Abstract
Cyclin-dependent kinase 5 (Cdk5) is emerging as a neuronal protein kinase involved in multiple aspects of neurotransmission in both post- and presynaptic compartments. Within the reward/motor circuitry of the basal ganglia, Cdk5 regulates dopamine neurotransmission via phosphorylation of the postsynaptic signal transduction pathway integrator, DARPP-32 (dopamine- and cyclic AMP-regulated phosphoprotein, Mr 32 000). Cdk5 has also been implicated in regulating various steps in the presynaptic vesicle cycle. Here we report that Cdk5 phosphorylates tyrosine hydroxylase (TH), the key enzyme for synthesis of dopamine. Using phosphopeptide mapping, site-directed mutagenesis, and phosphorylation state-specific antibodies, the site was identified as Ser31, a previously defined extracellular signal-regulated kinases 1/2 (ERK1/2) site. The phosphorylation of Ser31 by Cdk5 versus ERK1/2 was investigated in intact mouse striatal tissue using a pharmacological approach. The results indicated that Cdk5 phosphorylates TH directly and also regulates ERK1/2-dependent phosphorylation of TH through the phosphorylation of mitogen-activated protein kinase kinase 1 (MEK1). Finally, phospho-Ser31 TH levels were increased in dopaminergic neurons of rats trained to chronically self-administer cocaine. These results demonstrate direct and indirect regulation of the phosphorylation state of a Cdk5/ERK1/2 site on TH and suggest a role for these pathways in the neuroadaptive changes associated with chronic cocaine exposure.
Keywords: Cdk5, cocaine, dopamine, MAPK, phosphorylation, tyrosine hydroxylase
Tyrosine hydroxylase (TH), the rate-limiting enzyme in catecholamine biosynthesis, was first shown to be regulated by protein phosphorylation 30 years ago (Morgenroth et al. 1975). It is a tetrameric 56 kDa mixed-function monooxygenase that is phosphorylated at four serine residues in the N-terminus (Fig. 1a) (Zigmond et al. 1989; Kumer and Vrana 1996; Fitzpatrick 1999). TH activity is enhanced upon phosphorylation at Ser40 by cAMP-dependent protein kinase (PKA) as well as cGMP-dependent protein kinase (PKG), protein kinase C (PKC) and Ca2+-calmodulin-dependent protein kinase II (CaMKII). Phosphorylation of TH at Ser19 by CaMKII (Campbell et al. 1986) has also been associated with increased TH activity (Haycock et al. 1998). Both Ser40 and Ser19 are phosphorylated by mitogen-activated protein kinase-activated protein (MAPKAP) kinase 2. TH is also phosphorylated by Cdk1 at Ser8 in cultured cells (Hall et al. 1991).
Fig. 1.
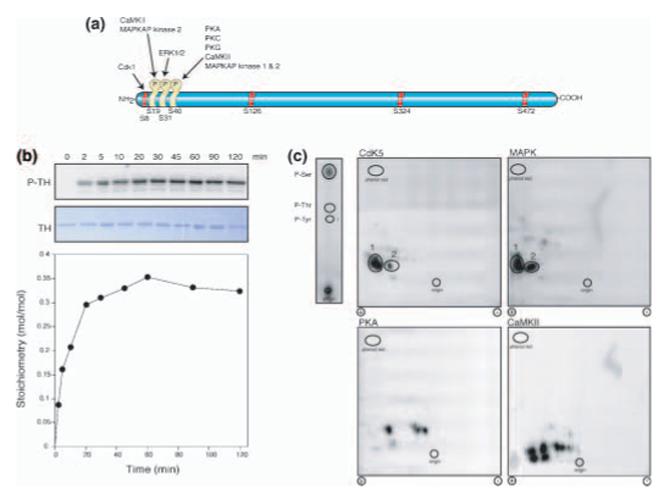
Phosphorylation of TH by Cdk5 and phosphoamino acid and phosphopeptide map analysis. (a) Schematic diagram of TH with sites of in situ phosphorylation indicated (yellow) along with protein kinases. Positions of all serine residues followed by proline are indicated in red and labeled, except for Ser31. (b) Time-course of in vitro phosphorylation reaction. Radiolabeled (P-TH) and Coomassie stained (TH) TH phosphorylated by Cdk5 and quantified stoichiometry (bottom). (c) Phosphoamino acid analysis of 32P-labeled TH phosphorylated by Cdk5 is shown, top left. The positions of comigrating phosphoserine, phosphothreonine and phosphotyrosine standards and the origin are indicated. Tryptic phosphopeptide maps of TH phosphorylated in vitro with the indicated protein kinases are shown in the four larger panels. The positions of comigrating phosphopeptides are indicated by numbers. The positions of the origins and phenol red markers are also indicated.
Phosphorylation of Ser31 by ERK1/2 has also been suggested to activate TH (Haycock et al. 1992). Interestingly, phosphorylation of Ser31 in humans may be affected through alternative splicing that results in differences in adjacent amino acid sequences (Sutherland et al. 1993b; Haycock 2002). Phospho-Ser31 TH levels have been shown to be increased by neuronal depolarization, phorbol esters, and nerve growth factor treatment in rat brain slices (Lindgren et al. 2002). Clear evidence also exists to suggest that ERK1/2 phosphorylates Ser31 in brain tissue (Lindgren et al. 2002). However, all basal Ser31 phosphorylation may not be accounted for by ERK1/2.
Cdk5 and its specific neuronal activators p35 and p39 are essential to neural progenitor cell migration and contribute to neuronal plasticity and associative learning during adulthood (Fischer et al. 2003). Cdk5 has been shown to regulate dopamine neurotransmission through phosphorylation of the striatal-enriched dopamine target DARPP-32 (Bibb et al. 1999), to increase in expression and activity in the striatum in response to chronic cocaine, and to mediate some of the effects of chronic cocaine (Bibb et al. 2001a; Bibb 2003). Furthermore, Cdk5 has been localized in both post- and presynaptic compartments (Norholm et al. 2003) and is emerging as an important regulator of presynaptic events such as synaptic vesicle endocytosis (Nguyen and Bibb 2003; Tan et al. 2003; Tomizawa et al. 2003; Lee et al. 2004; Sahin and Bibb 2004).
Here we show that Cdk5 also phosphorylates TH at Ser31 in vitro and in intact striatal tissue. Furthermore, we demonstrate that Cdk5 may modulate this site both directly and indirectly, through the regulation of the MEK1-ERK1/2 pathway. We also show that this site is modulated by chronic self-administration of cocaine in rats, thus raising the possibility that presynaptic regulation of Cdk5 in dopaminergic neuron terminals is an additional mechanism by which this protein kinase may mediate cocaine-induced neural and behavioral plasticity.
