Abstract
It was reported previously that, in the presence of water, a commercially available light-activated BisGMA/HEMA adhesive underwent physical separation into solid BisGMA-rich particles and a fluid-like HEMA-rich phase. The HEMA-rich phase exhibited limited monomer conversion suggesting that the photoinitiator is localized to the hydrophobic phase or that the photoinitiator is not compatible with the hydrophilic HEMA. The objective of the present study was to identify photoinitiators that are compatible with the hydrophilic HEMA-rich phase, when the mixtures are prepared without and with water added. The photoinitiator was camphoquinone (CQ, 0.5 mol %), and the coinitiators (0.5 mol %) were 2,2′-dihydroxyethyl-para-toluidine (DHEPT), dimethylaminoethyl methacrylate (DMAEMA), and N-phenylgly-cine (NPG), and (1 wt %) diphenyliodonium chloride (DPIC). Reactivities were evaluated using photodifferential scanning calorimetry, at 37°C, using visible light (>418 nm), with the parameters determined being enthalpy (ΔH), the induction time (herein defined as the time for 1% of the photopolymerization to be complete), and the time at which the maximum exotherm occurred. The degree of monomer conversion was measured using micro-Raman spectroscopy. It was shown that the reactivity ranking (based on time to exotherm peak maximum and total enthalpy) was HEMA/CQ/DHEPT < HEMA/CQ/DMAEMA < HEMA/CQ/NPG. Reactivity was dramatically increased for CQ/DMAEMA and CQ/NPG in the presence of DPIC, but not for CQ/DHEPT. Water has a major effect on HEMA conversion. At 10% of water, the conversion level of HEMA formulated with CQ/DMEMA dropped from ~100% to 86%. In comparison, the conversion in 10% of water increased to nearly 96% when DPIC was used. The results suggest that DHEPT, which is commonly used in commercial adhesives, is not compatible with HEMA. Both NPG and DMAEMA appear compatible with the HEMA. The ionic hydrophilic iodonium salt, DPIC, enhances the polymerization of HEMA, even in the presence of water. Future studies on water-compatible photoinitiators should be performed to address the detrimental effects of water on dentin adhesive systems.
Keywords: dental/craniofacial material, pHEMA, polymerization, photoreactivity, DSC
INTRODUCTION
The current generation of commercially-available adhesive systems that acid-etch the dentin characteristically bond via resin penetration and entanglement of collagen in the demineralized zone.1–3 With removal of the mineral phase by acid-etching, collagen fibrils are suspended and are thus supported by water. If the water is removed by drying, the collagen fibrils will collapse into a dense mat that impedes adhesive infiltration.4,5 A wet bonding technique has been adopted as a viable technique for addressing the problems associated with collagen collapse.6,7 This protocol involves keeping the dentin moist throughout the bonding procedure, thereby allowing the channels between the demineralized dentin collagen fibrils to be filled with water and/or oral fluids. Under these conditions, the only path available for adhesive resin in-filtration is diffusion of the resin monomers into whatever fluid is in the spaces of the substrate during bonding. Residual water on the dentin surface may result in the dilution of hydrophilic monomers.8 –10 Previous reports of the sensitivity of our current commercial dentin adhesives to excess moisture have included morphologic evidence of water blisters in adhesives applied to overwet surfaces.11,12
Applying hydrophobic adhesive resins onto these wet dentin surfaces may inhibit penetration of monomers.13,14 Hydrophilic monomers, such as hydroxyethyl methacrylate (HEMA), are included in both primer resins and adhesive formulations to improve the infiltration of the resin into the wet dentin surface. Micro-Raman spectra collected from a commercially-available BisGMA-based adhesive indicated that at 25 vol % of water, this material experienced phase separation and the composition of the phases included particles that were primarily hydrophobic BisGMA and the surrounding matrix of hydrophilic HEMA that exhibits limited monomer/polymer conversion.8 The limited conversion of the HEMA-rich phase suggests that either the photoinitiator is localized to the hydrophobic phase or it is incompatible with the hydrophilic HEMA. The results from this investigation highlight the need for characterization of reactant mixtures prepared with and without water when these mixtures are proposed for application as dentin adhesives. To date, very little work has been reported on such characterizations. Thus, the objective of the present work was to investigate the influence of four widely-used coinitiators, in combination with the photoinitiator (camphoquinone) and water on the photopolymerization of HEMA. Using the results from the photodifferential scanning calorimetry (Photo-DSC) and micro-Raman spectroscopic analyses, efforts are directed towards identifying photoinitiators that are compatible with the adhesive systems. This study tested the null hypothesis that there is no difference in photo reactivity and photo polymerization of HEMA formulated with initiators currently used in commercial BisGMA/HEMA adhesives.
