Abstract
The main purpose of this study was to determine whether experimental enhancement of oxidative stress by exposure to hyperoxia is an appropriate model for the acceleration of the normal aging process or for establishing a causal association between oxidative stress and aging. Insect tissues are directly exposed to ambient air via the tracheolar invaginations and are thus highly susceptible to oxidative stress under hyperoxic conditions. Amounts of glutathione (GSH), glutathione disulfide (GSSG) and protein mixed disulfides (PrSSG) were compared under normoxic and 100% ambient oxygen in males of two different strains of Drosophila melanogaster (Oregon R (WT) and y w strains). The reason for using two different strains was to preclude the effects of genetic background and to determine whether variations in longevity of the two strains are associated with resistance to oxidative stress. Amounts of GSSG and PrSSG increased whereas GSH:GSSG ratios declined as a function of age in both strains. Under hyperoxia, y w flies did not exhibit an increase in GSSG amount or a decline in GSH:GSSG ratio, whereas WT flies showed a decline in GSH:GSSG ratio only during the later part of hyperoxic exposure. In neither strain there was a progressive increase in PrSSG amount under hyperoxia. Results indicate that hyperoxia (100% oxygen) neither reproduces nor accelerates the pattern of alterations in glutathione redox state and PrSSG content observed during aging under normoxic conditions, although some other indicators of oxidative stress may be affected.
Keywords: Aging, oxidative stress, hyperoxia, glutathione, protein mixed disulfides, Drosophila
1. Introduction
Although the nature of the mechanisms causing age-related deleterious alterations is unclear, reactive oxygen species (ROS) are widely speculated to play a causal role. This notion, often referred to as the oxidative stress hypothesis, postulates that accumulation of ROS induced structural damage to various macromolecules underlies the aging-associated losses in cellular functions (Harman, 1956; Sohal and Weindruch, 1996; Beckman and Ames, 1998). The main supportive evidence is that rates of mitochondrial production of superoxide anion radical and hydrogen peroxide and the amounts of macromolecular oxidative damage (i) increase with age, (ii) show an inverse relationship with longevity of different species, and (iii) are attenuated by experimental regimens that extend life span, such as caloric restriction in laboratory rodents and decrease in metabolic rate in poikilotherms (reviewed in Sohal and Weindruch, 1996; Beckman and Ames, 1998; Sohal et al., 2002). Although such evidence is consistent with the predictions of the oxidative stress hypothesis, it is insufficient to establish a causal association. To establish the existence of such a relationship would require the demonstration that a decrease in the level of oxidative stress attenuates the rate of accrual of aging-specific oxidative damage and prolongs the life span, and reciprocally an elevation in oxidative stress would accelerate the age-related biochemical/physiological changes and shorten the life span.
The main purpose of the present study was to address the issue whether or not enhancement of oxidative stress quickens the progression of the changes that normally occur during the aging process. Hyperoxia is known to cause an increase in the rates of mitochondrial generation of ROS (Turrens et al., 1982a,Turrens et al., 1982b) as well as accelerate the accrual of macromolecular oxidative damage to tissues (Sohal and Dubey, 1994; Agarwal and Sohal, 1994). A recent study by Landis et al. (2004) showed a large overlap in aging and hyperoxia responses; for instance, 38% of the genes whose expression changed during aging were also affected by exposure to 100% oxygen in the same direction. However, expression of other genes during aging differed from that in response to hyperoxia. The data were interpreted to be consistent with the hypothesis that oxidative stress is a significant component of aging.