Materials and methods
Materials
All chemicals for solutions were purchased from Sigma, except where indicated. Restriction and DNA modifying enzymes were from New England Biolabs (Beverly, MA, USA). Cloning and expression vectors were from Novagen (Madison, WI, USA). Oligonucleotides were from Integrated DNA Technologies (Coralville, IA, USA). Heparin-Sepharose was from Amersham Pharmacia Biotech (Piscataway, NJ, USA) and glycerol from Invitrogen (Carlsbad, CA, USA). Protease inhibitors and ATP were from Roche (Indianapolis, IN, USA). MAPK, U0126 and roscovitine were from Calbiochem (San Diego, CA, USA). [γ-32P]ATP was from Perkin Elmer Life Sciences (Boston, MA, USA). Cdk5 and p35 or p25 were coexpressed and purified from Sf9 cultures using baculovirus vectors, as previously described (Saito et al. 2003). MEK1, in inactive and active form, CaMKII, and calmodulin were from Upstate Biotechnologies (Lake Placid, NY, USA). The catalytic subunit of PKA was purified from bovine heart as previously described (Kaczmarek et al. 1980). Affinity-purified polyclonal antibodies that selectively detect phospho-Ser19, phospho-Ser31, and phospho-Ser40 TH have previously been described (Salvatore et al. 2001).
Site-directed mutagenesis
Site-directed mutagenesis was performed using the Stratagene QuikChange kit, with the oligonucleotides 5′-ccc agc gcc ccg gcg cca cag ccc aag ggc-3′ for S8A, and 5′-gag gct gtc acg gcg caa ggt tca tcg g-3′ for S31A, the corresponding complementary strand oligos, and Pfu Turbo polymerase. Quick screening for mutated plasmids was carried out by restriction digest. Two plasmids were chosen after restriction screening for complete sequencing and were called pETYHS8A and pETYHS31A. Generation of the S40E TH expression plasmid was previously described (Daubner et al. 1992).
Bacterial expression and purification of TH
The Escherichia coli strain C41(DE3) (Avidis SA, France) was used for the expression of recombinant rat TH. After 6 h of incubation at 37°C, a 25-mL culture of Luria Bertani broth/ampicillin was used to inoculate 1-L cultures grown at 37°C until the A600 reached 0.8. The culture temperature was lowered to 30°C and expression was induced with 60 mg of isopropyl-β-d-thiogalactopyranoside (Inalco, Milan) for 15 h. TH was purified as described previously (Ellis et al. 2000); in short, the protocol consisted of lysis by sonication, centrifugation to remove cellular debris and unbroken cells, streptomycin sulfate precipitation to remove nucleic acids, ammonium sulfate fractionation and heparin-Sepharose chromatography. Proteins precipitating between 0 and 45% ammonium sulfate saturation were collected and subjected to chromatography; elution from the heparin column was via NaCl gradient. All steps were performed at 4°C in 50 mm HEPES, pH 7.0 to minimize pH changes upon freezing and thawing. Diethylenetriaminepentaacetic acid was kept in the buffers at a low level (75 μm) to prevent proteolysis without loss of enzyme-bound iron. Glycerol (10%, final concentration) was added to all buffers.
In vitro phosphorylation reactions
All reactions were carried out at 30°C in a final volume of at least 30 μL typically containing 10 μm TH, 200 μm ATP, and 0.2 Ci/mL [γ-32P] ATP. Cdk5 reactions were conducted in 30 mm 3-(N-morpholino)propanesulfonic acid (MOPS), pH 7.2 and 5 mm MgCl2. MAPK reactions were conducted in 50 mm Tris-HCl, pH 7.4, 10 mm MgCl2 and 20 mm EGTA; PKA reactions in 50 mm HEPES, pH 7.4, 1 mm EGTA, 10 mm magnesium acetate and 0.2 mg/mL bovine serum albumin (BSA); CaMKII reactions in 20 mm MOPS, pH 7.3, 25 mm β-glycerol phosphate, 1 mm sodium orthovanadate, 1 mm 1,4-dithiothreitol (DTT), 1 mm CaCl2, 10 mm MgCl2 and 8 μg/mL calmodulin. Time-course reactions (Figs 1a and 2a) were performed by removing 10-μL aliquots from the reaction solution at the designated time points and adding an equal volume of 5× sodium dodecyl sulfate (SDS)/DTT sample buffer. [32P]Phosphate incorporation was assessed by SDS-polyacrylaminde gel electrophoresis (PAGE) and PhosphorImager analysis of dried gels. Radioactive ATP was omitted from the time-course reactions analyzed by immunoblots (Fig. 3b), and 6 pmol of TH was loaded per lane for immunoblots.
Fig. 2.

Identification of Ser31 as the major site on TH phosphorylated by Cdk5 by site-directed mutagenesis and phosphorylation state-specific antibodies. (a) Time-course of in vitro phosphorylation of wild-type versus S31A TH by Cdk5. Coomassie stained and 32P-labeled TH are shown in the top two panels with quantification of stoichiometry in the lower panel. (b) Immunoblots of reaction mixtures from in vitro phosphorylation of TH with Cdk5, ERK1/2, and PKA stopped at the indicated time points. Detection of phospho-Ser31, phospho-Ser40 and total TH are shown in the top, middle, and bottom panels, respectively.
Fig. 3.
Intramolecular effects of phosphorylation at Ser40 and phospho-mimetic mutations at Ser40 and 19 on phosphorylation of TH by Cdk5. (a) Histogram depicting quantitation of V/K-values for phosphorylation of the indicated forms of TH by Cdk5. Error bars (SEM) were derived from two separate determinations for each reaction. (b) Quantitative kinetic values for phosphorylation of the indicated forms of TH by Cdk5. *nd, not determined as described in the text.
For assessment of the effects of phosphorylation-mimetic mutations at Ser19 and Ser40 and of TH phosphorylation at Ser40 upon steady-state kinetic constants for Cdk5, 10 μL reactions were conducted in 100 μm ATP, 10 mm MgCl2, 1 mm EDTA, 1 mm EGTA, 1 mm DTT, 5–10 μCi [γ32P]ATP and 2 μm Cdk5 in 25 mm HEPES, pH 7.4 at 30°C. The range of TH concentrations was 1.5 μm to 45 μm. Each 5 min reaction was spotted onto a 1.5 cm disk of phosphocellulose (P81) filter paper. Filters were washed three times for 5 min with 75 mm H3PO4 (250 mL per 10 filters), dehydrated by swirling in acetone, air-dried and subjected to scintillation counting. Nanomoles of phosphorylated TH versus [TH] were plotted using Kaleidagraph and the data were fit to the Michaelis–Menten equation (Synergy Software). TH phosphorylated at Ser40 was obtained by preparative phosphorylation of TH with PKA in a 5 mL reaction containing 80 μm TH, 560 μm ATP, 15 mm MgCl2 and 200 nm PKA in 37.5 mm HEPES, pH 7.0. After 1 h on ice the PKA concentration was increased to 400 nm and the ATP concentration was increased to 840 μm. The reaction was incubated on ice a further 2 h then loaded onto a monoQ column. The column was washed with 50 mm HEPES, 10% glycerol, pH 7.0, and TH was eluted from the column with a gradient of 0–0.5 m NaCl in the same buffer. The fractions containing TH (eluting at about 0.35 m NaCl) were pooled and dialyzed against 50 mm HEPES, 10% glyercol, pH 7.0. Preparative phosphorylation of TH at Ser40 was confirmed by further incubation of the sample with PKA and [γ-32P]ATP, as described (Sura et al. 2004). Samples that failed to incorporate radiolabeled ATP were deemed to be completely phosphorylated at Ser40.