MATERIALS AND METHODS
Specimen preparation
The HEMA was purchased from Acros Organics, Morris Plains, NJ, USA. The photoinitiator [camphoquinone (CQ, 0.5 mol %)] and the coinitiators [2,2′-dihydroxyethyl-para-toluidine (DHEPT, 0.5 mol %), dimethylaminoethyl methacrylate (DMAEMA, 0.5 mol %), N-phenylglycine (NPG, 0.5 mol %), and diphenyliodonium chloride (DPIC, 1 wt %)] were purchased from Aldrich, Milwaukee, WI, USA. CQ was chosen because it is a visible light activated photosensitizer in all commercial methacrylate dental systems. All the materials in this study were used as received. The solutions of the monomers, photoinitiators, and coinitiators were prepared in the absence of visible light and kept there until use on the same day.
Photo-differential scanning calorimeter
The polymerization of each reactant mixture (defined as HEMA + CQ + coinitiator) during irradiation with visible light was monitored using a Photo-DSC system, which comprised a differential scanning calorimeter (Dupont model 910; TA Instruments, New Castle, DE, USA) and a differential photocalorimeter that contains a 200-W mercury lamp which was filtered so as to emit light at a wavelength >418 nm (930 unit; TA Instruments, New Castle, DE, USA). About 15 mg of the reactant mixture was placed in the sample pan of the calorimeter and then irradiated for 20–40 min, at 37°C, in flowing nitrogen gas (flow rate: 40 mL/min) after 1 min of equilibration at ambient temperature. An empty sample pan was used in the reference position in the calorimeter. The intensity of the light was about 8 mW/cm2 at both the sample and the reference surfaces. Each reactant mixture studied was prepared under dry conditions as well as under wet conditions (addition of 10–40% of water). Measurements of mass before and after polymerization indicated that water loss was not a significant issue.
The following parameters were measured: enthalpy of the photopolymerization (ΔH); the induction time, which was defined as the time for 1% of the photopolymerization to be complete; the time at which the maximum exotherm temperature occurred. For each reactant mixture, these measurements were made in triplicate.
Micro-Raman spectroscopy
The reactant mixture(0.06 g) was cast on glass slides with wells, covered with mylar film, and light-cured for 40 s with a commercial visible-light-curing unit Spectrum® 800 (Densply, Milford, DE, USA). The intensity of the light was about 450 mW/cm2. Following polymerization, the mylar film was removed and the specimens were placed at the focus of a ×50 objective of an optical microscope (Nikon ME 600, Melville, NY, USA) and the Raman spectra were collected using a Jasco NRS 2000 Raman spectrometer equipped with Olympus lenses and a liquid-nitrogen-cooled CCD detector (Jasco Inc., Easton, MD, USA). The optical microscope allowed for visual identification of the position at which the Raman spectrum was obtained. The excitation source was an Argon laser, operating at 514.5 nm. The estimated power at the laser was 100 mW after passing through the bandpass filter and condensing optics, and ~3 mW power was incident on the sample.
RESULTS
Photo-DSC studies
Typical exotherm profiles for all the reactant mixtures studied are represented in Figures 1–4, with the full set of the photoreactivity parameters being given in Tables I–III. Comparison of the exotherms in Fig. 1 revealed notable differences in the photoreactivities of HEMA. The HEMA/CQ/DHEPT showed no appreciable exotherm, and thus reveals no obvious evidence of reaction under these test conditions. Combination of DHEPT and DMAEMA increased the efficiency. Efficiencies of NPG for initiation of HEMA polymerization were greater than DMAEMA. In the HEMA/CQ/DHEPT and HEMA/CQ/DMAEMA mixtures, the time to peak exotherm was ~1,410 and ~446 s, respectively; the enthalpy of the photoreaction was ~13 and ~98 J/g, respectively. The values when NPG was used in place of DHEPT or DMAEMA were ~297 s and ~145 J/g (Table I). The time to peak exotherm and enthalpy of the photoreaction provided a relative comparison of the rate and extent of the polymerization of each mixture. A reduced time to peak exotherm and increased enthalpy indicated increased reactivity.
Figure 1.
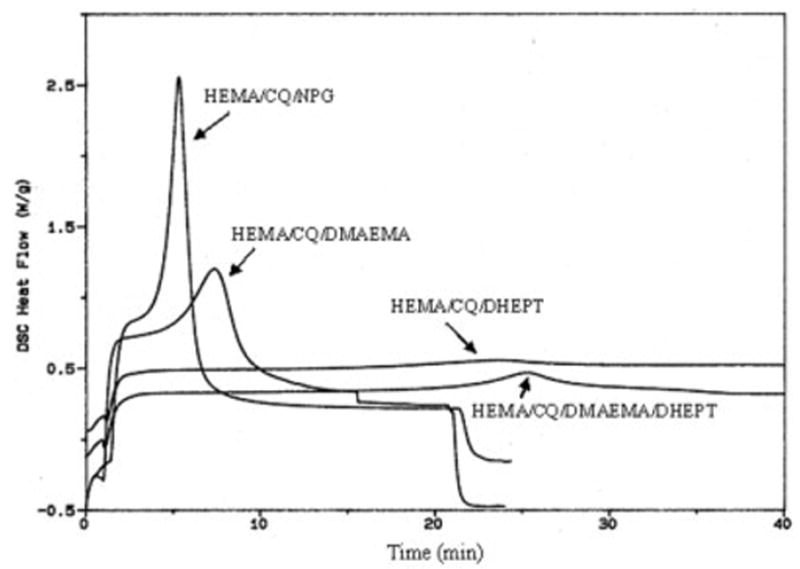
Photo-DSC exotherms for the photopolymerization of HEMA with different coinitiators. Sample weight, 14–15 mg and light intensity of 8 mW/cm2.
