Abstract
Coevolutionary theory proposes that the diversity of chemical structures found in plants is, in large part, the result of selection by herbivores. Because herbivores often feed on chemically similar plants, they should impose selective pressures on plants to diverge chemically or bias community assembly toward chemical divergence. Using a coevolved interaction between a group of chrysomelid beetles and their host plants, I tested whether coexisting plants of the Mexican tropical dry forest tend to be chemically more dissimilar than random. Results show that some of the communities are chemically overdispersed and that overdispersion is related to the tightness of the interaction between plants and herbivores and the spatial scale at which communities are measured. As coevolutionary specialization increases and spatial scale decreases, communities tend to be more chemically dissimilar. At fairly local scales and where herbivores have tight, one-to-one interactions with plants, communities have a strong pattern of chemical disparity.
Keywords: herbivore specialization, insect–herbivore interaction, plant chemical diversity
Researchers have long been interested in the question of whether coevolution, the reciprocal evolutionary influences of interacting groups of organisms, can shape patterns of divergence among related species within communities. Studies of interspecific competition have often looked at community-wide patterns of divergence among related species (1, 2). Likewise, a number of studies of pollinators and plants have focused on community-wide patterns of floral traits (3, 4). Yet, relatively less work has been focused on the role of plant–herbivore coevolution on community patterns of plant divergence. One interesting example is the work of Gilbert (5), who proposed that coevolution of Heliconius butterflies and Passiflora vines resulted in the diversification of leaf shapes of cooccurring Passiflora species.
Coevolution has been proposed as a major factor promoting the diversity of chemical compounds in plants (6, 7). The continuous selective effects of herbivore attack and plant defense are thought to be largely responsible for the incremental elaboration, proliferation, and intricacy of plant secondary compounds and insect detoxification mechanisms (8–11). Yet, the study of plant–herbivore coevolution and its impact on plant chemistry has focused primarily on interactions that involve a small number of species or populations. Such studies provide evidence that plants often produce distinctive chemicals that protect them against herbivory (11, 12). However, this approach has shed little light on the question of whether coevolution has created patterns of chemistry and interactions powerful enough to structure plant chemical diversity (6, 13).
Because related phytophagous insects often feed on plants that share common chemical compounds to which they are adapted (14–16), it is meaningful to ask whether herbivory might structure community chemical profiles, either biasing community assembly or imposing selective pressure for divergence of defensive compounds. This issue can be addressed by looking for community-level patterns caused by herbivory and coevolution (17, 18). If host shifts tend to occur between plants with similar chemistry, herbivory could limit the coexistence of plants that share common chemical compounds. If chemically similar plants cooccur, selection would favor divergence. Likewise, if a plant in a regional species pool is chemically similar to an already present species, the probability of successful invasion would be lower. Such processes of divergence and assembly should lead to chemically overdispersed communities, that is, communities with chemical defenses that are more dissimilar than expected by chance.
The effect of herbivory on diversification of chemistry should be stronger for narrowly coevolved systems involving fewer interacting species. In such associations, species tend to develop specific adaptations to the features of their counterparts (19). Thus, a chemically similar plant should be more vulnerable to a specialized herbivore able to handle many of its compounds. More diffuse associations, involving groups of herbivores interacting with a group of plants, may have more generalized adaptations because selection pressures on chemistry may conflict with multiple herbivores (20, 21). In such systems, chemical divergence is less likely to have an effect on herbivore host invasion, and community-level overdispersion is less likely to occur.
Resource competition is an alternative factor that is widely thought to influence the attributes of species and community assembly. If closely related species require the same limiting resources (i.e., water, nutrients, and pollinators), competitive exclusion may limit their coexistence (1). Several studies have shown that sympatric species are more phylogenetically distant than expected, presumably because related species have similar competitive niches (1, 22).
Here, I use the tropical genus Bursera to test whether coevolution with specialized herbivores could have resulted in community-level chemical overdispersion. I also test for community-level phylogenetic overdispersion which could suggest that resource competition is a structuring factor.