For the phosphorylation of MEK1 by Cdk5p/25, either inactive recombinant rabbit MEK1 or active human MEK1 purified from E. coli by Upstate Biotechnology was used. Both had N-terminal glutathione-S-transferase and C-terminal 6× histidine tags. Activation was achieved via coexpression with B-raf and MEKK1. MEK1 at a final concentration of 1.2 μm was incubated with Cdk5/p25 for 60 min using the experimental conditions described above for the reactions with Cdk5. [32P]Phosphate incorporation was assessed by SDS–PAGE and PhosphorImager analysis.
Two-dimensional phosphopeptide map and phosphoamino acid analysis
Dry excised gel fragments containing 32P-labeled phospho-TH were rehydrated in 35% methanol, 15% acetic acid, washed overnight in 50% methanol, dehydrated by SpeedVac and rehydrated/incubated for 20 h at 37°C in 50 mm NH4HCO3, pH 8.0 containing 75 ng/mL trypsin. Supernatants containing the tryptic digestion products were lyophilized, washed once with H2O and once with electrophoresis buffer (10 : 1% acetic acid–pyridine), resuspended in electrophoresis buffer, pH 3.5, and 1/10 was set aside for phosphoamino acid analysis. The remainder was spotted onto microcrystalline cellulose TLC plates (Analtech) for electrophoresis in the first dimension. Separation in the second dimension was achieved by TLC in high pyridine buffer containing 25% butanol, 7.5% acetic acid, 30% H2O, 37.5% pyridine. A trace amount of phenol red was used as marker. Phosphoamino acid analysis was conducted with the aliquot set aside as has been previously described (Sahin et al. 2004).
Immunocytochemistry
Male C57BL/6 mice (8 weeks old) were deeply anesthetized with sodium pentobarbital [100 mg/kg body weight, intraperironeally (i.p.)], and perfused quickly through ascending aorta with 4% paraformaldehyde in sodium cacodylate buffer. After 1-h fixation in situ, brains were removed and placed in fixative overnight. Forty-micrometer thick coronal sections were cut using a vibrating blade microtome, VT1000S (Leica Microsystems, Nussloch, Germany). Sections containing the striatum were processed for immunocytochemistry using the free-floating method, as described previously (Fukuda et al. 1996). Sections were incubated for 7 days at 20°C with a mixture of primary antibodies against DARPP-32 (C25–5a mouse monoclonal antibody; 1 : 20 000 dilution) and TH (Chemicon AB1542 sheep polyclonal antibody; 1 : 500 dilution) in 50 mm Tris-buffered saline containing 0.1% Triton X-100 and 1% BSA, followed by the incubation with fluorescein isothiocyanate-conjugated donkey anti-sheep IgG (1 : 100; Jackson ImmunoResearch, West Grove, PA, USA) and Cy5-conjugated donkey anti-mouse IgG (1 : 200; Jackson ImmunoResearch). Sections were mounted in Vectashield (Vector Laboratories, Burlingame, CA, USA) and examined with a TCS confocal microscope system equipped with a krypton-argon ion laser mounted on a light microscope (DMRE) (Leica Microsystems). Images were acquired separately in each channel to eliminate the possibility of signal bleed-over from one channel to the other.
Regulation of phosphorylation of TH in striatal slices
C57BL/6 mouse (8 weeks old) striatal slices were acutely microdissected and treated as described (Nishi et al. 2002). Following equilibration in oxygenated Kreb's bicarbonate buffer, slices were incubated with roscovitine at indicated concentrations or 40 μm U0126 for 1 h. Where indicated, slices were simultaneously treated with both 40 μm U0126 and 50 μm roscovitine. For treatment of slices with KCl, slices were incubated for 1 h in absence or presence of 50 μm roscovitine, 40 μm U0126 or both for 1 h followed by treatment with 40 mm KCl (final concentration) for 5 min. Control slices were not treated with the inhibitors or KCl. After incubation, slices were transferred to microfuge tubes, snap-frozen in liquid nitrogen and homogenized by sonication in boiling 1% SDS, 50 mm NaF. Equal amounts of protein (as determined by BCA assay, Pierce) from homogenates were subjected to SDS-PAGE and electrophoretic transfer to nitrocellulose membranes. Immunoreactive proteins were detected and quantified using an Odyssey infrared imaging system (LI-COR). The levels of phospho-Ser31 TH were normalized to the relative levels of total TH protein, mean control values defined as 1.0, and data were analyzed statistically by the unpaired Student's t-test, or anova and Newman–Keuls test using Statview.
Cocaine self-administration studies
Rats were trained to self-administer intravenous cocaine (1 mg/kg/infusion) for 10 days as was previously described (Lu et al. 2003). Each earned reward was paired with a discrete tone-light cue. During the withdrawal phase (1–90 days), rats were housed in the animal facility and were not given any treatment with the exception of handling 2–3 times each week. Control groups of naive rats (n = 10; weight range 400–500 g) were treated identically. At the designated withdrawal day (1, 30 or 90 days), rats from the different groups were decapitated and the brains were removed, frozen in − 50°C isopentane, and stored at − 80°C. Subsequently, bilateral tissue punches of the ventral tegmental area (VTA) were obtained from approximately 1-mm thick coronal sections cut in a Reichert-Jung 2800 Frigocut E cryostat at − 20°C. Tissue was processed for SDS–PAGE and immunoblotting for phospho-Ser31 and total TH levels as previously described (Jedynak et al. 2002; Lu et al. 2003). Quantitated data were analyzed by one-way anova on all four groups before normalizing to naive control values. Significance was obtained and this was followed by post hoc analysis using Fisher's PLSD test. All animal experiments were approved by the institutional animal care and research advisory committees of UT Southwestern, Kurume University, and the National Institutes of Health.
Results
Characterization of the phosphorylation of TH by Cdk5 and identification of the site phosphorylated by Cdk5
Cdk1, ERK1/2 and Cdk5 are proline-directed protein kinases that have overlapping consensus substrate site specificities and sometimes share sites of phosphorylation (Bibb et al. 2001b). TH, which was previously demonstrated to be phosphorylated by both Cdk1 and ERK1/2, was tested as a substrate for Cdk5. Pure Cdk5/p25 (Fig. 1b) as well as Cdk5/p35 (data not shown) efficiently phosphorylated recombinant TH in vitro. TH was typically phosphorylated to a stoichiometry of 0.35–0.5 mol/mol. The reaction achieved maximal stoichiometry by 60 min.
Phosphoamino acid analysis indicated that Cdk5 phosphorylated TH at a serine residue (Fig. 1c). Cdk5 is closely related in structure to Cdk1, and the two kinases often share substrate sites in vitro (Bibb et al. 1999; Bibb et al. 2001b). Therefore, it was considered likely that Ser8 would be the substrate for Cdk5. Site-directed mutagenesis, a proven approach to the identification of phosphorylation sites (Bibb and da Cruz e Silva 1996), was used to generate a S8A form of TH. However, the S8A site-directed mutant form of TH was found to be an equally efficient substrate for Cdk5 in comparison to the wild-type form (data not shown), eliminating Ser8 as a possible site of phosphorylation. Sequence analysis indicated that there are five potential sites for serine phosphorylation by proline-directed kinases (Fig. 1a). One of these is the previously defined ERK1/2 site, Ser31. To further characterize the phosphorylation of TH by Cdk5 and to evaluate the possibility that the Cdk5-dependent phosphorylation also occurred at the ERK1/2 site, tryptic phosphopeptide maps of TH phosphorylated by PKA, CaMKII, MAPK and Cdk5 were generated (Fig. 1c). The maps generated from TH phosphorylated by the four kinases were somewhat similar, suggesting that all the sites of phosphorylation were located closely within the primary structure of the protein, and that the digestion with trypsin was incomplete. However, the maps of TH phosphorylated by Cdk5 and MAPK were the most similar, suggesting that they both phosphorylated the same site.