Our previous studies have shown that the redox state of tissues, reflected by the GSH:GSSG ratio, becomes progressively more oxidizing during aging in Drosophila as well as in mice (Rebrin et al., 2003, 2004). Furthermore, the pro-oxidizing shifts in glutathione redox state were attenuated by decreasing the ambient temperature of flies (Rebrin et al., 2004) or by caloric restriction of mice (Rebrin et al., 2003). Both of these regimens are also known to extend the life spans of the respective species. In these studies, protein mixed disulfide (PrSSG) content was found to follow an age-related pattern similar to that of GSH:GSSG ratio. Accordingly, in the present study a comparison of the amounts of GSH, GSSG and PrSSG, and GSH:GSSG ratios was made under normoxic and hyperoxic conditions (100% oxygen partial pressure) in two different strains of D. melanogaster, WT (Oregon R) and y w. The rationale for using 100% oxygen to create a hyperoxic environment was not only that a previous study reporting a similarity in gene expression during aging and under hyperoxia also employed this concentration (Landis et al., 2004), but also that the studies on the resistance of insects to withstand oxidative stress have often employed this oxygen concentration (Agarwal and Sohal, 1994). Two different strains of flies were used in order to preclude the possibility that the observed changes may occur only in a specific genetic background. Thus the main question being addressed in this investigation is whether hyperoxia causes an accelerated recapitulation of the normal age-related pattern of changes in glutathione redox state.
2. Materials and methods
2.1. Reagents
GSH and GSSG were purchased from Sigma Chemical Co. (St Louis, MO); acetonitrile, meta-phosphoric acid and 1-octane sulfonic acid were from EM Science (Gibbstown, NJ). All other chemicals were HPLC grade or of the highest purity available commercially.
2.2. Animals
Male D. melanogaster of Oregon-R wild type (WT) and y w strains were collected under mild CO2 anaesthesia in groups of 25, approximately one day post-eclosion, and subsequently maintained at 25°C on a cornmeal-sucrose-yeast medium, as described (Mockett et al., 2002).
2.3. Sample preparation and measurements of GSH, GSSG and protein mixed disulfides by HPLC with electrochemical detection
Flies were immobilized on ice for 1-2 min, weighed, and homogenized in 10 vol of freshly prepared ice-cold 5% (w/v) meta-phosphoric acid (MPA), using 1.5 ml plastic tubes and pestles obtained from RPI (Mt. Prospect, IL). The homogenates were incubated for 30 min on ice and centrifuged at 18,000 g for 20 min at 4°C. Supernatants were filtered using 0.45 μm PTFE Acrodisc® CR 4 mm syringe filters, obtained from Gelman Laboratory (Ann Arbor, MI); filtrates were transferred to sampling vials and either analyzed immediately or stored at -80°C for up to 1 month. Pellets from the acid precipitation were washed 3 times in 5% MPA to remove the free (non-protein bound) GSH and GSSG. Protein mixed disulfides were measured as GSH, bound to proteins, which was subsequently released by incubation of protein pellets in 100 mM phosphate buffer, pH 7.4 containing 0.1 mM DTT for 1.5 h at 37°C.
The procedure for detection and quantification of GSH and GSSG used here and the precautionary measures taken to minimize spontaneous GSH oxidation have been described by us previously (Rebrin et al., 2003, 2004). Briefly, GSH and GSSG were separated by HPLC, fitted with a Shimadzu Class VP solvent delivery system, using a reverse phase C18 Luna (II) column (3μ; 4.6 × 150 mm), obtained from Phenomenex (Torrance, CA). The mobile phase for isocratic elution consisted of 25 mM monobasic sodium phosphate, 0.5 mM of the ion-pairing agent 1-octane sulfonic acid, 1% (v/v) acetonitrile, pH 2.7, adjusted with 85% phosphoric acid. The flow rate was 0.7 ml/min. Under these conditions, the separation was completed in 35 min; GSSG was the last eluting peak, with a retention time of approximately 30 min. Calibration standards were prepared in 5% (w/v) MPA. GSH and GSSG were detected with a model 5600 CoulArray® electrochemical detector (ESA Inc., Chelmsford, MA), equipped with a four-channel analytical cell, using potentials of +400, +600, +750 and +875 mV. GSH was monitored at +600 mV and GSSG at +750 mV. Each sample was injected twice, and the average of the peak areas was used for quantification.
2.4. Statistical analysis
Two factor analysis of variance with age and fly strain as the factors was performed using Microsoft® EXCEL software. Significance of the age- and hyperoxia-dependent trends was determined by linear regression using SYSTAT 10 (SYSTAT Software, Richmond, CA, USA). Results at each specific time point are mean ± SD of four independent experiments, each consisting of 25-50 flies.
