Abstract
A six-month study was conducted in p53(+/-) mice to evaluate the possible oncogenicity of resveratrol (3,5,4’-trihydroxy-trans-stilbene), a cancer chemopreventive agent present in grapes and other foods. p53(+/-) mice (25/sex/group) received daily gavage exposure to vehicle only (negative control), resveratrol doses of 1000, 2000, or 4000 mg/kg/day, or p-cresidine (400 mg/kg/day; positive control). No mortality was seen in mice receiving the low dose of resveratrol. However, the mid and high doses induced mortality associated with impaction of the test article in the gastrointestinal tract. Resveratrol had no effect on body weight, food consumption, or clinical signs in surviving mice in any dose group, but induced dose-related increases in liver weight and serum cholesterol in both sexes. Mild anemia was seen in male mice at the high dose only; hematologic effects were not seen in females. Histopathology identified the kidney (hydronephrosis) and urinary bladder (epithelial hyperplasia) as target tissues for resveratrol toxicity. The incidences of both benign and malignant tumors in mice exposed to resveratrol were comparable to those in vehicle controls. By contrast, the positive control article, p-cresidine, induced urinary bladder cancer in both sexes. When administered to p53(+/-) mice at its maximum tolerated dose, resveratrol demonstrates no evidence of oncogenicity.
Keywords: Resveratrol, Cancer chemopreventive agent, Oncogenicity, p53(+/-) mouse
1. Introduction
Resveratrol (3,5,4’-trihydroxy-trans-stilbene) is a naturally occurring polyphenol that is present in grapes, peanuts, and other foods that are commonly consumed as part of the human diet (Sanders et al., 2000). Grapes (particularly grape skins) provide a particularly abundant source of resveratrol, and the compound is found in concentrations of up to 10 ppm in red wine (Celotti et al., 1996). An expanding body of scientific evidence suggests that resveratrol has a broad range of desirable biological actions, including cardioprotection (Hung et al., 2000), cancer prevention (Jang and Pezzuto, 1999), and prolongation of life span in several species (Howitz et al., 2003; Valenzano et al., 2006).
The results of cancer chemoprevention studies in laboratory animal models demonstrate that resveratrol can inhibit carcinogenesis in several organ sites (Gescher and Steward, 2003). Although the compound appears to be inactive in chemoprevention in the mouse lung (Hecht et al., 1999; Berge et al., 2004), resveratrol confers significant protection against cancer induction in the rat and mouse mammary gland (Banerjee et al., 2002; Provinciali et al., 2005), rat and mouse colon (Sale et al., 2005), mouse skin (Jang et al., 1997; Kapadia et al., 2002; Aziz et al., 2005), and rat esophagus (Li et al., 2002). Extending these experimental data are the results of a recent epidemiology study suggesting that resveratrol consumption is inversely related to breast cancer risk: in that study, 50% or greater reductions in breast cancer odds ratios were observed in women with the highest levels of resveratrol consumption (Levi et al., 2005).
The key mechanism(s) underlying the chemopreventive activity of resveratrol have not been identified. However, resveratrol demonstrates a broad range of biological effects that could result in anticarcinogenic efficacy. Mechanisms of resveratrol action in cancer chemoprevention may include: modulation of the activity of carcinogen-metabolizing enzymes (Jang et al., 1997; Chun et al., 1999; Ciolino and Yeh, 1999); scavenging of free radicals (Leonard et al., 2003); inhibition of malignant transformation (She et al., 2003); inhibition of cell proliferation (Sgambato et al., 2001; Joe et al., 2002; Lanzilli et al., 2006); suppression of cyclooxygenase activity (Subbaramaiah et al., 1998; Banerjee et al., 2002; Szewczuk et al., 2004); induction of apoptosis (Surh et al., 1999; Joe et al., 2002; Dong, 2003); and inhibition of angiogenesis (Tseng et al., 2004; Garvin et al., 2006).
The chemopreventive activity of resveratrol in experimental models, when considered with its relatively benign toxicity profile in laboratory animals (Juan et al., 2002; Crowell et al., 2004), suggests that resveratrol merits consideration for efficacy evaluation in human cancer prevention trials. Clinical evaluation of the chemopreventive efficacy of resveratrol will require the conduct of randomized trials involving chronic administration protocols. Prior to the conduct of such trials, it is essential to demonstrate that resveratrol is not, in itself, carcinogenic. A priori, one might consider carcinogenesis to be an unlikely result of chronic exposure to an antioxidant polyphenol. However, recent reports from several groups demonstrate that resveratrol induces micronuclei, sister chromatid exchanges, and other evidence of DNA damage in in vitro genotoxicity assays (Matsuoka et al., 2001; Schmitt et al., 2002; Matsuoka et al., 2002; Fukuhara et al., 2006). As a result, evaluation of the possible carcinogenicity of resveratrol is an essential element of its preclinical development.
The present studies were performed to evaluate the possible oncogenic activity of resveratrol in the TSG-p53(+/-) mouse (p53 knockout mouse), and to characterize the plasma pharmacokinetics of resveratrol and its major metabolites in mice following oral (gavage) dosing. The p53 knockout mouse is accepted by several regulatory agencies as an alternative model for oncogenicity bioassays (McDonald et al., 2004), and demonstrates a >85% concordance with the results of chronic rodent oncogenicity bioassays (Storer et al., 2001). These studies address a key regulatory requirement for the entry of resveratrol into clinical trials for cancer prevention, and will provide critical laboratory data relevant to the possible utility of resveratrol as a cancer preventive agent in humans.
2. Materials and methods
2.1. Animal welfare
Prior to the initiation of experimentation, study protocols were reviewed and approved by the IIT Research Institute Animal Care and Use Committee. All work involving experimental animals was performed in full compliance with NIH Guidelines for the Care and Use of Laboratory Animals.
2.2. Animals and animal husbandry
C57BL/6 and TSG-p53(+/-) (heterozygous p53 knockout) mice were purchased from Taconic, Germantown, NY. The p53 knockout mouse is a genetically engineered animal strain that is accepted by the United States Food and Drug Administration (FDA) as an alternative model for oncogenicity bioassays of agents that demonstrate either clear or equivocal genotoxic activity. The European Committee for Proprietary Medicinal Products accepts oncogenicity studies in this model for all agents, regardless of demonstrated genotoxicity (McDonald et al., 2004). The C57BL/6 mouse is the non-transgenic parental strain of the TSG-p53(+/-) mouse, and is commonly used for range-finding and pharmacokinetics studies conducted in association with oncogenicity studies performed in the TSG-p53(+/-) mouse model system.