Figure 4.
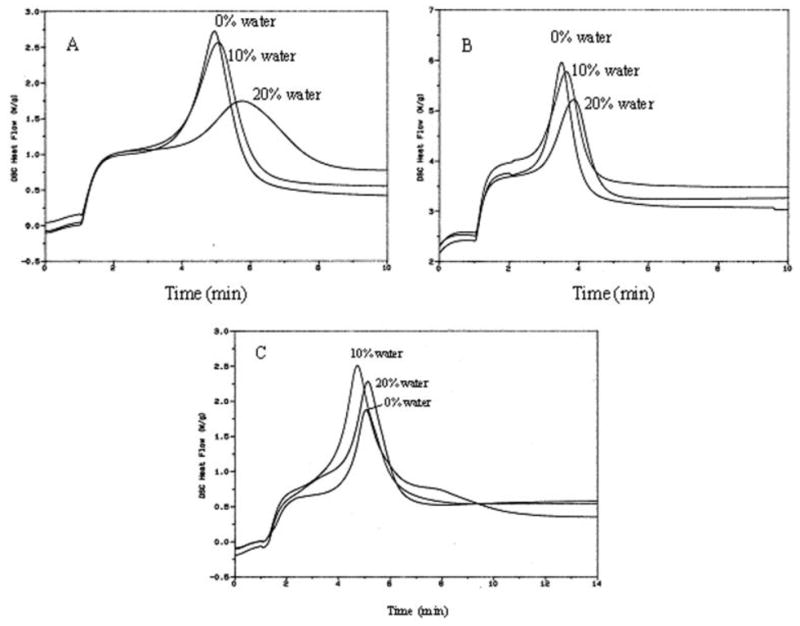
Influence of added water on the Photo-DSC exotherms for the photopolymerization of HEMA/CQ/NPG (A), HEMA/CQ/NPG/DPIC (B), and HEMA/CQ/DHEPT/DMAEMA/DPIC (C).
TABLE I.
Summary of Values of Photo-DSC Parameters of HEMA/CQ-Based Reactant Mixtures Prepared with No Water Added: Influence of Coinitiator
| Photoinitiators | Induction time (s) | Time to peak (s) | Enthalpy ΔH (J/g) |
|---|---|---|---|
| CQ/DHEPT | 1039 (31) | 1410 (38) | 12.7 (0.6) |
| CQ/DHEPT/DMAEMA | 1154 (24) | 1516 (28) | 47.4 (1.1) |
| CQ/DMAEMA | 254 (7) | 446 (14) | 98.3 (3.6) |
| CQ/NPG | 189 (8) | 297 (11) | 145.2 (4.5) |
Values given are mean values (standard deviations).
TABLE III.
Summary of Values of Photo-DSC Parameters of HEMA/CQ-Based Reactant Mixtures: Influence of Coinitiator and Added Water
| Photoinitiators | Water (%) | Induction time (s) | Time to peak (s) | Enthalpy ΔH (J/g) |
|---|---|---|---|---|
| CQ/NPG | 0 | 189 (8) | 297 (11) | 145.2 (4.5) |
| 10 | 181 (6) | 304 (9) | 149.9 (3.4) | |
| 20 | 239 (7) | 346 (10) | 117.9 (2.1) | |
| CQ/NPG/DPIC | 0 | 148 (5) | 210 (8) | 113.3 (2.3) |
| 10 | 145 (4) | 217 (12) | 113.5 (1.9) | |
| 20 | 174 (12) | 231 (9) | 109.0 (1.3) | |
| CQ/DHEPT/DMAEMA/DPIC | 0 | 189 (10) | 305 (13) | 156.4 (3.5) |
| 10 | 155 (4) | 283 (11) | 171.3 (4.1) | |
| 20 | 144 (7) | 307 (9) | 170.8 (3.3) |
Values given are mean values (standard deviations).
Incorporation of the third component DPIC to the system had a marked accelerating effect on the polymerization rate (Figs. 2 and 3). In the HEMA/CQ/DMAEMA mixture, the induction time was reduced dramatically from ~254 to ~102 s in the presence of DPIC. The enthalpy of the photoreaction was also increased from ~98 to ~124 J/g (Tables I and II). In the HEMA/CQ/DHEPT/DPIC mixture the time to peak exotherm was ~515 s, and the enthalpy of the photoreaction was ~12 J/g (Table II). The values when NPG was used in place of DHEPT or DMAEMA and in combination with DPIC were ~210 s and ~113 J/g. The time to peak exotherm in the HEMA/CQ/DMAEMA/DHEPT mixture was ~1,516 s, and the enthalpy of the photoreaction was ~47 J/g. When DPIC was included in this mixture, the time to peak exotherm was reduced to ~305 s, and the enthalpy of the photoreaction increased to ~156 J/g.