3. Results
3.1. Age-associated changes in glutathione redox state and protein mixed disulfides under normoxia
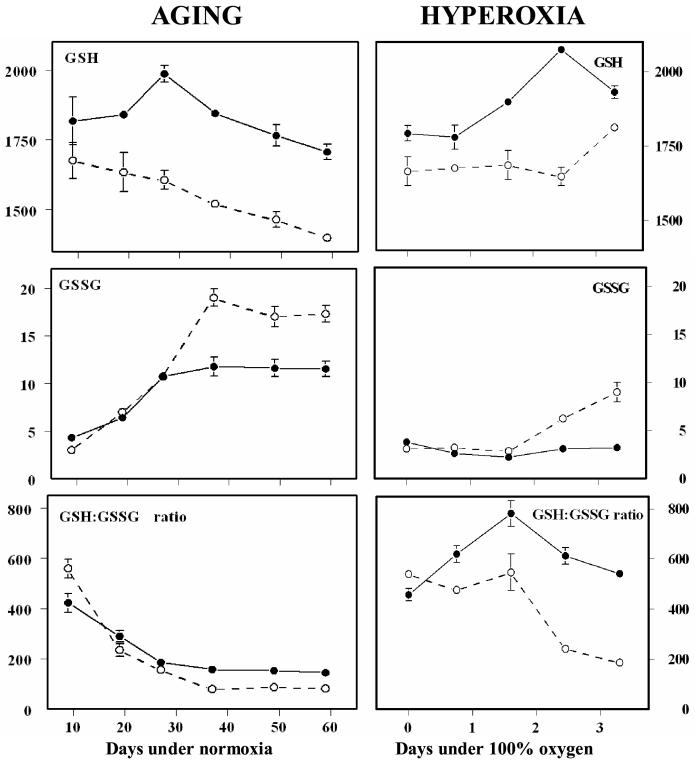
Representative survivorship curves of WT and y w flies are shown in Fig. 1. The mean life spans were 51 and 67 days, respectively. Biochemical measurements were made at several different ages, ranging from 9 to 59 days of age. Concentrations of glutathione (GSH) and glutathione disulfide (GSSG) were determined in whole-body homogenates of WT and y w flies at 9, 19, 27, 37, 49 and 59 days of age (Fig. 2). The WT flies exhibited a 17% decline (P < 0.001) in GSH content between 9 and 59 days of age; however, there was no such loss in the y w flies. During the same period there was a 5.5-fold elevation in GSSG content in the WT flies and a 2.7-fold rise in the y w flies (P < 0.001).
Figure 1.
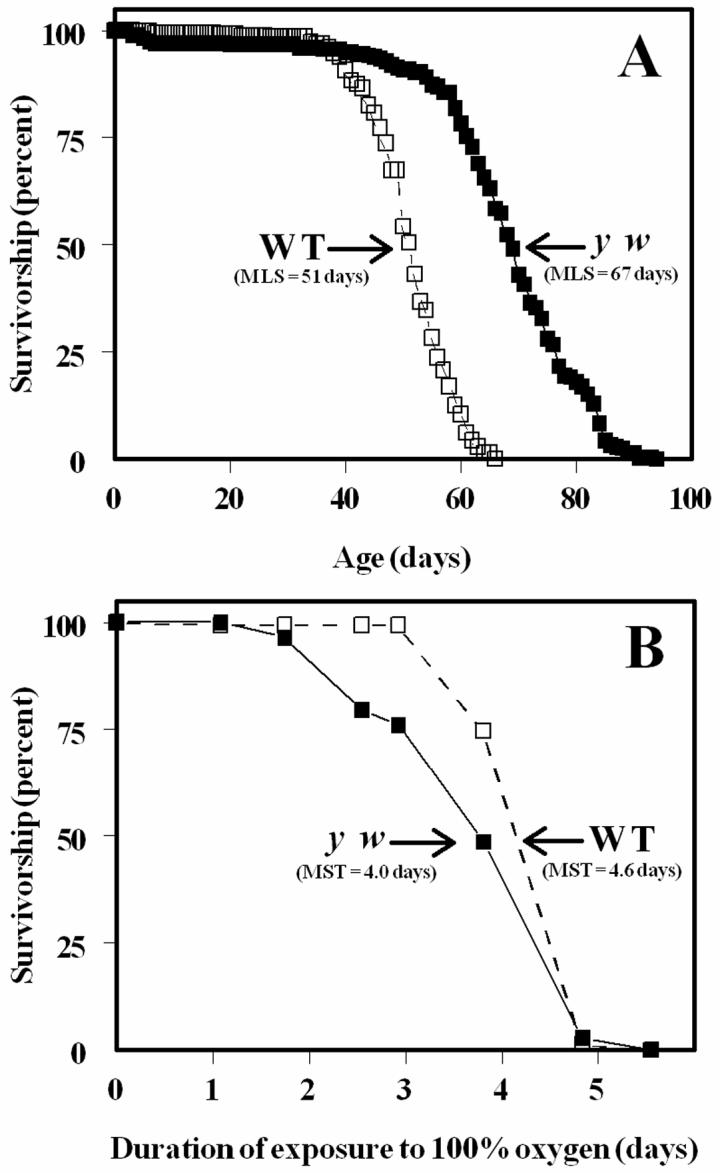
Life spans of WT and y w Drosophila melanogaster. A: Male flies (WT, open symbols, n=343; y w, closed symbols, n=277) were maintained in groups of 25 under constant light at 25.0 ± 0.5°C. Mortality was recorded and flies transferred to fresh food vials every 1-2 days. B: Survival curves of male flies (WT, open symbols, n=150; y w, closed symbols, n=142) under 100% ambient oxygen. Mortality was recorded every 10-12 h and flies transferred to fresh food vials every 24 h. Results are representative of two or more separate experiments.
Figure 2.