Mice were received at 6 to 8 weeks of age and were quarantined for one week prior to the administration of test or control articles. Mice were housed individually in suspended stainless steel cages in a windowless, climate-controlled room that was maintained on a 12-hour light/12-hour dark cycle. At all times during the quarantine and dosing periods, animals were permitted free access to certified Purina Laboratory Chow 5002 (PMI Feeds, Richmond, IN) and coarse-filtered City of Chicago water (delivered via an automatic watering system). After randomization into experimental groups, mice were identified by tail tattoo; no transponders were used to identify animals in any study.
2.3. Test and control articles
Resveratrol (3,5,4’-trihydroxy-trans-stilbene; C14H12O3; CAS No. 501-36-0; purity = 99.5%) was obtained from Royalmount Pharma, Inc. (Montreal, Quebec, Canada), and was administered in a vehicle of 0.5% (w/v) aqueous methylcellulose containing 0.2% (w/v) Tween 80 (Sigma Chemical Co., St. Louis, MO). Mice in the vehicle control groups in the range-finding and definitive oncogenicity studies received daily gavage administration of vehicle only. Mice in the positive control group in the oncogenicity study received daily oral (gavage) exposure to p-cresidine (Sigma). p-Cresidine (3-amino-4-methoxytoluene, 2-methoxy-5-methylaniline; C8H11NO; CAS No. 120-71-8; purity = 98.5%) induces urinary bladder cancers in TSG-p53(+/-) mice, and has been used previously as a positive control article for oncogenicity bioassays in this animal model (Storer et al. 2001; Johnson et al., 2006).
2.4. Dose range-finding study design
A four-week dose range-finding study was conducted in mice to support dose selection for the definitive oncogenicity evaluation. Using a computerized randomization procedure that blocks for body weights, C57BL/6 mice were randomly assigned to groups of 10/sex. After randomization, mice received daily gavage exposure to resveratrol at doses of 0 (vehicle control), 1000, 2000, 3000, 4000, or 5000 mg/kg/day for 28 consecutive days. All study animals were observed twice daily for mortality or evidence of moribundity, and were weighed weekly. On Study Day 29, all mice were bled for clinical pathology evaluations, were euthanized by CO2 asphyxiation, and received a limited gross necropsy with collection of gross lesions only. Histopathology was not performed.
2.5. Pharmacokinetics study design
To characterize the plasma pharmacokinetics of resveratrol and its primary metabolites, 60 female C57BL/6 mice received a single oral (gavage) dose of 4000 mg resveratrol per kg body weight. Six animals per time point were euthanized for blood collection at pre-dose, 0.25, 0.5, 1, 2, 4, 8 and 24 hours after resveratrol administration. Plasma was collected and stored at -80°C prior to analysis for resveratrol, resveratrol glucuronide, and resveratrol sulfate.
To quantitate plasma levels of resveratrol, an internal standard (carbamazepine) was added to each plasma sample, and the sample was extracted into ethyl acetate. The ethyl acetate extract was then evaporated, and the samples were reconstituted in mobile phase (0.13% acetic acid:acetonitrile [75:25, v/v]). Reconstituted samples were analyzed by HPLC, using a Phenomenex Spherisorb ODS C18, 5μ, 250 × 4.6 mm i.d. column and a flow rate of 1.0 ml/min. Elution time of resveratrol was 9.2 min. The resveratrol content of each sample was determined by measuring absorbance at 310 nm, and was then quantified by comparison to a standard curve prepared on each day of analysis.
Previous studies have demonstrated that the primary plasma metabolites of resveratrol are its glucuronide and sulfate conjugates (Sale et al., 2004). To determine the concentration of resveratrol glucuronide, plasma samples were incubated with β-glucuronidase (E. coli type X-A, 424 units/sample [Sigma]) for one hour at 40°C, according to the method of Marier et al. (2002). Samples were then extracted, and resveratrol was quantitated by HPLC as described above. The quantity of resveratrol glucuronide in each sample was calculated by subtracting the quantity of resveratrol present in an aliquot of the sample that had not been incubated with β-glucuronidase from the quantity of resveratrol present in samples after incubation with β-glucuronidase.
Plasma levels of resveratrol sulfate were quantitated using the method of Yu et al. (2002). Plasma containing internal standard (carbamazepine) was extracted with acetonitrile. After centrifugation, the supernatant was evaporated to dryness, and then redissolved in enzyme solution containing sulfatase (Patella vulgata Type V, 0.5 units per sample [Sigma]) dissolved in 0.15 M NaCl. After incubation for 18.5 hr at 37°C, samples were extracted for quantitation of resveratrol as described above. The quantity of resveratrol sulfate present in each sample was calculated by subtracting the quantity of resveratrol present in samples that had not been incubated with sulfatase from the quantity of resveratrol present in samples that had undergone sulfatase treatment.
2.6. Oncogenicity study design
The definitive oncogenicity study was conducted in full compliance with FDA Good Laboratory Practice Regulations (Title 21, Code of Federal Regulations, Part 58). In the definitive oncogenicity study, groups of 25 TSG-53(+/-) mice per sex received daily oral (gavage) exposure to resveratrol at doses of 1000, 2000, or 4000 mg/kg body weight/day for six months. As a result of mortality in the high dose group, the 4000 mg/kg/day dose level was reduced to 3000 mg/kg/day at the end of study week 4. Resveratrol was administered in a vehicle of 0.5% (w/v) aqueous carboxymethylcellulose containing 0.2% (w/v) Tween 80, using a dosing volume of 10 ml/kg. Mice in the vehicle control group (25 per sex) received daily gavage exposure to vehicle only (10 ml/kg body weight). Mice in the positive control group (25 per sex) received daily gavage exposure to p-cresidine (400 mg/kg body weight/day) for the same period.
Mice were observed twice daily throughout the quarantine and dosing periods for mortality or evidence of toxicity. Body weights and food consumption were measured individually for each animal once per week, and each animal underwent a detailed, hand-held clinical and physical observation on a weekly basis. Blood samples for clinical pathology evaluations were collected immediately prior to the terminal necropsy. Clinical chemistry assays were performed using an automated clinical chemistry analyzer (Synchron LX-20, Beckman Coulter, Inc., Fullerton, CA); hematology assays were performed using an automated hematology analyzer (Advia 120, Bayer Healthcare, Tarrytown, NY).
