Abstract
Aging is associated with a change in the relationship between the timing of sleep and circadian rhythms, such that the rhythms occur later with respect to sleep than in younger adults. To investigate whether a difference in the phase-delaying response to evening light contributes to this, we conducted a 9-day inpatient study in 10 healthy older (≥ 65 y.o.) subjects. We assessed circadian phase in a constant routine, exposed each subject to a 6.5-h broad spectrum light stimulus beginning in the early biological night, and reassessed circadian phase. The stimuli spanned a range from very dim (~2 lux) to very bright (~8,000 lux) indoor light. We found a significant dose-response relationship between illuminance and the phase shift of the melatonin rhythm, with evidence that sensitivity, but not the maximal response to light, differed from that of younger adults. These findings suggest an age-related reduction in the phase-delaying response to moderate light levels. However, our findings alone do not explain the altered phase relationship between sleep and circadian rhythms associated with aging.
Suggested keywords: biological rhythm, circadian, aging, melatonin, chronobiology, light, phase shift
1. Introduction
Healthy aging is associated with changes in sleep quality, duration, and timing. These changes include less of the deeper sleep stages (stages 3 and 4, slow wave sleep), an increase in the number of awakenings during the night, and earlier sleep-wake times. These age-related changes in sleep occur even in the absence of clinically-significant sleep disorders such as sleep disordered breathing or periodic limb movement disorder.
The circadian timing system is one of the two major regulatory processes influencing sleep [20;22;67], the other being the homeostatic pressure for sleep. It is a major determinant of sleep timing, allows for consolidation of sleep towards the end of the sleep episode, and influences the distribution of sleep stages within a sleep episode. Because of these influences on sleep, age-related changes in the circadian timing system have been hypothesized to contribute to the observed age-related changes in sleep.
The circadian system in humans has an average period (cycle length) that is longer than 24 h [15;18;34;61], and is entrained (synchronized) to the 24 h day by regular exposure to light and darkness [60]. Light has a phase-dependent effect on the circadian system [21], with light exposure during the late subjective day/early subjective night causing phase delay shifts (to a later hour), light during the late subjective night/early subjective day causing phase advance shifts (to an earlier hour), and light exposure during the subjective daytime causing very small phase shifts [35;39;47]. The effects of light on the circadian system are determined not only by the timing of light exposure, but by other factors including the intensity [64], wavelength [7; 45;56], duration [49;50] and prior light exposure history [19;32;54]. We and others have reported previously that the relative timing between the phase of circadian rhythms and the timing of the nocturnal sleep episode is significantly different between healthy older and young adults [26;27;44], and we have found that this change in phase relationship does not appear to be related to an age-related difference in the length or lability of the endogenous circadian period [18]. As light exposure is required to maintain entrainment of the non-24 h circadian clock to the 24 h day, and the relative phase alignment of the internal clock to the external day is directly dependent upon the qualities of the light, age-related changes in light sensitivity could contribute to the observed age-related differences in the phase relationship between sleep and the circadian timing system.
There is evidence from animal studies that there is a reduction in light sensitivity of the circadian system with advancing age, including smaller phase shifts in response to light [4;66] (see [59] for review), a smaller range of entrainment [48], greater light levels necessary for stable entrainment [43], and changes in the rate of re-entrainment [11;62]. These reductions at the whole animal level may be due to observed age-related changes in the response to light at the cellular level in the suprachiasmatic nucleus (SCN, the locus of the central circadian pacemaker in mammals), including a higher threshold for cellular responses [66] and/or decreased response to light [4;42;55]. There is also evidence that age-related structural changes in the circadian and/or visual systems may contribute to a reduction in light sensitivity, including reduced light transmission through the eye [16;65] (especially of shorter wavelengths [8]), and a reduction in the number of circadian photoreceptors [52].
A change in the photic sensitivity of the human circadian timing system might manifest as a change in sleep patterns. Some reports suggest that older individuals living in their home environments receive lower levels of light exposure and fewer minutes of bright light exposure per day than do young adults [14;28;51], although not all studies agree on this [37]. Institutionalized elderly have been reported to receive even less bright light than healthy elders [53], and there is also an association between daytime light exposure and nighttime sleep quality and consolidation in both institutionalized and healthy older people [36;53;57] with exposure to greater amounts of daytime light associated with better nighttime sleep quality. A recent study comparing light exposure between young and middle-aged subjects found that while the daily exposure to different levels of light did not differ with age, the pattern of light exposure across the day was different [38]. Timed artificial light exposure has been shown to improve sleep maintenance insomnia in community-dwelling older people [13], and increasing the duration and strength of daytime lighting has been reported to be associated with greater nighttime sleep consolidation and improved sleep efficiency in institutionalized elders [2;29;58].
Together, these reports suggest that reduced light exposure levels and/or a decreased sensitivity to light with aging might contribute to age-related increases in sleep disruption and the age-related alteration in the phase relationship between sleep timing and the timing of the biological clock that have been reported previously. The current study was designed to determine whether the sensitivity or capacity of the human circadian system to respond to a single, phase-delaying, broad spectrum white light pulse was reduced with age.
2. Methods
2.1. Subjects
Subjects were recruited for the study from newspaper advertisements directed to people age 65 and older. Subjects were not taking medications and had no acute or chronic medical problems at the time of study. Subjects were in good health as determined by medical screening (serum chemistry, complete blood count, urinalysis, chest radiograph, physical examination), ophthalmologic screening (including ruling out color-blindness, glaucoma, and a history of eye trauma, as well as an examination of the lens using the LOCSIII classification system [17] to ensure significant cataracts were not present), were in good psychological health (as determined by the Geriatric Depression Scale, Mattis Dementia Rating Scale, Folstein Mini-Mental State Exam, and by clinical interview), and were free from clinically significant sleep disorders (as determined by an all-night polysomnographic study). Each gave written informed consent prior to study; the study was reviewed and approved by the Partners HealthCare Human Subjects Committee and were conducted in accordance with the principles outlined in the Declaration of Helsinki.
A total of 14 subjects began the inpatient portion of the study. Two withdrew consent prior to the first circadian phase estimation procedure and two were disempaneled by the investigators prior to the first circadian phase estimation procedure (one for borderline hypertension, the other developed an upper respiratory infection on the second baseline day in the laboratory). The remaining 10 subjects (2 women, 8 men; age 68.3 ± 3.7 years) completed the study between late 1999 and early 2003.
