Abstract
The goal of this work was to assess the effect of the controlled delivery of neurotrophin-3 (NT-3) from an affinity-based delivery system in fibrin scaffolds on regeneration following spinal cord injury (SCI). A heparin-based delivery system (HBDS) was used to immobilize NT-3 within fibrin scaffolds via non-covalent interactions. The fibrin scaffolds were implanted in lesions immediately after injury in an adult rat model of SCI (complete ablation of a 2 mm segment of the cord at T9). Delivery of NT-3 was controlled by an affinity-based delivery system that limits drug loss by diffusion and releases the drug via cell-mediated processes. Twelve weeks after injury and treatment, animals treated with fibrin scaffolds and NT-3, with or without the delivery system, did not show functional improvement over saline controls. Substantial cavitation at edges of the lesion was present, and while neuronal fibers were present inside the lesion, traced corticospinal and dorsal sensory tracts did not regenerate into the lesion. Therefore, while previous studies indicate that the controlled delivery of NT-3 from fibrin scaffolds may increase the short term regenerative response the continued degeneration of the cord, indicative of the severity of the injury, limits the long term regeneration stimulated by this treatment. Chronic or repeated treatments or a less severe injury model may prove useful in assessing the utility of controlled delivery systems for the treatment of spinal cord injury
Keywords: growth factor, nerve injury
Introduction
The delivery of neurotrophin-3 (NT-3) has the potential to improve functional regeneration after spinal cord injury (SCI). Implantation of fibroblasts genetically modified to overexpress NT-3 in a rat dorsal hemisection model of SCI increases functional recovery after 3 months, as measured by the number of footfalls in a grid task [1]. The implantation of a collagen scaffold containing NT-3 in the same injury model resulted in greater functional regeneration than control collagen scaffolds after 4 weeks [2]. In these studies, NT-3 treatment was given directly after the injury of the spinal cord. However, delayed treatment with NT-3 has also been shown to promote functional recovery. The implantation of fetal spinal cord and the infusion of NT-3 and BDNF with an osmotic mini-pump increased functional regeneration (as assessed by stair ascension and treadmill tests) when given 2-4 weeks after the complete transaction of the spinal cord [3]. When the transplantation of fibroblasts genetically modified to produce NT-3 was delayed 3 months after dorsal hemisection, slight functional improvement (as assessed by the Basso-Beattie-Bresnehan, or BBB, scale) were observed 3 months after treatment [4]. These studies indicate that NT-3 delivery can enhance regeneration acutely and chronically following SCI.
Previously, the effect of the controlled delivery of NT-3 from fibrin scaffolds containing a heparin-based delivery system (HBDS) on spinal cord regeneration after 9 days was tested [5] (Taylor, et al. submitted JCR). This delivery system consists of a synthetic bidomain peptide that contains a Factor XIIIa substrate that allows it to be cross-linked into fibrin scaffolds. The peptide also contains a heparin-binding domain that allows it to interact with heparin via electrostatic interactions. Heparin can then interact with strong heparin-binding growth factors, such as bFGF, or growth factors with basic domains, such as NGF or NT-3 [6, 7]. This series of interactions limits the diffusion of growth factors from fibrin scaffolds and allows the local delivery of growth factors based on cell-mediated degradation of the fibrin scaffold. In the previous chapter, the delivery of 1000 ng/mL of NT-3 with the HBDS was found to enhance neuronal fiber sprouting into the lesion to a greater extent than the same concentration of NT-3 without the HBDS. In addition, fibrin scaffolds were found to improve the regenerative environment by decreasing the formation of the glial scar at white matter borders of the lesion. However, functional regeneration as assessed by the BBB scale was not observed after 12 weeks.
In this study, we further investigate the effect of controlled delivery of NT-3 from fibrin scaffolds containing the HBDS on regeneration of neuronal tracts that are key to functional recovery. After SCI, the same suction ablation model of SCI, in which a 2-mm section of the thoracic level 9 cord was aspirated to create a complete gap between the rostral and caudal cord segments was employed in a 12-week study. Immediately after injury, three different treatment groups, either tris-buffered saline (TBS), fibrin containing 1000 ng/mL NT-3, or fibrin containing the HBDS and 1000 ng/mL NT-3 were implanted in the lesion site. In this study, behavioral analysis, anterograde tracing of the corticospinal tract, retrograde tracing of the ascending sensory dorsal columns, as well as immunohistochemistry were used to compare the three treatment groups.