All mice underwent a complete necropsy with tissue collection. Intercurrent deaths were necropsied immediately after their discovery. At the end of the six-month dosing period, surviving animals were euthanized in random order by CO2 asphyxiation; necropsy was initiated within five minutes of euthanasia. At the terminal necropsy, weights of the adrenals, brain, heart, kidneys, liver, spleen, testes/ovaries, and thymus were collected individually for each animal. Histopathologic evaluations on approximately 45 tissues per animal were performed on all animals in the vehicle control group and on all animals in the groups receiving the low and middle doses of resveratrol. In consideration of the number of early deaths in the group receiving the high dose of resveratrol, no histopathologic evaluation of tissues was performed on this dose group. Histopathologic evaluation of tissues from animals in the positive control group was limited to the kidney and urinary bladder, known target tissues for the oncogenicity of p- cresidine (Storer et al., 2001).
2.7. Statistical analysis
Statistical analysis of continuous data (body weight, food consumption, clinical pathology, organ weights) was performed using analysis of variance, with post-hoc comparisons made using Dunnett′s test. Incidence data (survival, clinical observations, histopathology) were compared using X2 analysis. A minimum significance level of p < 0.05 was used for all comparisons.
3. Results
3.1. Dose range-finding study of resveratrol
The results of the range-finding study demonstrated that the maximum dose of resveratrol that can be delivered to mice on a daily basis is limited by mortality associated with impaction of unabsorbed test material in the gastrointestinal tract, rather than by any specific toxicity of the agent itself. In this study, 40% mortality was observed in male C57BL/6 mice receiving the highest dose of resveratrol (5000 mg/kg/day). In all early deaths in this group, a large mass of unabsorbed test article was identified at necropsy in the stomach and/or intestines. No pattern of early deaths was seen in male mice receiving lower doses of resveratrol, or in female mice at any resveratrol dose level.
Aside from the mortality associated with the impaction of resveratrol in the gastrointestinal tract, no clear evidence of resveratrol toxicity was identified in any dose group. Group mean body weights were comparable in all groups (data not shown), and a regular schedule of clinical and physical observations signs provided no evidence of agent toxicity. Similarly, clinical chemistry and hematology assays failed to identify any pattern of resveratrol-associated toxicity. Other than impaction of unabsorbed test article in the gastrointestinal tract and associated organ dilatation, no pattern of gross lesions that was related to resveratrol administration was identified in any dose group in the range-finding study.
On the basis of these data, it was concluded that the mortality observed in male mice exposed to the high dose of resveratrol was related to physical factors associated with the large quantity of test article that was administered, rather than to any specific toxicity of resveratrol. However, the results of this study clearly demonstrated that impaction of unabsorbed test material limits the quantity of resveratrol that can be administered in repeat-dose studies. In consideration of the mortality seen in C57BL/6 mice receiving resveratrol at a dose of 5000 mg/kg/day, the 4000 mg/kg/day dose was selected for use in the pharmacokinetics study and as the high dose in the definitive oncogenicity study.
3.2. Pharmacokinetics study of resveratrol
The results of the pharmacokinetics study demonstrated that resveratrol is rapidly absorbed after an oral (gavage) dose, and is almost completely metabolized to conjugated metabolites very soon after absorption.
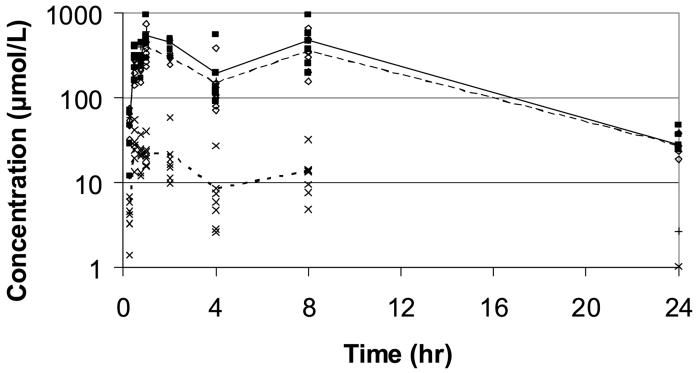
Fifteen min after administration of a single dose of 4000 mg resveratrol per kg body weight, plasma concentrations of resveratrol glucuronide and resveratrol sulfate were comparable (∼ 47 ± 25 μmol/L) and were approximately ten-fold greater than the plasma concentration of the parent compound (4.2 ± 2.0 μmol/L; Fig. 1). Plasma concentration of resveratrol reached a maximum of 30.2 ± 15.3 μmol/L at 30 min post-dosing, and declined thereafter. By contrast, plasma levels of both conjugated metabolites continued to increase to maxima at 1 hour (resveratrol glucuronide: 534 ± 228 μmol/L; resveratrol sulfate: 386 ± 188 μmol/L). From their maximum values at 30 min (parent) or 60 min (metabolites), plasma levels of the parent drug and both metabolites were decreased at 2 and 4 hours, but demonstrated small increases between 4 and 8 hours (Fig. 1); the increases in plasma levels seen between 4 and 8 hours could reflect enterohepatic circulation. Between 1 and 8 hours post-dosing, concentrations of both conjugated resveratrol metabolites ranged from approximately 15- to 40-times the concentration of the parent drug.
Fig. 1.
Plasma concentration profiles of resveratrol (--X--), resveratrol glucuronide (—■—), and resveratrol sulfate (--◇--) in mice following a single oral (gavage) dose of 4000 mg resveratrol per kg body weight.
At 24 h post-dosing, plasma levels of both resveratrol conjugates were in the range of 25 to 30 μmol/L, while plasma levels of the parent drug were below the limit of quantitation (0.5 μmol/L) in five of the six animals sampled. It is interesting to note that mean plasma levels of resveratrol glucuronide and resveratrol sulfate at 24 h post-dosing were comparable to the peak plasma levels of unconjugated resveratrol seen at 30 min post-dosing.
3.3. Oncogenicity study of resveratrol
Daily gavage administration of resveratrol at the mid and high doses (2000 and 4000 mg/kg/day) induced mortality in both sexes of p53(+/-) mice. At necropsy, essentially all intercurrent deaths demonstrated masses of impacted test article in the gastrointestinal tract; no gastrointestinal hemorrhage or other evidence of local resveratrol toxicity was seen. As such, early mortality in groups receiving the mid and high doses of resveratrol appears to be a function of the mass of resveratrol administered, rather than a result of any agent-specific toxicity.