2.2. Study protocol
The 9-day inpatient study began with three baseline days, with 8 hours of nocturnal sleep and 16 hours wake scheduled at each subject’s habitual times as determined from a diary they kept during the week prior to entering the lab. Upon waking after the third baseline night, subjects began a 26.7-h constant routine procedure to assess their initial circadian phase (see description below and Figure 1). Following an 8-h recovery sleep, the subjects woke in the late afternoon to a 16-h waking episode during which the experimental light exposure was presented (see below). After the light exposure day, the subjects woke to a 52-h constant routine procedure to reassess their circadian phase. Following an 11.3-h recovery sleep episode, the subjects were discharged home.
Figure 1.
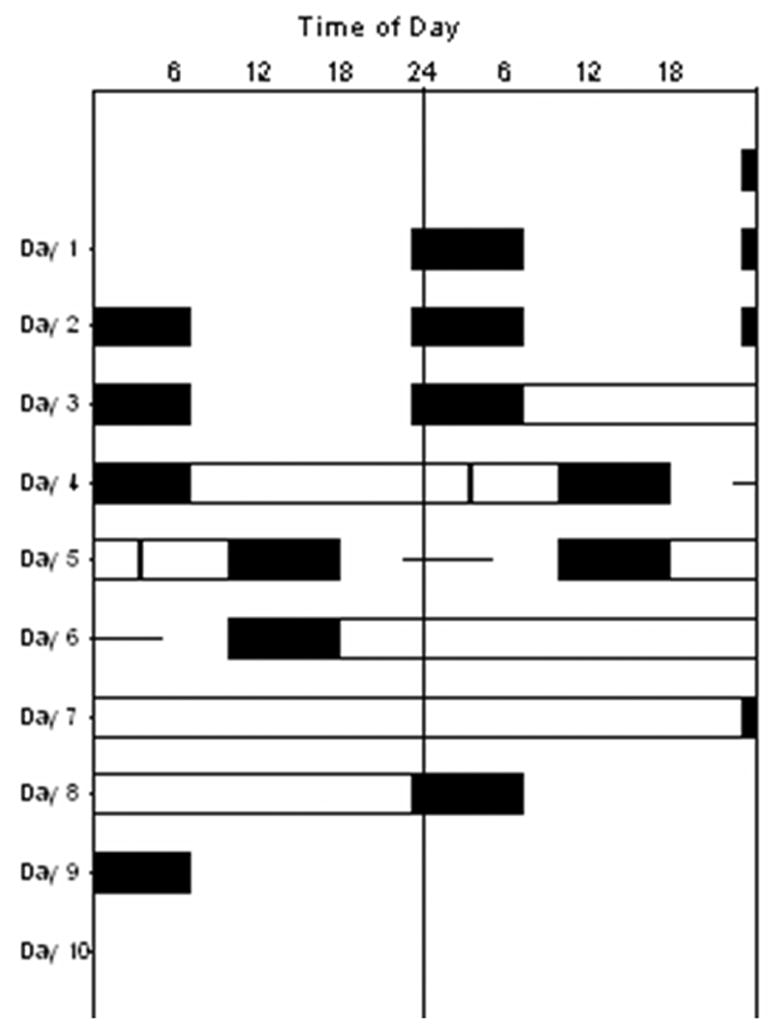
Double raster plot of study protocol. Time of day is presented on the horizontal axis, with each day of the experiment shown both to the right of, and beneath, the previous day. Scheduled sleep episodes are shown in the black boxes, constant routine (CR) circadian phase estimation procedures are shown in the open bars on Days 4–5 (CR1) and Days 6–8 (CR2), the predicted timing of the melatonin phase (MELmax) is shown with the vertical bar on Day 5, and the 6.5-h experimental light exposure session is shown with the horizontal line on Day 5–6. The timing of each subject’s protocol was adjusted to his/her own habitual sleep-wake times during the week prior to study.
2.3. Circadian phase assessment
Circadian phase was assessed using core body temperature and melatonin collected throughout the two constant routine (CR) procedures. The constant routine is a protocol designed to assess endogenous circadian phase by eliminating or distributing across the 24-h day periodic changes in behavior and the environment that can obscure the underlying contribution from the circadian pacemaker on the data being recorded [25;46]. Throughout the CR, the subjects were restricted to semi-recumbent bed rest in constant dim light (see below), were required to remain awake, and were given hourly snacks designed to spread their caloric and fluid intake evenly across the 24-h day. A technician remained in the room with the subject throughout the CR to converse with them and ensure they remained awake.
Throughout each CR, core body temperature was collected each minute via a rectal thermistor, blood was collected every 30 minutes via an indwelling intravenous catheter, and saliva was collected hourly. Blood samples were placed into tubes containing EDTA and placed on ice for up to an hour before being centrifuged and the resulting plasma was frozen until analysis. Saliva samples were placed on ice for up to an hour before being frozen. Plasma samples were assayed for melatonin using a radioimmunoassay (assay sensitivity 0.7 pg/mL; intra-assay coefficient of variation 12.1% at 16.5 pg/mL, 5.7% at 68.7 pg/mL; inter-assay coefficient of variation 13.2% at 17.3 pg/mL, 8.4% at 69 pg/mL; Pharmasan Labs, Osceola, WI). In one subject from whom we were unable to obtain blood samples, saliva samples were assayed for melatonin using the same radioimmunoassay.
2.4. Light conditions and experimental light exposure
All lighting was provided by ceiling-mounted fluorescent lamps (T8 or T12 lamps with CCT of 4100K, Philips Lighting Eindhoven, the Netherlands; see Figure 2) transmitted through ultraviolet (UV)-shielding ceiling filters (Lexan, GE Plastics, Pittsfield, MA). All lighting was controlled by the experimenters at all times and subjects had no access to any other lighting. During the first two complete baseline days, the ambient lighting was approximately 0.23 W/m2 (~89 lux) at 137 cm from the floor in the horizontal angle and had a maximum of 0.48 W/m2 (150 lux) at 187 cm from the floor in the horizontal angle anywhere in the room. Throughout the two constant routine circadian phase assessment procedures, the ambient lighting was reduced to approximately 0.0087 W/m2 (~3.3 lux) at 137 cm from the floor in the horizontal angle and had a maximum of 0.048 W/m2 (15 lux) at 187 cm from the floor in the vertical angle anywhere in the room. Ambient lighting on the experimental light exposure day was further reduced to approximately 0.0048 W/m2 (~1.8 lux) at 137 cm from the floor in the horizontal angle and had a maximum of 5 lux at 187 cm from the floor in the vertical angle anywhere in the room. During scheduled sleep episodes, all lights were switched off.
