Abstract
In human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus (SIV) the gag gene encodes the precursor polyprotein Pr55Gag, which is cleaved by the viral protease to produce the major structural proteins. Recently, it has been shown that HIV and SIV gag RNAs contain internal ribosome entry sites (IRESs) that mediate translation of Pr55Gag [Pr57Gag in HIV type 2 (HIV-2)] isoforms. Previously, we demonstrated that SIVmac239 p43(–), a mutant that does not express the Pr55Gag isoform, SIV p43, replicates more efficiently than wild-type (WT) SIVmac239 in cell culture. In this study, we characterize SIVmac239 p43(–) virion production and demonstrate that, in the absence of SIV p43, cleavage of Pr55Gag is increased in budded virions, resulting in a higher percentage of mature particles. Additionally, intracellular cleavage of Pr55Gag is increased in SIVmac239 p43(–), suggesting that SIV p43 suppresses premature cleavage of Pr55Gag by the viral protease.
Introduction
In human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus (SIV) the structural proteins and viral enzymes are encoded by the gag and pol genes, respectively, and translated as the precursor polyproteins, Pr55Gag and Pr160Gag-Pol (Henderson et al., 1990; Jacks et al., 1988; Ratner et al., 1985). Late in the HIV/SIV lifecycle, Pr55Gag and Pr160Gag-Pol polyproteins oligomerize in the cytoplasm (Cen et al., 2004; Chiu, Yao, and Wang, 2002; Huang and Martin, 1997; Park and Morrow, 1992) and are targeted to the plasma membrane of the host cell (Bryant and Ratner, 1990; Gonzalez et al., 1993; Henderson et al., 1988; Yuan et al., 1993) where they initiate budding of the virion. During and after virion budding, the polyproteins are cleaved by the viral protease (PR) into their constitutive domains in a process referred to as maturation (Henderson et al., 1990; Kaplan, Manchester, and Swanstrom, 1994; Rue et al., 2005; Vogt, 1996). Maturation of virions begins when PR, initially present as a domain within Pr160Gag-Pol, cleaves itself from the polyprotein (Debouck et al., 1987; Meek et al., 1989). Subsequently, PR cleaves Pr55Gag into its constituent domains: matrix (MA), capsid (CA), nucleocapsid (NC), and p6 (Henderson et al., 1990). During maturation, the virion undergoes a major morphological rearrangement in which CA forms a cone shaped core around the NC-bound viral RNA, and MA remains associated with the viral envelope [reviewed in (Gelderblom, Ozel, and Pauli, 1989)]. PR also releases the other viral enzymes, reverse transcriptase (RT) and integrase (IN) from Pr160Gag-Pol. Maturation of virions is an absolute requirement for the production of infectious particles (Kaplan et al., 1993; Kohl et al., 1988; Peng et al., 1989; Pettit et al., 1994).
In addition to expression of Pr55Gag, we and others have reported the discovery of N-terminally truncated Pr55Gag (Pr57Gag in HIV-2) isoforms expressed in HIV-1, HIV-2 and SIV (Buck et al., 2001; Herbreteau et al., 2005; Mervis et al., 1988; Nicholson et al., 2006). Translation of these isoforms is promoted by internal ribosome entry sites (IRESs) (Buck et al., 2001; Herbreteau et al., 2005), which are structural RNA elements that mediate translation initiation via a cap-independent mechanism [for reviews, see (Hellen and Sarnow, 2001; Vagner, Galy, and Pyronnet, 2001)]. An interesting feature of the IRESs that drive expression of the HIV and SIV Pr55Gag isoforms is that they reside entirely within the coding region of their respective gag mRNAs. Typically, IRESs are found in the 5’ untranslated region (UTR) of mRNAs.
An additional and intriguing feature of the HIV gag IRESs (as they will be referred to herein) in particular is that, in addition to driving expression of the N-terminally truncated Pr55Gag isoforms, they promote translation of full-length Pr55Gag from the upstream gag start codon (Buck et al., 2001; Herbreteau et al., 2005). In contrast, our data suggested that the SIV gag IRES was not able to efficiently drive expression of Pr55Gag from the upstream start codon: in vitro translation of a leaderless gag construct demonstrated that expression of Pr55Gag was dependent on the 5’ m7G cap structure and was inhibited in the presence of excess free 5’ m7G (Nicholson et al., 2006). Within the same construct, SIV p43 was translated efficiently in both cases, indicating that the SIV gag IRES only functioned to drive expression of SIV p43.
Besides the difference in the ability of the HIV gag IRESs and the SIV gag IRES to drive expression from an upstream codon, the HIV Pr55Gag isoforms appear to function quite differently than SIV p43. The HIV Pr55Gag isoforms, HIV-1 p40, HIV-2 p50 and HIV-2 p44, seem to be required for efficient viral replication (Buck et al., 2001; Herbreteau et al., 2005). In contrast, an SIVmac239 mutant that does not express SIVp43 replicates more efficiently in cell culture than wild-type (WT) SIVmac239 (Nicholson et al., 2006). As of yet, no specific function(s) have been described for any of the HIV/SIV Pr55Gag isoforms.
In this study, we identify one mechanism by which SIV p43 functions in the viral lifecycle. Characterization of SIVmac239 p43(–) demonstrates that, compared to populations of WT SIVmac239 virions, populations of SIVmac239 p43(–) virions have a higher percentage of mature virions. We also demonstrate that, in the absence of SIV p43, intracellular cleavage of Pr55Gag is increased, suggesting that SIV p43 suppresses premature cleavage of full-length Pr55Gag by the viral protease.