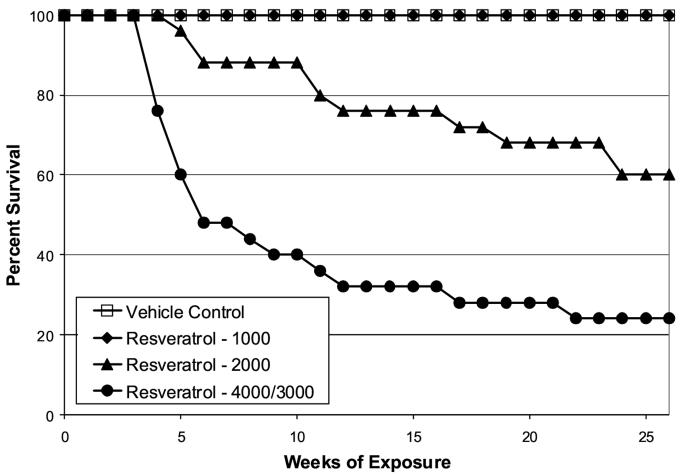
In male mice in the high dose group (4000 mg/kg/day), significant mortality was encountered early in the study (Fig. 2). This mortality necessitated a reduction in the high dose of resveratrol from 4000 to 3000 mg/kg/day at the end of study week 4. Significant mortality was first seen in male mice in the middle dose group (2000 mg/kg/day) after five weeks of resveratrol administration; this dose of resveratrol was continued until study termination. Mortality patterns in female mice (Fig. 3) demonstrated dose- and time-related mortality patterns that were comparable to those seen in males.
Fig. 2.
Survival of male TSG-p53(+/-) mice receiving daily oral exposure to resveratrol. Essentially all mortality in mice receiving resveratrol doses > 2000 mg/kg/day was associated with impaction and accumulation of resveratrol in the gastrointestinal tract.
Fig. 3.
Survival of female TSG-p53(+/-) mice receiving daily oral exposure to resveratrol. Essentially all mortality in mice receiving resveratrol doses > 2000 mg/kg/day was associated with impaction and accumulation of resveratrol in the gastrointestinal tract.
At the termination of the study at 26 weeks, survival in male mice was 28% in the high dose group and 64% in the middle dose group. Similarly, in female mice, survival rates in the high and middle dose groups were 24% and 60%, respectively. By contrast, survival was 100% in animals exposed to the low dose of resveratrol (1000 mg/kg/day), and 100% in mice receiving the positive control article (p-cresidine, 400 mg/kg/day). Survival rates in male and female mice in the vehicle control group were 96% and 100%, respectively.
Weekly clinical observations, body weight measurements, and quantitation of food intake failed to identify any evidence of toxicity in surviving animals exposed to resveratrol. In male mice, mean terminal body weights in resveratrol-treated groups ranged from 94.7% to 103.1% of vehicle control, while terminal body weights in female mice receiving resveratrol ranged from 97.7% to 104.5% of vehicle control (p > 0.05 for all comparisons). By contrast, statistically significant reductions in food consumption were seen during all study weeks in groups exposed to the positive control article, p-cresidine. These reductions in food consumption were correlated with reductions in mean body weight and body weight gain: statistically significant reductions in group mean body weight in mice exposed to p-cresidine were first seen in males at week 4 and in females at week 5; these reductions persisted throughout the study. At study termination, group mean body weights in male and female mice receiving p-cresidine were 82.4% and 89.0% of mean body weight in sex-matched vehicle controls (p < 0.05 for both comparisons).
Clinical pathology evaluations performed on samples collected at the terminal necropsy provided little evidence of resveratrol toxicity. Modest (<40%) but dose-related and statistically significant increases in serum cholesterol were seen in both sexes (Table 1). Small (<15%) but statistically significant reductions in red blood cell count, hematocrit, and hemoglobin were seen in male mice in the high dose group (Table 1), but were not present in male mice receiving lower doses of resveratrol or in females in any dose group. Occasional statistically significant differences from vehicle control were found in several other clinical pathology parameters in mice exposed to resveratrol. However, these changes were generally small (<10%), not dose-related, observed in one sex only, and involved test values that remained within normal limits of the assay. As such, these differences are interpreted as reflecting the large number of comparisons that were made in the study, and are not considered to be biologically significant.
Table 1.
Selected clinical pathology parameters in p53(+/-) (p53 knockout) mice receiving chronic oral exposure to resveratrol
| Resveratrol Dose (mg/kg/day) | Male Cholesterol (mg/dL) | Male RBC (106/μl) | Male Hemoglobin (g/dL) | Male Hematocrit (%) | Female Cholesterol (mg/dL) | Female RBC (106/μl) | Female Hemoglobin (g/dL) | Female Hematocrit (%) |
|---|---|---|---|---|---|---|---|---|
| 0 (Vehicle Control) | 82 ± 8 | 10.8 ± 0.3 | 15.5 ± 0.4 | 53.4 ± 1.3 | 61 ± 11 | 10.3 ± 0.9 | 14.8 ± 1.3 | 50.2 ± 4.4 |
| Resveratrol - 1000 | 79 ± 15 | 11.0 ± 0.4 | 15.6 ± 0.5 | 53.6 ± 2.3 | 64 ± 10 | 10.5 ± 0.4 | 15.1 ± 0.5 | 51.7 ± 2.0 |
| Resveratrol - 2000 | 85 ± 13 | 10.9 ± 0.6 | 15.3 ± 1.3 | 53.7 ± 3.2 | 81 ± 12 a | 10.2 ± 0.5 | 14.2 ± 1.1 | 49.1 ± 3.2 |
| Resveratrol - 4000/3000 | 101 ± 21 a | 9.8 ± 0.8 a | 13.7 ± 1.5 a | 46.6 ± 5.7 a | 83 ± 30 a | 10.0 ± 0.2 | 14.9 ± 0.6 | 50.0 ± 1.5 |
p < 0.05 versus vehicle control.
As discussed above, masses of unabsorbed test article and dilatation of the stomach and intestines were consistent necropsy findings in early deaths from groups receiving the high and middle doses of resveratrol. By contrast to these findings in early deaths, necropsy findings were unremarkable in surviving animals in the high and middle dose groups, and in all animals in the low dose and vehicle control groups. Organ weight data collected at the terminal necropsy demonstrated statistically significant, dose-related increases in absolute and relative liver weights in both sexes of mice exposed to resveratrol (Table 2), but no other biologically significant changes.
Table 2.