Figure 2.
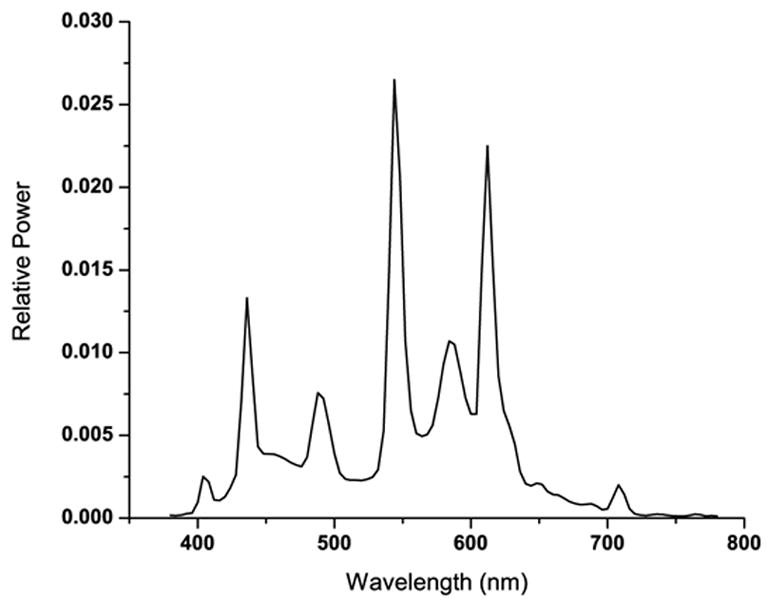
Spectral distribution of the fluorescent lamps used in the studies.
The 6.5-h experimental light exposure was timed such that it was centered in the middle of the waking episode, and occurred during the early biological nighttime (beginning 30 min before habitual bedtime and continuing until 2 h before habitual wake time). This resulted in the light exposure day being scheduled at an inverted time relative to the subject’s usual schedule (see Figure 1). Approximately 30 min before the start of the experimental light exposure, the subject was seated in a chair in a fixed position within the study room where they remained until the 6.5-h light exposure was completed. Throughout the 6.5-h light exposure, the subject wore clear, UV-excluding glasses (UVEX Ultraspec 2000, UVEX Safety, Smithfield, RI). In alternating 6-min segments throughout the light exposure session, the subject was instructed to gaze at a target on the wall which had been calibrated prior to the study to achieve the experimental light level. At other times, the subject was free to direct their gaze around the room, except during brief intervals (<1 min) every 30 min, when they looked at a computer screen to rate their alertness on a 9-point scale. A technician was present in the room for the duration of the light exposure session to ensure the subject remained seated with their eyes open and gazed at the target during the alternating 6-min segments, and to record the actual light level with a research photometer (IL1400 radiometer/powermeter with an SEL-033/F/W detector, International Light, Inc., Newburyport, MA). Throughout each experimental light exposure day, blood or saliva was collected and later assayed for melatonin as described above in order to assess the acute suppressive effects of the light on melatonin.
A total of ten target experimental light levels were chosen to span the range from 5 lux to 8,000 lux (see Table 1). Subjects were assigned at random to an experimental light level on the day of their admission to the laboratory; in cases where a study was not completed the next subject admitted to the study was assigned to that randomized light level.
Table 1.
Individual subject data.
| Subject | Age | Sex | Habitual Bed Time | Habitual Wake Time | Initial MELmax | Initial CBTmin | Illuminance (lux) | MELmax Shift (h) | CBTmin Shift (h) | Melatonin Suppression (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2021 | 65 | M | 23:32 | 7:32 | 2:33 | 3:36 | 1.35 | −0.56 | −0.63 | −3.4 |
| 1993 | 67 | F | 22:32 | 6:31 | 2:13 | 4:56 | 22.8 | −0.29 | 0.66 | 49.8 |
| 19F6 | 66 | M | 22:36 | 6:36 | 4:48 | 7:35 | 65.6 | −1.02 | 0 | 17.4 |
| 2215 | 70 | M | 23:01 | 7:04 | 3:26 | 5:31 | 122 | −0.68 | −0.63 | 0 |
| 22AA | 65 | M | 21:27 | 5:32 | 3:42 | 6:02 | 207 | −1.68 | −1.79 | 56.4 |
| 22L1 | 69 | M | 23:05 | 7:10 | 2:58 | 7:43 | 319 | −1.7 | −0.3 | 78.6 |
| 2002 | 76 | M | 23:30 | 7:25 | 3:40 | 7:39 | 1,570 | −2.97 | −1.6 | 61.1 |
| 19G7 | 65 | M | 23:14 | 7:16 | 3:21 | 6:03 | 3,527 | −2.32 | −2.03 | 78.3 |
| 2033 | 67 | M | 23:51 | 8:06 | 4:06 | * | 6,464 | −2.71 | * | 57.1 |
| 2001 | 73 | F | 21:59 | 5:59 | 0:05 | 1:56 | 7,960 | −3.22 | −1.84 | 50.9 |
2.5. Data analysis
The subject’s pre-study bedtime and wake time were determined by averaging the times recorded in the sleep diary from the seven nights prior to entering the laboratory.
Core body temperature phase was assessed by the maximum likelihood fit of a 2-harmonic regression model with first-order autoregressive noise [9;10], and melatonin phase was assessed by the maximum likelihood fit of a 3-harmonic regression model. The first 5 h and the final 30 min of data from the CR were excluded from analysis due to the potential masking effects of waking and changing posture at the beginning and end of the CR. Core body temperature phase was defined as the minimum of the fitted waveform (CBTmin) and melatonin phase was defined as the maximum of the fitted waveform (MELmax). Phase shift was defined as CR2 phase – CR1 phase (see Figure 3, lower panel).
Figure 3.