Materials and Methods
DNA constructs
The WT SIVmac239 and SIVmac239 p43(–) viral clones were previously described (Nicholson et al., 2006). Briefly, SIVmac239 p43(–) was generated by in vitro mutagenesis of SIVmac239 pBS− and encoded an ATG → ATC mutation (mutated base underlined) at ATG118 of the MA domain of gag. Vectors M(118)IMA and FSMA are based on the gag-alone expression vector, SIV Pr55GagpCI, and have been previously described (Nicholson et al., 2006). Briefly, M(118)IMA contains an ATG → ATC mutation at ATG118 of the MA domain of gag and has been shown to eliminate expression of SIV p43 (Nicholson et al., 2006). FSMA contains a −1 frameshift at base 53 of the MA domain of SIV gag and has been shown to express SIV p43 but not SIV Pr55Gag.
Cell culture and metabolic labeling
Human embryonic kidney 293T cells (American Type Culture Collection, Manassas, Virginia) were maintained as previously described (Rue et al., 2003). 293T cells at ~40% confluence were transfected in T-75 flasks with 9.75 μg DNA or T-150 flasks with 21 μg DNA using Lipofectamine Plus (Invitrogen) according to the manufacturer’s instructions. Twenty-four hours post-transfection, cells were labeled with [35S]Met/Cys for 4 h as previously described (Nicholson et al., 2006). Radiolabeled cells were washed in phosphate-buffered saline (PBS), lysed in modified radioimmunoprecipitation assay (RIPA) buffer (PBS with 1% [vol/vol] Igepal [NP-40], 0.5% [wt/vol] sodium deoxycholate, 0.1% [wt/vol] sodium dodecyl sulfate [SDS], 1 mM sodium orthovanadate, and protease inhibitor cocktail III [Calbiochem]), and clarified by sonication and centrifugation. Cell supernatants were filtered through a Millex 0.45-μm-pore-size syringe-tip filter (Millipore), and radiolabeled virus was pelleted through a 20% sucrose cushion in TNE buffer (20mM Tris [pH 8.0], 150 mM NaCl, and 2 mM EDTA) at 133,000 x g for 2 h at 4°C. Virus pellets were then lysed in RIPA buffer.
For immunoprecipitations, radiolabeled cell (500 μg) and virus lysates were incubated with 3 μg of immunoglobulin G (IgG)-purified rabbit SIV CA polyclonal antiserum (HRP) for 1 h at 4°C, followed by an overnight incubation with Protein A-agarose beads (Sigma-Aldrich) at 4°C. Protein A-agarose beads were pelleted and washed with RIPA buffer and proteins were resolved by SDS-PAGE using 12.5% Tris-HCl Criterion pre-cast gels (Bio-Rad). Fixed gels were analyzed using a Typhoon 9210 phosphorimager and Molecular Dynamics ImageQuant version 5.5 software (Amersham Pharmacia).
Transmission electron microscopy
293 T cells were transfected as described above with proviral DNA for either WT SIVmac239 or SIVmac239 p43(–). One day post-transfection, the cells were pelleted and fixed at 4°C in 2.5% glutaraldehyde in Millonig’s sodium phosphate buffer for 2 h and washed three times with Millonig’s sodium phosphate buffer. Samples were submitted to Electron Microscopy Bioservices, LLC (EMBS, Frederick, MD) for ultra-thin sectioning and analysis.
Virion morphology comparison
The proportion of mature virions in WT SIVmac239 and SIVmac239 p43(–) samples was determined as follows: completely budded virions in 25 photomicrographs of each virus sample were assessed as either immature or mature. In order to minimize bias in the study, two individuals analyzed photomicrographs that were randomly ordered and numbered by an outside individual. The proportion of mature particles for each viral clone were compared using a two-sided, two sample test of proportions (Rosner, 2000).
Analysis of proteolytic processing of Pr55Gag in budded virions
Proteolytic processing of Pr55Gag in budded virions was carried out as previously described (Rue et al., 2005). Briefly, 293T cells were transfected as described above with proviral DNA for either WT SIVmac239 or SIVmac239 p43(–) and labeled with [35S]Met/Cys as described above, except for only 1 h. Virus-containing supernatants were filtered, aliquoted, and incubated at 37ºC for different periods of time. At each time point, an aliquot was immediately frozen at −80°C in order to stop processing. At the end of the time course, all aliquots were thawed in an ice bath. Virus from each aliquot was purified by centrifugation through a sucrose cushion, lysed and immunoprecipitated with antibody to CA as described above.
Co-transfections and SIV p27 ELISA
For co-transfection experiments, 293T cells were transfected in 6-well plates with 2 μg total DNA (1 μg each plasmid) using Lipofectamine Plus (Invitrogen) according to the manufacturer’s instructions. 24 hours post-transfection, virus-containing supernatants were pelleted briefly to remove cell debris and assayed for p27 production with the SIV core antigen assay kit (Coulter).