Figure 2.
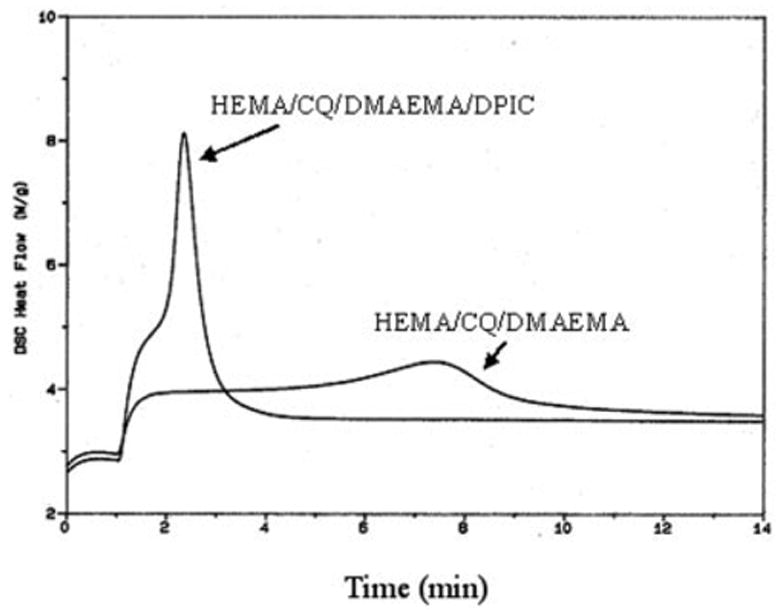
Photo-DSC exotherms for the photopolymerization of HEMA/CQ/DMAEMA with or without the third component DPIC.
Figure 3.
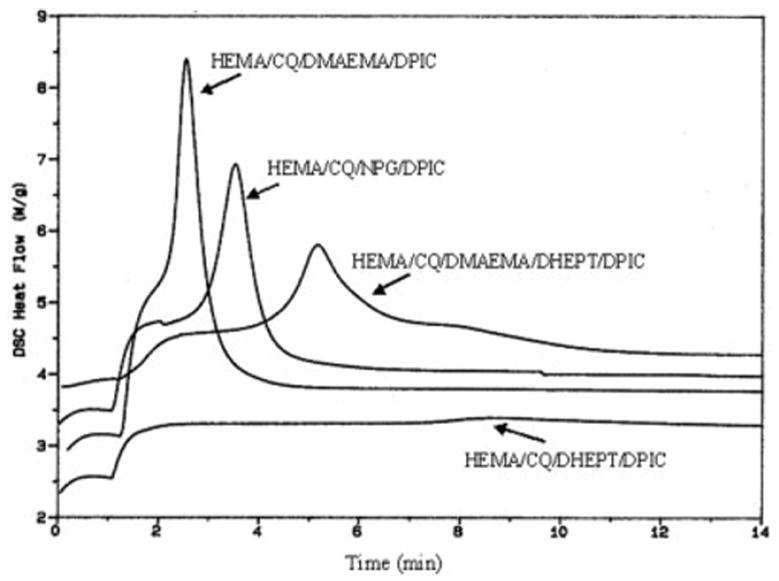
Effect of DPIC addition on Photo-DSC exotherms for the photopolymerization of HEMA with different coinitiators
TABLE II.
Summary of Values of Photo-DSC Parameters of HEMA/CQ-Based Reactant Mixtures Prepared with No Water Added: Influence of Addition of DPIC
| Photoinitiators | Induction time (s) | Time to peak (s) | Enthalpy ΔH (J/g) |
|---|---|---|---|
| CQ/DHEPT/DPIC | 445 (22) | 515 (24) | 11.5 (0.5) |
| CQ/DHEPT/DMAEMA/DPIC | 189 (10) | 305(13) | 156.4 (3.5) |
| CQ/DMAEMA/DPIC | 102 (7) | 141(12) | 124.1 (2.8) |
| CQ/NPG/DPIC | 148 (5) | 210(8) | 113.3 (2.3) |
Values given are mean values (standard deviations).