Because the site of TH phosphorylation by MAPK has been demonstrated to be Ser31, this residue was next targeted for site-directed mutagenesis. Mutation of Ser31 to Ala prevented Cdk5 from phosphorylating TH (Fig. 2a). To further evaluate the possibility that the site of phosphorylation by Cdk5 was Ser31, immunoblot analyses were conducted using the phosphorylation state-specific antibody to this site (Fig. 2b). The phospho-Ser31 antibody detected TH phosphorylated in vitro by either MAPK or Cdk5, but not TH that was phosphorylated by PKA. In contrast the phospho-Ser40 antibody detected TH phosphorylated by PKA (Fig. 2b). These results demonstrate that the site at which Cdk5 phosphorylates TH is Ser31.
Characterization of the intramolecular effects of phosphorylation of TH at different sites
It has been suggested that phosphorylation at Ser19 enhances phosphorylation at Ser40 (Bevilaqua et al. 2001). However, the effects of phosphorylation at one site on TH upon subsequent phosphorylations at different sites have not been further evaluated. Kinetic analyses were performed (Fig. 3) and the V/K-value, a steady-state kinetic parameter indicating substrate specificity, for phosphorylation of wild-type TH by Cdk5 was found to be 280 ± 8 mm/min. The V/K-value for the phosphorylation of TH by ERK2 has recently been reported to be 519 ± 6 mm/min (Royo et al. 2004). Furthermore, the V/K-value for human TH phosphorylated by PKA is also similar, having a value of 380 mm/min (Almas et al. 1992). The kinetic parameters for phosphorylation of phospho-Ser40 TH, the phospho-mimetic mutants S40E and S19E, and a form of TH in which both Ser19 was mutated to Glu and Ser40 was preparatively phosphorylated (S19E/P-S40) were also defined. The Vmax and KM values for dephospho-wild-type TH as a substrate of Cdk5 could not be determined due to the high concentration of substrate required for saturation, and a lower limit of 225 μm, or five times the maximum concentration of TH (45 μm) was set for the KM value. Mutation of serines at 19 or 40 to Glu, as well as PKA-dependent preparative phosphorylation at Ser40 did not affect the V/K-values. In contrast the V/K-value for S19E/P-S40 TH was 490 ± 50 mm/min, suggesting that the substrate specificity of Cdk5-dependent phosphorylation was increased by the presence of negative charges at both of these residues. In addition, phospho-Ser40, S40E and S19E/P-S40, all of which contain negative charges at amino acid 40, exhibited KM values at least fivefold lower than that of the dephospho-wild-type form of TH. Because of the increased binding efficiency of the phosphorylation reactions using these substrates, Vmax values were also defined for phospho-Ser40, S40E, and S19E/P-S40. Interestingly, the Vmax value for S19E/P-S40 TH (18.8 ± 4.5 min−1) was significantly higher than that for phospho-Ser40 (8.5 ± 1.6 min−1), indicating that the higher V/K-value for this form of TH was due to an increase in the catalytic efficiency with which Cdk5 phosphorylated TH when a negative charge was present at amino acid 19, in addition to phosphorylation at Ser40.
Phosphorylation of TH by Cdk5 in intact striatal tissue and interactions between ERK 1/2 and Cdk5 pathways
A number of studies indicate that Cdk5 is present both in the postsynaptic and presynaptic compartments (Tomizawa et al. 2002; Norholm et al. 2003). In captured laser-scanning, confocal, microscope images of mouse striatal tissue (Fig. 4a) that were costained for TH (red) and the striatal-specific postsynaptic Cdk5 substrate DARPP-32 (green), TH localized exclusively to the presynaptic compartment of striato-nigral dopaminergic synapses, and no colocalization with DARPP-32 was detected. To determine if Cdk5 catalyzes the phosphorylation of Ser31 TH within this compartment, striatal slices from mouse brain were treated with various concentrations of the selective Cdk5 inhibitor roscovitine. Homogenates from these slices were analyzed by immunoblotting for phospho-Ser31 and total TH levels (Fig. 4b). A low concentration of roscovitine (5 μm) caused a small (5.1 ± 0.1%) but significant increase in phosho-Ser31 levels. No effect was observed using 10 μm roscovitine while higher concentrations of 25 and 50 μm caused reductions to 88.2 ± 0.5% and 86.5 ± 0.3%, respectively, in basal phospho-Ser31 levels.
Fig. 4.

Location of TH in the presynaptic compartment and phosphorylation by Cdk5 in the striatum. (a) Mouse striatal tissue was immunostained for TH (red) and postsynaptic DARPP-32 (green), and fluorescent images were collected by confocal laser scanning microscopy. Immunoreactivity for DARPP-32 occurs in somata and dendrites of caudatoputamen. In contrast, TH immunoreactivity is localized to neuropili and neural processes originating from nigral tracts. No overlapping signal (yellow) was detected in any images examined. Scale bar represents 25 μm. (b) Cdk5 phosphorylates TH in striatum. Striatal slices were incubated for 60 min with the Cdk5 inhibitor, roscovitine at the indicated concentrations. Homogenates were subjected to SDS-PAGE and immunoblotted for detection of phospho-Ser31 and total TH. Quantitation of phopho-Ser31 from multiple experiments are shown, *p < 0.05, Student's unpaired t-test, n = 4
In an effort to determine the relative contributions of ERK1/2 and Cdk5 to the basal level of phosphorylation of Ser31 TH, striatal slices were treated with 50 μm roscovitine, 40 μm U0126 (a selective inhibitor of ERK1/2-activating kinase MEK1) or both (Fig. 5a,b). Roscovitine treatment caused a reduction in phospho-Ser31 to 86.1 ± 0.4% of basal levels. Treatment with U0126 caused a reduction to 58.1 ± 0.1%, similar to that previously reported using a different MAPK inhibitor, PD98059 (Lindgren et al. 2002). Treatment with both compounds caused a reduction in phospho-Ser31 levels to 32.5 ± 0.4%.
Fig. 5.

Cdk5 both directly phosphorylates Ser31 TH in striatum and regulates its phosphorylation at the same site by ERK1/2. (a) Effect of Cdk5 and MEK1 inhibitors on phospho-Ser31. Striatal slices were incubated for 60 min with roscovitine (Ros), the MEK1 inhibitor U0126 or both. Homogenates were immunoblotted for phospho-Ser31 or total TH. Quantitation of multiple experiments is shown. *p = 0.0069 versus control, **p < 0.001 versus control, †p < 0.02 versus roscovitine, §p < 0.04 versus U0126, Student's unpaired t-test, n = 4–6. (b) Effects of Cdk5 and MEK1 inhibitors on the phosphorylation states of ERK1 and 2. The same membrane used in (a) was also blotted for phospho-Thr202/phospho-Tyr204 levels and total ERK2. ERK1 was not detectable with the total ERK antibody. *p < 0.05 versus control, †p < 0.05 versus P-ERK1, Student's unpaired t-test, n = 4. (c) Phosphorylation of MEK1 by Cdk5 in vitro. Either inactive or active MEK1 was phosphorylated by Cdk5 or incubated in the absence of Cdk5 for 60 min and reaction mixtures were subjected to SDS–PAGE, the gel was Coomassie stained, and the radiographic image was derived using a PhosphorImager.
