patel V.V., Zhou R., Ferrari V.A., Epstein J.A., Patel V.V., Ferrari V.A., Epstein J.A., Patel V.V., Epstein J.A., Patel V.V., Patel V.V. Homeobox protein Hop functions in the adult cardiac conduction system. Circ. Res. 2005;96:898–903. doi: 10.1161/01.RES.0000163108.47258.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn L., Sadoshima J., Greene A., Parker C., Morgan K.G., Izumo S., Sadoshima J., Greene A., Parker C., Morgan K.G., Izumo S., Greene A., Parker C., Morgan K.G., Izumo S., Parker C., Morgan K.G., Izumo S., Morgan K.G., Izumo S., Izumo S. Conditional differentiation of heart- and smooth muscle-derived cells transformed by a temperature-sensitive mutant of SV40 T antigen. J. Cell Sci. 1996;109:397–407. doi: 10.1242/jcs.109.2.397. [DOI] [PubMed] [Google Scholar]
- Keith A., Flack M., Flack M. The form and nature of the muscular connections between the primary divisions of the vertebrate heart. J. Anat. Physiol. 1907;41:172–189. [PMC free article] [PubMed] [Google Scholar]
- Kreuzberg M.M., Schrickel J.W., Ghanem A., Kim J.S., Degen J., Janssen-Bienhold U., Lewalter T., Tiemann K., Willecke K., Schrickel J.W., Ghanem A., Kim J.S., Degen J., Janssen-Bienhold U., Lewalter T., Tiemann K., Willecke K., Ghanem A., Kim J.S., Degen J., Janssen-Bienhold U., Lewalter T., Tiemann K., Willecke K., Kim J.S., Degen J., Janssen-Bienhold U., Lewalter T., Tiemann K., Willecke K., Degen J., Janssen-Bienhold U., Lewalter T., Tiemann K., Willecke K., Janssen-Bienhold U., Lewalter T., Tiemann K., Willecke K., Lewalter T., Tiemann K., Willecke K., Tiemann K., Willecke K., Willecke K. Connexin30.2 containing gap junction channels decelerate impulse propagation through the atrioventricular node. Proc. Natl. Acad. Sci. 2006;103:5959–5964. doi: 10.1073/pnas.0508512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei M., Jones S.A., Liu J., Lancaster M.K., Fung S.S.M., Dobrzynski H., Camelliti P., Maier S.K.G., Noble D., Boyett M.R., Jones S.A., Liu J., Lancaster M.K., Fung S.S.M., Dobrzynski H., Camelliti P., Maier S.K.G., Noble D., Boyett M.R., Liu J., Lancaster M.K., Fung S.S.M., Dobrzynski H., Camelliti P., Maier S.K.G., Noble D., Boyett M.R., Lancaster M.K., Fung S.S.M., Dobrzynski H., Camelliti P., Maier S.K.G., Noble D., Boyett M.R., Fung S.S.M., Dobrzynski H., Camelliti P., Maier S.K.G., Noble D., Boyett M.R., Dobrzynski H., Camelliti P., Maier S.K.G., Noble D., Boyett M.R., Camelliti P., Maier S.K.G., Noble D., Boyett M.R., Maier S.K.G., Noble D., Boyett M.R., Noble D., Boyett M.R., Boyett M.R. Requirement of neuronal- and cardiac-type sodium channels for murine sinoatrial node pacemaking. J. Physiol. 2004;559:835–848. doi: 10.1113/jphysiol.2004.068643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Dobrzynski H., Yanni J., Boyett M.R., Lei M., Dobrzynski H., Yanni J., Boyett M.R., Lei M., Yanni J., Boyett M.R., Lei M., Boyett M.R., Lei M., Lei M. Organisation of the mouse sinoatrial node: Structure and expression of HCN channels. Cardiovasc. Res. 2006;73:729–738. doi: 10.1016/j.cardiores.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Mangoni M.E., Traboulsie A., Leoni A.L., Couette B., Marger L., Le Q.K., Kupfer E., Cohen-Solal A., Vilar J., Shin H.S., Traboulsie A., Leoni A.L., Couette B., Marger L., Le Q.K., Kupfer E., Cohen-Solal A., Vilar J., Shin H.S., Leoni A.L., Couette B., Marger L., Le Q.K., Kupfer E., Cohen-Solal A., Vilar J., Shin H.S., Couette B., Marger L., Le Q.K., Kupfer E., Cohen-Solal A., Vilar J., Shin H.S., Marger L., Le Q.K., Kupfer E., Cohen-Solal A., Vilar J., Shin H.S., Le Q.K., Kupfer E., Cohen-Solal A., Vilar J., Shin H.S., Kupfer E., Cohen-Solal A., Vilar J., Shin H.S., Cohen-Solal A., Vilar J., Shin H.S., Vilar J., Shin H.S., Shin H.S., et al. Bradycardia and slowing of the atrioventricular conduction in mice lacking Cav3.1/α1G T-type calcium channels. Circ. Res. 2006;98:1422–1430. doi: 10.1161/01.RES.0000225862.14314.49. [DOI] [PubMed] [Google Scholar]