Materials and Methods
All materials were purchased from Fisher Scientific (Pittsburgh, PA) unless otherwise noted.
Preparation of Fibrin Scaffolds
Fibrin scaffolds were made as described previously [8] by mixing the following components: human plasminogen-free fibrinogen (4 mg/mL, Sigma, St. Louis, MO), CaCl2 (2.5 mM), and thrombin (2 NIH units/mL, Sigma) in tris-buffered saline (TBS, 137 mM NaCl, 2.7 mM KCl, 33 mM Tris, pH 7.4). The bidomain peptide, denoted α2PI1-7-ATIII121-134, was synthesized by standard solid phase Fmoc chemistry (amino acids from Nova Biochem, San Diego, CA and solvents from Applied Biosystems, Foster City, CA) with the amino acid sequence dLNQEQVSPK(βA)FAKLAARLYRKA-NH2, where dL denotes dansyl leucine (Sigma), bold residues indicate the transglutaminase substrate (from the N-terminus of α2-plasmin inhibitor [9]), and the italicized residues indicate the heparin-binding sequence that was modified from antithrombin III [10]. In scaffolds containing the delivery system, α2PI1-7-ATIII121-134 peptide (0.23 mM) and heparin (6 μM, Sigma, sodium salt from porcine intestinal mucosa) were added to the fibrin polymerization mixture (total volume of 200 μl). This concentration of peptide corresponds to the cross-linking efficiency of 8 mole peptide per mole fibrinogen (approximately 25%) [8, 11]. Recombinant human neurotrophin-3 (NT-3) (varying concentrations, see below, Peprotech, Rocky Hill, NJ) was added in some groups to the polymerization mixture. Small beads of fibrin (1 mm diameter) were formed by ejecting the polymerization mixture from a 29-gauge needle onto a sterile surface. The beads were allowed to polymerize at room temperature for 30 min before implantation.
In vivo Studies - Rat Suction Ablation Spinal Cord Injury Model
All experimental procedures on animals complied with the Guide for the Care and Use of Laboratory Animals and were performed under the supervision of the Division of Comparative Medicine at Washington University. Adult, female Long Evan rats (250-275 g, Charles River, Wilmington, MA) were anesthetized with a cocktail of Ketaset (ketamine, 66 mg/kg, Wyeth, Madison, NJ) and Domitor (medetomidine, 0.44 mg/kg, Pfizer, New York, NY), and a dorsal laminectomy at T9 was performed to expose the spinal cord. The spinal column was immobilized by clamps attached to the spinous processes and a rigid frame. The dura was slit 1 mm longitudinally, just lateral to the midline. A 2 mm section of the spinal cord was aspirated using a suction tube (Baron, 3 French, Biomedical Research Instruments, Rockville, MD) attached to a vacuum pump, leaving a complete gap between the rostral and caudal spinal cord segments. A sterile fibrin scaffold with or without the delivery system and/or NT-3 was placed in the gap The three treatment groups received sterile TBS injection, fibrin containing NT-3 (1000 ng/mL), and fibrin containing the delivery system and NT-3 (1000 ng/mL) (total of 3 groups, 8-10 animals per group). The dural slit was covered with a small piece of fat, and the animal was removed from the clamps. The muscle and fascia were sutured, and the skin was closed with surgical staples (Fine Science Tools, Foster City, CA). Antisedan (0.3 mg, Pfizer) was given to reverse the effects of Domitor. Animals were given cefazolin (15 mg/kg, twice a day) for the duration of the 9-day study and for the first 14 days of the 12-week study as a prophylactic against urinary tract infections. Bladders were expressed manually twice a day; animals did not regain bladder function within 12 weeks.
The animals were euthanized 12 weeks post-operatively with an overdose of Euthasol (0.15 mL, Midlothian, VA). They were perfused transcardially with 150 mL 0.1 M phosphate buffer containing heparin (100,000 IU/L) followed by 400 mL 4% paraformaldehyde (Sigma) in 0.1 M phosphate buffer. The spinal cord was dissected and postfixed in the same fixative solution for 4 hr, followed by cryopreservation with 30% sucrose overnight. The spinal cord was embedded in Tissue-Tek OCT compound Mounting Media and sectioned with a cryostat. Twenty μm parasaggital sections, respectively, were mounted on SuperFrost Plus glass slides and processed for immunohistochemistry.