Burseras are typically low- to medium-size trees (Fig. 1). The genus includes ≈100 species distributed from the southern United States to Peru (23). It reaches its maximum diversity and abundance in the tropical dry forests of Mexico where, with ≈85 endemic species, it is one of the major elements of the flora (24, 25). The predominance of Bursera is particularly striking along the deep canyons of the Balsas River basin, which is one of the major extensions of the dry forest. On the floors of these canyons, this genus often becomes the absolute dominant woody taxon, surpassing legumes and other groups in diversity and abundance and validating the name “cuajiotales” given to many of these forests, from the Aztec name “cuajiote” (leprous tree) given to Bursera species (25–27). In the eastern region of the Balsas depression, for example, in an area of ≈50,000 km2 that includes Zopilote Canyon, ≈45 species of Bursera occur and 9–15 Bursera species commonly coexist in single localities. There are high levels of endemism in the genus, and 65% of the species have a geographic distribution of <50,000 km2. Because the genus is old, highly adapted to the ecological and climatic conditions of these forests, and of great physiognomic importance, its evolution and diversification has been linked to the history of the Mexican dry forests (24).
Fig. 1.

Bursera aptera. The genus Bursera reaches its maximum diversity and abundance in the tropical dry forests of Mexico where, with ≈85 endemic species, it is one of the major elements of the flora.
Bursera produces an array of terpenes, mostly mono- and sesquiterpenes, and alkanes (28–30). These compounds are toxic or repellent to insect herbivores and, in Bursera, decrease the survival and growth of their specialized herbivores, the chrysomelid genus Blepharida (31, 32). The impact of Blepharida on Bursera often depends on the defensive status of the plants, and poorly defended individuals with relatively low concentration of terpenes can be completely defoliated by these beetles (33).
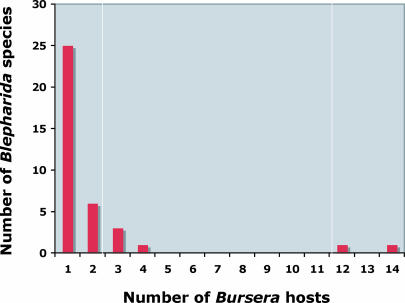
Blepharida includes ≈45 species that feed on Bursera [Fig. 2 and supporting information (SI) Table 2]. Blepharida species have been observed to be the most frequent and abundant herbivores of Bursera in visits to multiple field sites in Mexico over the past 15 years. Most Blepharida are narrowly specialized with one Bursera host (which I will refer to as “monophagous”) or two to four hosts (“oligophagous”) (Fig. 3) (32). A few, relatively more generalized (“polyphagous”) beetles feed on many Bursera hosts (32). Time-calibrated phylogenies of these insects and plants suggest that they have interacted for at least 112 million years (8) and that plant defensive chemical traits and the insects' counterdefensive feeding strategies have evolved in response to concurrent reciprocal selective pressures (32, 34).
Fig. 2.

Blepharida pallida. The Blepharida genus includes ≈45 species, which all feed only on Bursera.
Fig. 3.
Number of hosts attacked by Blepharida species. Most Blepharida species are highly specialized and are known to feed on only one (monophagous) or up to four hosts (oligophagous). But two of them are more generalized, feeding on 12 or 14 hosts (here called “polyphagous”).
These herbivores show a preference for colonizing chemically similar plants that are not necessarily phylogenetically close (14). This preference for chemically similar plants should impose pressures on plants to develop divergent chemistries. Therefore, this is a good system by which to examine whether chemical overdispersion is an aspect of plant community structure. Because related Bursera species are often chemically dissimilar, hypotheses about chemical traits can be tested without the concern that chemistry is completely correlated with plant phylogeny (14).
Results and Discussion
Testing for Community-Level Chemical Overdispersion.