Results
Replication kinetics of SIVmac239 p43(–)
Previously, we reported that SIVmac239 p43(–) exhibited increased replication kinetics in CEMX174 cells compared to WT SIVmac239 [Fig. 1, reprinted from (Nicholson et al., 2006) with permission from Elsevier]. Initially, we focused our analysis on the last 10 days of the growth curve and reported that, between days 6 and 16 post-transfection, SIVmac239 p43(–) replication was increased 2-fold compared to WT SIVmac239. However, re-examination of these data revealed an aspect of the growth curve that warranted further investigation: at day 2 post-transfection, SIVmac239 p43(–) replication is reduced approximately 75% compared to WT SIVmac239 (Fig. 1). To investigate the decreased replication of SIVmac239 p43(–) at day 2 in the growth curve, we simplified our experimental system in order to characterize a single round of SIVmac239 p43(–) replication. In addition to accounting for the decreased replication of SIVmac239 p43(–) at the early time point in the growth curve, it was hoped that characterization of a single round of SIVmac239 p43(–) replication might provide insight into the later stage increase in replication kinetics of SIVmac239 p43(–) in cell culture compared to WT SIVmac239.
FIG. 1.
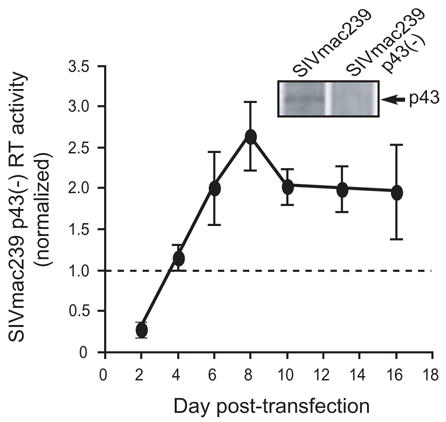
Replication kinetics of SIVmac239 p43(–). Cell-free supernatants of CEMX174 cells transfected with wild-type (WT) SIVmac239 or SIVmac239 p43(–) were analyzed on the indicated days post-transfection using a standard reverse transcription (RT) assay. The values plotted represent the RT activity of SIVmac239 p43(–) normalized to WT SIVmac239 RT activity at each time-point from three independent experiments. Inset. Lysates prepared from COS-1 cells that were transfected with either WT SIVmac239 or SIVmac239 p43(–) were labeled with [35S]Met/Cys and immunoprecipitated as described in Materials and Methods. Note the absence of SIV p43 in SIVmac239 p43(–). Figure reprinted from (Nicholson et al., 2006) with permission from Elsevier.
Cleavage of Pr55Gag is enhanced in SIVmac239 p43(–)
To examine a single round of virus replication, 293T cells were transfected with equal amounts of proviral DNA of either WT SIVmac239 or SIVmac239 p43(–) and metabolically labeled with [35S]Met/Cys. Virus particles were purified from cell supernatants, and both cell lysates and virus lysates were immunoprecipitated with antibody to SIV capsid (CA) and resolved by SDS-PAGE (Fig. 2A). Although we transfected the cells with an equal amount of proviral DNA, we consistently observed less Pr55Gag in both cell and virus lysates derived from cells transfected with SIVmac239 p43(–). However, despite the observed lower levels of Pr55Gag in SIVmac239 p43(–), it appeared that the CA levels were approximately equivalent in SIVmac239 p43(–) and WT SIVmac239. Since CA is the final product of Pr55Gag processing (Pettit et al., 1994), the CA/Pr55Gag ratio is an indicator of the efficiency of Pr55Gag processing by the viral protease; therefore, we calculated the ratio of CA to Pr55Gag in each lane (Fig. 2B). In both cell and virus lysates derived from cells transfected with SIVmac239 p43(–), the ratio of CA/Pr55Gag was increased approximately 2-fold compared to WT SIVmac239, indicating that proteolytic cleavage of Pr55Gag is increased in SIVmac239 p43(–).
FIG. 2.
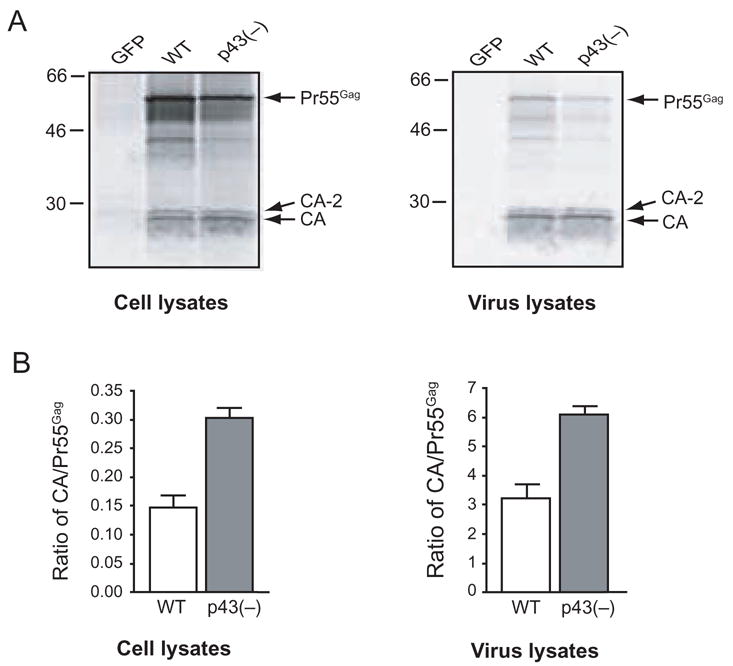
Cleavage of Pr55Gag is enhanced in SIVmac239 p43(–). A. Cell or virus lysates prepared from 293T cells that were transfected with the control vector pEGFP-N1, WT SIVmac239 (WT) proviral DNA, or SIVmac239 p43(–) [p43(–)] proviral DNA and labeled with [35S]Met/Cys were immunoprecipitated with IgG-purified SIV CA polyclonal antiserum. Immunoprecipitates were resolved by SDS-PAGE and visualized by phosphorimager analysis. B. CA and Pr55Gag levels were quantitated by band densitometry of gels from at least three independent immunoprecipitation experiments, and the ratio of CA/Pr55Gag was determined.