- Marionneau C., Couette B., Liu J., Li H., Mangoni M.E., Nargeot J., Lei M., Escande D., Demolombe S., Couette B., Liu J., Li H., Mangoni M.E., Nargeot J., Lei M., Escande D., Demolombe S., Liu J., Li H., Mangoni M.E., Nargeot J., Lei M., Escande D., Demolombe S., Li H., Mangoni M.E., Nargeot J., Lei M., Escande D., Demolombe S., Mangoni M.E., Nargeot J., Lei M., Escande D., Demolombe S., Nargeot J., Lei M., Escande D., Demolombe S., Lei M., Escande D., Demolombe S., Escande D., Demolombe S., Demolombe S. Specific pattern of ionic channel gene expression associated with pacemaker activity in the mouse heart. J. Physiol. 2005;562:223–234. doi: 10.1113/jphysiol.2004.074047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneghini V., Odent S., Platonova N., Egeo A., Merlo G.R., Odent S., Platonova N., Egeo A., Merlo G.R., Platonova N., Egeo A., Merlo G.R., Egeo A., Merlo G.R., Merlo G.R. Novel TBX3 mutation data in families with Ulnar-Mammary syndrome indicate a genotype–phenotype relationship: Mutations that do not disrupt the T-domain are associated with less severe limb defects. Eur. J. Med. Genet. 2006;49:151–158. doi: 10.1016/j.ejmg.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Miake J., Marban E., Nuss H.B., Marban E., Nuss H.B., Nuss H.B. Biological pacemaker created by gene transfer. Nature. 2002;419:132–133. doi: 10.1038/419132b. [DOI] [PubMed] [Google Scholar]
- Milanesi R., Baruscotti M., Gnecchi-Ruscone T., DiFrancesco D., Baruscotti M., Gnecchi-Ruscone T., DiFrancesco D., Gnecchi-Ruscone T., DiFrancesco D., DiFrancesco D. Familial sinus bradycardia associated with a mutation in the cardiac pacemaker channel. N. Engl. J. Med. 2006;354:151–157. doi: 10.1056/NEJMoa052475. [DOI] [PubMed] [Google Scholar]
- Mommersteeg M.T., Hoogaars W.M., Prall O.W., de Gier-de Vries C., Wiese C., Clout D.E., Papaioannou V.E., Brown N.A., Harvey R.P., Moorman A.F., Hoogaars W.M., Prall O.W., de Gier-de Vries C., Wiese C., Clout D.E., Papaioannou V.E., Brown N.A., Harvey R.P., Moorman A.F., Prall O.W., de Gier-de Vries C., Wiese C., Clout D.E., Papaioannou V.E., Brown N.A., Harvey R.P., Moorman A.F., de Gier-de Vries C., Wiese C., Clout D.E., Papaioannou V.E., Brown N.A., Harvey R.P., Moorman A.F., Wiese C., Clout D.E., Papaioannou V.E., Brown N.A., Harvey R.P., Moorman A.F., Clout D.E., Papaioannou V.E., Brown N.A., Harvey R.P., Moorman A.F., Papaioannou V.E., Brown N.A., Harvey R.P., Moorman A.F., Brown N.A., Harvey R.P., Moorman A.F., Harvey R.P., Moorman A.F., Moorman A.F., et al. Molecular pathway for the localized formation of the sinoatrial node. Circ. Res. 2007;100:354–362. doi: 10.1161/01.RES.0000258019.74591.b3. [DOI] [PubMed] [Google Scholar]
- Moorman A.F.M., Christoffels V.M., Christoffels V.M. Cardiac chamber formation: Development, genes and evolution. Physiol. Rev. 2003;83:1223–1267. doi: 10.1152/physrev.00006.2003. [DOI] [PubMed] [Google Scholar]