Dose-related increases in absolute and relative liver weights in p53(+/-) (p53 knockout) mice receiving chronic oral exposure to resveratrol
| Resveratrol Dose (mg/kg/day) | Absolute Liver Weight (Males) (g, Mean ± S.D.) | Absolute Liver Weight (Females) (g, Mean ± S.D.) | Relative Liver Weight a (Males) (Mean ± S.D.) | Relative Liver Weight a (Females) (Mean ± S.D.) |
|---|---|---|---|---|
| 0 (Vehicle Control) | 1.67 ± 0.13 | 1.41 ± 0.28 | 5.17 ± 0.24 | 5.29 ± 0.69 |
| Resveratrol - 1000 | 1.72 ± 0.15 | 1.49 ± 0.14 | 5.31 ± 0.39 | 5.59 ± 0.33 |
| Resveratrol - 2000 | 1.85 ± 0.17 b | 1.50 ± 0.20 | 5.61 ± 0.25 b | 5.80 ± 0.54 b |
| Resveratrol - 4000/3000 | 1.85 ± 0.11 b | 1.67 ± 0.18 b | 6.05 ± 0.26 b | 6.11 ± 0.50 b |
As percent of body weight.
p < 0.05 versus vehicle control.
Histopathologic evaluation of tissues provided no evidence of oncogenicity of resveratrol in p53(+/-) mice. Although benign and malignant lesions (primarily lymphomas) were seen in all study groups, the incidences of neoplasms in groups exposed to resveratrol did not differ from incidences of neoplasia observed in sex-matched vehicle controls. A summary of neoplastic lesions identified in study animals is provided in Table 3.
Table 3.
Neoplastic lesions in p53(+/-) (p53 knockout) mice receiving chronic oral exposure to resveratrol a or positive control article (p-cresidine)
| Resveratrol Dose |
p-Cresidine Dose |
||||
|---|---|---|---|---|---|
| 0 | 1000 | 2000 | 400 | ||
| Lesion Site | Lesion Type | (Vehicle Control) | mg/kg/day | mg/kg/day | mg/kg/day |
| Male Mice | |||||
| Lymph Node | Lymphoma | 10/24 b | 9/25 | 9/25 | -- |
| Thymus | Lymphoma | 1/25 | 0/25 | 0/25 | -- |
| Spleen | Lymphoma | 0/25 | 0/25 | 2/25 | -- |
| Adrenal Cortex | Adenoma | 0/25 | 1/25 | 0/25 | -- |
| Prostate | Sarcoma | 0/25 | 1/25 | 0/25 | -- |
| Urinary Bladder | Transitional Cell Carcinoma | 0/25 | 0/25 | 0/25 | 8/25 |
| Urinary Bladder | Squamous Cell Carcinoma | 0/25 | 0/25 | 0/25 | 2/25 |
| Urinary Bladder | Mesenchymal Tumor | 0/25 | 0/25 | 0/25 | 1/25 |
| Urinary Bladder | Transitional Cell Papilloma | 0/25 | 0/25 | 0/25 | 3/25 |
| Female Mice | |||||
| Lymph Node | Lymphoma | 11/25 | 9/25 | 12/25 | -- |
| Spleen | Lymphoma | 2/25 | 1/25 | 1/25 | -- |
| Pancreas | Acinar Cell Carcinoma | 1/25 | 0/25 | 0/25 | -- |
| Lung | Alveolar Cell Adenoma | 0/25 | 1/25 | 0/25 | -- |
| Urinary Bladder | Transitional Cell Carcinoma | 1/25 | 0/25 | 0/25 | 9/25 |
| Urinary Bladder | Mesenchymal Tumor | 0/25 | 0/25 | 0/25 | 3/25 |
| -- | Rhabdomyosarcoma | 0/25 | 0/25 | 0/25 | 1/25 |
Histopathologic evaluation of tissues from mice receiving the high dose of resveratrol was not performed due to high mortality in this dose group. These animals were not considered to be at risk of oncogenesis.
Number of lesions identified/Number of tissues evaluated.
-- Not evaluated
The positive control article, p-cresidine, induced urinary bladder neoplasms in both sexes of p53(+/-) mice (Table 3). In the positive control group, 44% (11/25) of male mice were diagnosed with malignancies in the urinary bladder (eight transitional cell carcinomas, two squamous cell carcinomas, and one mesenchymal tumor). Similarly, 48% (12/25) of female mice exposed to p-cresidine demonstrated urinary bladder neoplasms (nine transitional cell carcinomas and three mesenchymal tumors). In addition to these malignant bladder lesions, three male mice in the positive control group demonstrated transitional cell papillomas, a benign lesion that is generally considered to be a precursor lesion to transitional cell carcinoma.
Dose-related non-neoplastic changes were identified in the kidney and urinary bladder of mice exposed to resveratrol. Hydronephrosis of mild to moderate severity was a common, treatment-related finding in surviving mice exposed to either the 1000 or 2000 mg/kg/day dose levels of resveratrol. Hydronephrosis was present in 18% of mid dose males, 60% of mid dose females, 20% of low dose males, and 12% of low dose females. By contrast, hydronephrosis was not diagnosed in any animal in the vehicle control group. There was no evidence of drug-related inflammation in the urinary tract of animals with or without hydronephrosis, and no evidence of calculi or other accumulation of urinary solids was identified. Several animals with hydronephrosis also demonstrated tubular necrosis and infarction, suggesting that at least some instances of hydronephrosis may have resulted from non-lethal loss of renal tissue rather than from pressure atrophy associated with urinary tract obstruction.
Epithelial hyperplasia of the urinary bladder was diagnosed in 44% of surviving males and 33% of surviving females in the group exposed to the mid dose of resveratrol. This lesion was not seen in any animal in the low dose resveratrol group, and was present in 0% of males and 4% of females in the vehicle control group. By contrast, epithelial hyperplasia of the urinary bladder was present in 100% of male mice and 96% of female mice in the positive control group exposed to p-cresidine.
4. Discussion
Data from an expanding body of carcinogenesis studies in experimental models demonstrate that resveratrol is an attractive candidate for efficacy evaluation in chemoprevention clinical trials. In addition to its anticarcinogenic activity, resveratrol has a number of cardioprotective, anti-aging, and other biological activities that suggest its possible utility in the prevention of cardiovascular and other chronic diseases. The present oncogenicity study in p53(+/-) mice addresses critical scientific and regulatory steps in the preclinical development of resveratrol for possible clinical study as a cancer chemopreventive agent, and may also support the initiation of Phase II and Phase III clinical trials to evaluate its utility in the prevention of other diseases.
An essential part of the preclinical development of any potential chemopreventive agent to which humans may receive chronic exposure is the demonstration that the agent is not carcinogenic in experimental model systems. Bacterial mutagenesis studies (Ames Tests) with resveratrol have produced negative results, and thus provide no evidence to support the hypothesis that resveratrol is likely to be mutagenic and/or carcinogenic in humans. By contrast, however, genotoxic effects of resveratrol have been reported in mutagenesis and DNA damage studies conducted using mammalian cell models. In studies conducted using L5178 mouse lymphoma cells and Chinese hamster V79 cells, Schmitt and colleagues (2002) reported that resveratrol increases the incidence of sister chromatid exchanges, induces micronuclei, and increases the incidence of spindle distortions and dislocated chromosomes. In considering the sum of the available genetic toxicology data, resveratrol must be considered to be an agent with, at a minimum, equivocal genotoxic activity. In consideration of this demonstrated activity in well-studied genetic toxicology model systems, in vivo studies of the possible carcinogenicity of resveratrol become an increasingly important component of its preclinical data base.