Comparison of GSH and GSSG content and GSH:GSSG ratios between the shorter lived WT (open circles) and longer lived y w (black circles) flies as a function of age under normoxic conditioons (left column) and under 100% ambient oxygen (right column). Results at each time point are mean ± SD of four independent experiments, each consisting of 25-50 flies, and are expressed as pmol/mg body weight.
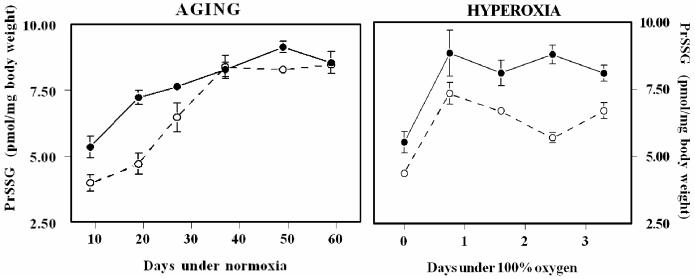
The amount of PrSSG, measured as glutathionyl adducts with protein, also increased with age in both strains of flies (Fig. 3). Although the total PrSSG content was lower in the WT flies at younger ages, the age-related increase was faster in WT than y w flies (117% vs. 57% between 9 and 37 days in WT and y w flies, respectively). The amounts of PrSSG at older ages were similar in the two strains. Analysis of variance showed that there were significant differences between strains and among age groups, as well as a significant interaction between these factors (P<0.001 for all comparisons).
Figure 3.
Comparison of protein-glutathione mixed disulfides (PrSSG) between the shorter-lived WT (open circles) and longer-lived y w (black circles) male flies as a function of age under normoxia (left panel) and under 100% ambient oxygen (right panel). Results at each time point are mean ± SD of four independent experiments, each consisting of 25-50 flies, and are expressed as pmol/mg body weight.
3.2. Effects of hyperoxia on survival, glutathione redox state and protein mixed disulfide content
To determine the effects of hyperoxia, 10-day-old flies were exposed to 100% oxygen partial pressure until death. Survivorship data, presented in Fig. 1B, indicated that WT flies survived longer than the y w flies (P<0.0001), which is in contrast to the result observed under normoxic conditions.