- Moorman A.F.M., Houweling A.C., de Boer P.A.J., Christoffels V.M., Houweling A.C., de Boer P.A.J., Christoffels V.M., de Boer P.A.J., Christoffels V.M., Christoffels V.M. Sensitive non-radioactive detection of mRNA in tissue sections: Novel application of the whole-mount in situ hybridization protocol. J. Histochem. Cytochem. 2001;49:1–8. doi: 10.1177/002215540104900101. [DOI] [PubMed] [Google Scholar]
- Naiche L.A., Harrelson Z., Kelly R.G., Papaioannou V.E., Harrelson Z., Kelly R.G., Papaioannou V.E., Kelly R.G., Papaioannou V.E., Papaioannou V.E. T-Box genes in vertebrate development. Annu. Rev. Genet. 2005;39:219–239. doi: 10.1146/annurev.genet.39.073003.105925. [DOI] [PubMed] [Google Scholar]
- Novak A., Guo C., Yang W., Nagy A., Lobe C.G., Guo C., Yang W., Nagy A., Lobe C.G., Yang W., Nagy A., Lobe C.G., Nagy A., Lobe C.G., Lobe C.G. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- Papadatos G.A., Wallerstein P.M., Head C.E., Ratcliff R., Brady P.A., Benndorf K., Saumarez R.C., Trezise A.E., Huang C.L., Vandenberg J.I., Wallerstein P.M., Head C.E., Ratcliff R., Brady P.A., Benndorf K., Saumarez R.C., Trezise A.E., Huang C.L., Vandenberg J.I., Head C.E., Ratcliff R., Brady P.A., Benndorf K., Saumarez R.C., Trezise A.E., Huang C.L., Vandenberg J.I., Ratcliff R., Brady P.A., Benndorf K., Saumarez R.C., Trezise A.E., Huang C.L., Vandenberg J.I., Brady P.A., Benndorf K., Saumarez R.C., Trezise A.E., Huang C.L., Vandenberg J.I., Benndorf K., Saumarez R.C., Trezise A.E., Huang C.L., Vandenberg J.I., Saumarez R.C., Trezise A.E., Huang C.L., Vandenberg J.I., Trezise A.E., Huang C.L., Vandenberg J.I., Huang C.L., Vandenberg J.I., Vandenberg J.I., et al. Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a. Proc. Natl. Acad. Sci. 2002;99:6210–6215. doi: 10.1073/pnas.082121299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi D.J., Rentschler S., Gourdie R.G., Fishman G.I., Mikawa T., Rentschler S., Gourdie R.G., Fishman G.I., Mikawa T., Gourdie R.G., Fishman G.I., Mikawa T., Fishman G.I., Mikawa T., Mikawa T. Induction and patterning of the cardiac conduction system. Int. J. Dev. Biol. 2002;46:765–775. [PubMed] [Google Scholar]
- Rodriguez C.I., Buchholz F., Galloway J., Sequerra R., Kasper J., Ayala R., Stewart A.F., Dymecki S.M., Buchholz F., Galloway J., Sequerra R., Kasper J., Ayala R., Stewart A.F., Dymecki S.M., Galloway J., Sequerra R., Kasper J., Ayala R., Stewart A.F., Dymecki S.M., Sequerra R., Kasper J., Ayala R., Stewart A.F., Dymecki S.M., Kasper J., Ayala R., Stewart A.F., Dymecki S.M., Ayala R., Stewart A.F., Dymecki S.M., Stewart A.F., Dymecki S.M., Dymecki S.M. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat. Genet. 2000;25:139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- Rosen M.R., Brink P.R., Cohen I.S., Robinson R.B., Brink P.R., Cohen I.S., Robinson R.B., Cohen I.S., Robinson R.B., Robinson R.B. Genes, stem cells and biological pacemakers. Cardiovasc. Res. 2004;64:12–23. doi: 10.1016/j.cardiores.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Santoro B., Chen S., Luthi A., Pavlidis P., Shumyatsky G.P., Tibbs G.R., Siegelbaum S.A., Chen S., Luthi A., Pavlidis P., Shumyatsky G.P., Tibbs G.R., Siegelbaum S.A., Luthi A., Pavlidis P., Shumyatsky G.P., Tibbs G.R., Siegelbaum S.A., Pavlidis P., Shumyatsky G.P., Tibbs G.R., Siegelbaum S.A., Shumyatsky G.P., Tibbs G.R., Siegelbaum S.A., Tibbs G.R., Siegelbaum S.A., Siegelbaum S.A. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J. Neurosci. 2000;20:5264–5275. doi: 10.1523/JNEUROSCI.20-14-05264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram G., Pourrier M., Melnyk P., Nattel S., Pourrier M., Melnyk P., Nattel S., Melnyk P., Nattel S., Nattel S. Differential distribution of cardiac ion channel expression as a basis for regional specialization in electrical function. Circ. Res. 2002;90:939–950. doi: 10.1161/01.res.0000018627.89528.6f. [DOI] [PubMed] [Google Scholar]