To control for the possibility that this observation was due to an unexpected change in antibody recognition due to the M → I substitution in SIVmac239 p43(–) we also subjected non-immunoprecipitated, [35S]-labeled virus lysates of WT SIVmac239 and SIVmac239 p43(–) to SDS-PAGE and phosphorimager analysis. Non-immunoprecipitated, virus lysates exhibited the same increase in CA/Pr55Gag ratio in SIVmac239 p43(–) compared to WT SIVmac239 (data not shown), confirming that cleavage of Pr55Gag is increased in SIVmac239 p43(–) compared to WT SIVmac239.
Transmission electron microscopy of SIVmac239 p43(–) virions
To further characterize a single round of SIVmac239 p43(–) replication, we examined 293T cells transfected with either WT SIVmac239 or SIVmac239 p43(–) by transmission electron microscopy (TEM) (Fig. 3). TEM analysis allowed for the clarification of several features of SIVmac239 p43(–) observed in Figure 2. First, TEM analysis could confirm the decrease in SIVmac239 p43(–) virion production suggested by the lower levels of Pr55Gag in SIVmac239 p43(–) virus lysates compared to WT SIVmac239 virus lysates in Figure 2. Second, we could examine the effect of the increased Pr55Gag cleavage observed in SIVmac239 p43(–) on virion morphology. It was not clear whether the increased CA/Pr55Gag ratio in SIVmac239 p43(–) virus lysates indicated that Pr55Gag was being cleaved at a higher rate within budded virions, or if, due to the enhanced cleavage of Pr55Gag in the cell, virions were released with a higher CA/Pr55Gag ratio. If SIVmac239 p43(–) virions had a higher CA/Pr55Gag ratio prior to budding, the presence of excess CA could affect virion or core morphology. Alternatively, if cleavage of Pr55Gag was increased in budded virions, it might be reflected by the presence of more mature particles in a given population of SIVmac239 p43(–) virions compared to a population of WT SIVmac239 virions.
FIG. 3.
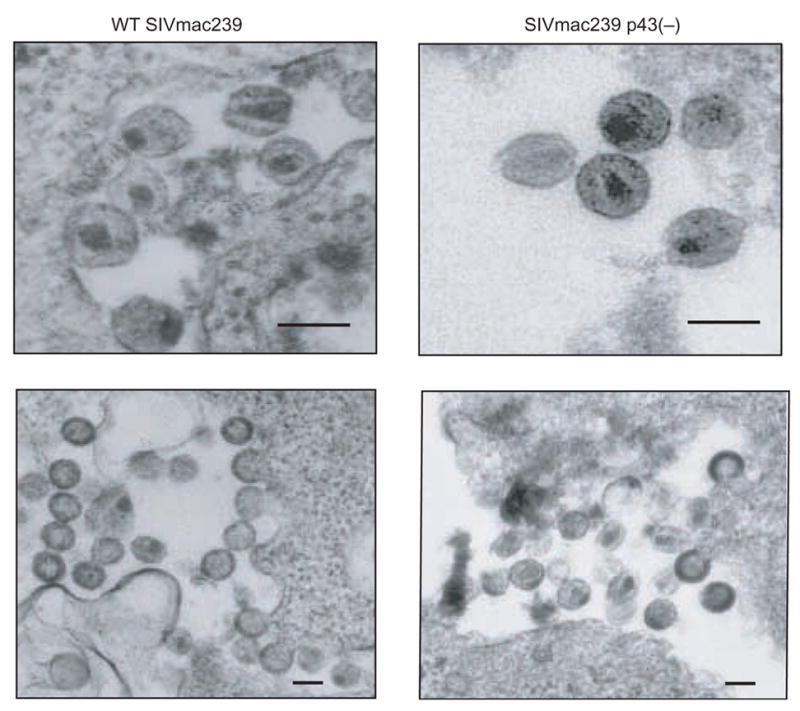
Transmission electron micrographs of WT SIVmac239 and SIVmac239 p43(–). 293T cells transfected with WT SIVmac239 or SIVmac239 p43(–) were harvested 24 h post-transfection, ultra thin-sectioned, and analyzed by transmission electron microscopy. Bars represent 100nm.
293T cells were transfected with equal amounts of proviral DNA for either WT SIVmac239 or SIVmac239 p43(–), and 24 hours post-transfection cells were fixed and submitted for TEM analysis. In both samples we observed examples of typical lentivirus virions: budding virions, immature virions and mature virions (Fig. 3). We did not observe any gross morphological differences between WT SIVmac239 and SIVmac239 p43(–) virions, suggesting that the enhanced cleavage of Pr55Gag in SIVmac239 p43(–) did not result in production of aberrant virus particles. We also did not observe any differences in core morphology in SIVmac239 p43(–) virions compared to WT SIVmac239 virions. However, consistent with the data from the immunoprecipitation experiments presented in Figure 2, there did appear to be fewer virions in the SIVmac239 p43(–) samples. Additionally, it appeared that in the SIVmac239 p43(–) samples, there was a higher percentage of mature particles than in WT SIVmac239 (Fig. 3).