Immunohistochemistry
Sections were washed with PBS and permeabilized with 0.1% Triton-X 100 for 30 min. After thorough washes in PBS, the sections were blocked with 10% BSA and 3% normal goat serum (NGS, Sigma) for 5 min. The following primary antibodies were used: glial fibrilliary acidic protein (GFAP, rabbit polyclonal, recognizing astrocytes, 1:4, ImmunoStar, Hudson, WI), Sections were incubated with neuronal class III β-tubulin (Tuj1, mouse monoclonal, recognizing neurons, 1:500, Covance Research Products, Inc., Berkeley, CA). Antibodies were diluted in 2% NGS and incubated with the sections overnight at 4° C. The sections were washed with PBS and treated with goat anti-rabbit or goat anti-mouse secondary antibodies (Alexa Fluor 488 conjugated, 1:300, Molecular Probes) with 2% NGS for 1 hr at room temperature. The sections were washed and stained with Hoechst 33258 (1:1000, Molecular Probes) for 10 min. Sections were then washed and mounted with ProLong Antifade reagent.
Quantification of Cavitation and Neuronal Fiber Density - 12 week study
The lesion area in saggital sections near the central canal was imaged at 40 x magnification, and individual images were spliced together using Photoshop to yield a complete image of the lesion area and surrounding intact cord. Images were analyzed with Ia32 software. The borders of the lesion area were defined manually by referencing the astrocyte scar in adjacent GFAP-stained sections. The total lesion area was defined as the total number of pixels within those borders. Encroaching dorsal and ventral roots and cystic cavities were manually outlined, and total root and cavity areas were defined as the sum of all pixels within all outlined regions of each type. The regenerated tissue area was calculated as the total lesion area minus the area of roots and cavities. Within the regenerated tissue area, neuronal fiber area was defined as the total number of pixels in which the staining intensity was above an intensity threshold (set to identify only Tuj1-positive neuronal fibers). Neuronal fiber density was calculated in two ways. In one calculation, the neuronal fiber area was divided by the total lesion area. In the second calculation, the neuronal fiber area was divided by the regenerated tissue area.
Corticospinal Tract Tracing
In the 12-week study, the corticospinal tract was bilaterally anterogradely labeled with biotinylated dextran amine (BDA, MW 10,000, Molecular Probes, Eugene, OR) in 6 animals per treatment group at 10 weeks post injury. After being anesthetized as above, the rats were mounted in a stereotaxic frame, and an incision was made along the midline of the skull. The skin was retracted laterally. The skull was scraped of all periosteum, and a motorized trephine was used to drill a 3 mm diameter craniotomy over the sensorimotor area of both right and left cortices. A micromanipulator holding a pulled glass micropipette (about 50 μm diameter) was lowered, puncturing the dura over the brain. The pipette was advanced into the brain, over the course of 2 minutes, to a depth of 2 mm. 400 nL of 10% w/v BDA in TBS was injected through the pipette over 3 min using positive pressure from a 10 μl Hamilton syringe (Sigma). Injection was performed at 5 sites in sensorimotor cortex (anterior-posterior coordinates spanning from -0.5 mm to -3.0 mm from Bregma, lateral coordinates 2 mm on either side of Bregma). Coordinates were taken from Paxinos and Watson (Paxinos and Watson, 1998). Liquid alginate (2% weight/volume, gift of Dr. Daryl Kipke, Arizona State University) was used to fill in the cranial defect, and calcium chloride (5% weight/volume) was added to cross-link the alginate yielding a hydrogel that sealed the trephine hole. Dental cement (Henry Schein, Melville, NY) was used to create a hard cap over the site of craniotomy, and the overlying skin was sutured.
Every 5th sagittal section of the spinal cord was processed for BDA histology. Sections were washed with phosphate buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.4) and incubated with PBS with 0.1% Triton-X for 30 min. After PBS washes, sections were blocked with 10% bovine serum albumin (BSA) in PBS for 5 min. Sections were then incubated with AlexaFluor 488-conjugated streptavidin (Molecular Probes, 1:500 dilution, with 2% BSA) for 2 hours. Sections were then washed with PBS three times and mounted with Prolong Anti-Fade Reagent (Molecular Probes).