- Schulze-Bahr E., Neu A., Friederich P., Kaupp U.B., Breithardt G., Pongs O., Isbrandt D., Neu A., Friederich P., Kaupp U.B., Breithardt G., Pongs O., Isbrandt D., Friederich P., Kaupp U.B., Breithardt G., Pongs O., Isbrandt D., Kaupp U.B., Breithardt G., Pongs O., Isbrandt D., Breithardt G., Pongs O., Isbrandt D., Pongs O., Isbrandt D., Isbrandt D. Pacemaker channel dysfunction in a patient with sinus node disease. J. Clin. Invest. 2003;111:1537–1545. doi: 10.1172/JCI16387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stieber J., Herrmann S., Feil S., Loster J., Feil R., Biel M., Hofmann F., Ludwig A., Herrmann S., Feil S., Loster J., Feil R., Biel M., Hofmann F., Ludwig A., Feil S., Loster J., Feil R., Biel M., Hofmann F., Ludwig A., Loster J., Feil R., Biel M., Hofmann F., Ludwig A., Feil R., Biel M., Hofmann F., Ludwig A., Biel M., Hofmann F., Ludwig A., Hofmann F., Ludwig A., Ludwig A. The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc. Natl. Acad. Sci. 2003;100:15235–15240. doi: 10.1073/pnas.2434235100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieber J., Hofmann F., Ludwig A., Hofmann F., Ludwig A., Ludwig A. Pacemaker channels and sinus node arrhythmia. Trends Cardiovasc. Med. 2004;14:23–28. doi: 10.1016/j.tcm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Tse H.F., Xue T., Lau C.P., Siu C.W., Wang K., Zhang Q.Y., Tomaselli G.F., Akar F.G., Li R.A., Xue T., Lau C.P., Siu C.W., Wang K., Zhang Q.Y., Tomaselli G.F., Akar F.G., Li R.A., Lau C.P., Siu C.W., Wang K., Zhang Q.Y., Tomaselli G.F., Akar F.G., Li R.A., Siu C.W., Wang K., Zhang Q.Y., Tomaselli G.F., Akar F.G., Li R.A., Wang K., Zhang Q.Y., Tomaselli G.F., Akar F.G., Li R.A., Zhang Q.Y., Tomaselli G.F., Akar F.G., Li R.A., Tomaselli G.F., Akar F.G., Li R.A., Akar F.G., Li R.A., Li R.A. Bioartificial sinus node constructed via in vivo gene transfer of an engineered pacemaker HCN channel reduces the dependence on electronic pacemaker in a sick-sinus syndrome model. Circulation. 2006;114:1000–1011. doi: 10.1161/CIRCULATIONAHA.106.615385. [DOI] [PubMed] [Google Scholar]
- Verheijck E.E., van Kempen M.J., Veereschild M., Lurvink J., Jongsma H.J., Bouman L.N., van Kempen M.J., Veereschild M., Lurvink J., Jongsma H.J., Bouman L.N., Veereschild M., Lurvink J., Jongsma H.J., Bouman L.N., Lurvink J., Jongsma H.J., Bouman L.N., Jongsma H.J., Bouman L.N., Bouman L.N. Electrophysiological features of the mouse sinoatrial node in relation to connexin distribution. Cardiovasc. Res. 2001;52:40–50. doi: 10.1016/s0008-6363(01)00364-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Dobrzynski H., Tellez J., Niwa R., Billeter R., Honjo H., Kodama I., Boyett M.R., Dobrzynski H., Tellez J., Niwa R., Billeter R., Honjo H., Kodama I., Boyett M.R., Tellez J., Niwa R., Billeter R., Honjo H., Kodama I., Boyett M.R., Niwa R., Billeter R., Honjo H., Kodama I., Boyett M.R., Billeter R., Honjo H., Kodama I., Boyett M.R., Honjo H., Kodama I., Boyett M.R., Kodama I., Boyett M.R., Boyett M.R. Extended atrial conduction system characterised by the expression of the HCN4 channel and connexin45. Cardiovasc. Res. 2006;72:271–281. doi: 10.1016/j.cardiores.2006.07.026. [DOI] [PubMed] [Google Scholar]