To investigate whether Bursera communities are chemically overdispersed, I analyzed the chemistry of 57 species by using gas chromatography–mass spectrometry (see Methods). Analyses were directed at volatile terpenes and alkanes that are the most abundant secondary compounds in Bursera and have known impact on Blepharida (28–30). A matrix of chemical dissimilarity among species was constructed based on the presence and relative concentration of 74 chemical compounds found in these plants.
I quantified the average chemical distance among species in 18 Bursera communities in four areas of the tropical dry forests located in the Balsas basin, and three communities in one area in the Papaloapan river basin (Fig. 4). These five areas were selected because they contain the highest diversity of Bursera and Blepharida, and because the Blepharida species found there tend to be monophagous or oligophagous (35).
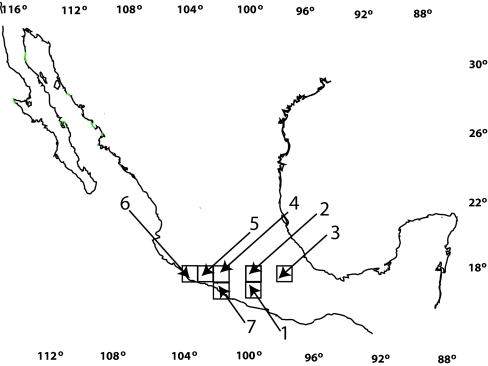
Fig. 4.
Geographic localization of selected areas of study. Areas 1–5 contain the highest diversity of Bursera and monophagous and oligophagous Blepharida. In areas 6 and 7, polyphagous Blepharida are more frequent. 1, Chilpancingo; 2, Cañón del Zopilote; 3, Tehuacán; 4, Infiernillo; 5, Aguililla; 6, Zihuatanejo; 7, Aquila.
Six other Bursera communities from two coastal areas in the Pacific were also included in the analysis (Fig. 4). These two areas were chosen because they have high numbers of Bursera species and many of them are attacked by the polyphagous Blepharida, B. alternata, and B. pallida, which are abundant there (35). All areas were ≈10,650 km2 (1° latitude and longitude), except areas in the Pacific that were smaller because part of the targeted 1° latitude and longitude was occupied by the Pacific Ocean.
Bursera tends to grow in deep canyons. Species occupy different altitudinal ranges with the highest diversity usually occurring at the canyon bottoms (35). Within areas, I used communities with increasingly large altitudinal bands (and hence geographic area) with the smaller ones nested within the larger. For all areas, the smallest altitudinal band is located at the bottom of the canyons and increasing bands add species and habitats by moving up in altitude, still within the 1° target area. Finally, for each area, I expanded the geographic extent to 3° of latitude and longitude using the full altitudinal range.
To determine which species were present in an area and at what elevations, species lists for all of the communities were constructed by using information from the major Mexican herbaria [Herbario Nacional de Mexico (MEXU), Herbario de la Escuela Nacional de Ciencias Biológicas, and Herbario del Cantro de Ecología, region del Bajío], from the on-line biodiversity information of the Mexican Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (www.conabio.gob.mx), and from repeated visits over 15 years to these areas.
I also estimated the degree of specialization in the interaction between Bursera and Blepharida species in the communities studied. For this, I calculated the percent of Bursera species that are attacked by monophagous or oligophagous Blepharida.
The percent of Bursera species that are attacked by monophagous or oligophagous Blepharida tended to be high in all communities located in the Balsas and Papaloapan river basins [Table 1; Chilpancingo (area 1), Cañón del Zopilote (area 2), Tehuacán (area 3), Infiernillo (area 4), and Aguililla (area 5)]. In most of these communities, this percentage was >58, and in most communities of small geographical scale (situated below 1,000 m of altitude), the percentage was >78. The highest level of specialization was at the community located at the bottom of the canyon in Chilapancingo, in which only one Bursera species hosts a polyphagous beetle species and all other burseras are attacked by a single monophagous or oligophagous Blepharida species each. Considerably lower levels of specialization were found in areas 6 and 7 (Zihuatanejo and Aquila) (Table 1), where the percentage of Bursera species that are attacked by monophagous or oligophagous Blepharida ranged from 22 to 53. These results are congruent with previous observations that polyphagous species tend to attack Bursera species distributed along the Pacific coast (35).