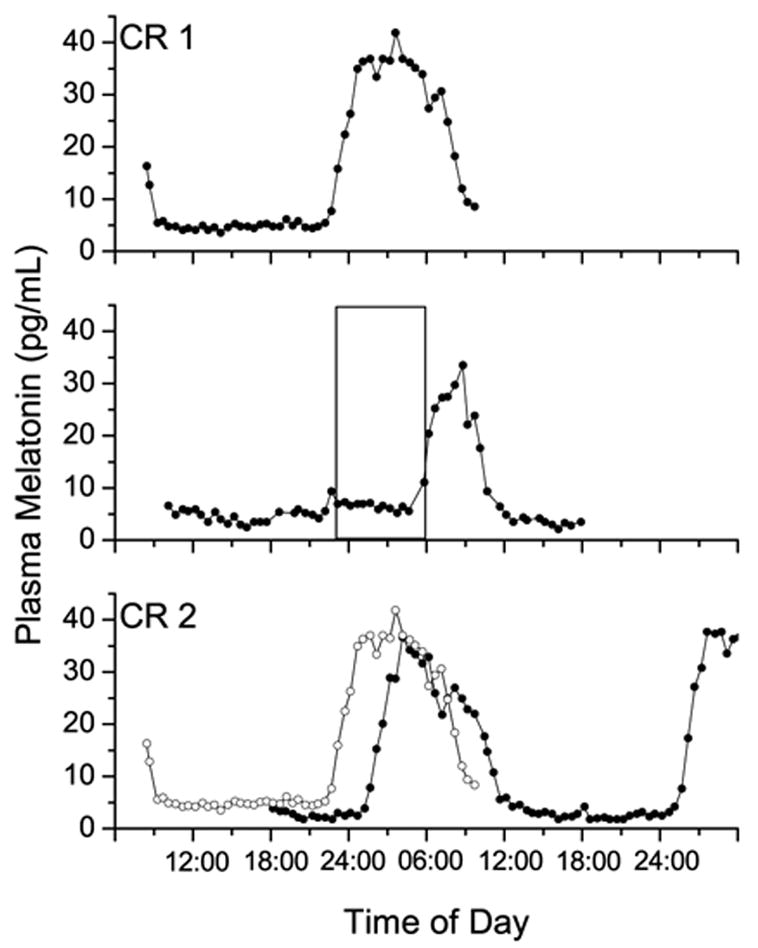
Plasma melatonin data from subject 19G7, a 65 year old man who was exposed to a 3,527 lux light stimulus. Upper panel: plasma melatonin data from the initial circadian phase estimation procedure (CR1); middle panel: plasma melatonin data from the intervention day, with the 6.5-h experimental light exposure indicated by the open box; lower panel: plasma melatonin data from the final circadian phase estimation procedure (CR2) shown in the solid symbols, with data from CR1 replotted from above in the open symbols. During CR1, the fitted peak of the melatonin secretion (MELmax) occurred at 03:45, 3.5 h before habitual wake time. During CR2 MELmax occurred at 06:30, a 3.5 h phase delay. Melatonin was suppressed by 78% during the 6.5-h 3,527 lux light stimulus.
Melatonin suppression was calculated by determining the area under the curve (AUC) during the 6.5-h experimental light exposure (see Figure 3, middle panel) and comparing it with the AUC from the same clock hours on the previous night (during CR1). Suppression was calculated as (AUCCR1 – AUClight exposure)/AUCCR1.
Dose-response analysis was done by fitting the data with a four-parameter logistic model: y = [(a–c)/(1+(x/b)d)]+c [64], a model derived from the Michaelis-Menton equation. In this model, a is the estimated response of the system to an illuminance of 0 lux, b is the illuminance at which 50% of the estimated maximum response occurs, c is the asymptotic maximum estimated response of the system, and d is a factor related to the steepness of the rise of the linear portion of the curve. Goodness-of-fit is estimated by the adjusted R2, which takes into account the number of parameters used to fit the data. The R term is also given as a familiar comparison.
The actual experimental light level to which each subject was exposed was estimated by averaging the light readings taken after each of the 33 fixed gaze episodes during the 6.5-h light exposure session. All analyses and figures are presented with respect to these averaged readings, rather than the target levels. All data are presented as average ± SEM, except for model parameters which are presented as average ± SD.
3. Results
The average bed- and wake times of the subjects were 22:53 ± 0:15 and 06:55 ± 0:15, respectively. Initial melatonin phase (MELmax) occurred at 03:05 ± 0:24, an average of 3.83 ± 0. 4 h before habitual wake time, while initial core temperature phase occurred at 05:40 ± 0:39 (n=9), an average of 1.12 ± 0.63 h before habitual wake time.
The 6.5-h light exposure session following CR1 was scheduled to begin 0.5 h before the subjects’ usual bedtimes (see Figure 1) so as to be centered 3.5 h before the predicted CBTmin, at the same relative circadian phase as in our study of younger adults [64]. Because of the variability between bedtime, CBTmin, and MELmax between individuals, this resulted in the light exposure session beginning 4.67 ± 0.39 h before MELmax (range 2.6 to 6.7 h). Actual light exposure levels were within 5% of target levels for all but the two lowest target levels (5 lux and 25 lux targets), in which cases lower than targeted levels were achieved (1.35 lux and 21.8 lux, respectively).
Core body temperature phase delay shifts in response to the light exposure ranged from +40 to −122 min, with the size of the shift having a dose-response relationship with the illuminance of the experimental light exposure (4-parameter log model, R = −0.80, adj. R2 = 0.27, p<0.01). Melatonin phase delay shifts in response to the experimental light exposure ranged from −17 to −193 min, with the size of the phase delay shift significantly related to the illuminance of the 6.5-h stimulus (4-parameter log model, R = −0.96, adj. R2 = 0.86, p<0.01; see Figure 4). The circadian rhythms of core body temperature and plasma melatonin shifted in parallel in response to the light exposure (Pearson correlation, r = 0.79, p<0.02; see Table 1).
Figure 4.
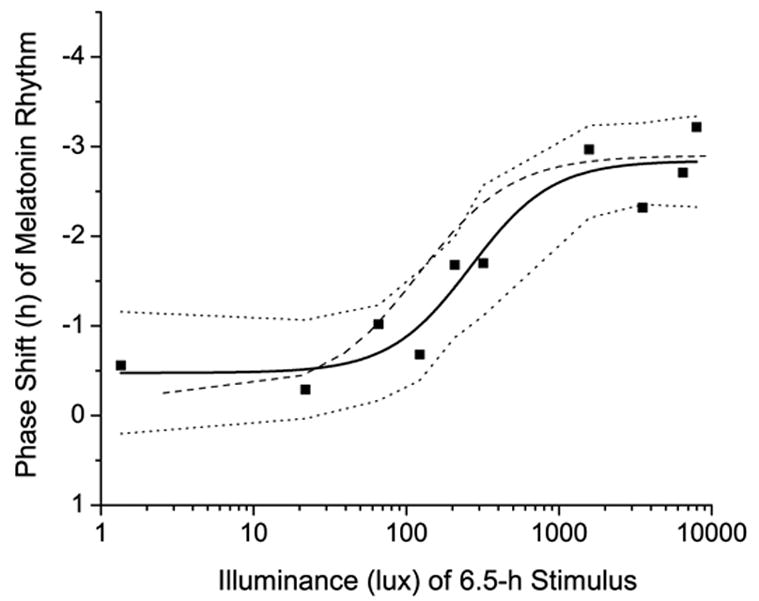
Phase shift of fitted plasma melatonin peak (MELmax) vs. illuminance of experimental light stimulus. Data from each of the ten subjects are plotted individually and shown with square symbols. Solid line represents the 4-parameter logistic model fit to the data, with the 95% confidence interval of the model shown in the dotted lines. For comparison, the 4-parameter logistic model fit to the data from our previous study in younger adults [64] is shown in the dashed line.