While these results indicate that both Cdk5 and MAPK phosphorylate TH in intact brain tissue, the responses to Cdk5 inhibition were less than that observed for other postsynaptic substrate sites phosphorylated exclusively by Cdk5 (Bibb et al. 1999; Bibb et al. 2001b). It has previously been suggested that MEK1 serves as a substrate of Cdk5 and that this phosphorylation inhibits the ability of MEK1 to activate ERK1/2 (Sharma et al. 2002). To evaluate the possibility that inhibiting Cdk5 caused a concomitant activation of ERK1/2 while reducing Cdk5-dependent phosphorylation of TH, the phosphorylation states of phospho-Thr202/phospho-Tyr-204 ERK1/2 were evaluated in the same homogenates (Fig. 5b). Inhibition of Cdk5 by roscovitine treatment of slices caused a 40 ± 10% increase in the phosphorylation state of ERK1 and a 102 ± 9% increase in the phosphorylation state of ERK2. In contrast, treatment of slices with U0126 caused 70 ± 4 and 89 ± 3% reductions in phospho-ERK1 and phospho-ERK2 levels, respectively. Slices treated with both roscovitine and U0126 exhibited levels of reduction similar to slices treated with U0126 alone.
To further evaluate the possibility that Cdk5 regulates ERK1/2 activity, in vitro phosphorylation reactions were conducted using MEK1 as a substrate (Fig. 5c). Cdk5/p25 was found to phosphorylate both inactive and active forms of recombinant MEK1. No phosphate incorporation was observed in the absence of active Cdk5. Taken together, these results indicate Cdk5 directly phosphorylates TH at Ser31 and also controls the phosphorylation of this site indirectly through the regulation of the MEK1-ERK1/2 pathway.
Stimuli such as membrane depolarization caused by treatment of slices with KCl have been shown to activate (increase phosphorylation) ERK1/2 via MEK-dependent phosphorylation and to increase Ser31 phosphorylation (Lindgren et al. 2002). Thus, the relative contributions of Cdk5 and ERK1/2 to the basal state of phosphorylation of TH at Ser31 and their respective roles in membrane depolarization-dependent responses were evaluated. Slices were treated with either roscovitine or U0126, or both followed by a 5-min treatment with KCl (Fig. 6). Treatment of slices with KCl alone caused a 27 ± 8% increase in the levels of phospho-Ser31 TH compared with untreated controls. As expected, this KCl treatment also elicited increases of 175 ± 8 and 43 ± 3% in the levels of phospho-ERK2 and phospho-ERK1, respectively. The KCl-induced increase in phospho-Ser31 was attenuated to 111 ± 6% in slices preincubated in the presence of roscovitine. The level of phospho-Ser31 in roscovitine-treated slices stimulated by KCl was significantly lower than that detected in slices treated with KCl alone and statistically indistinguishable from the basal level in control slices. Furthermore, phospho-Ser31 levels were reduced to 64 ± 3% of control levels in slices treated with U0126 prior to KCl stimulation. This reduction was significantly enhanced to 41 ± 2% in slices treated with both roscovitine and U0126 prior to KCl treatment. Roscovitine had virtually no additional effect on the phosphorylation levels of ERK1/2 compared to KCl treatment alone. Treatment of the slices with U0126 or U0126 plus roscovitine prior to incubation with KCl, lowered phospho-ERK1/2 to almost undetectable levels. These results confirm that KCl-induced depolarization causes an increase in phospho-Ser31 levels. This increase correlated with a large increase in phospho-ERK1/2 levels. While roscovitine reduced the response to stimulation by KCl, this reduction was approximately the same as the reduction in basal levels produced by roscovitine. Therefore, it is unlikely that Cdk5 mediates any of the increase in phospho-Ser31 levels in response KCl-induced depolarization.
Fig. 6.

The effect of KCl-induced membrane depolarization on TH Ser31 and ERK1/2 phosphorylation. Striatal slices were treated with roscovitine and/or U0126, followed by treatment with 40 mm KCl for 5 min. (a) Striatal slice homogenates were immunoblotted for phospho-Ser31 and total TH. Quantitation of multiple experiments is shown. *p < 0.001 versus control, †p < 0.05, ††p < 0.01 versus KCl, §p < 0.001 versus KCl + U0126, anova and Newman-Keuls test, n = 4. (b) Homogenates were immunoblotted for phospho-ERK1/2 and total ERK2. The ERK2 protein band appears as a doublet in two of the lanes in the total ERK2 blot, where ERK2 was highly activated by KCl. *p < 0.001 versus controls, anova and Newman-Keuls test, n = 4.
Effect of cocaine self-administration on phospho-Ser31 levels in rat dopaminergic neurons
Cdk5 levels have been shown to increase in response to chronic exposure to cocaine in rats, and it has been suggested that Cdk5 mediates some of the behavioral effects of chronic cocaine through regulation of postsynaptic signaling pathways in dopaminoceptive neurons (Bibb et al. 2001a). The findings reported here raise the interesting possibility that Cdk5 may also be involved in the presynaptic regulation of TH associated with animal paradigms of cocaine addiction. Hope and colleagues have shown Cdk5 levels are increased in dopaminergic neurons of rats trained to chronically self-administer cocaine for 10 days (Lu et al. 2003). The level of phospho-Ser31 TH was evaluated in VTA tissue lysates from these animals at 1, 30 and 90 days after withdrawal following cocaine self-administration (Fig. 7). Phospho-Ser31 levels were increased 90 ± 40% following 1 day of withdrawal. A trend toward increased levels (140 ± 20%) was observed, although not significant statistically, after 30 days withdrawal. Phospho-Ser31 returned to levels indistinguishable from that of naive control animals by 90 days. These results suggest that phospho-Ser31 TH levels are modulated by chronic self-administration of cocaine, and that the increase in levels may persist for substantial periods of time following cocaine exposure. Because the increase in phospho-Ser31 levels corresponds to the previously observed increase in Cdk5 levels, it is possible that this effect is the result of Cdk5-dependent phosphorylation of TH. In support of this, no effect of cocaine self-administration was observed for phospho- or total ERK1/2 levels in the same lysates, as determined by immunoblots (data not shown).
Fig. 7.
Effect of cocaine self-administration on phospho-Ser31 TH levels following withdrawal. Mean (± SEM) percentage change from naïve rats at each withdrawal period. *p = 0.04, one-way anova (n = 6–8).
Discussion
We report here that Cdk5 efficiently phosphorylates TH at Ser31. It has been shown that while ERK1/2 predominantly phosphorylates Ser31, it also is capable of contributing to the phosphorylation of Ser8, another proline-directed protein kinase site in vitro (Royo et al. 2004). Cdk5 would appear to specifically phosphorylate only Ser31, as mutation of Ser8 to Ala had no effect on Cdk5-dependent phosphorylation while mutation of Ser31 to Ala effectively eliminated phosphate incorporation.