Ascending Sensory Tract Tracing
Four days before the endpoint of the 12-week study, ascending sensory tracts were labeled bilaterally by injection of cholera toxin B (CTB, List Biological) into right and left sciatic nerves in 6 animals per treatment group. After anesthetization, a 4-cm incision was made 0.5 cm dorsal and parallel to the femur. The sciatic nerve was exposed through a dorsal/gluteal splitting incision. 4 μL of a 1% solution of CTB was injected over 1 min into the nerve 2 mm proximal to its trifurcation. The pipette tip was left in place for 1 min before withdrawal. The wound was closed by suturing the muscle and skin layers.
Every 5th sagittal section of the spinal cord was processed for CTB immunohistochemistry. Sections were washed, incubated with PBS with 0.1% Triton X-100 for 30 minutes, blocked for 5 min with PBS with 10% BSA and 3% rabbit serum (RS), and incubated overnight with goat anti-choleragenoid (List Biological, 1:10000, with 2% RS). After thorough PBS washes, sections were incubated with rabbit anti-goat IgG conjugated AlexaFluor 555 (Molecular Probes, 1:300, with 2% RS), washed, and mounted with Prolong Anti-Fade Reagent.
Basso-Beattie-Bresnahan (BBB) Open Field Locomotor Testing
Hindlimb function was assessed weekly after injury using the BBB locomotor rating scale in an open field (2 x 4 feet) (Basso et al., 1995). Rats were observed for 4 min by a trained observer, and each hindlimb was scored individually from 0 (no observable movements) to 21 (normal gait).
Results
The ability of fibrin scaffolds containing the HBDS and NT-3 to influence regeneration was evaluated at 12 weeks after spinal cord injury by suction ablation at level T9.
Long Term 12 week Study - Immunohistological Assessment
Based on the short term study of NT-3 delivery from the HBDS, we concluded that, of the doses tested, a dose of 1000 ng/mL resulted in the greatest increase in neuronal fiber sprouting into the lesion area. To test the effect of the delivery system at a longer time point after implantation, regeneration was assessed at 12 weeks. Immediately after injury by suction ablation at T9, one of three treatments was administered - TBS, F-NT3(1000 ng/mL), or F-DS-NT3(1000 ng/mL). Because of the requirement for prolonged animal care and the requirement of larger group sizes for statistical comparison of BBB scores, a complete set of control treatments (including F-only, F-DS groups) was not performed. The TBS control was chosen because it provided a baseline for the extent of the injury without treatment; this was particularly important because this model of SCI has not been studied at 12 weeks. The F-NT3(1000 ng/mL) control was chosen in order to compare the effects of controlled and uncontrolled delivery of NT-3 (with or without the HBDS, respectively).
Neuronal fiber staining of each of the three treatment groups was performed to assess the effect of fibrin scaffold treatment on neuronal fiber sprouting (Figure 1). In all treatment groups, large cavities were present, especially at the rostral and caudal ends of the lesion, which were not observed 9 days after injury [5]. The length of the lesion in all treatment groups was observed to be about 5 mm. Some neuronal fiber sprouting was seen from the rostral and caudal borders with the intact cord, and neuronal fibers were present in the middle of the lesion (near the fibrin implantation site) in nearly every cord. To assess the gross anatomical aspects of the lesions and the neuronal fiber sprouting into the lesion area, the areas of the lesion, the cavities, the encroaching roots, and the total area staining positive for neuronal fibers (Tuj1 staining) were quantified. The area of each anatomical feature, including roots within the lesion area, cavities present inside the lesion area, the total lesion area, and the lesion area free of both roots and cavities are shown in Figure 2A. No differences were found between any of the groups.
Figure 1.