The addition of 10% of water had little influence on the photoreactivity of both the HEMA/CQ/NPG and HEMA/CQ/NPG/DPIC mixtures [Figs. 4(A,B)]. For example, in the HEMA/CQ/NPG mixture, the time to peak exotherm and the enthalpy of reaction were changed slightly from ~297 s and ~145 J/g to ~304 s and ~150 J/g, respectively (Table III). When the water content was increased to 20%, the photoreactivity in the HEMA/CQ/NPG mixture decreased, but only slightly decreased in the HEMA/CQ/NPG/DPIC mixture. The presence of the DPIC increased the compatibility under aqueous conditions. Interestingly, the presence of 10 and even 20% of water had little effect on the reactivity of the HEMA/CQ/DMAEMA/DHEPT/DPIC mixture [Fig. 4(C)]. In Table III, at 0% of water the time to peak exotherm was ~305 s and the enthalpy of the photoreaction was ~156 J/g. When the mixture was combined with 10 and 20% water the time to peak exotherm was ~283 and ~307 s, respectively. The enthalpy of the photoreaction was ~171 and ~171 J/g at 10 and 20% of water, respectively. The reactivity of the reaction actually increased in the presence of water although further experimentation is required to fully characterize the differences between 10 and 20% of H2O. These results clearly suggested that the HEMA/CQ/DMAEMA/DHEPT/DPIC mixture was compatible with hydrophilic conditions.
Raman spectroscopic studies
Typical micro-Raman spectra for various reactant mixtures are represented in Figures 5 and 6. To determine the effect of water on the degree of cure in the HEMA formulated with CQ/DMAEMA, Raman spectral data were collected on the following water/HEMA (wt/wt) mixtures: 0/100, 10/90, 20/80, and 40/60 (Fig. 5). Raman spectral data were acquired on polymerized and unpolymerized HEMA. The CH2 band was used as an internal standard. The degree of cure was determined by ratioing the relative integrated intensities of the 1640 and 1452 cm−1 spectral features. The ratio of these features for the unpolymerized HEMA was set as the baseline; 0% conversion was assigned to this ratio. Noted in the spectrum labeled 0% water, there was no apparent contribution from the spectral feature at 1640 cm−1. The conversion level of HEMA was ~100% in this spectrum. Based on the relative integrated intensities of the 1640 and 1452 spectral features, at 10% of water, the conversion level of HEMA dropped to ~86%. When water was added to HEMA at a concentration of 20 and 40%, the conversion level (based on the relative integrated intensities of the 1640 and 1452 spectral features) dropped to ~74 and ~35%, respectively.
Figure 5.
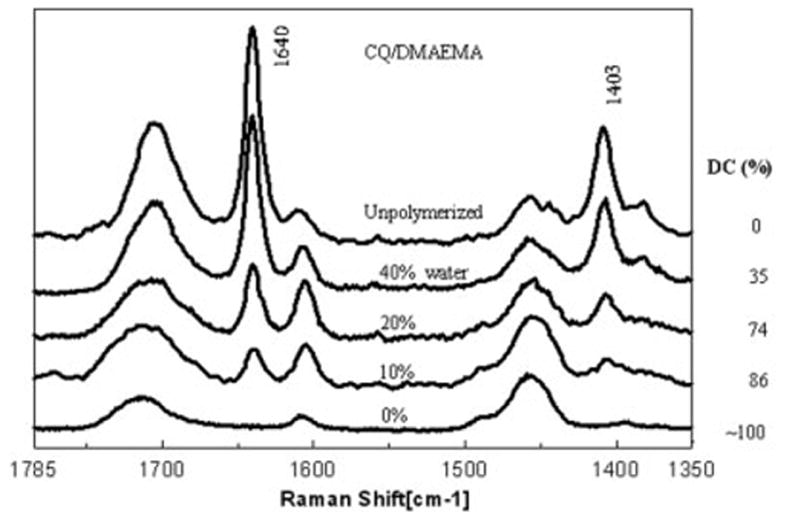
Typical Raman spectra of the reactant mixture, HEMA/CQ/DMAEMA, prepared with different amounts of added water (the spectrum of the unpolymerized HEMA system is also shown).
Figure 6.
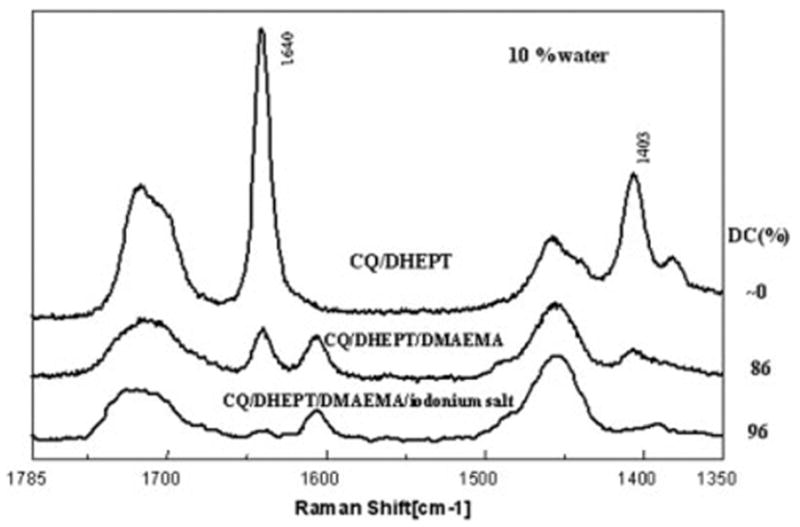
Typical Raman spectra of HEMA/CQ-based reactants prepared with addition of 10% of water, influence of coinitiator, and DPIC addition.