Table 1.
Results of randomization tests on phylogenetic and chemical overdispersion of Bursera communities
| Area | Localization | Elevation, m | Phylogenetic distance |
Chemical distance |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Observed, mean | Random, mean | Z score | P | No. of species sampled | Observed, mean | Random, mean | Z score | P | No. of species sampled | % species attacked by monophagous or oligophagous Blepharida | |||
| Chilpancingo | lat 17–18°, long 99–100° | 400–650 | 40.530 | 38.795 | 0.473 | 0.338 | 12 | 0.909 | 0.815 | 1.548 | 0.031** | 13 | 92 |
| 400–1,000 | 38.614 | 38.705 | −0.034 | 0.548 | 18 | 0.879 | 0.815 | 1.404 | 0.058* | 19 | 78 | ||
| 400–2,200 | 37.020 | 38.735 | −0.993 | 0.847 | 29 | 0.814 | 0.814 | −0.009 | 0.500 | 28 | 65 | ||
| lat 16–19°, long 98–101° | 400–2,200 | 36.674 | 38.726 | −1.428 | 0.913 | 34 | 0.817 | 0.815 | 0.066 | 0.435 | 36 | 61 | |
| Cañón del Zopilote | lat 18–19°, long 99–100° | 400–500 | 40.800 | 38.700 | 0.288 | 0.355 | 5 | 0.960 | 0.184 | 1.280 | 0.060* | 5 | 89 |
| 400–750 | 40.056 | 38.796 | 0.276 | 0.424 | 9 | 0.904 | 0.815 | 1.321 | 0.064* | 11 | 80 | ||
| 400–1,000 | 39.181 | 38.714 | 0.151 | 0.469 | 15 | 0.896 | 0.815 | 1.480 | 0.044 ** | 15 | 79 | ||
| 400–2,200 | 38.537 | 38.785 | −0.110 | 0.577 | 22 | 0.812 | 0.815 | −0.073 | 0.562 | 23 | 72 | ||
| lat 17–20°, long 98–101° | 400–2,200 | 39.389 | 38.717 | 0.636 | 0.275 | 42 | 0.817 | 0.815 | 0.076 | 0.489 | 39 | 69 | |
| Tehuacán | lat 18–19°, long 97–98° | 550–800 | 37.000 | 38.788 | −0.393 | 0.696 | 9 | 0.935 | 0.815 | 1.413 | 0.036** | 9 | 86 |
| 550–2,200 | 36.894 | 38.687 | −0.487 | 0.726 | 12 | 0.884 | 0.815 | 1.08 | 0.125 | 12 | 80 | ||
| lat 17–20°, long 96–99° | 550–2,200 | 39.330 | 38.719 | 0.282 | 0.415 | 24 | 0.849 | 0.815 | 0.749 | 0.236 | 19 | 82 | |
| Infiernillo | lat 18–19°, long 101–102° | 200–500 | 43.267 | 38.588 | 0.734 | 0.234 | 6 | 0.957 | 0.816 | 1.709 | 0.007** | 8 | 86 |
| 200–1,000 | 44.278 | 38.690 | 1.222 | 0.084* | 9 | 0.927 | 0.816 | 1.776 | 0.011** | 12 | 64 | ||
| 200–2,200 | 40.627 | 38.710 | 0.719 | 0.223 | 18 | 0.876 | 0.815 | 1.328 | 0.075* | 19 | 71 | ||
| lat 17–20°, long 100–103° | 200–2,200 | 37.403 | 38.723 | −0.757 | 0.784 | 29 | 0.843 | 0.815 | 0.835 | 0.209 | 27 | 68 | |
| Aguililla | lat 18–19°, long 102–103° | 150–300 | 43.867 | 38.888 | 0.781 | 0.221 | 6 | 0.981 | 0.813 | 1.445 | 0.028** | 5 | 80 |
| 150–600 | 42.607 | 38.636 | 0.788 | 0.204 | 8 | 0.954 | 0.816 | 1.659 | 0.015** | 8 | 89 | ||
| 150–1,000 | 43.033 | 38.685 | 1.350 | 0.073* | 14 | 0.888 | 0.814 | 1.204 | 0.094* | 13 | 58 | ||
| 150–2,000 | 43.331 | 38.755 | 1.879 | 0.017** | 20 | 0.818 | 0.814 | 0.087 | 0.506 | 17 | 69 | ||
| lat 17–20°, long 101–104° | 150–2,000 | 39.786 | 38.714 | 0.685 | 0.254 | 32 | 0.821 | 0.815 | 0.200 | 0.444 | 30 | 59 | |