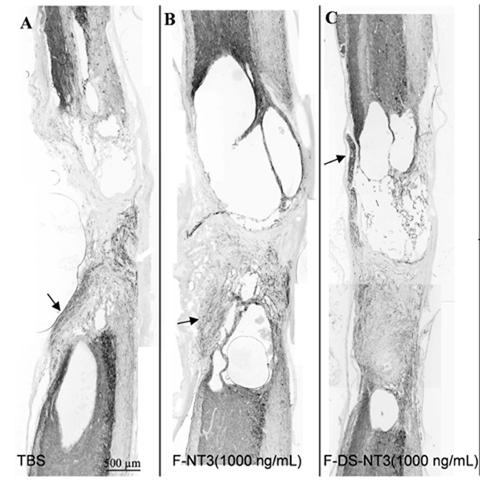
A-C. Neuronal fiber (Tuj1) staining of sagittal sections of lesion areas treated with (A) TBS, (B) F-NT3 (1000 ng/mL), and (C) F-DS-NT3(1000 ng/mL) after 12 weeks. Substantial cavitation and expansion of the lesion site occurred in all groups. Parasagittal sections, with rostral cord oriented toward top, ventral surface toward right. Encroaching dorsal and ventral roots (arrows).
Figure 2.

A. Areas of anatomical features within the lesion. No statistical difference between groups. Error bars represent standard error of the mean. B. Neuronal fiber density. Neuronal density is calculated as the area of Tuj1-positive fibers divided by either the area of the whole lesion (area=whole lesion. Neuronal density, calculated using the area of the lesion without roots and cavities, is enhanced in the F-DS-NT3 group over the TBS control group, but not over the F-NT3 group. * indicates p<0.05 vs. the TBS group. Error bars represent standard error of the mean.
Neuronal fiber density was calculated in two ways (Figure 2B). First, the total area of Tuj1-positive fibers was divided by the area of the whole lesion (including cavities and roots), as was done in the short term studies. There were no differences between groups using this analysis. In the second density calculation, the total area of Tuj1-positive fibers was divided by the area of the lesion minus the area of cavities and roots. This density calculation represents a more direct measure of the neuronal density in areas of interest in the lesion. With this calculation, the neuronal density was statistically greater in the F-DS-NT3 (1000 ng/mL) group (14%) vs. the TBS group (8%), but not vs. the F-NT3 (1000 ng/mL) group (13%). Therefore, NT-3 delivery may increase neuronal fiber density slightly, but HBDS-mediated delivery of NT-3 from fibrin scaffolds does not enhance neuronal fiber density compared to NT-3 delivery without the HBDS. For reference, the Tuj1 density of normal cords was measured to be ∼5% in white matter, ∼80% in gray matter, and 40% overall.
Corticospinal and Dorsal Sensory Tract Regeneration
The regeneration of the corticospinal tract was examined by tracing with biotin dextran amine (BDA). Descending fibers of the dorsal corticospinal tract (CST) lie at the base of the dorsal funiculus. In cross-sections of the thoracic cord above the lesion, CST axons traced using BDA appeared at the base of the dorsal funiculus (not shown). In Figure 3A, BDA-positive fibers traveled in the dorsal funiculus of the rostral cord, just dorsal to gray matter. As the fibers approached the lesion, the fibers were truncated or diverted to more dorsal white matter (Figure 3A, arrow). Fibers were infrequently seen in the spared dorsal matter encircling rostral lesion cavities (Figure 3A, arrowhead), but not inside the lesion (in all groups). No BDA-traced fibers were present in cross-sections of the lumbar cord below the lesion, indicating the complete lesion of the corticospinal tract and a failure to regenerate CST axons across the lesion.
Figure 3.
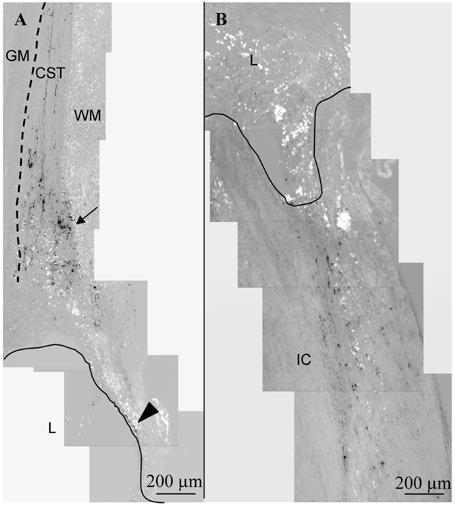
Corticospinal and ascending sensory tract tracing at 12 weeks. Parasagittal sections, with rostral cord oriented toward top, ventral surface toward left. A. Biotin dextran amine (BDA) anterograde tracing of the corticospinal tract (CST) in a F-DS-NT3 (1000 ng/mL)-treated cord. The CST extends from the rostral intact cord toward the lesion site. The CST is located just dorsal to the gray matter (GM, indicated by dotted line), in the most ventral part in the white matter (WM) of the dorsal funiculus. In all groups, as fibers approached the lesion (L, border indicated by line), the fibers were truncated or diverted to more dorsal white matter (arrow). Infrequently, CST-positive fibers were seen in the spared dorsal matter encircling the rostral lesion cavity (arrowhead). B. Tracing of ascending sensory neurons with cholera toxin B (CTB) in a n F-DS-NT3(1000 ng/mL) treated cord. In all groups, CTB staining ended abruptly at the lesion border with the intact cord (IC).