The results of the present study demonstrate that resveratrol is not oncogenic in TSG-p53(+/-) (p53 knockout) mice when the agent is administered at its maximum tolerated dose for six months. Because the six-month oncogenicity study in the p53 knockout mouse is accepted by the United States Food and Drug Administration and other regulatory agencies as a suitable preclinical model for carcinogenicity evaluations, the lack of oncogenicity of resveratrol in the p53 knockout mouse is generally accepted as adequate evidence of lack of carcinogenicity in murine model systems.
The maximum dose of resveratrol that could be administered to mice was limited by a physical factor (impaction of drug substance in the gastrointestinal tract) rather than by any chemical-specific toxicity. Differential mortality patterns associated with impaction of the test article appeared to be related to animal body weight. At each dose level, animals with higher body weights received a greater total mass of resveratrol than did animals with lower body weights. Because the male mice in both studies were larger than the female mice, the males received a larger mass of test article each day; this could explain the sex difference in mortality observed in the range-finding study. Similarly, the female TSG-p53(+/-) (p53 knockout) mice used in the oncogenicity study were larger than the female C57BL/6 mice used in the range-finding study, and therefore received a greater mass of the test article at each dose. Essentially all of the mortality due to resveratrol impaction in females in the oncogenicity study was seen in mice whose body weights were in the upper portion of the range.
Although resveratrol did not increase the incidence of any neoplasm in the present study, a number of non-neoplastic effects were seen in resveratrol-treated animals. In evaluating the possible oncogenicity of resveratrol, perhaps the most significant of these was the finding of urothelial hyperplasia (generally of minimal to mild severity) in more than one-third of surviving animals of both sexes in the group exposed to the mid dose of resveratrol. Urothelial hyperplasia was not identified in animals in the low dose group. Because urothelial hyperplasia could be secondary to physical factors such as hydronephrosis or urinary calculi, a post-hoc analysis of individual animal data was performed in the attempt to identify such an association. No link between hydronephrosis and urothelial hyperplasia was found, and urinary calculi were not commonly seen in any experimental group. Because no physical mechanism can be proposed to explain the increased incidence of urothelial hyperplasia in mice receiving the mid dose of resveratrol, this is considered to be a direct, dose-related effect of the test article.
The Cmax of free resveratrol measured in mouse plasma in the present PK study ranged from 20 to 40 μM, and plasma levels of resveratrol conjugates in mice were more than 10-fold higher than levels of free resveratrol. The Cmax for free resveratrol in mice was reached at 1 to 2 hours after dosing, and plasma resveratrol levels remained above 10 μM for at least at 8 hours. By comparison, the Cmax of free resveratrol measured in the plasma of human volunteers enrolled in a Phase I clinical trial ranged from 0.5 to 2 μM (Royalmount Pharma, unpublished data). As in mice, Cmax in humans was reached between 1 and 2 hours post-dosing. The Cmax for resveratrol in humans also increased in a reasonably linear fashion with dose. On this basis, the low and mid dose levels of resveratrol administered in the murine oncogenicity study could be expected to yield plasma levels of free resveratrol that are at least 5- to 20-fold higher than the levels that were measured in humans enrolled in the Phase I clinical trial. These data suggest that plasma drug levels in mice in the low dose group (where no urothelial hyperplasia was seen) are likely to exceed those achieved in the clinic by a factor of at least 5 to 10. Similarly, plasma drug levels in mice in the middle dose group (the lowest dose group in which urothelial hyperplasia was seen) are likely to exceed those achieved in the clinic by a factor of at least 10, and perhaps by as much as 20. In consideration of these data, it is concluded that urothelial hyperplasia in p53 knockout mice was seen only at plasma drug levels that substantially exceed those that are likely to be reached in humans.
Clinical pathology studies identified small but statistically significant elevations in serum cholesterol in both sexes of mice exposed to resveratrol, and a mild anemia (as indicated by decreases in RBC count, hematocrit, and hemoglobin) in male mice exposed to the high dose only. In consideration of the small magnitude of the increases in serum cholesterol seen in resveratrol-treated mice, it is considered unlikely that these increases are of toxicologic significance. The mechanisms underlying the mild anemia induced by resveratrol in male mice are unknown; however, the observed anemia could reflect a minimal toxic effect of resveratrol, or could result from the activity of resveratrol as a phytoestrogen (Schmitt et al., 2002).
Statistically significant, dose-related increases in liver weight were seen in surviving animals exposed to the mid and high doses of resveratrol. This hepatomegaly was not associated with any microscopic changes in the liver, but could reflect the induction by resveratrol of hepatic Phase II enzymes involved in carcinogen detoxification (Jang et al., 1997). As discussed above, resveratrol-treated animals also demonstrated dose-related hydronephrosis and epithelial cell hyperplasia of the urinary bladder. Although neither hydronephrosis nor urothelial hyperplasia were linked to any functional alteration, these findings do identify the kidney and urinary bladder as possible targets for resveratrol toxicity. In this regard, the renal and urinary bladder toxicity of resveratrol identified in the present study is consistent with the findings of Crowell et al. (2004) who conducted a 4-week toxicity study of resveratrol in rats. By contrast to these data from rodent models, a recently completed study conducted in our laboratory did not identify hydronephrosis, urothelial hyperplasia, or any other significant histopathologic changes in the urinary tract of beagle dogs receiving daily oral exposure to resveratrol for 4 weeks. To our knowledge, no toxicology studies of resveratrol have been conducted in non-human primates.
The results of the present study demonstrate that chronic oral administration of resveratrol at its maximum tolerated dose does not increase the incidence of any malignant or benign neoplasm in TSG-p53(+/-) (p53 knockout) mice. Chronic oral administration of resveratrol to mice at a dose of 2000 mg/kg/day did induce a statistically significant increase in the incidence of urothelial hyperplasia, a proliferative lesion in the urinary bladder. However, mice exposed to resveratrol at 1000 mg/kg/day demonstrated no evidence of urothelial hyperplasia, and pharmacokinetic data suggest that this effect was seen only at plasma resveratrol levels that exceed targeted human plasma levels by a factor of at least 10. In consideration of the demonstrated chemopreventive activity of resveratrol in animal models for cancer in the breast, colon, and other sites; its relative lack of toxicity at doses generating plasma drug levels that are similar to targeted drug levels in humans; and its lack of oncogenicity in predictive animal model systems, resveratrol clearly merits further consideration for possible evaluation in human cancer chemoprevention trials.