Melatonin suppression during the 6.5-h light exposure ranged from 0 to 79% (see Table 1). While the lowest intensity of light (1.35 lux) had little effect on melatonin and many of the higher intensities caused robust suppression (e.g., 207 lux-6,464 lux elicited greater than 50% suppression), there were striking discrepancies. The subject exposed to 22 lux had 50% suppression while the subject exposed to 7,960 lux, the highest intensity tested, had only 51% suppression. There was no detectable relationship between melatonin suppression and illuminance over the range of illuminances tested, nor was there a correlation between melatonin suppression and phase shift.
We conducted a comparison of the illuminance-resetting response results from these older subjects to that observed in a group of younger subjects studied in our laboratory between late 1997 and late 1998 [64]. That group of younger subjects had a similar sex distribution (82% male) as our older group, and their number of baseline days and the baseline and CR lighting conditions were also the same. The 6.5-h experimental light exposure began on average 5.21 ± 0.21 h before MELmax (range 4.12 to 7.58 h) in the younger subjects, a phase of light exposure that was not significantly different from that in the current group of older subjects (p = 0.19, T-test).
Model estimates for illuminance response changes in melatonin phase in the older subjects were: a = −0.475 ± 0.279, b = 263 ± 88.9, c = −2.84 ± 0.224, and d = 1.63 ± 1.02. Model estimates for illuminance response changes in melatonin phase in the younger subjects were: a = −0.240 ± 0.409, b = 119 ± 43.1, c = −2.90 ± 0.238, and d = 1.42 ± 0.661 [64]. The a, c, and d model terms were statistically indistinguishable between the two age groups (p>0.3). This indicates that the response to 0 lux of light (the a model term, representing the drift in phase in response to a longer than 24-h circadian period and non-photic cues), the maximum phase shift that was achieved (the c model term), and the steepness of the slope (the d model term, a measure of the “squareness” of the sigmoidal waveform) were similar in the young and older groups. In contrast, the sensitivity term (b) of the older group was significantly greater than that of the younger subjects (p<0.005), indicating that the older subjects were less sensitive to light of moderate illumination, and required higher illumination to evoke the same response when the illumination was non-saturating.
4. Discussion
Our current study examined the relationship between light intensity and the circadian phase delay response to a single 6.5-h light stimulus in healthy older people. We found that in healthy older people, the circadian rhythms of both core body temperature and plasma melatonin were shifted in parallel in response to the 6.5-h experimental light stimulus, and that there was a significant relationship between illuminance and the phase-delay shift of both the melatonin and temperature rhythms. The illuminance-response relationship using melatonin data provided a much better model fit (adj. R2 = 0.86) than did the core body temperature data (adj. R2 = 0.27), likely due to the greater confidence in the accuracy of the melatonin phase data [41].
We also compared the results of our study in older subjects to a previous study we had conducted in younger adults in order to determine if there was an age-related difference in the response to this phase-delaying light stimulus. We found that some aspects of the circadian response to the light stimulus in older subjects did not differ from responses observed previously in young adults. The maximum phase shift obtainable to a single 6.5-h light stimulus in the older adults in this study (the c term in the 4-parameter model) was equivalent to that obtained in young adults in similar studies [31;45;64]. The progressive delay drift of phase due to the 24.2 h average intrinsic period and non-photic influences on the circadian timing system was also similar between the healthy older and younger adults, as evidenced by the similar values obtained for the a term in the 4-paramater models fit to the data, representing the predicted shift in response to a 0 lux stimulus.
We did observe a difference in the sensitivity of the system (the b term in the 4-parameter model, representing the illuminance level at which 50% of the asymptotic maximum response is observed) between the older and young subjects (Figure 4, compare solid and dashed lines). The greater b term reflects a rightward shift of the model fit to the data from the older subjects, indicating that they are less responsive to low-to-moderate levels of light (~50–1,000 lux). Given that pupil dynamics and lens opacity can change with aging, corneal light exposure may be quite different from retinal light exposure in older subjects, and the change we observed may be due to a change in the effective retinal illumination, rather than representing an age-related reduction in circadian light sensitivity. Future studies focused on this narrow illuminance range, with larger numbers of older and young participants may be able to address this question.
Our current findings are consistent with several previous studies in humans that also did not find significant differences in the magnitude of phase delay shifts elicited by late evening/early night light exposure when those light levels were very high or very low. In a previous study we conducted in which subjects were exposed to three consecutive nights of exposure to 5 h bright indoor light, we did not find significant differences between young and older subjects in the size of the phase delay shifts [40]. The intensity of light used in that study, however, was so large (9,500 lux) as to saturate the circadian timing system’s ability to respond to light. It would therefore only have been able to detect a change in the maximum response to light, as opposed to any change in sensitivity. A recent study of the melatonin-suppressing effects of monochromatic evening light in young and older females found no change in response to long wavelength (548 nm) visible light [33]. Another recent study that compared circadian phase-shifting responses to dim (10 lux) and bright (3,500 lux) light in young and older adults found no differences between the two age groups [3], consistent with our finding of no difference in the response to very dim light or to very bright light.