To quantitatively confirm that SIVmac239 p43(–) produced a higher proportion of mature particles compared to WT SIVmac239, we compared the percentage of mature virions in both samples as described in the Materials and Methods. Briefly, in a blinded study, 25 electron micrographs of WT SIVmac239 and SIVmac239 p43(–) were examined, and every completely budded virion was judged as either immature or mature. Any virion containing an electron dense outer shell was designated as immature and any virion containing a condensed core (conical, centric, or acentric) was designated as mature (Gelderblom et al., 1987). Consistent with the apparent reduction in Pr55Gag levels in both cell and virus lysates in our immunoprecipitation experiments (Fig. 2), we observed approximately 50% fewer budded SIVmac239 p43(–) virus particles than WT SIVmac239 particles (Fig. 4). We found that SIVmac239 p43(–) contained 85% mature particles, which was significantly higher (p<0.001) than the 57% observed in WT SIVmac239 (Fig. 4). These data clearly demonstrate that the increased processing of Pr55Gag in SIVmac239 p43(–) observed in virus lysates (Fig. 2) reflects an increase in virion maturation in SIVmac239 p43(–) virions compared to WT SIVmac239 virions.
FIG. 4.
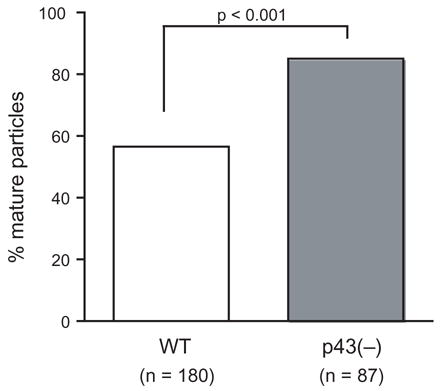
Percentage of mature virus particles observed for WT SIVmac239 and SIVmac239 p43(–). The p-value was calculated from a two sample test for the difference of proportions.
Characterization of a single round of virus replication by immunoprecipitation experiments and TEM analysis prompted us to propose a model that could account for the increased replication kinetics of SIVmac239 p43(–). In early rounds of replication in the growth curve experiments (up to day 2 post-transfection), SIVmac239 p43(–) produces fewer virions than WT SIVmac239, resulting in lower RT activity observed in the supernatant. However, the SIVmac239 p43(–) virions that are produced mature faster and thus are able to spread more quickly than WT SIVmac239 virions. If the increased rate of spread compensates and eventually overcomes the decrease in virion production, the result would be increased virus replication, which is observed by day 6 post-transfection.
Pr55Gag processing in SIVmac239 p43(–) virions
To further examine the increase in maturation of SIVmac239 p43(–) virions, we used an assay previously described by our laboratory to examine processing of Pr55Gag in budded WT SIVmac239 and SIVmac239 p43(–) virions over time (Rue et al., 2005). Briefly, 293T cells transfected with WT SIVmac239 or SIVmac239 p43(–) were labeled with [35S]Met/Cys and virus-containing supernatants were aliquoted and incubated at 37ºC for different periods of time. At each time point an aliquot was immediately frozen at −80°C to stop processing of Pr55Gag. At the end of the time course all aliquots were thawed, and virus from each aliquot was purified by centrifugation through a sucrose cushion, lysed, immunoprecipitated with antibody to CA and resolved by SDS-PAGE (Fig. 5A, B). It is important to note that there is a crucial difference in experimental design between this experiment and the immunoprecipitation experiment in Figure 2. In the immunoprecipitation experiments, cells were labeled with [35S] for 4 hours to generally characterize SIV protein expression and virion production. However, in this next experiment, we wanted to track the maturation of a specific population of budded virions and therefore reduced the labeling time to 1 hour.
FIG. 5.

Proteolytic cleavage of Pr55Gag in cell-free SIV virions. 293T cells were transfected with either the control vector pEGFP-N1, (A) WT SIVmac239 proviral DNA, or (B) SIVmac239 p43(–) proviral DNA and labeled with [35S]Met/Cys. Virus-containing supernatants were aliquoted and incubated at 37°C for the indicated times. At the end of the time-course, virus was purified through a sucrose cushion, lysed, immunoprecipitated with IgG-purified SIV CA polyclonal antiserum, resolved by SDS-PAGE and visualized using a phosphorimager. Gels are representative of three independent experiments. (C) CA and Pr55Gag levels were quantitated by band densitometry of gels from at least three independent intravirion cleavage experiments, and the ratio of CA/Pr55Gag was determined for each time point.
We examined the CA/Pr55Gag ratio at each time point in the 6 hour time course and found that between 0 and 30 minutes, the CA/Pr55Gag ratio was essentially the same in WT SIVmac239 and SIVmac239 p43(–). However, by 1 h, the CA/Pr55Gag ratio was increased slightly in SIVmac239 p43(–) virions, and had increased to approximately 1.4-fold by 2 hrs (Fig. 5C). At the end of the time course, the CA/Pr55Gag ratio remained approximately 1.4-fold higher in SIVmac239 p43(–) virions compared to WT SIVmac239 virions. These data confirm that the CA/Pr55Gag ratio increases over time in SIVmac239 p43(–) virions at a faster rate than in WT SIVmac239 virions, and further support our model to explain the observed replication kinetics of SIVmac239 p43(–) in cell culture.