Acknowledgements
This work was supported by contract N01-CN-05135 from the Chemopreventive Agent Development Research Group, Division of Cancer Prevention, National Cancer Institute. The authors thank Leigh Ann Senoussi for assistance in preparing the manuscript.
Abbreviation
- FDA
Food and Drug Administration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement for Authors
T. L. Horn - None
M. J. Cwik - None
R. L. Morrissey - None
I. Kapetanovic - None
J. A. Crowell - None
T. D. Booth - employee of Royalmount Pharma, producers of resveratrol
D. L. McCormick - None
References
- Aziz MH, Reagan-Shaw S, Wu J, Longley BJ, Ahmad N. Chemoprevention of skin cancer by grape constituent resveratrol: relevance to human disease? FASEB J. 2005;19:1193–1195. doi: 10.1096/fj.04-3582fje. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002;62:4945–4954. [PubMed] [Google Scholar]
- Berge G, Ovrebo S, Eilertsen E, Haugen A, Mollerup S. Analysis of resveratrol as a lung cancer chemopreventive agent in A/J mice exposed to benzo[a]pyrene. Br. J. Cancer. 2004;91:1380–1383. doi: 10.1038/sj.bjc.6602125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celotti E, Ferrarini R, Zironi R, Conte LS. Resveratrol content of some wines obtained from dried Valpolicella grapes: Recioto and Amarone. J. Chromatogr. A. 1996;730:47–52. doi: 10.1016/0021-9673(95)00962-0. [DOI] [PubMed] [Google Scholar]
- Chun YJ, Kim MY, Guengerich FP. Resveratrol is a selective human cytochrome P-450 1A1 inhibitor. Biochem. Biophys. Res. Commun. 1999;262:20–24. doi: 10.1006/bbrc.1999.1152. [DOI] [PubMed] [Google Scholar]
- Ciolino HP, Yeh GC. Inhibition of aryl hydrocarbon-induced cytochrome P-450 1A1 enzyme activity and CYP1A1 expression by resveratrol. Mol. Pharmacol. 1999;56:760–767. [PubMed] [Google Scholar]
- Crowell JA, Korytko PJ, Morrissey RL, Booth TD, Levine BS. Resveratrol-associated renal toxicity. Toxicol. Sci. 2004;82:614–619. doi: 10.1093/toxsci/kfh263. [DOI] [PubMed] [Google Scholar]
- Dong Z. Molecular mechanism of the chemopreventive effect of resveratrol. Mutat. Res. 2003;523:524–145. doi: 10.1016/s0027-5107(02)00330-5. [DOI] [PubMed] [Google Scholar]
- Fukuhara K, Nagakawa M, Nakanishi I, Ohkubo K, Imai K, Urano S, Fukuzumi S, Ozawa T, Ikota N, Mochizuki M, Miyata N, Okuda H. Structural basis for DNA-cleaving activity of resveratrol in the presence of Cu(II) Bioorg. Med. Chem. 2006;14:1437–1443. doi: 10.1016/j.bmc.2005.09.070. [DOI] [PubMed] [Google Scholar]
- Garvin S, Ollinger K, Dabrosin C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006;231:113–122. doi: 10.1016/j.canlet.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Gescher AJ, Steward WP. Relationship between mechanisms, bioavailability, and preclinical chemopreventive efficacy of resveratrol: a conundrum. Cancer Epidemiol. Biomarkers Prev. 2003;12:953–957. [PubMed] [Google Scholar]
- Hecht SS, Kenney PM, Wang M, Trushin N, Agarwal S, Rao AV, Upadhyaya P. Evaluation of butylated hydroxyanisole, myo-inositol, curcumin, esculetin, resveratrol and lycopene as inhibitors of benzo[a]pyrene plus 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice. Cancer Lett. 1999;137:123–130. doi: 10.1016/s0304-3835(98)00326-7. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Hung LM, Chen JK, Huang SS, Lee RS, Su MJ. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc. Res. 2000;47:549–555. doi: 10.1016/s0008-6363(00)00102-4. [DOI] [PubMed] [Google Scholar]
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- Jang M, Pezzuto JM. Cancer chemopreventive activity of resveratrol. Drugs Exp. Clin. Res. 1999;25:65–77. [PubMed] [Google Scholar]
- Joe AK, Liu H, Suzui M, Vural ME, Xiao D, Weinstein IB. Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin. Cancer Res. 2002;8:893–903. [PubMed] [Google Scholar]
- Johnson WD, Dooley L, Morrissey RL, Arp L, Kapetanovic I, Crowell JA, McCormick DL. Oncogenicity evaluations of chemopreventive soy components in p53(+/-) (p53 knockout) mice. Int. J. Toxicol. 2006 doi: 10.1080/10915810600683366. in press. [DOI] [PubMed] [Google Scholar]
- Juan ME, Vinardell MP, Planas JM. The daily oral administration of high doses of trans-resveratrol to rats for 28 days is not harmful. J. Nutr. 2002;132:257–260. doi: 10.1093/jn/132.2.257. [DOI] [PubMed] [Google Scholar]
- Kapadia GJ, Azuine MA, Tokuda H, Takasaki M, Mukainaka T, Konoshima T, Nishino H. Chemopreventive effect of resveratrol, sesamol, sesame oil and sunflower oil in the Epstein-Barr virus early antigen activation assay and the mouse skin two-stage carcinogenesis. Pharmacol. Res. 2002;45:499–505. doi: 10.1006/phrs.2002.0992. [DOI] [PubMed] [Google Scholar]
- Lanzilli G, Fuggetta MP, Tricarico M, Cottarelli A, Serafino A, Falchetti R, Ravagnan G, Turriziani M, Adamo R, Franzese O, Bonmassar E. Resveratrol down-regulates the growth and telomerase activity of breast cancer cells in vitro. Int. J. Oncol. 2006;28:641–648. [PubMed] [Google Scholar]
- Leonard SS, Xia C, Jiang BH, Stinefelt B, Klandorf H, Harris GK, Shi X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem. Biophys. Res. Commun. 2003;309:1017–1026. doi: 10.1016/j.bbrc.2003.08.105. [DOI] [PubMed] [Google Scholar]