| Zihuatanejo | lat 17–18°, long 101–102° | 0–1,000 | 38.611 | 38.682 | −0.014 | 0.547 | 9 | 0.786 | 0.815 | −0.369 | 0.699 | 9 | 22 |
| 0–2,000 | 39.439 | 38.767 | 0.186 | 0.461 | 12 | 0.778 | 0.813 | −0.570 | 0.745 | 12 | 33 | ||
| lat 17–18°, long 101–102° | 0–2,000 | 42.053 | 38.739 | 1.851 | 0.018** | 28 | 0.806 | 0.814 | −0.213 | 0.612 | 26 | 53 | |
| Aquila | lat 18–19°, long 103–104° | 0–1,000 | 40.717 | 38.682 | 0.693 | 0.245 | 16 | 0.837 | 0.815 | 0.373 | 0.415 | 15 | 43 |
| 0–2,000 | 41.987 | 38.704 | 0.466 | 0.054* | 22 | 0.826 | 0.815 | 0.256 | 0.433 | 19 | 50 | ||
| lat 17–20°, long 102–105° | 0–2,000 | 39.468 | 38.711 | 0.431 | 0.349 | 29 | 0.806 | 0.814 | −0.237 | 0.620 | 26 | 52 | |
For each community examined, the observed average chemical or phylogenetic distance between species was compared to a distribution of average chemical or phylogenetic distances of an equal number of randomly selected species (the null model). P values are reported for one-tailed tests, i.e., the observed chemical distance was greater than all but the proportion P of 1,000 null communities. ∗, P < 0.1; ∗∗, P < 0.05.
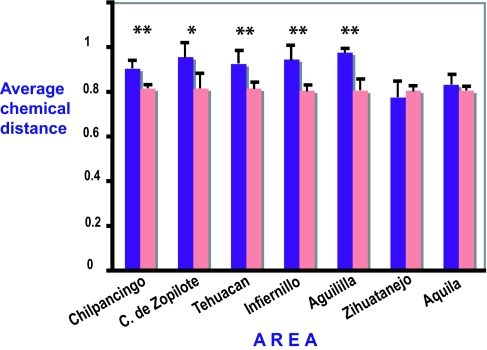
I tested for chemical overdispersion with randomization tests. I examined whether the particular configurations of species found in these real communities are more chemically diverse than communities randomly chosen from the same overall set of species. The average chemical distance for a given community was compared with the distribution of average chemical distances of many random communities constructed by choosing the same number of species randomly from the full list of species for all communities. Results show that in those areas where coevolutionary specialization with Blepharida is high (as measured by the percent of Bursera species that are attacked by monophagous or oligophagous Blepharida), communities were significantly overdispersed (Table 1). High specialization and overdispersion occur at smaller scales in all of the areas of the Balsas and Papaloapan basins: Chilpancingo, Cañón del Zopilote, Tehuacán, Infiernillo, and Aguililla (Table 1 and Fig. 5). This pattern is consistent with the idea that beetles impose stronger selective pressure for chemical divergence of the locally present plant species. However, increasing scale in this study also results in increasing habitat diversity by including higher-elevation habitats. So, the decline in overdispersion with scale could be due to weaker species interactions or a greater importance of overdispersion at canyon bottoms than at higher elevations. In both areas on the Pacific coast (Zihuatanejo and Aquila) where coevolutionary specialization is low, average chemical distances did not differ much from the corresponding random communities at any scale (Table 1 and Fig. 5).