Overexpression of SIV p43 increase particle production in WT SIVmac239
Because increased intracellular processing suppresses particle release (17, 20, 31), a prediction of the model we propose is that increased levels of SIV p43 would further suppress intracellular processing thereby increasing particle production by WT SIVmac239. To test this prediction, we co-transfected 293T cells with WT SIVmac239 and either an SIV p43 expression vector (FSMA) or GFP as a negative control and measured virus release into the supernatant by p27 ELISA. Compared to WT SIVmac239 co-transfected with GFP, WT SIVmac239 co-transfected with SIV p43 produced approximately 50% more particles, confirming the prediction of our model (Fig. 6). We wanted to determine whether the observed increase in particle production was due exclusively to SIV p43, or if any “Gag-like” protein would have a similar effect. Since the gag gene expresses not only Pr55Gag, but SIV p43 as well, we co-transfected WT SIVmac239 with M(118)IMA, a previously described gag-alone expression vector that carries the mutation of the start codon of SIV p43 to ATC (Nicholson et al., 2006). Thus, the use of this expression vector allowed us to examine the effect of excess Pr55Gag on particle production without increasing levels of SIV p43. Co-transfection of WT SIVmac239 with M(118)IMA resulted in similar levels of particle production observed in co-transfections of WT SIVmac239 with GFP, strongly indicating that the observed increase in particle production in the presence of increased levels of SIV p43 was due exclusively to SIV p43 (Fig. 6).
FIG. 6.

Overexpression of SIV p43 result in increased particle production from WT SIVmac239. 293T cells were co-transfected with WT SIVmac239 and either the control vector pEGFP-N1, an SIV p43 expression vector, or the Pr55Gag-alone expression vector, M(118)IMA. Virus-containing supernatants were assayed for p27 content and data represent the mean and standard deviation from 6 independent experiments.
Discussion
Previously we, and others, identified IRESs within HIV-1, HIV-2 and SIV gag RNAs that mediate expression of N-terminally truncated Pr55Gag (Pr57Gag in HIV-2) isoforms (Buck et al., 2001; Herbreteau et al., 2005; Nicholson et al., 2006). Here, we report the first function attributed to any of the HIV/SIV Pr55Gag isoforms and present compelling evidence that demonstrates SIV p43 acts to suppress proteolytic processing of Pr55Gag by the viral protease.
This study was initiated to investigate the early replication kinetics of SIVmac239 p43(–) in cell culture, and our analysis of a single round of replication has lead us to propose the following model to describe the observed SIVmac239 p43(–) replication kinetics over time in cell culture. We propose that SIVmac239 p43(–) replication is decreased compared to WT SIVmac239 in the early phase of the growth curve due to the decreased production of virions by SIVmac239 p43(–). However, the increased rate of SIVmac239 p43(–) virion maturation results in a faster spread of the virus through the cell culture, ultimately offsetting the decrease in virion production over time.
While the increased replication kinetics of SIVmac239 p43(–) after day 6 of the CEMX174 growth curve experiment are intriguing, the most important aspect of SIV p43 observed in this study may be the apparent decrease in virion production observed in cells transfected with SIVmac239 p43(–) likely due to the increase in intracellular cleavage of Pr55Gag in the absence of SIV p43. Previous reports have demonstrated that overexpression of the HIV-1 viral protease (PR) increases intracellular cleavage of Pr55Gag, and results in a dramatic reduction in virion budding and infectivity (Karacostas et al., 1993; Luukkonen, Fenyo, and Schwartz, 1995; Shehu-Xhilaga, Crowe, and Mak, 2001). While the frameshift mechanism that allows for translation of PR limits the amount of PR synthesized relative to Pr55Gag, further regulation of PR activity may be required to ensure cleavage of Pr55Gag does not occur prematurely (Krausslich, 1991). Thus, we propose that the major function of SIV p43 may be to suppress intracellular cleavage of Pr55Gag by the viral protease. SIV p43 is expressed at low levels relative to Pr55Gag and the effect it has on cleavage of Pr55Gag are subtle. However, a subtle effect is most likely all that is required of SIV p43; since cleavage of Pr55Gag is required for the production of infectious particles, greater suppression of Pr55Gag processing by SIV p43 could be detrimental to the virus.
What we have contemplated throughout our investigation of SIV p43, is why SIVmac239 p43(–) exhibits such different replication kinetics than the HIV mutants that did not express their respective Pr55Gag isoforms. If we presume that the HIV IRES- driven Pr55Gag isoforms function similarly to SIV p43 to suppress intracellular cleavage of Pr55Gag, the difference in replication kinetics may be reconciled simply by comparing the processing of Pr55Gag in budded HIV-1 and SIV virions. In this study and an earlier report, we demonstrate that processing of SIV Pr55Gag can occur over time in budded virions (Rue et al., 2005). However, in HIV-1 it is not clear whether intravirion processing of Pr55Gag occurs; one study suggested that processing of Pr55Gag occurred either before the virion was released from the cell, or very shortly after budding (Kaplan, Manchester, and Swanstrom, 1994). Also, it is generally agreed that populations of HIV- 1 virions contain very few, if any immature budded virions (compared to the approximate 40% we observe in WT SIVmac239), strongly supporting the claim that the vast majority of Pr55Gag processing in HIV-1 occurs prior to virion release. Thus, if the HIV-1 IRES-driven Pr55Gag isoform, HIV-1 p40, inhibits processing of Pr55Gag, we would expect that in the absence of HIV-1 p40, processing would occur at a faster rate. Due to an increase in the rate of intracellular Pr55Gag processing, it is possible that Pr55Gag could be cleaved in the host cell before virions could properly assemble and begin budding, resulting in a profound decrease in virus replication as observed in the case of HIV-1 p40(–).