On comparing the spectra presented in Figures 5 and 6, it was clear that in the presence of 10% of water HEMA formulated with 0.5 mol % CQ and DHEPT did not polymerize. These results suggested that DHEPT, one of the commonly used amines in the dental bonding systems, was a poor photoinitiator for HEMA. In comparison, at the same concentration of water, i.e., 10%, the HEMA formulated with CQ/DHEPT/DMAEMA showed conversion at the level of about ~86%. The HEMA formulated with CQ/DHEPT/DMAEMA and DPIC showed nearly 96% conversion in 10% of water.
DISCUSSION
The most commonly employed photoinitiators are those in which radicals are formed in a bimoleclular process containing an excited state of a dye, such as CQ, and a coinitiator that acts as an electron donor.15,16 The majority of commercial dentin resins contain camphorquinone/amine pairs as photoinitiating systems.15,17 Radical photoinitiation proceeds as follows.18 Under visible light excitation, the photoinitiator(I) is promoted to its first excited singlet state (1I*) then via fast intersystem crossing it converts into its triplet state (3I*). This transient state can undergo either direct cleavage of the molecule (according to a Norrish Type I photoscission process) or hydrogen abstraction with amine compounds. These reactions yield reactive radical species which attack monomer molecules and initiate the polymerization.18 The effectiveness of the CQ/amine systems depends on the H-atom donor ability of amines used as coinitiators and the compatibility of initiator systems with monomers.
It was shown that there were notable differences in photopolymerization and photoreactivity of HEMA using different coinitiators. The comparison of these results has clearly indicated that DHEPT is not compatible with the hydrophilic HEMA. Both NPG and DMAEMA appeared compatible with the hydrophilic HEMA. It was surprisingly noticed that the visible light photoinitiator system CQ/DHEPT, which is used in most commercial adhesive/composite systems, did not initiate the polymerization of HEMA (Fig. 1). Apparently, the excited state for DHEPT or its exciplex with CQ is quenched because it is at a relatively higher (less stable) energy level compared to that of the alternative amine dimethylaminoethyl methacrylate (DMAEMA).19 Among the coinitiators, the reactivity ranking is listed as follows: DHEPT<DMAEMA<NPG.
The addition of DPIC to the photoinitiator systems had a marked accelerating effect on the polymerization rate (Figs. 2, 3). It was shown that even in the presence of the iodonium salt (DPIC), the aromatic DHEPT is still not compatible with the hydrophilic HEMA (Fig. 3). The addition of the iodonium salt (DPIC) to a photoinitiator system significantly increased the rate of free radical polymerization in comparison with that of systems in which just a dye and an amine were used.20 It was proposed that the iodonium salt might act as an electron scavenger from the dye radical.21 In these three-component photoinitiator systems, the resulting amine-based radical is active for polymerization, but the dye-based radical is a terminating radical. The third component, the iodonium salt, is thought to oxidize this inactive dye-based radical, regenerating the original dye and producing an additional active radical. This is an extra source of radicals for initiating polymerization. The rationale for including DPIC in our photoinitiator systems was that in addition to its ability to increase the reactivity of the free radical cure, it should also be more soluble in hydrophilic solvents due to its ionic nature.
Water or saliva is ubiquitous in the mouth of healthy patients, and thus it routinely interferes with our efforts to bond to dental tissues. The results, presented here, clearly indicated that water can substantially affect the reactivity of components within the dentin adhesive system. The addition of water dramatically inhibited the polymerization of HEMA (Fig. 5). This result is also supported by Paul et al.22 Using tensile properties as a determinant for adequate/inadequate polymerization, it has been previously reported that intrinsic water at concentrations of >5% inhibited the light polymerization of HEMA, even with a 10-fold increase in the initiators CQ/DMAEMA.22
The type of coinitiator affects the rate of polymerization and the final conversion level of HEMA in the presence of water. In the presence of water, HEMA formulated with CQ/DHEPT did not polymerize. The addition of 10% of water had little influence on the photoreactivity of the HEMA/CQ/NPG mixture (Fig. 4). It was reported previously that the addition of water actually promoted the polymerization of HEMA in the presence of N-phenylglycine (NPG) itself.23 Glycine derivatives have been previously proposed as coinitiators for polymeric dental formulations.24 The principal advantage of a CQ/NPG system is that it should be biologically less toxic than the amines.24 The fact that the NPG could promote polymerization in the presence of water is extremely beneficial for bonding to moist dentin surfaces. The effects of DPIC on the photopolymerization of HEMA were also investigated in aqueous mixtures. The salt DPIC notably increased the polymerization rate. These results clearly suggested that the presence of the DPIC increased the compatibility under aqueous conditions (Fig. 6). The results have also indicated that studies of photoinduced reactions in aqueous mixtures are critical to our efforts to identify adhesive formulations that will provide durable bonds to wet dentin substrates.