The pattern of changes in glutathione redox state and PrSSG content was determined in 10-day-old flies exposed to 100% ambient oxygen for 0, 0.75, 1.6, 2.5, and 3.3 days, after which the flies undergo rapid mortality. In contrast to the age-related trend observed under normoxic conditions, hyperoxia had no marked effect on GSH concentration in either of the two strains of flies (Fig.2). While GSSG content in the WT flies increased 2.8-fold after 3.3 days of hyperoxia (versus control), there was no discernible increase in the y w flies. Furthermore, unlike the pattern observed under normoxia, where GSH:GSSG ratios tended to decline progressively with age in both strains, under hyperoxia this ratio increased initially in y w flies, followed by a decline, whereas the WT flies showed no change initially, followed by a steep decline. As was also observed under normoxic conditions, the PrSSG content was higher in y w than in WT flies (Fig.3); however, the overall pattern of changes varied under hyperoxia. After an initial rise during the first 0.75 days of exposure to 100% oxygen, the amounts of PrSSG remained relatively constant in both strains. Quantitatively, the increase in the amount of PrSSG was greater during aging in a normoxic environment compared to the increase under hyperoxia.
4. Discussion
Results of the present study indicate that irrespective of the genetic background the overall pattern of changes in glutathione redox state observed under hyperoxia was, in both strains of flies, dissimilar to that occurring during the normal aging process.
Although there is presently no validated, single measure or a quantitative scale for expressing the absolute or an overall level of “oxidative stress” in tissues, the glutathione redox state, represented by the GSH:GSSG ratio, is widely used as a surrogate for determining the direction of the shift in the level of oxidative stress in tissues (de la Asuncion et al., 1996; Schafer and Buettner, 2001; Dröge 2002). Enhanced in vivo fluxes of ROS are known to cause oxidation of GSH into GSSG by direct interaction with ROS or by being a substrate for the enzymatic elimination of peroxides, among others (reviewed in Schafer and Buettner, 2001; Reed 1990; Dickinson and Forman, 2002). Nascent GSSG is either reconverted enzymatically to GSH or extruded from the cell, or it forms PrSSG via thiol-disulfide exchange reactions. Hence, shifts in the GSH:GSSG ratio and the amount of PrSSG are widely regarded as indicators of changes in the level of oxidative stress in tissues (Cotgreave and Gerdes, 1998; Schafer and Buettner, 2001; Dickinson and Forman, 2002).
The finding that the age-related decline in GSH content occurred only in the WT flies and not in the y w flies suggests that loss of GSH is dependent on genotype and is not an invariable characteristic of the aging process; however, the severity of oxidative stress, indicated by the amounts of GSSG and PrSSG and ratio of GSH/GSSG, increased with age in both strains of flies. Previous studies on mammals (Wang et al., 2003) and insects (Richie and Lang, 1998) have also found contradictory trends in age-related alterations in GSH content. Some tissues and species exhibited a decrease whereas others showed no age related variation in GSH amounts (Rebrin et al., 2003; Hagen, 2003; Liu et al., 2004). As pointed out previously (Rebrin et al., 2003, 2004), technical problems associated with tissue processing, handling and analytical methodology, among others, can potentially cause errors in the recovery, yield, and quantification of GSH/GSSG. A widely recognized hazard is the artifactual oxidation of GSH to GSSG. The methodology used here resulted in minimal GSH oxidation, and the GSH:GSSG ratios obtained were among the highest values that have been reported (Rebrin et al., 2003; Jones, 2002). Another notable feature of the present study is that to ascertain the pattern of age-related changes, measurements were made at five different ages rather than two or three, as is often the case.
It can be reasoned that the oxidative stress hypothesis would be relatively more directly supported provided it were true that enhanced fluxes of ROS cause an accelerated recapitulation of the various changes that occur during normal aging. Hyperoxia, which increases the rates of mitochondrial ROS generation (Turrens et al., 1982a,Turrens et al., 1982b), is widely considered to be an appropriate experimental model for the elevation of oxidative stress, especially in comparison to the frequently used redox cyclers such as paraquat, which generate O -.2 but also deplete NADPH (Bus et al., 1976). Notwithstanding these considerations, the present findings indicated that hyperoxia did not reproduce the pattern of changes observed in glutathione redox state during normal aging. For instance, unlike aging under normoxia, hyperoxic exposure did not cause a loss of GSH in WT flies, or a rise in GSSG level or a decline in GSH:GSSG ratio in y w flies. Furthermore, in contrast to an almost progressive increase in PrSSG content during normal aging, under hyperoxia, PrSSG amounts remained unchanged after an initial rise in both strains of flies. Since the patterns of age-related changes in glutathione redox state and PrSSG content transcend phylogenetic boundaries they can be considered to be a characteristic of the aging process.