Thus, although the long term replication kinetics of the HIV mutants are exactly opposite of what is observed in SIVmac239 p43(–), they are consistent with the proposed model of SIV p43 function. If it is confirmed that the HIV Pr55Gag isoforms prevent premature intracellular cleavage of Pr55Gag, they may provide attractive targets for antiretroviral therapeutic agents.
Acknowledgments
We would like to thank Dr. Sarah Rue for her assistance with counting and classifying virus particles and Dr. Patrick Tarwater for the statistical analysis of the TEM study. Additionally, we thank the members of the Retrovirus lab for helpful discussions. This work was supported by grants to J.E.C. from the National Institutes of Health (NS47984, NS07392, MH70306 and HL75840).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bryant M, Ratner L. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc Natl Acad Sci U S A. 1990;87(2):523–7. doi: 10.1073/pnas.87.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CB, Shen X, Egan MA, Pierson TC, Walker CM, Siliciano RF. The human immunodeficiency virus type 1 gag gene encodes an internal ribosome entry site. J Virol. 2001;75(1):181–91. doi: 10.1128/JVI.75.1.181-191.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cen S, Niu M, Saadatmand J, Guo F, Huang Y, Nabel GJ, Kleiman L. Incorporation of pol into human immunodeficiency virus type 1 Gag virus-like particles occurs independently of the upstream Gag domain in Gag-pol. J Virol. 2004;78(2):1042–9. doi: 10.1128/JVI.78.2.1042-1049.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu HC, Yao SY, Wang CT. Coding sequences upstream of the human immunodeficiency virus type 1 reverse transcriptase domain in Gag-Pol are not essential for incorporation of the Pr160(gag-pol) into virus particles. J Virol. 2002;76(7):3221–31. doi: 10.1128/JVI.76.7.3221-3231.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debouck C, Gorniak JG, Strickler JE, Meek TD, Metcalf BW, Rosenberg M. Human immunodeficiency virus protease expressed in Escherichia coli exhibits autoprocessing and specific maturation of the gag precursor. Proc Natl Acad Sci U S A. 1987;84(24):8903–6. doi: 10.1073/pnas.84.24.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom HR, Hausmann EH, Ozel M, Pauli G, Koch MA. Fine structure of human immunodeficiency virus (HIV) and immunolocalization of structural proteins. Virology. 1987;156(1):171–6. doi: 10.1016/0042-6822(87)90449-1. [DOI] [PubMed] [Google Scholar]
- Gelderblom HR, Ozel M, Pauli G. Morphogenesis and morphology of HIV. Structure-function relations. Arch Virol. 1989;106(1–2):1–13. doi: 10.1007/BF01311033. [DOI] [PubMed] [Google Scholar]
- Gonzalez SA, Affranchino JL, Gelderblom HR, Burny A. Assembly of the matrix protein of simian immunodeficiency virus into virus-like particles. Virology. 1993;194(2):548–56. doi: 10.1006/viro.1993.1293. [DOI] [PubMed] [Google Scholar]
- Hellen CU, Sarnow P. Internal ribosome entry sites in eukaryotic mRNA molecules. Genes Dev. 2001;15(13):1593–612. doi: 10.1101/gad.891101. [DOI] [PubMed] [Google Scholar]
- Henderson LE, Benveniste RE, Sowder R, Copeland TD, Schultz AM, Oroszlan S. Molecular characterization of gag proteins from simian immunodeficiency virus (SIVMne) J Virol. 1988;62(8):2587–95. doi: 10.1128/jvi.62.8.2587-2595.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LE, Sowder RC, Copeland TD, Oroszlan S, Benveniste RE. Gag precursors of HIV and SIV are cleaved into six proteins found in the mature virions. J Med Primatol. 1990;19(3–4):411–9. [PubMed] [Google Scholar]
- Herbreteau CH, Weill L, Decimo D, Prevot D, Darlix JL, Sargueil B, Ohlmann T. HIV-2 genomic RNA contains a novel type of IRES located downstream of its initiation codon. Nat Struct Mol Biol. 2005;12(11):1001–7. doi: 10.1038/nsmb1011. [DOI] [PubMed] [Google Scholar]
- Huang M, Martin MA. Incorporation of Pr160(gag-pol) into virus particles requires the presence of both the major homology region and adjacent C-terminal capsid sequences within the Gag-Pol polyprotein. J Virol. 1997;71(6):4472–8. doi: 10.1128/jvi.71.6.4472-4478.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T, Power MD, Masiarz FR, Luciw PA, Barr PJ, Varmus HE. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature. 1988;331(6153):280–3. doi: 10.1038/331280a0. [DOI] [PubMed] [Google Scholar]
- Kaplan AH, Manchester M, Swanstrom R. The activity of the protease of human immunodeficiency virus type 1 is initiated at the membrane of infected cells before the release of viral proteins and is required for release to occur with maximum efficiency. J Virol. 1994;68(10):6782–6. doi: 10.1128/jvi.68.10.6782-6786.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan AH, Zack JA, Knigge M, Paul DA, Kempf DJ, Norbeck DW, Swanstrom R. Partial inhibition of the human immunodeficiency virus type 1 protease results in aberrant virus assembly and the formation of noninfectious particles. J Virol. 1993;67(7):4050–5. doi: 10.1128/jvi.67.7.4050-4055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karacostas V, Wolffe EJ, Nagashima K, Gonda MA, Moss B. Overexpression of the HIV-1 gag-pol polyprotein results in intracellular activation of HIV-1 protease and inhibition of assembly and budding of virus-like particles. Virology. 1993;193(2):661–71. doi: 10.1006/viro.1993.1174. [DOI] [PubMed] [Google Scholar]