In the commercial adhesive mixtures, such as Bis-GMA/HEMA, HEMA acts as a coreactant, a penetrant, and a solubility enhancer. It reacts with the comonomer BisGMA in free radical fashion because they are both methacrylates. It helps the adhesive penetrate more effectively into the wet demineralized dentin. This hydrophilic, small, polar molecule also dissolves the BisGMA in a wide variety of concentrations and allows for increased mixing of the BisGMA and water before phase separation occurs. It was previously reported that the monomer/polymer conversion of BisGMA/HEMA resins decreased from 53.5% to 22.7% using FTIR, when 0.2 mL of water was added per ml of resin.9 In other words, the addition of water reduced the degree of conversion to half its dry value. It was found that these mixtures underwent phase separation (BisGMA-rich and hydrophilic HEMA-rich phases) as they mixed with water.8 The conversion level of the HEMA-rich phase was dramatically lower than that of the BisGMA-rich phase.8 The results of this study indicated that in the presence of 10% of water, the degree of conversion (DC) was only ~86% in the HEMA/CQ/DHEPT/DMAEMA mixture after 40 s of light exposure; the DC was almost zero in the HEMA/CQ/DHEPT mixture (Fig. 6), in which the photoinitiator is one of the commonly-used photoinitiator systems in the dental bonding systems. It was indicated that photoinitiators that can efficiently initiate the polymerization of BisGMA/HEMA mixtures might not be effective in initiating HEMA. Relatively small water contamination could have dramatic effects on the degree of cure within the phase of HEMA, since it mixes well with water.
Light curing of current dental resins is normally optimized based on the proper selection and combination of photoinitiator/coinitiator on the lab bench under dry conditions. Our results indicated that the polymerization behavior should be studied in the presence of water and/or solvents. This study indicates that the selection and combination of photoinitiator/coinitiator are specific to the different adhesive systems. The development of dental adhesive that are suitable for wet bonding applications should be based on the curing behavior of resins under both dry and wet conditions. In the mouth of patients, the photopolymerization is usually performed in the presence of water and/or saliva. It is doubtful whether microphase separation in adhesives applied to wet, demineralized dentin matrices can be eliminated. To improve the efficiency of the photoinitiator systems for photopolymerization in water and/or solvents, one aspect of our approach should include water-compatible photoinitiators to ensure that photoinitiators are present in both hydrophilic and hydrophobic layers, if phase separation occurs.
Currently, adhesives such as all-in-one bonding systems containing acidic, self-etching and more hydrophilic monomers have been developed to address the problems associated with bonding to wet dentin such as phase separation and technique sensitivity. However, our recent results indicate that water is still a major interfering factor in polymerization, and as a result, unpolymerized acidic monomers could continue to etch the dentin, leading to a detrimental impact on the bond.10,25 Since there is little control over the presence of water in the tooth, investigators must study more efficient water-compatible photoinitiators to address the problems associated with incomplete conversion in these dental adhesive systems. The results suggest that in the presence of water the third component, ionic hydrophilic iodonium salt, enhances the polymerization of HEMA. Based on our review of the literature, this is the first time that water-compatible photoinitiators have been used to address the detrimental effects of water on dentin adhesives and/or the components within these systems. The Raman results support the Photo-DSC data and reflect the complementary nature of these techniques.
CONCLUSIONS
The reactivity ranking is listed as follows: DHEPT<DMAEMA<NPG.
DHEPT, which is commonly used in commercial adhesives, is not compatible with HEMA.
Reactivity was dramatically increased for DMAEMA/DPIC and NPG/DPIC, even in the presence of water, but not for DHEPT/DPIC.
It was indicated that studies on water-compatible photoinitiators should be performed to address the detrimental effects of wet environments.
Acknowledgments
The authors thank Dr. Cecil C. Chappelow and Charles S. Pinzino of the Midwest Research Institute, Kansas City, MO, USA for permission to use the Photo-DSC equipment.