The regeneration of ascending sensory neurons of the dorsal column was examined by tracing with cholera toxin B (CTB). CTB staining was seen in cross sections below the lesion at an enlargement of the area at the medial base of the dorsal horn (gray matter). This area is Clarke’s nucleus (located from C8 to L2), where sensory axons from the periphery synapse on neurons whose axons form the ascending dorsal spinocerebellar tract. In sagittal sections, CTB-positive fibers existed both in the gray matter of the dorsal horn and in the white matter of the dorsal funiculus. As shown in Figure 3B, these extending fibers were interrupted by the lesion area and did not sprout into the lesion. Sprouting of CTB-positive fibers into the lesion area was not seen in any group. Additionally, cross-sections of the thoracic cord above the lesion did not show any CTB staining, indicating the complete lesion of ascending axons and their failure to regenerate beyond the lesion.
Assessment of Functional Regeneration
In order to assess the effect of treatment on functional ability, BBB open field motor testing [12] was performed weekly (Figure 4). Immediately after injury, animals had no hindlimb function (score of 0). One week after injury, animals had regained slight or extensive movement of two or three hindlimb joints (BBB score of 2-3). At 12 weeks, most animals had regained sweeping motion or plantar paw placement of their hindlimbs (BBB score of 8). Some animals had regained plantar placement of their hindpaw with weight support or occasional weight supported plantar steps (score of 9-10). However, no differences were observed between the treatment groups.
Figure 4.
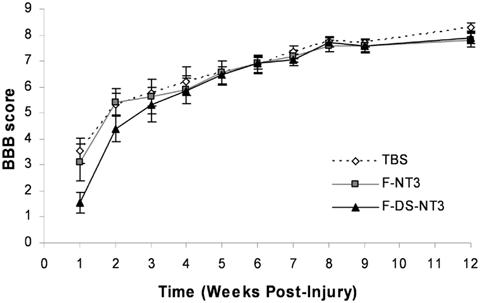
Functional assessment of SCI with BBB testing in 12-week study. One week after injury, slight or extensive movement of two or three joints was regained, as indicated by a BBB score of 2-3 (a score of 21 indicates unimpaired hindlimb movement). At 12 weeks, most animals had regained sweeping motion or plantar paw placement of hindlimbs (score of 8), some had regained plantar placement with weight-support or occasional weight supported plantar steps (score of 9-10). No statistical differences were seen between groups. Error bars represent standard error of the mean.
Discussion
The promising results of the short-term (9 day) in vivo study we performed previously (Taylor, et al, submitted JCR) led us to examine the ability of the HBDS to influence regeneration over a longer term (12 weeks). Because the F-DS-NT3(1000 ng/mL) treatment was the most effective in the short-term study, we focused our efforts on three treatment groups for the long-term study: TBS, F-NT3(1000 ng/mL), and F-DS-NT3(1000 ng/mL). Functional ability as assessed by the BBB scale did not improve with treatment; however, the BBB scale is not capable of reporting subtle changes in regeneration, particularly if regeneration of key neuronal tracts has not achieved functional connection with the their previous targets. Therefore, we conducted tracing studies of the corticospinal tract and the ascending dorsal sensory tract, two key tracts for control of motor and sensory function, respectively. Twelve weeks after injury, the lesion in all groups was much larger than at 9 days, largely due to formation of cystic cavities devoid of cells. These cystic cavities occurred in all treatment groups, particularly at the rostral and caudal borders of the lesion, but were not evident at 9 days post-injury. At 12 weeks, the lesion was approximately 5 mm in length (vs. 2-3 mm at 9 days). The difference in lesion size and cavity formation between 9 days post injury and 12 weeks post injury indicates that the lesion site continues to expand, possibly due to inflammatory processes, which agrees with other studies using severe injury models [13].