Our study also found a difference in the degree of melatonin suppression between the older and young subjects’ responses to light. The greatest amount of melatonin suppression that was observed in this study was 78.6%. In response to the same experimental light exposure procedures, all young subjects in our previous study who were exposed to at least 350 lux of light showed at least 92% melatonin suppression [64]. Those young subjects also displayed a strong dose-response relationship between illuminance and melatonin suppression, with robust melatonin suppression occurring at illuminances at least as bright as room light (>100 lux) and little suppression occurring at illuminances less than room light [64]. Our current cohort of healthy older individuals, in addition to having a lower capacity to suppress melatonin at any intensity of light, did not show a dose response relationship between melatonin suppression and illuminance. As there was a normal responsiveness of the circadian timing system to the phase delaying effects of light, the melatonin suppression data suggest that there may be an age-related change in the light transmission system between the SCN and the source of plasma melatonin (pineal gland). Alternatively, there may be a pathway from the retina to the SCN or other hypothalamic target that is involved in the acute cessation of melatonin production, but not in entrainment, and this pathway may be selectively affected by aging. In fact, sympathetic innervation of the eye, which is also responsible for sympathetic innervation of the pineal gland [63], may have diminished capacity with aging [6] and could account for the variability in melatonin suppression we observed in our subjects.
A recent study by Herljevic et al. reported that melatonin suppression in older women was reduced compared with younger women in response to short wavelength (456 nm) visible light, but they found no age-related change in melatonin suppression in response to longer wavelength (548 nm) light [33]. That finding is of interest due to the fact that the circadian system of humans and other mammals is most sensitive to shorter wavelengths of visible light [7;45;56], and the specialized retinal ganglion cells that serve as the primary circadian photoreceptors [5;30] have a peak sensitivity in that same range. While our broad-spectrum white light stimulus was quite different than the stimulus used by Herljevic et al., it is also the case that both our older and young study populations were predominantly male (80% and 82% respectively), which may explain why we observed age-related differences in melatonin suppression and they did not.
It is important to note that in our study, subjects were extremely healthy, had no sleep complaints, and were screened for major visual deficits and age-related changes in lens pigmentation. Despite this, they had the common features of an aging circadian system, including early habitual wake times (before 07:00), melatonin and core body temperature phases occurring in the latter half of their habitual sleep time, and baseline night sleep efficiencies below 80% [23;26;27]. While we found no significant differences in the response to dim or very bright light in our very healthy older subjects when compared with young adults, we did find differences in the response to moderate levels of light. It should be noted that results from the older subjects in the current study were compared with results from a group of young subjects whose study was completed before the current study began. However, the lighting conditions in both studies were highly controlled, and the protocol and lighting conditions in the current study of older subjects was designed to be the same as that in the prior study in young adults in order that such a comparison could be performed.
It is possible that in older individuals with ocular problems (e.g., cataracts), light transmission to the circadian pacemaker could be altered, which in turn could further reduce their responsiveness to moderate levels of light, and even reduce their response to bright light. Even with an intact circadian responsiveness to light, older individuals are likely to be less responsive to the use of light as a treatment for transient circadian rhythm sleep disorders such as jet lag and shift work disorder [1], due to their reduced ability to sleep at adverse circadian phases [24]. In a study that used a bright light treatment regimen in middle-aged subjects scheduled to a night work schedule, while the subjects were found to phase-shift by >6 hours in response to the bright light treatment, they did not fully adapt to the 9h shift in sleep timing and therefore had high rates of sleep disruption [12].
Our current finding that older subjects show a reduced circadian phase shifting response to moderate levels of nighttime light exposure cannot itself explain the difference in entrained circadian phase we and others have observed in older people [26;27;44]. We have also reported that a change in circadian period does not occur with healthy aging [18]. Together, these findings suggest that the observed age-related difference in the phase relationship between the timing of sleep and the timing of circadian rhythmicity, and the increased variability in this relationship, are more likely due to differences in the phase-advancing response to morning light and/or differences in light exposure patterns across the waking day between young and older adults. Further investigations focused on the response to morning light and to light exposure patterns across the 24-h day should provide a better understanding of these observations.
Acknowledgments
We wish to thank the study participants; the subject recruiters (C. O’Brien, K. Malvey, D. McCarthy); K.B. Librera-McKay and the staff of the DSM Chronobiology Core for staffing the CRs and the light exposure sessions; the BWH GCRC staff; E.J. Silva, R.F. Dimanche, and M.J. Duverne for assistance with the data processing; J.M. Ronda for Information Systems support; and Dr. K. Scheuermaier for helpful comments on the manuscript.
The studies were supported by NIH grant R01 AG06072 and were conducted in the BWH GCRC supported by M01 RR02635.
Disclosure Statement. The authors do not have any actual or potential conflicts of interest that could inappropriately bias this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.ICSD-2. Diagnostic and coding manual. 2. Westchester: American Academy of Sleep Medicine; 2005. The international classification of sleep disorders. [Google Scholar]