Footnotes
Contract grant sponsor: National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD, USA; contract grant numbers: DE014392 and DE015281
References
- 1.Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16:265–273. doi: 10.1002/jbm.820160307. [DOI] [PubMed] [Google Scholar]
- 2.Eick JD, Gwinnet AJ, Pashley DH, Robinson SJ. Current concepts on adhesion to dentin. Crit Rev Oral Bio Med. 1997;8:306–335. doi: 10.1177/10454411970080030501. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Spencer P. Hybridization efficiency of the adhesive dentin interface with wet bonding. J Dent Res. 2003;82:141–145. doi: 10.1177/154405910308200213. [DOI] [PubMed] [Google Scholar]
- 4.Pashley DH, Ciucchi B, Sano H, Horner JA. Permeability of dentin to adhesive agents. Quintessence Int. 1993;24:618–631. [PubMed] [Google Scholar]
- 5.Gwinnett AJ. Quantitative contribution of resin infiltration/hybridization to dentin bonding. Am J Dent. 1993;6:7–9. [PubMed] [Google Scholar]
- 6.Kanca J. Improved bond strength through acid etching of dentin and bonding to wet dentin surfaces. J Am Dent Assoc. 1992;123:235–243. doi: 10.14219/jada.archive.1992.0248. [DOI] [PubMed] [Google Scholar]
- 7.Gwinnett AJ. Dentin bond strength after air drying and rewetting. Am J Dent. 1994;7:144–148. [PubMed] [Google Scholar]
- 8.Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. J Biomed Mater Res. 2002;62:447–456. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 9.Jacobsen T, Soderholm K-J. Some effects of water on dentin bonding. Dent Mater. 1995;11:132–136. doi: 10.1016/0109-5641(95)80048-4. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Spencer P. Continuing etching of an all-in-one adhesive in wet dentin tubules. J Dent Res. 2005;84:350–354. doi: 10.1177/154405910508400411. [DOI] [PubMed] [Google Scholar]
- 11.Tay FR, Gwinnett AJ, Wei SHY. The overwet phenomenon: A transmission electron microscopic study of surface moisture in the acid-conditioned, resin-dentin interface. Am J Dent. 1996;9:161–166. [PubMed] [Google Scholar]
- 12.Tay FR, Gwinnett AJ, Pang KM, Wei SHY. An optical, micro-morphological study of surface moisture in the total etched resin-dentin interface. Am J Dent. 1996;9:43–48. [PubMed] [Google Scholar]
- 13.Wang Y, Spencer P. Evaluation of the interface between one-bottle adhesive systems and dentin by Goldner’s trichrome stain. Am J Dent. 2005;18:66–72. [PubMed] [Google Scholar]
- 14.Wang Y, Spencer P. Quantifying adhesive penetration in adhesive/dentin interface using confocal Raman microspectroscopy. J Biomed Mater Res. 2002;59:46–55. doi: 10.1002/jbm.1215. [DOI] [PubMed] [Google Scholar]
- 15.Cook WD. Photopolymerization kinetics of dimethacrylates using the camphorquinone/amine initiator system. Polymer. 1992;33:600–609. [Google Scholar]
- 16.Jakubiak J, Allonas X, Fouassier JP, Sionkowska A, Andrzejewska E, Linden LA, Rabek JF. Camphorquinone-amines photoinitiating systems for the initiation of free radical polymerization. Polymer. 2003;44:5219–5226. [Google Scholar]
- 17.Sionkowska A, Kaminska A, Linden LA, Rabek JF. Comparison of the photopolyerization kinetics of triethyleneglycol dimethacrylate initiated by camphorquinone/N-phenylglycine and camphorquinone/DL-α-phenylglycine. Polym Bull. 1999;43:349–355. [Google Scholar]
- 18.Bonamy A, Fouassier JP, Lougnot DJ. Novel and efficient water-soluble photoinitiator for polymerization. J Polym Sci Polym Lett Ed. 1982;20:315–320. [Google Scholar]
- 19.Chandra R, Soni RK. Recent developments in thermally curable and photocurable systems. Prog Polym Sci. 1994;19:137–169. [Google Scholar]
- 20.Padon KS, Scranton AB. A mechanistic investigation of a three-component radical photoinitiator system comprising methylene blue, N-methyldiethanolamine, and diphenyliodonium chloride. J Polym Sci Part A: Polym Chem. 2000;38:2057–2066. [Google Scholar]
- 21.Kim D, Scranton AB. The role of diphenyl iodonium salt (DPI) in three-component photoinitiator systems containing methylene blue (MB) and an electron donor. J Polym Sci Part A: Polym Chem. 2004;42:5863–5871. [Google Scholar]
- 22.Paul SJ, Leach M, Rueggeberg FA, Pashley DH. Effect of water content on the physical properties of model dentine primer and bonding resins. J Dent. 1999;27:209–214. doi: 10.1016/s0300-5712(98)00042-6. [DOI] [PubMed] [Google Scholar]
- 23.Imai Y, Suzuki A. Effects of water and carboxylic acid monomer on polymerization of HEMA in the presence of N-phenylglycine. Dent Mater. 1994;10:275–277. doi: 10.1016/0109-5641(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 24.Kucybala Z, Pietrzak M, Paczkowski J. Kinetic studies of a new photointiator hybrid system based on camphorquinone-N-phenylglycine derivatives for laser polymerization of dental restorative and stereolithographic (3D) formulations. Polymer. 1996;37:4585–4591. [Google Scholar]
- 25.Wang Y, Spencer P. Physicochemical interactions at the interfaces between self-etch adhesive systems and dentin. J Dent. 2004;32:567–579. doi: 10.1016/j.jdent.2004.06.005. [DOI] [PubMed] [Google Scholar]