In all groups, some areas of the lesion (other than areas of encroaching roots) were populated by cells and contained neuronal fibers. Neuronal fiber density in this area was greater in the F-DS-NT3 group vs. the TBS group, but not versus the F-NT3 group. When neuronal fiber density was measured using the whole lesion area (including cavities and roots, as in short term studies), neuronal fiber density was not significantly different between groups. This indicates that fibrin scaffolds containing NT-3 may show slight enhancement over TBS controls, but this data does not show an effect of the controlled delivery of NT-3 from HBDS at 12 weeks.
While neuronal fibers were present inside the lesion, descending corticospinal tract axons and ascending dorsal sensory tract axons were not. The presence of these fibers at the rostral and caudal borders of the lesion, respectively, indicates that continued degeneration of the cord caused these axonal tracts to degenerate as well.
It is possible that the HBDS did not prolong the availability of NT-3 long enough to counteract the continuing degeneration of important axonal tracts and to promote their regeneration. Previous studies indicate that fibrin implanted after dorsal hemisection SCI is completely degraded by 14 days (Sakiyama and Schwab, unpublished results). If the lesion continued to expand significantly after 14 days, no NT-3 would have been available to aid the regenerative response.
Therefore, while controlled delivery of NT-3 from fibrin scaffolds enhanced neuronal fiber sprouting and decreased glial scar formation 9 days after injury, the efficacy of this treatment was limited at 12 weeks due to continued degeneration of spinal cord. Further investigation of regenerative potential of controlled delivery of NT-3 from fibrin scaffolds might call for concurrent treatment with drugs that limit secondary injury due to inflammation, the use of a less severe initial injury (such as a dorsal hemisection model or a contusion model). In particular the later model has greater similarity to injuries most commonly seen clinically and thus might give more insight into potential clinical benefit.
An alternative approach would be to perform the treatment in the chronic phase after the lesion site has stabilized. This would allow the implanted fibrin to have direct contact with the intact cord after the secondary injury had occurred. Many neurotrophin and cell transplantation therapies have show increased regeneration with chronic treatment (2 -8 weeks post injury) [3, 4, 14-18] and thus this approach may also prove beneficial for controlled delivery therapies as well. In addition, currently most patients do not have access to advance treatments during the chronic stage of injury, so chronic treatment would be more relevant for insight into potential clinic benefits for patients. Finally, a combination approach could be used with both acute and chronic administration of the fibrin system to gain the benefits of reduction of scar formation in the early phase of injury, as well as a prolonged time of interaction with a later chronic phase treatment..
Acknowledgements
The authors thank Amanda McKee for surgical assistance, Dr. Daniel Becker and Dr. Michael Howard for instruction on the spinal cord injury model, Dr. Lawrence Schramm, Dr. Frank Schottler, and Urvi Lee for helpful discussions, Daniel Hunter for analysis assistance, and Suellen Greco and Isabel Acevado for help with veterinary care. The authors acknowledge the Whitaker Foundation for graduate fellowship support (SJT) and the NIH-NINDS for funding (R01 NS51454).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grill R, Murai K, Blesch A, Gage FH, Tuszynski MH. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J Neurosci. 1997;17(14):5560–72. doi: 10.1523/JNEUROSCI.17-14-05560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houweling DA, Lankhorst AJ, Gispen WH, Bar PR, Joosten EA. Collagen containing neurotrophin-3 (NT-3) attracts regrowing injured corticospinal axons in the adult rat spinal cord and promotes partial functional recovery. Exp Neurol. 1998b;153(1):49–59. doi: 10.1006/exnr.1998.6867. [DOI] [PubMed] [Google Scholar]