- 2.Ancoli-Israel S, Gehrman P, Martin JL, Shochat T, Marler M, Corey-Bloom J, Levi L. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer's disease patients. Behav Sleep Med. 2003;1:22–36. doi: 10.1207/S15402010BSM0101_4. [DOI] [PubMed] [Google Scholar]
- 3.Benloucif S, Green K, L'Hermite-Balériaux M, Weintraub S, Wolfe LF, Zee PC. Responsiveness of the aging circadian clock to light. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2005.10.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benloucif S, Masana MI, Dubocovich ML. Light-induced phase shifts of circadian activity rhythms and immediate early gene expression in the suprachiasmatic nucleus are attenuated in old C3H/HeN mice. Brain Res. 1997;747:34–42. doi: 10.1016/s0006-8993(96)01182-1. [DOI] [PubMed] [Google Scholar]
- 5.Berson DM. Strange vision: Ganglion cells as circadian photoreceptors. Trends Neurosci. 2003;26:314–320. doi: 10.1016/S0166-2236(03)00130-9. [DOI] [PubMed] [Google Scholar]
- 6.Bitsios P, Prettyman R, Szabadi E. Changes in autonomic function with age: A study of pupillary kinetics in healthy young and old people. Age Ageing. 1996;25:432–438. doi: 10.1093/ageing/25.6.432. [DOI] [PubMed] [Google Scholar]
- 7.Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brainard GC, Rollag MD, Hanifin JP. Photic regulation of melatonin in humans: Ocular and neural signal transduction. J Biol Rhythms. 1997;12:537–546. doi: 10.1177/074873049701200608. [DOI] [PubMed] [Google Scholar]
- 9.Brown EN, Choe Y, Luithardt H, Czeisler CA. A statistical model of the human core-temperature circadian rhythm. Am J Physiol. 2000;279:E669–E683. doi: 10.1152/ajpendo.2000.279.3.E669. [DOI] [PubMed] [Google Scholar]
- 10.Brown EN, Czeisler CA. The statistical analysis of circadian phase and amplitude in constant-routine core-temperature data. J Biol Rhythms. 1992;7:177–202. doi: 10.1177/074873049200700301. [DOI] [PubMed] [Google Scholar]
- 11.Burešová M, Benešová O, Illnerová H. Aging alters resynchronization of the circadian system in rats after a shift of the light-dark cycle. Experientia. 1990;46:75–77. doi: 10.1007/BF01955421. [DOI] [PubMed] [Google Scholar]
- 12.Campbell SS. Effects of timed bright-light exposure on shift-work adaptation in middle-aged subjects. Sleep. 1995;18:408–416. doi: 10.1093/sleep/18.6.408. [DOI] [PubMed] [Google Scholar]
- 13.Campbell SS, Dawson D, Anderson MW. Alleviation of sleep maintenance insomnia with timed exposure to bright light. J Am Geriatr Soc. 1993;41:829–836. doi: 10.1111/j.1532-5415.1993.tb06179.x. [DOI] [PubMed] [Google Scholar]
- 14.Campbell SS, Kripke DF, Gillin JC, Hrubovcak JC. Exposure to light in healthy elderly subjects and Alzheimer's patients. Physiol Behav. 1988;42:141–144. doi: 10.1016/0031-9384(88)90289-2. [DOI] [PubMed] [Google Scholar]
- 15.Carskadon MA, Labyak SE, Acebo C, Seifer R. Intrinsic circadian period of adolescent humans measured in conditions of forced desynchrony. Neurosci Lett. 1999;260:129–132. doi: 10.1016/s0304-3940(98)00971-9. [DOI] [PubMed] [Google Scholar]
- 16.Charman WN. Age, lens transmittance, and the possible effects of light on melatonin suppression. Ophthal Physiol Opt. 2003;23:181–187. doi: 10.1046/j.1475-1313.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- 17.Chylack LT, Jr, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, Friend J, McCarthy D, Wu S-Y for the Longitudinal Study of Cataract Study Group. The lens opacities classification system III. Arch Ophthalmol. 1993;111:831–836. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 18.Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 19.Czeisler CA, Kronauer RE, Allan JS, Duffy JF, Jewett ME, Brown EN, Ronda JM. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–1333. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- 20.Czeisler CA, Weitzman ED, Moore-Ede MC, Zimmerman JC, Knauer RS. Human sleep: Its duration and organization depend on its circadian phase. Science. 1980;210:1264–1267. doi: 10.1126/science.7434029. [DOI] [PubMed] [Google Scholar]
- 21.Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents. II. The variability of phase response curves. J Comp Physiol [A] 1976;106:253–266. [Google Scholar]
- 22.Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–68. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- 23.Dijk DJ, Duffy JF. Circadian regulation of human sleep and age-related changes in its timing, consolidation and EEG characteristics. Ann Med. 1999;31:130–140. doi: 10.3109/07853899908998789. [DOI] [PubMed] [Google Scholar]
- 24.Dijk DJ, Duffy JF, Czeisler CA. Age-related increase in awakenings: Impaired consolidation of nonREM sleep at all circadian phases. Sleep. 2001;24:565–577. doi: 10.1093/sleep/24.5.565. [DOI] [PubMed] [Google Scholar]
- 25.Duffy JF, Dijk DJ. Getting through to circadian oscillators: Why use constant routines? J Biol Rhythms. 2002;17:4–13. doi: 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]
- 26.Duffy JF, Dijk DJ, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am J Physiol. 1998;275:R1478–R1487. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]
- 27.Duffy JF, Zeitzer JM, Rimmer DW, Klerman EB, Dijk DJ, Czeisler CA. Peak of circadian melatonin rhythm occurs later within the sleep of older subjects. Am J Physiol. 2002;282:E297–E303. doi: 10.1152/ajpendo.00268.2001. [DOI] [PubMed] [Google Scholar]
- 28.Espiritu RC, Kripke DF, Ancoli-Israel S, Mowen MA, Mason WJ. Natural light exposure of adults 40–64 years old. Sleep Res. 1992;21:374. [Google Scholar]
- 29.Fetveit A, Skjerve A, Bjorvatn B. Bright light treatment improves sleep in institutionalised elderly--An open trial. Int J Geriatr Psychiatry. 2003;18:520–526. doi: 10.1002/gps.852. [DOI] [PubMed] [Google Scholar]
- 30.Freedman MS, Lucas RJ, Soni B, von Schantz M, Muñoz M, David-Gray Z, Foster R. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- 31.Gronfier C, Wright KP, Jr, Kronauer RE, Jewett ME, Czeisler CA. Efficacy of a single sequence of intermittent bright light pulses for delaying circadian phase in humans. Am J Physiol. 2004;287:E174–E181. doi: 10.1152/ajpendo.00385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hébert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herljevic M, Middleton B, Thapan K, Skene DJ. Light-induced melatonin suppression: Age-related reduction in response to short wavelength light. Exp Gerontol. 2005;40:237–242. doi: 10.1016/j.exger.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Hiddinga AE, Beersma DGM, van den Hoofdakker RH. Endogenous and exogenous components in the circadian variation of core body temperature in humans. J Sleep Res. 1997;6:156–163. doi: 10.1046/j.1365-2869.1997.00047.x. [DOI] [PubMed] [Google Scholar]
- 35.Honma K, Honma S. A human phase response curve for bright light pulses. Jpn J Psychiatry Neurol. 1988;42:167–168. [Google Scholar]