Phosphorylation of TH by Cdk5 occurs with similar kinetic parameters to the phosphorylation of this site by ERK1/2 as well as the phosphorylation of Ser40 by PKA in vitro. Although TH was not phosphorylated stoichiometrically by Cdk5, similar results have been observed for ERK1/2 (Haycock et al. 1992; Sutherland et al. 1993a; Halloran and Vulliet 1994), as well as CaMKII and PKC (Funakoshi et al. 1991). In contrast PKA reactions generally are capable of achieving a stoichiometry of 1 mol/mol. While TH was readily phosphorylated by Cdk5 in vitro, the reaction did not achieve a maximal rate at concentrations of dephospho-wild-type TH well above the physiological range. Similar results have also been observed with ERK2 (Royo et al. 2004) and MAPKAP2 (Sutherland et al. 1993a). Interestingly, the addition of a negative charge, either via PKA-dependent preparative phosphorylation or site-directed phospho-mimetic mutation enhanced the binding efficiency of the Cdk5-dependent reaction such that saturation was achieved within the physiological range of TH concentration. Furthermore, while mutation of Ser19, to Glu had no effect on binding efficiency, PKA-dependent preparative phosphorylation of S19E TH caused an increase in the catalytic efficiency of the Cdk5-dependent reaction. Thus it is possible that phosphorylation at Ser40 by the first messenger-responsive protein kinases (e.g. PKA, PKG, PKC and CaMKII) may convert TH into a better substrate for Cdk5 in dopaminergic neurons. Moreover, an additional phosphorylation at Ser19 by CaMKII or MAPKAP kinase 2 may serve to further increase the rate at which Cdk5 converts TH into its triply phosphorylated form.
Inhibition of MEK1 by U0126 effectively eliminated phosphorylation of ERK1 and 2, while phospho-Ser31 TH was only reduced to half the level observed in untreated slices. If ERK1/2 and Cdk5 were the only two protein kinases targeting this site, it might be expected that inhibition of Cdk5 by roscovitine would result in a 50% reduction in phospho-Ser31. However, Cdk5 inhibition produced a much smaller reduction and, moreover, phospho-Ser31 could still be detected after treatment with both Cdk5 and MEK1 inhibitors. These observations could result from residual phospho-Ser31 that was not dephosphorylated during the time-course of the experiment or from inefficient inhibition of either or both kinases. It is also feasible that another, as yet unidentified, proline-directed kinase phosphorylates Ser31.
If Cdk5 and ERK1/2 are the only kinases involved, another possible explanation for the smaller effect of roscovitine alone is that cross-talk occurs between Cdk5 and ERK1/2 pathways. It has been suggested that Cdk5 phosphorylates MEK1, thereby inhibiting ERK1/2 activation (Sharma et al. 2002). In support of this mechanism, treatment of striatal tissue with roscovitine not only reduced phospho-Ser31 levels less than expected, but also activated ERK1/2. In further support of this pathway, both inactive and active MEK1 were found to serve as substrates for Cdk5 derived from eukaryotic cells. This result would appear to be in contrast to the earlier report that suggested that inactivated MEK1 did not serve as a substrate for recombinant Cdk5 derived from bacteria (Sharma et al. 2002).
Various methods of cell stimulation such as KCl-mediated membrane depolarization have previously been shown to increase phospho-Ser31 levels, and this has been attributed to the corresponding activation of ERK1/2 (Lindgren et al. 2002). Cdk5 phosphorylates dephosphins, which are involved in synaptic vesicle recycling and are dephosphorylated in response to membrane depolarization (Nguyen and Bibb 2003). However, Cdk5 has not been reported to be activated by membrane depolarization. Indeed, comparison of the effects of roscovitine on basal versus KCl-stimulated levels of phospho-Ser31 TH does not suggest that Cdk5 is activated to phosphorylate TH in response to membrane depolarization. Taken together, these neuropharmacological and biochemical studies suggest a complex regulatory scheme in which Cdk5 contributes to phospho-Ser31 levels through direct phosphorylation and indirectly via regulation of the MEK-ERK1/2 pathway.
Cocaine-induced regulation of TH has been well documented both in VTA dopamine neurons and in dopamine terminals in the nucleus accumbens (Trulson and Ulissey 1987; Trulson et al. 1987; Beitner-Johnson and Nestler 1991; Sorg et al. 1993; Vrana et al. 1993; Masserano et al. 1996; Todtenkopf and Stellar 2000; Todtenkopf et al. 2000). The effects of cocaine on TH activity would appear to depend upon the administration regimen and withdrawal time. Acute cocaine inhibits dopamine synthesis in a dose-dependent manner via a putative negative feedback mechanism. Furthermore, acute dosage of cocaine (up to 30 mg/kg) was shown to cause a decrease in phospho-Ser19, 31 and 40 levels in the nucleus accumbens that corresponded with reduced l-DOPA production (Jedynak et al. 2002). Here we show that chronic cocaine self-administration causes an increase in phospho-Ser31 levels in dopaminergic neurons which corresponded to an increase in Cdk5 levels detected in the same tissue samples (Lu et al. 2003). The novel signaling events reported here provide additional mechanisms by which Cdk5 may modulate dopamine neurotransmission, and may have important implications to drug addiction and other aspects of dopamine neurobiology.
Acknowledgements
We would like to gratefully acknowledge the assistance of Jernej Ule, who conducted the first Cdk5/TH reaction, Bogachan Sahin for help with some of the experiments, Dr Takaichi Fukuda for use of the confocal microscope, Dr Yavin Shaham for the use of his laboratory for drug self-administration, Atsuko Horiuchi for PKA and Paul Greengard for providing the antibody for DARPP-32. This work was supported by funding from the National Institute of Drug Abuse, the National Alliance for Research on Schizophrenia and Depression, the Ella McFadden Charitable Trust Fund at the Southwestern Medical Foundation (JAB), NIH Grant GM 47291 (PFF), NIH Grant NS25134 (JWH) and a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (AN).