- 3.Coumans JV, Lin TT, Dai HN, MacArthur L, McAtee M, Nash C, Bregman BS. Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J Neurosci. 2001;21(23):9334–44. doi: 10.1523/JNEUROSCI.21-23-09334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuszynski MH, Grill R, Jones LL, Brant A, Blesch A, Low K, Lacroix S, Lu P. NT-3 gene delivery elicits growth of chronically injured corticospinal axons and modestly improves functional deficits after chronic scar resection. Exp Neurol. 2003;181(1):47–56. doi: 10.1016/s0014-4886(02)00055-9. [DOI] [PubMed] [Google Scholar]
- 5.Taylor SJ, McDonald JW, 3rd, Sakiyama-Elbert SE. Controlled release of neurotrophin-3 from fibrin gels for spinal cord injury. J Control Release. 2004;98(2):281–94. doi: 10.1016/j.jconrel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000b;65(3):389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 7.Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J Control Release. 2000a;69(1):149–58. doi: 10.1016/s0168-3659(00)00296-0. [DOI] [PubMed] [Google Scholar]
- 8.Schense JC, Hubbell JA. Cross-linking exogenous bifunctional peptides into fibrin gels with factor XIIIa. Bioconjug Chem. 1999;10(1):75–81. doi: 10.1021/bc9800769. [DOI] [PubMed] [Google Scholar]
- 9.Ichinose A, Tamaki T, Aoki N. Factor XIII-mediated cross-linking of NH2-terminal peptide of alpha 2-plasmin inhibitor to fibrin. FEBS Lett. 1983;153(2):369–71. doi: 10.1016/0014-5793(83)80645-0. [DOI] [PubMed] [Google Scholar]
- 10.Tyler-Cross R, Sobel M, Marques D, Harris RB. Heparin binding domain peptides of antithrombin III: analysis by isothermal titration calorimetry and circular dichroism spectroscopy. Protein Sci. 1994;3(4):620–7. doi: 10.1002/pro.5560030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakiyama SE, Schense JC, Hubbell JA. Incorporation of heparin-binding peptides into fibrin gels enhances neurite extension: an example of designer matrices in tissue engineering. Faseb J. 1999;13(15):2214–24. doi: 10.1096/fasebj.13.15.2214. [DOI] [PubMed] [Google Scholar]
- 12.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12(1):1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 13.Patist CM, Mulder MB, Gautier SE, Maquet V, Jerome R, Oudega M. Freeze-dried poly(D,L-lactic acid) macroporous guidance scaffolds impregnated with brain-derived neurotrophic factor in the transected adult rat thoracic spinal cord. Biomaterials. 2004;25(9):1569–82. doi: 10.1016/s0142-9612(03)00503-9. [DOI] [PubMed] [Google Scholar]
- 14.von Meyenburg J, Brosamle C, Metz GA, Schwab ME. Regeneration and sprouting of chronically injured corticospinal tract fibers in adult rats promoted by NT-3 and the mAb IN-1, which neutralizes myelin-associated neurite growth inhibitors. Exp Neurol. 1998;154(2):583–94. doi: 10.1006/exnr.1998.6912. [DOI] [PubMed] [Google Scholar]
- 15.Ye JH, Houle JD. Treatment of the chronically injured spinal cord with neurotrophic factors can promote axonal regeneration from supraspinal neurons. Exp Neurol. 1997;143(1):70–81. doi: 10.1006/exnr.1996.6353. [DOI] [PubMed] [Google Scholar]
- 16.Tobias CA, Shumsky JS, Shibata M, Tuszynski MH, Fischer I, Tessler A, Murray M. Delayed grafting of BDNF and NT-3 producing fibroblasts into the injured spinal cord stimulates sprouting, partially rescues axotomized red nucleus neurons from loss and atrophy, and provides limited regeneration. Exp Neurol. 2003;184(1):97–113. doi: 10.1016/s0014-4886(03)00394-7. [DOI] [PubMed] [Google Scholar]
- 17.Grill RJ, Blesch A, Tuszynski MH. Robust growth of chronically injured spinal cord axons induced by grafts of genetically modified NGF-secreting cells. Exp Neurol. 1997;148(2):444–52. doi: 10.1006/exnr.1997.6704. [DOI] [PubMed] [Google Scholar]
- 18.Shumsky JS, Tobias CA, Tumolo M, Long WD, Giszter SF, Murray M. Delayed transplantation of fibroblasts genetically modified to secrete BDNF and NT-3 into a spinal cord injury site is associated with limited recovery of function. Exp Neurol. 2003;184(1):114–30. doi: 10.1016/s0014-4886(03)00398-4. [DOI] [PubMed] [Google Scholar]