Another relevant question is whether variations in longevity between flies of different genetic background can be predicted solely on the basis of their resistance to oxidative stress. Results indicate that while the WT flies had a shorter life span than y w flies under normoxic conditions, they survived longer than y w flies under hyperoxia, even though the WT flies displayed a relatively higher degree of oxidative stress under hyperoxia, as reflected by the GSSG content and GSH:GSSG ratio. This finding indicates that, contrary to the widely held view, resistance to induced oxidative stress is not a reliable predictor or determinant of longevity under normoxic contitions. Comparable findings have been made previously. For instance, a comparison of short and long lived lines of Drosophila, obtained by selection of progeny from either young or old parents, as described by Luckinbill et al. (1988), showed that the short-lived strains of flies survived longer under hyperoxia than the long-lived strains (Mockett et al., 2001). Similarly, transgenic flies overexpressing Cu,Zn- or Mn-superoxide dismutase or thioredoxin reductase lived longer under hyperoxia than their respective controls, but the life span under normoxic conditions was similar (Orr and Sohal, 1993; Mockett et al., 1999).
Another issue arising from this study is why the y w flies do not exhibit a decrease in GSH:GSSG ratio under hyperoxia, which is well established to cause a state of severe oxidative stress. Although it is beyond the scope of this study to identify the specific reasons, the presence of relatively more efficient mechanisms for antioxidative defense, rapid extrusion of GSSG or induction of GSH synthesis in y w flies may play a role. It seems probable that mechanisms and manifestations of enhanced oxidative stress during aging differ from those under hyperoxia, as also suggested by the observations of Landis et al. (2004). Nevertheless, the present results clearly demonstrate that differences in the life span and glutathione redox state, observed during normal aging in the two strains of flies, are mostly not duplicated under hyperoxic conditions. It is therefore inferred that hyperoxic exposure neither accelerates the normal aging process nor can life expectancy be predicted on the basis of resistance to hyperoxia.
Acknowledgements
This research was supported by grants RO1 AG7657 from the National Institute on Aging-National Institutes of Health. We are grateful to Dr. Robin J. Mockett for assistance in experimental studies.
Abbreviations
- GSH
glutathione
- GSSG
oxidized glutathione or glutathione disulfide
- MPA
meta-phosphoric acid
- PrSSG
protein-glutathione mixed disulfides
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal S, Sohal RS. DNA oxidative damage and life expectancy in houseflies. Proc. Natl. Acad. Sci. USA. 1994;91:12332–12335. doi: 10.1073/pnas.91.25.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Bus JS, Cagen SZ, Olgaard M, Gibson JE. A mechanism of paraquat toxicity in mice and rats. Toxic. Appl. Pharmacol. 1976;35:501–513. doi: 10.1016/0041-008x(76)90073-9. [DOI] [PubMed] [Google Scholar]
- Cotgreave IA, Gerdes RG. Recent trends in glutathione biochemistry - Glutathioneprotein interactions: A molecular link between oxidative stress and cell proliferation? Biochem. Biophys. Res. Commun. 1998;242:1–9. doi: 10.1006/bbrc.1997.7812. [DOI] [PubMed] [Google Scholar]
- de la Asuncion GJ, Millan A, Pla R, Bruseghini L, Esteras A, Pallardo FV, Sastre J, Vina J. Mitochondrial glutathione oxidation correlates with age-associated oxidative damage to mitochondrial DNA. FASEB J. 1996;10:333–338. doi: 10.1096/fasebj.10.2.8641567. [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Forman HJ. Cellular glutathione and thiols metabolism. Biochem. Pharmacol. 2002;64:1019–1026. doi: 10.1016/s0006-2952(02)01172-3. [DOI] [PubMed] [Google Scholar]
- Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Hagen TM. Oxidative stress, redox imbalance, and the aging process. Antioxid. Redox Signal. 2003;5:503–506. doi: 10.1089/152308603770310149. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Meth. Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- Landis GN, Abdueva D, Skvortsov D, Yang J, Rabin BE, Carrick J, Tavare S, Tower J. Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 2004;101:7663–7668. doi: 10.1073/pnas.0307605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Wang H, Shenvi S, Hagen TM, Liu R-M. Glutathione metabolism during aging and in Alzheimer desease. Ann. NY Acad. Sci. 2004;1019:346–349. doi: 10.1196/annals.1297.059. [DOI] [PubMed] [Google Scholar]
- Luckinbill LS, Graves JL, Reed AH, Koetsawang S. Localizing genes that defer senescence in Drosophila melanogaster. Heredity. 1988;60:367–374. doi: 10.1038/hdy.1988.54. [DOI] [PubMed] [Google Scholar]
- Mockett RJ, Sohal RS, Orr WC. Overexpression of glutathione reductase extends survival in transgenic Drosophila melanogaster under hyperoxia but not normoxia. FASEB J. 1999;13:1733–1742. doi: 10.1096/fasebj.13.13.1733. [DOI] [PubMed] [Google Scholar]
- Mockett RJ, Orr WC, Rahmandar JJ, Sohal BH, Sohal RS. Antioxidant status and stress resistance in long- and short-lived lines of Drosophila melanogaster. Exp. Geront. 2001;36:441–463. doi: 10.1016/s0531-5565(00)00258-8. [DOI] [PubMed] [Google Scholar]
- Mockett RJ, Orr WC, Sohal RS. Overexpression of Cu,ZnSOD and MnSOD in transgenic Drosophila. Meth. Enzymol. 2002;349:213–220. doi: 10.1016/s0076-6879(02)49336-6. [DOI] [PubMed] [Google Scholar]
- Orr WC, Sohal RS. Effects of overexpression of Cu-Zn superoxide dismutase on life span and response to oxidative stress. Arch. Biochem. Biophys. 1993;301:34–40. doi: 10.1006/abbi.1993.1111. [DOI] [PubMed] [Google Scholar]
- Reed DJ. Glutathione: toxicological implifications. Ann. Rev. Pharmacol. Toxicol. 1990;30:603–631. doi: 10.1146/annurev.pa.30.040190.003131. [DOI] [PubMed] [Google Scholar]
- Rebrin I, Kamzalov S, Sohal RS. Effects of age and caloric restriction on glutathione redox state in mice. Free Radic. Biol. Med. 2003;35:626–635. doi: 10.1016/s0891-5849(03)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebrin I, Bayne A-CV, Mockett RJ, Orr WC, Sohal RS. Free aminothiols, glutathione redox state and protein mixed disulphides in aging Drosophila melanogaster. Biochem. J. 2004;382:131–136. doi: 10.1042/BJ20040506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richie JP, Jr., Lang CA. A decrease in cysteine levels causes the glutathione deficiency in aging of the mosquito. Proc. Soc. Exp. Biol. Med. 1988;187:235–240. doi: 10.3181/00379727-187-42660. [DOI] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Dubey A. Mitochondrial oxidative damage, hydrogen peroxide release, and aging. Free Radic. Biol. Med. 1994;16:621–626. doi: 10.1016/0891-5849(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Mockett RJ, Orr WC. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic. Biol. Med. 2002;33:575–586. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens JF, Freeman BA, Levitt JG, Crapo JD. Hyperoxia increases H2O2 release by lung mitochondria and microsomes. Arch. Biochem. Biophys. 1982a;217:401–410. doi: 10.1016/0003-9861(82)90519-7. [DOI] [PubMed] [Google Scholar]
- Turrens JF, Freeman BA, Crapo JD. Hyperoxia increases H2O2 release by lung mitochondria and microsomes. Arch. Biochem. Biophys. 1982b;217:411–421. doi: 10.1016/0003-9861(82)90519-7. [DOI] [PubMed] [Google Scholar]
- Wang H, Liu H, Liu R-M. Gender differences in glutathione metabolism during aging in mice. Exp. Geront. 2003;38:507–517. doi: 10.1016/s0531-5565(03)00036-6. [DOI] [PubMed] [Google Scholar]