- 36.Hood B, Bruck D, Kennedy G. Determinants of sleep quality in the healthy aged: The role of physical, psychological, circadian and naturalistic light variables. Age Ageing. 2004;33:159–165. doi: 10.1093/ageing/afh051. [DOI] [PubMed] [Google Scholar]
- 37.Jean-Louis G, Kripke DF, Ancoli-Israel S, Klauber MR, Sepulveda RS, Mowen MA, Assmus JD, Langer RD. Circadian sleep, illumination, and activity patterns in women: Influences of aging and time reference. Physiol Behav. 2000;68:347–352. doi: 10.1016/s0031-9384(99)00186-9. [DOI] [PubMed] [Google Scholar]
- 38.Kawinska A, Dumont M, Selmaoui B, Paquet J, Carrier J. Are modifications of melatonin circadian rhythm in the middle years of life related to habitual patterns of light exposure? J Biol Rhythms. 2005;20:451–460. doi: 10.1177/0748730405280248. [DOI] [PubMed] [Google Scholar]
- 39.Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klerman EB, Duffy JF, Dijk DJ, Czeisler CA. Circadian phase resetting in older people by ocular bright light exposure. J Investig Med. 2001;49:30–40. doi: 10.2310/6650.2001.34088. [DOI] [PubMed] [Google Scholar]
- 41.Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17:181–193. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- 42.Kolker DE, Fukuyama H, Huang DS, Takahashi JS, Horton TH, Turek FW. Aging alters circadian and light-induced expression of clock genes in golden hamsters. J Biol Rhythms. 2003;18:159–169. doi: 10.1177/0748730403251802. [DOI] [PubMed] [Google Scholar]
- 43.Labyak SE, Turek FW, Wallen EP, Zee PC. Effects of bright light on age-related changes in the locomotor activity of Syrian hamsters. Am J Physiol. 1998;274:R830–R839. doi: 10.1152/ajpregu.1998.274.3.R830. [DOI] [PubMed] [Google Scholar]
- 44.Lewy AJ, Bauer VK, Singer CM, Minkunas DV, Sack RL. Later circadian phase of plasma melatonin relative to usual waketime in older subjects. Sleep. 2000;23:A188–A189. [Google Scholar]
- 45.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88:4502–4505. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 46.Mills JN, Minors DS, Waterhouse JM. Adaptation to abrupt time shifts of the oscillator[s] controlling human circadian rhythms. J Physiol. 1978;285:455–470. doi: 10.1113/jphysiol.1978.sp012582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minors DS, Waterhouse JM, Wirz-Justice A. A human phase-response curve to light. Neurosci Lett. 1991;133:36–40. doi: 10.1016/0304-3940(91)90051-t. [DOI] [PubMed] [Google Scholar]
- 48.Morin LP. Age-related changes in hamster circadian period, entrainment, and rhythm splitting. J Biol Rhythms. 1988;3:237–248. [Google Scholar]
- 49.Nelson DE, Takahashi JS. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus) J Physiol. 1991;439:115–145. doi: 10.1113/jphysiol.1991.sp018660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rimmer DW, Boivin DB, Shanahan TL, Kronauer RE, Duffy JF, Czeisler CA. Dynamic resetting of the human circadian pacemaker by intermittent bright light. Am J Physiol. 2000;279:R1574–R1579. doi: 10.1152/ajpregu.2000.279.5.R1574. [DOI] [PubMed] [Google Scholar]
- 51.Savides TJ, Messin S, Senger C, Kripke DF. Natural light exposure of young adults. Physiol Behav. 1986;38:571–574. doi: 10.1016/0031-9384(86)90427-0. [DOI] [PubMed] [Google Scholar]
- 52.Semo M, Lupi D, Peirson SN, Butler JN, Foster RG. Light-induced c-fos in melanopsin retinal ganglion cells of young and aged rodless/coneless (rd/rd cl) mice. Eur J Neurosci. 2003;18:3007–3017. doi: 10.1111/j.1460-9568.2003.03061.x. [DOI] [PubMed] [Google Scholar]
- 53.Shochat T, Martin J, Marler M, Ancoli-Israel S. Illumination levels in nursing home patients: Effects on sleep and activity rhythms. J Sleep Res. 2000;9:373–379. doi: 10.1046/j.1365-2869.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- 54.Smith KA, Schoen MW, Czeisler CA. Adaptation of human pineal melatonin suppression by recent photic history. J Clin Endocrinol Metab. 2004;89:3610–3614. doi: 10.1210/jc.2003-032100. [DOI] [PubMed] [Google Scholar]
- 55.Sutin EL, Dement WC, Heller HC, Kilduff TS. Light-induced gene expression in the suprachiasmatic nucleus of young and aging rats. Neurobiol Aging. 1993;14:441–446. doi: 10.1016/0197-4580(93)90102-h. [DOI] [PubMed] [Google Scholar]
- 56.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Someren EJW, Hagebeuk EEO, Lijzenga C, Scheltens P, de Rooij SEJA, Jonker C, Pot AM, Mirmiran M, Swaab DF. Circadian rest-activity rhythm disturbances in Alzheimer's disease. Biol Psychiatry. 1996;40:259–270. doi: 10.1016/0006-3223(95)00370-3. [DOI] [PubMed] [Google Scholar]
- 58.van Someren EJW, Kessler A, Mirmiran M, Swaab DF. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biol Psychiatry. 1997;41:955–963. doi: 10.1016/S0006-3223(97)89928-3. [DOI] [PubMed] [Google Scholar]
- 59.Weinert D. Age-dependent changes of the circadian system. Chronobiol Int. 2000;17:261–283. doi: 10.1081/cbi-100101048. [DOI] [PubMed] [Google Scholar]
- 60.Wright KP, Jr, Gronfier C, Duffy JF, Czeisler CA. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms. 2005;20:168–177. doi: 10.1177/0748730404274265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol. 1999;277:R1152–R1163. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 62.Zee PC, Rosenberg RS, Turek FW. Effects of aging on entrainment and rate of resynchronization of circadian locomotor activity. Am J Physiol. 1992;263:R1099–R1103. doi: 10.1152/ajpregu.1992.263.5.R1099. [DOI] [PubMed] [Google Scholar]
- 63.Zeitzer JM, Ayas NT, Wu AD, Czeisler CA, Brown R. Bilateral oculosympathetic paresis associated with loss of nocturnal melatonin secretion in patients with spinal cord injury. J Spinal Cord Med. 2005;28:55–59. doi: 10.1080/10790268.2005.11753798. [DOI] [PubMed] [Google Scholar]
- 64.Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y, Brainard GC, Zee PC, Pinto LH, Takahashi JS, Turek FW. Effects of aging on lens transmittance and retinal input to the suprachiasmatic nucleus in golden hamsters. Neurosci Lett. 1998;258:167–170. doi: 10.1016/s0304-3940(98)00887-8. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, Kornhauser JM, Zee PC, Mayo KE, Takahashi JS, Turek FW. Effects of aging on light-induced phase-shifting of circadian behavioral rhythms, FOS expression and CREB phosphorylation in the hamster suprachiasmatic nucleus. Neuroscience. 1996;70:951–961. doi: 10.1016/0306-4522(95)00408-4. [DOI] [PubMed] [Google Scholar]
- 67.Zulley J, Wever R, Aschoff J. The dependence of onset and duration of sleep on the circadian rhythm of rectal temperature. Pflügers Arch. 1981;391:314–318. doi: 10.1007/BF00581514. [DOI] [PubMed] [Google Scholar]