Abbreviations used
- BSA
bovine serum albumin
- CaMKII
Ca2+-calmodulin-dependent protein kinase II
- Cdk5
cyclin-dependent protein kinase 5
- DARPP-32
dopamine-and cyclic AMP-regulated phosphoprotein, relative molecular mass 32 000
- DTT
1,4-dithiothreitol
- ERK1/2
extracellular signal-regulated kinases 1/2
- MAPK
mitogen-activated protein kinase
- MAPKAP kinase 1 and 2
mitogen-activated protein kinase-activated protein kinases 1 and 2
- MEK1
mitogen-activated protein kinase kinase 1
- MEKK1
MAP/ERK kinase kinase 1
- MOPS
3-[N-morpholino]propanesulfonic acid
- PAGE
polyacrylamide gel electrophoresis
- PKA
cAMP-dependent protein kinase
- PKC
protein kinase C
- PKG
cGMP-dependent protein kinase
- SDS
sodium dodecyl sulfate
- TH
tyrosine hydroxylase
- VTA
ventral tegmental area
References
- Almas B, Le Bourdelles B, Flatmark T, Mallet J, Haavik J. Regulation of recombinant human tyrosine hydroxylase isozymes by catecholamine binding and phosphorylation. Structure/activity studies and mechanistic implications. Eur. J. Biochem. 1992;209:249–255. doi: 10.1111/j.1432-1033.1992.tb17283.x. [DOI] [PubMed] [Google Scholar]
- Beitner-Johnson D, Nestler EJ. Morphine and cocaine exert common chronic actions on tyrosine hydroxylase in dopaminergic brain reward regions. J. Neurochem. 1991;57:344–347. doi: 10.1111/j.1471-4159.1991.tb02133.x. [DOI] [PubMed] [Google Scholar]
- Bevilaqua LRM, Graham ME, Dunkley PR, von Nagy-Felsobuki EI, Dickson PW. Phosphorylation of Ser19 alters the conformation of tyrosine hydroxylase to increase the rate of phosphorylation of ser40. J. Biol. Chem. 2001;276:40 411–40 416. doi: 10.1074/jbc.M105280200. [DOI] [PubMed] [Google Scholar]
- Bibb JA. Role of cdk5 in neuronal signaling, plasticity, and drug abuse. Neurosignals. 2003;12:191–199. doi: 10.1159/000074620. [DOI] [PubMed] [Google Scholar]
- Bibb JA, da Cruz e Silva EF. Identification of posttranslational modification sites by site-directed mutagenesis. In: Hemmings HCJ, editor. Neuromethods. Vol. 30. Humana Press Inc.; Totowa, N.J.: 1996. pp. 275–307. Regulatory Protein Modification: Techniques and Protocols. [Google Scholar]
- Bibb JA, Snyder GL, Nishi A, et al. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signaling in neurons. Nature. 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi AGS, Yan Z, Sagawa ZK, Nairn AC, Nestler EJ, Greengard P. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001a;410:376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Nishi A, O'Callaghan JP, et al. Phosphorylation of protein phosphatase inhibitor-1 by Cdk5. J. Biol. Chem. 2001b;276:14 490–14 497. doi: 10.1074/jbc.M007197200. [DOI] [PubMed] [Google Scholar]
- Campbell DG, Hardie DG, Vulliet PR. Identification of the four phosphorylation sites in the N-terminal region of tyrosine hydroxylase. J. Biol. Chem. 1986;261:10 489–10 492. [PubMed] [Google Scholar]
- Daubner SC, Lauriano C, Haycock JW, Fitzpatrick PF. Site-directed mutagenesis of serine 40 of rat tyrosine hydroxylase. J. Biol. Chem. 1992;267:12 639–12 646. [PubMed] [Google Scholar]
- Ellis HR, Daubner SC, Fitzpatrick PF. Mutation of serine 395 of tyrosine hydroxylase decouples oxygen–oxygen bond cleavage and tyrosine hydroxylation. Biochemistry. 2000;39:4174–4181. doi: 10.1021/bi9928546. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Spiess J, Radulovic J. Cdk5: a novel role in learning and memory. Neurosignals. 2003;12:200–208. doi: 10.1159/000074621. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick PF. Tetrahydropterin-dependent amino acid hydroxylases. Annu. Rev. Biochem. 1999;68:355–381. doi: 10.1146/annurev.biochem.68.1.355. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Aika Y, Heizmann CW, Kosaka T. Dense GABAergic input on somata of parvalbumin-immunoreactive GABAergic neurons in the hippocampus of the mouse. Neurosci. Res. 1996;26:181–194. doi: 10.1016/s0168-0102(96)01102-9. [DOI] [PubMed] [Google Scholar]
- Funakoshi H, Okuno S, Fujisawa H. Different effects on activity caused by phosphorylation of tyrosine hydroxylase at serine 40 by three multifunctional protein kinases. J. Biol. Chem. 1991;266:15 614–15 620. [PubMed] [Google Scholar]
- Hall FL, Braun RK, Mihara K, Fung YK, Berndt N, Carbonaro-Hall DA, Vulliet PR. Characterization of the cytoplasmic proline-directed protein kinase in proliferative cells and tissues as a heterodimer comprised of p34cdc2 and p58cyclin A. J. Biol. Chem. 1991;266:17 430–17 440. [PubMed] [Google Scholar]
- Halloran SM, Vulliet PR. Microtubule-associated protein kinase-2 phosphorylates and activates tyrosine hydroxylase following depolarization of bovine adrenal chromaffin cells. J. Biol. Chem. 1994;269:30 960–30 965. [PubMed] [Google Scholar]
- Haycock JW. Species differences in the expression of multiple tyrosine hydroxylase protein isoforms. J. Neurochem. 2002;81:947–953. doi: 10.1046/j.1471-4159.2002.00881.x. [DOI] [PubMed] [Google Scholar]
- Haycock JW, Wakade AR. Activation and multiple-site phosphorylation of tyrosine hydroxylase in perfused rat adrenal glands. J. Neurochem. 1992;58:57–64. doi: 10.1111/j.1471-4159.1992.tb09276.x. [DOI] [PubMed] [Google Scholar]
- Haycock JW, Ahn NG, Cobb MH, Krebs EG. ERK1 and ERK2, two microtubule-associated protein 2 kinases, mediate the phosphorylation of tyrosine hydroxylase at serine-31 in situ. Proc. Natl Acad. Sci. USA. 1992;89:2365–2369. doi: 10.1073/pnas.89.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock JW, Lew JY, Garcia-Espana A, Lee KY, Harada K, Meller E, Goldstein M. Role of serine-19 phosphorylation in regulating tyrosine hydroxylase studied with site- and phosphospecific antibodies and site-directed mutagenesis. J. Neurochem. 1998;71:1670–1675. doi: 10.1046/j.1471-4159.1998.71041670.x. [DOI] [PubMed] [Google Scholar]
- Jedynak JP, Ali SF, Haycock JW, Hope BT. Acute administration of cocaine regulates the phosphorylation of serine-19–31 and -40 in tyrosine hydroxylase. J. Neurochem. 2002;82:382–388. doi: 10.1046/j.1471-4159.2002.00982.x. [DOI] [PubMed] [Google Scholar]
- Kaczmarek LK, Jennings KR, Strumwasser F, Nairn AC, Walter U, Wilson FD, Greengard P. Microinjection of catalytic subunit of cyclic AMP-dependent protein kinase enhances calcium action potentials of bag cell neurons in cell culture. Proc. Natl Acad. Sci. USA. 1980;77:7487–7491. doi: 10.1073/pnas.77.12.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumer SC, Vrana KE. Intricate regulation of tyrosine hydroxylase activity and gene expression. J. Neurochem. 1996;67:443–462. doi: 10.1046/j.1471-4159.1996.67020443.x. [DOI] [PubMed] [Google Scholar]