- Kohl NE, Emini EA, Schleif WA, Davis LJ, Heimbach JC, Dixon RA, Scolnick EM, Sigal IS. Active human immunodeficiency virus protease is required for viral infectivity. Proc Natl Acad Sci U S A. 1988;85(13):4686–90. doi: 10.1073/pnas.85.13.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krausslich HG. Human immunodeficiency virus proteinase dimer as component of the viral polyprotein prevents particle assembly and viral infectivity. Proc Natl Acad Sci U S A. 1991;88(8):3213–7. doi: 10.1073/pnas.88.8.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luukkonen BG, Fenyo EM, Schwartz S. Overexpression of human immunodeficiency virus type 1 protease increases intracellular cleavage of Gag and reduces virus infectivity. Virology. 1995;206(2):854–65. doi: 10.1006/viro.1995.1008. [DOI] [PubMed] [Google Scholar]
- Meek TD, Dayton BD, Metcalf BW, Dreyer GB, Strickler JE, Gorniak JG, Rosenberg M, Moore ML, Magaard VW, Debouck C. Human immunodeficiency virus 1 protease expressed in Escherichia coli behaves as a dimeric aspartic protease. Proc Natl Acad Sci U S A. 1989;86(6):1841–5. doi: 10.1073/pnas.86.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis RJ, Ahmad N, Lillehoj EP, Raum MG, Salazar FH, Chan HW, Venkatesan S. The gag gene products of human immunodeficiency virus type 1: alignment within the gag open reading frame, identification of posttranslational modifications, and evidence for alternative gag precursors. J Virol. 1988;62(11):3993–4002. doi: 10.1128/jvi.62.11.3993-4002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson MG, Rue SM, Clements JE, Barber SA. An internal ribosome entry site promotes translation of a novel SIV Pr55(Gag) isoform. Virology. 2006;349(2):325–34. doi: 10.1016/j.virol.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Park J, Morrow CD. The nonmyristylated Pr160gag-pol polyprotein of human immunodeficiency virus type 1 interacts with Pr55gag and is incorporated into viruslike particles. J Virol. 1992;66(11):6304–13. doi: 10.1128/jvi.66.11.6304-6313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, Ho BK, Chang TW, Chang NT. Role of human immunodeficiency virus type 1-specific protease in core protein maturation and viral infectivity. J Virol. 1989;63(6):2550–6. doi: 10.1128/jvi.63.6.2550-2556.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit SC, Moody MD, Wehbie RS, Kaplan AH, Nantermet PV, Klein CA, Swanstrom R. The p2 domain of human immunodeficiency virus type 1 Gag regulates sequential proteolytic processing and is required to produce fully infectious virions. J Virol. 1994;68(12):8017–27. doi: 10.1128/jvi.68.12.8017-8027.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L, Haseltine W, Patarca R, Livak KJ, Starcich B, Josephs SF, Doran ER, Rafalski JA, Whitehorn EA, Baumeister K, et al. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985;313(6000):277–84. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Rosner B. Fundamentals of Biostatistics. 5. Duxbury; Pacific Grove, CA: 2000. [Google Scholar]
- Rue SM, Roos JW, Amzel LM, Clements JE, Barber SA. Hydrogen bonding at a conserved threonine in lentivirus capsid is required for virus replication. J Virol. 2003;77(14):8009–18. doi: 10.1128/JVI.77.14.8009-8018.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rue SM, Roos JW, Tarwater PM, Clements JE, Barber SA. Phosphorylation and proteolytic cleavage of gag proteins in budded simian immunodeficiency virus. J Virol. 2005;79(4):2484–92. doi: 10.1128/JVI.79.4.2484-2492.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehu-Xhilaga M, Crowe SM, Mak J. Maintenance of the Gag/Gag-Pol ratio is important for human immunodeficiency virus type 1 RNA dimerization and viral infectivity. J Virol. 2001;75(4):1834–41. doi: 10.1128/JVI.75.4.1834-1841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagner S, Galy B, Pyronnet S. Irresistible IRES. Attracting the translation machinery to internal ribosome entry sites. EMBO Rep. 2001;2(10):893–8. doi: 10.1093/embo-reports/kve208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt VM. Proteolytic processing and particle maturation. Curr Top Microbiol Immunol. 1996;214:95–131. doi: 10.1007/978-3-642-80145-7_4. [DOI] [PubMed] [Google Scholar]
- Yuan X, Yu X, Lee TH, Essex M. Mutations in the N-terminal region of human immunodeficiency virus type 1 matrix protein block intracellular transport of the Gag precursor. J Virol. 1993;67(11):6387–94. doi: 10.1128/jvi.67.11.6387-6394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