Fig. 5.
Average chemical distance for Bursera communities that develop at the lowest altitudes in each of the seven areas studied (purple bars) compared with the average chemical distance of 10,000 random Bursera communities of equal size (pink bars). Black lines above bars indicate confidence intervals. ∗, P < 0.1; ∗∗, P < 0.05.
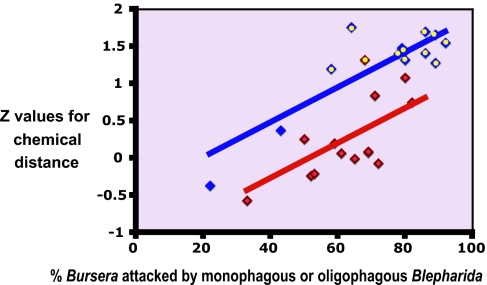
The key role of herbivore specialization for chemical distance can be appreciated by plotting the deviation of chemical distance from that expected for random communities (calculated as Z scores) (36) against the percentage of Bursera species that are attacked by monophagous or oligophagous Blepharida species (Fig. 6). As specialization increases, the Z scores for chemical distance increase. This happens equally for large areas and small areas (slope = 0.0234, P < 0.0001) although small areas have higher Z scores at any given percent monophagy/oligophagy (0.749 SD higher, P < 0.0003). Thus, whereas the degree of specialization also affects chemical distance at larger spatial scales, the lower average chemical distance at larger scales explains why fewer large areas had significant overdispersion.
Fig. 6.
Standardized average chemical distances for 27 Bursera communities in the seven geographic areas studied vs. percentage of Bursera species that are attacked by monophagous or oligophagous Blepharida. Blue squares indicate communities that develop at altitudes up to 1,000 m. Red squares indicate larger communities that develop at altitudes up to 2,200 m. Yellow dots indicate communities in which chemical defenses are statistically more dissimilar than random. Regression lines for small and large communities were fit simultaneously adjusting for correlated error structure (assuming local, compound symmetric error covariance; see Methods).
Testing for Phylogenetic Overdispersion.
A matrix of phylogenetic distance was also constructed for 58 Bursera species, and average phylogenetic distance was calculated for the same communities that were tested for chemical overdispersion. Randomization tests were also performed to test for phylogenetic overdispersion.
Higher average phylogenetic distances than those expected by chance were rare in the 27 communities studied. It never occurred at the smaller scales where resource competition might be expected to be strongest (22) (Table 1). Probability values even increased at smaller scales in some cases, as if closely related species were more likely to cooccur as communities became smaller. If we assume, as others have, that a close phylogenetic relationship reflects competitive similarity, resource competition does not seem to structure these communities. Alternatively, competitive niches among sympatric species may not be phylogenetically conservative.
Results show that there is a nonrandom pattern of chemical overdispersion in Bursera communities that seems to be tied to the tightness of the interaction with their herbivores. As coevolutionary specialization with Blepharida increases, communities tend to be more chemically dissimilar. A possible alternative explanation for this pattern of overdispersion of chemistry, phylogenetic overdispersion driven by plant competition, was tested and can be rejected as an explanation for the observed patterns. Terpenes may have alternative functions besides antiherbivore defense, and it is possible that chemical overdispersion could be due to other causes. Terpenes may have other physiological functions, such as protection from high temperatures (37). They may also have other ecological roles, such as attraction to pollinators (38). It is unlikely, however, that these physiological or ecological alternative functions are behind Bursera's community patterns of chemical differentiation. If the main function of Bursera's chemistry was physiological, then we would expect the community to exhibit the opposite pattern: plants should converge in chemistry to adapt to similar conditions, and communities should have a lower average distance than random. Likewise, there is little floral diversity in the genus, and pollination is accomplished by generalized insects. Thus, there is no a priori reason to expect a correlation between leaf chemistry and pollination. Hence, whereas it is not possible to completely exclude a role for other factors, degree of herbivore specialization seems to be the strongest candidate for explaining chemical overdispersion. This explanation is reinforced by the strong relationship of herbivore specialization and degree of overdispersion.