- Levi F, Pasche C, Lucchini F, Ghidoni R, Ferraroni M, La Vecchia C. Resveratrol and breast cancer risk. Eur. J. Cancer Prev. 2005;14:139–142. doi: 10.1097/00008469-200504000-00009. [DOI] [PubMed] [Google Scholar]
- Li ZG, Hong T, Shimada Y, Komoto I, Kawabe A, Ding Y, Kaganoi J, Hashimoto Y, Imamura M. Suppression of N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumorigenesis in F344 rats by resveratrol. Carcinogenesis. 2002;23:1531–1536. doi: 10.1093/carcin/23.9.1531. [DOI] [PubMed] [Google Scholar]
- Marier JF, Vachol P, Gritsas A, Zhang J, Moreau J-P, Ducharme MP. Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J. Pharmacol. Exper. Ther. 2002;302:369–373. doi: 10.1124/jpet.102.033340. [DOI] [PubMed] [Google Scholar]
- Matsuoka A, Furuta A, Ozaki M, Fukuhara K, Miyata N. Resveratrol, a naturally occurring polyphenol, induces sister chromatid exchanges in a Chinese hamster lung (CHL) cell line. Mutat. Res. 2001;494:107–113. doi: 10.1016/s1383-5718(01)00184-x. [DOI] [PubMed] [Google Scholar]
- Matsuoka A, Takeshita K, Furuta A, Ozaki M, Fukuhara K, Miyata N. The 4′-hydroxy group is responsible for the in vitro cytogenetic activity of resveratrol. Mutat. Res. 2002;521:29–35. doi: 10.1016/s1383-5718(02)00211-5. [DOI] [PubMed] [Google Scholar]
- McDonald J, French JE, Gerson RJ, Goodman J, Inoue T, Jacobs A, Kasper P, Keller D, Lavin A, Long G, McCullough B, Sistare FD, Storer R, van der Laan JW. The utility of genetically modified mouse assays for identifying human carcinogens: a basic understanding and path forward. The Alternatives to Carcinogenicity Testing Committee ILSI HESI. Toxicol. Sci. 2004;77:188–194. doi: 10.1093/toxsci/kfh037. [DOI] [PubMed] [Google Scholar]
- Provinciali M, Re F, Donnini A, Orlando F, Bartozzi B, Di Stasio G, Smorlesi A. Effect of resveratrol on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Int. J. Cancer. 2005;115:36–45. doi: 10.1002/ijc.20874. [DOI] [PubMed] [Google Scholar]
- Sale S, Tunstall RG, Ruparelia KC, Potter GA, Steward WP, Gescher AJ. Comparison of the effects of the chemopreventive agent resveratrol and its synthetic analog trans 3,4,5,4′-tetramethoxystilbene (DMU-212) on adenoma development in the Apc(Min+) mouse and cyclooxygenase-2 in human-derived colon cancer cells. Int. J. Cancer. 2005;115:194–201. doi: 10.1002/ijc.20884. [DOI] [PubMed] [Google Scholar]
- Sale S, Verschoyle RD, Boocock D, Jones DJ, Wilsher N, Ruparelia KC, Potter GA, Farmer PB, Steward WP, Gescher AJ. Pharmacokinetics in mice and growth-inhibitory properties of the putative cancer chemopreventive agent resveratrol and the synthetic analogue trans 3,4,5,4′-tetramethoxystilbene. Br. J. Cancer. 2004;90:736–744. doi: 10.1038/sj.bjc.6601568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders TH, McMichael RW, Jr., Hendrix KW. Occurrence of resveratrol in edible peanuts. J. Agric. Food Chem. 2000;48:1243–1246. doi: 10.1021/jf990737b. [DOI] [PubMed] [Google Scholar]
- Schmitt E, Lehmann L, Metzler M, Stopper H. Hormonal and genotoxic activity of resveratrol. Toxicol. Lett. 2002;136:133–142. doi: 10.1016/s0378-4274(02)00290-4. [DOI] [PubMed] [Google Scholar]
- Sgambato A, Ardito R, Faraglia B, Boninsegna A, Wolf FI, Cittadini A. Resveratrol, a natural phenolic compound, inhibits cell proliferation and prevents oxidative DNA damage. Mutat. Res. 2001;496:171–180. doi: 10.1016/s1383-5718(01)00232-7. [DOI] [PubMed] [Google Scholar]
- She QB, Ma WY, Wang M, Kaji A, Ho CT, Dong Z. Inhibition of cell transformation by resveratrol and its derivatives: differential effects and mechanisms involved. Oncogene. 2003;22:143–150. doi: 10.1038/sj.onc.1206370. [DOI] [PubMed] [Google Scholar]
- Storer RD, French JE, Haseman J, Hajian G, LeGrand EK, Long GG, Mixson LA, Ochoa R, Sagartz JE, Soper KA. p53+/- hemizygous knockout mouse: overview of available data. Toxicol. Pathol. 2001;29:30–50. doi: 10.1080/019262301753178465. [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K, Chung WJ, Michaluart P, Telang N, Tanabe T, Inoue H, Jang M, Pezzuto JM, Dannenberg AJ. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J. Biol. Chem. 1998;273:21875–21882. doi: 10.1074/jbc.273.34.21875. [DOI] [PubMed] [Google Scholar]
- Surh YJ, Hurh YJ, Kang JY, Lee E, Kong G, Lee SJ. Resveratrol, an antioxidant present in red wine, induces apoptosis in human promyelocytic leukemia (HL-60) cells. Cancer Lett. 1999;140:1–10. doi: 10.1016/s0304-3835(99)00039-7. [DOI] [PubMed] [Google Scholar]
- Szewczuk LM, Forti L, Stivala LA, Penning TM. Resveratrol is a peroxidase-mediated inactivator of COX-1 but not COX-2: a mechanistic approach to the design of COX-1 selective agents. J. Biol. Chem. 2004;279:22727–22737. doi: 10.1074/jbc.M314302200. [DOI] [PubMed] [Google Scholar]
- Tseng SH, Lin SM, Chen JC, Su YH, Huang HY, Chen CK, Lin PY, Chen Y. Resveratrol suppresses the angiogenesis and tumor growth of gliomas in rats. Clin. Cancer Res. 2004;10:2190–2202. doi: 10.1158/1078-0432.ccr-03-0105. [DOI] [PubMed] [Google Scholar]
- Yu C, Young GS, Chow A, Li Y, Kosmeder JW, Lee YS, Hirschelman WH, Pezzuto JM, Mehta RG, van Breeman RB. Human rat and mouse metabolism of resveratrol. Pharm. Res. 2002;19:1907–1914. doi: 10.1023/a:1021414129280. [DOI] [PubMed] [Google Scholar]
- Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr. Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]