- Lee SY, Wenk MR, Kim Y, Nairn AC, De Camilli P. Regulation of synaptojanin 1 by cyclin-dependent kinase 5 at synapses. Proc. Natl Acad. Sci. USA. 2004;101:546–551. doi: 10.1073/pnas.0307813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren N, Goiny M, Herrera-Marschitz M, Haycock JW, Hökfelt T, Fisone G. Activation of extracellular signal-regulated kinases 1 and 2 by depolarization stimulates tyrosine hydroxylase phosphorylation and dopamine synthesis in rat brain. Eur. J. Neurosci. 2002;15:769–773. doi: 10.1046/j.1460-9568.2002.01901.x. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Shaham Y, Hope BT. Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J. Neurochem. 2003;85:1604–1613. doi: 10.1046/j.1471-4159.2003.01824.x. [DOI] [PubMed] [Google Scholar]
- Masserano JM, Baker I, Natsukari N, Wyatt RJ. Chronic cocaine administration increases tyrosine hydroxylase activity in the ventral tegmental area through glutaminergic- and dopaminergic D2-receptor mechanisms. Neurosci. Lett. 1996;217:73–76. [PubMed] [Google Scholar]
- Morgenroth VH, Hegstrand LR, Roth RH, Greengard P. Evidence for involvement of protein kinase in the activation by adenosine 3′:5′-monophosphate of brain tyrosine 3-monooxygenase. J. Biol. Chem. 1975;250:1946–1948. [PubMed] [Google Scholar]
- Nguyen C, Bibb JA. Cdk5 and the mystery of synaptic vesicle endocytosis. J. Cell Biol. 2003;23:1189–1197. doi: 10.1083/jcb.200310038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Bibb JA, Matsuyama S, Hamada M, Higashi H, Nairn AC, Greengard P. Regulation of DARPP-32 dephosphorylation at PKA- and Cdk5-sites by NMDA and AMPA receptors: distinct roles of calcineurin and protein phosphatase-2A. J. Neurochem. 2002;81:832–841. doi: 10.1046/j.1471-4159.2002.00876.x. [DOI] [PubMed] [Google Scholar]
- Norholm S, Bibb JA, Ouimet CC, Nestler EJ, Taylor JR, Greengard P. Cocaine-induced proliferation of dendritic spines is dependent on the activity of cyclin-dependent kinase 5. Neuroscience. 2003;116:19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royo M, Dabner SC, Fitzpatrick PF. Specificity of MAP kinase ERK2 for phosphorylation of tyrosine hydroxylase. Arch. Biochem. Biophys. 2004;423:247–252. doi: 10.1016/j.abb.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Sahin B, Bibb JA. Protein kinases talk to lipid phosphatases at the synapse. Proc. Natl Acad. Sci. USA. 2004;101:112–113. doi: 10.1073/pnas.0308374101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin B, Kansy JW, Nairn AC, Spychala J, Ealich SE, Fienberg AA, Greene RW, Bibb JA. Molecular characterization of mouse adenosine kinase and its evaluation as a target for protein phosphorylation. Eur. J. Biochem. 2004;271:3547–3555. doi: 10.1111/j.1432-1033.2004.04291.x. [DOI] [PubMed] [Google Scholar]
- Saito T, Onuki R, Kusakawa G, Ishiguro K, Bibb JA, Kishimoto T, Hisanaga S. Developmental regulation of the proteolysis of the p35 cyclin-dependent kinase 5 activator by phosphorylation. J. Neurosci. 2003;23:1189–1197. doi: 10.1523/JNEUROSCI.23-04-01189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Waymire JC, Haycock JW. Depolarization-stimulated catecholamine biosynthesis: involvement of protein kinases and tyrosine hydroxylase phosphorylation sites in situ. J. Neurochem. 2001;79:349–360. doi: 10.1046/j.1471-4159.2001.00593.x. [DOI] [PubMed] [Google Scholar]
- Sharma P, Veeranna Sharma M, Amin ND, Sihag RK, Grant P, Ahn NG, Kulkarni AB, Pant HC. Phosphorylation of MEK1 by cdk5/p35 down-regulates the mitogen-activated protein kinase pathway. J. Biol. Chem. 2002;277:528–534. doi: 10.1074/jbc.M109324200. [DOI] [PubMed] [Google Scholar]
- Sorg BA, Chen S-Y, Kalivas PW. Time course of tyrosine hydroxylase expression after behavioral sensitization to cocaine. J. Pharmacol. Exp. Ther. 1993;266:424–430. [PubMed] [Google Scholar]
- Sura G, Daubner SC, Fitzpatrick PF. Effects of phosphorylation by protein kinase A on binding of catecholamines to the human tyrosine hydroxylase isoforms. J. Neurochem. 2004;90:970–978. doi: 10.1111/j.1471-4159.2004.02566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland C, Alterio J, Campbell DG, Le Bourdelles B, Mallet J, Haavik J, Cohen P. Phosphorylation and activation of human tyrosine hydroxylase in vitro by mitogen-activated protein (MAP) kinase and MAP-kinase-activated kinases 1 and 2. Eur. J. Biochem. 1993a;217:715–722. doi: 10.1111/j.1432-1033.1993.tb18297.x. [DOI] [PubMed] [Google Scholar]
- Tan TC, Valova VA, Malladi CS, et al. Cdk5 is essential for synaptic vesicle endocytosis. Nat. Cell. Biol. 2003;5:701–710. doi: 10.1038/ncb1020. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Stellar J. Assessment of tyrosine hydroxylase immunoreactive innervation in five subregions of the nucleus accumbens shell in rats treated with repeated cocaine. Synapse. 2000;38:261–270. doi: 10.1002/1098-2396(20001201)38:3<261::AID-SYN5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, De Leon KR, Stellar JR. Repeated cocaine treatment alters tyrosine hydroxylase in the rat nucleus accumbens. Brain Res. Bull. 2000;52:407–411. doi: 10.1016/s0361-9230(00)00277-x. [DOI] [PubMed] [Google Scholar]
- Tomizawa K, Ohta J, Matsushita M, Moriwaki A, Li S, Takei K, Matsui H. Cdk5/p35 regulates neurotransmitter release through phosphorylation and downregulation of P/Q-type voltage-dependent calcium channel activity. J. Neurosci. 2002;22:2590–2597. doi: 10.1523/JNEUROSCI.22-07-02590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa K, Sunada S, Lu YF, et al. Co-phosphorylation of amphiphysin I and dynamin I by Cdk5 regulates clathrin-mediated endocytosis of synaptic vesicles. J. Cell Biol. 2003;163:813–824. doi: 10.1083/jcb.200308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trulson ME, Ulissey MJ. Chronic cocaine administration decreases dopamine synthesis rate and increases [3H] spiroperidol binding in rat brain. Brain Res. Bull. 1987;19:35–38. doi: 10.1016/0361-9230(87)90162-6. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Joe JC, Babb S, Raese JD. Chronic cocaine administration depletes tyrosine hydroxylase immunoreactivity in the meso-limbic dopamine system in rat brain: quantitative light microscopic studies. Brain Res. Bull. 1987;19:39–45. doi: 10.1016/0361-9230(87)90163-8. [DOI] [PubMed] [Google Scholar]
- Vrana SL, Vrana KE, Koves TR, Smith JE, Dworkin SI. Chronic cocaine administration increases CNS tyrosine hydroxylase enzyme activity and mRNA levels and tryptophan hydroxylase enzyme activity levels. J. Neurochem. 1993;61:2262–2268. doi: 10.1111/j.1471-4159.1993.tb07468.x. [DOI] [PubMed] [Google Scholar]
- Zigmond RE, Schwarzschild MA, Rittenhous AR. Acute regulation of tyrosine hydroxylase by nerve activity and by neurotransmitters via phosphorylation. Annu. Rev. Neurosci. 1989;12:415–461. doi: 10.1146/annurev.ne.12.030189.002215. [DOI] [PubMed] [Google Scholar]