This investigation provides evidence that Blepharida can affect Bursera's community structure. Whereas other studies have also shown that the structure of vegetation may be modified by herbivores (39), the impact of plant–herbivore coevolution on plant chemical profiles at the community level has rarely been examined. My results suggest that coevolution, by arranging the chemical features of plants, may act in concert with herbivory to influence community structure. Previous research indicates that throughout their evolution, closely related species of Blepharida have invaded more chemically similar Bursera plants (14). The present study shows that Blepharida's selective pressures may have favored the evolution or assembly of chemically divergent Bursera communities.
That chemical overdispersion exists in areas inhabited by highly specialized, monophagous Blepharida species, but not in areas mostly inhabited by polyphagous species, suggests a possible positive feedback loop in the evolution of herbivore specialization. Monophagous species appear to favor the assembly or evolution of Bursera communities with chemically different species. But then, chemical disparity in the plant community would make the adoption of new hosts more difficult, thus reinforcing Blepharida's specialization. This positive feedback could also create a link between specialization and the strength of herbivory. High herbivore pressure may favor the assembly or evolution of dissimilar defensive chemistry among members of the plant community, thus perpetuating the cycle of specialization. Low herbivore pressure, such as when coevolution is diffuse and damage is inflicted by less virulent polyphagous herbivores (40, 41), may permit the assembly or evolution of more chemically similar coexisting plant species, perpetuating the cycle of generalization. Plants that are completely (or almost completely) defoliated by monophagous or oligophagous Blepharida species are frequently observed in the field. Yet, no instances of this kind of extreme damage have been observed with the polyphagous B. pallida or B. alternata (33). This observation suggests that highly specialized Blepharida tend to inflict greater damage to their hosts. However, more data are needed to ascertain whether herbivore pressure and plant community chemical structure feed back into Blepharida's feeding specialization.
Coevolutionary theory predicts that specialized herbivores select for chemical divergence (7, 10). Generalized herbivores are less likely to do so because they are adapted to a range of plant chemistries. Thus, the average chemical distance among plants in communities should depend on the proportion of specialized herbivores. Results here demonstrate that plant chemical overdispersion occurs in local communities in cases in which herbivores are more specialized and, thus, that plant–herbivore coevolution may be a significant factor promoting chemical diversity at a community level.
Methods
Collection of Plant Tissues.
Samples of leaves from 57 Bursera species were collected from live plants in natural populations in Mexico and immediately extracted in dichloromethane. Collection of species was concentrated in mainland Mexico and represents ≈82% of the species present in the area. Endemic species from the Baja California and Yucatan peninsulas were not sampled because Blepharida beetles from the mainland are not likely to encounter them. Because plant chemistry may vary within and among populations, I sampled several individuals and populations for each species. Samples consisted of three to five individuals in each of one to two populations for species of restricted geographic distributions and up to five populations for species of more widespread distributions. For a few of the species, sampling consisted of only two individuals because the species are rare and difficult to find in a limited amount of time. Sampling was restricted to mature, full-grown individuals, concentrating on new, fully developed leaves during time of active growth of plants (June, July, and August).
Chemical Analysis.
All extracts were analyzed by gas chromatography–mass spectrometry performed on a Hewlett–Packard 5890 gas chromatograph linked to a Hewlett–Packard 5970B mass selective detector (Hewlett–Packard, Palo Alto, CA) at 70 eV (1 eV = 1.602 × 10−19 J), m/z 40–600 full scan with a DB-5 column (J & W Scientific, Folsom, CA; 15 m long, 0.32-mm inside diameter, and 0.25-μm film). Helium was the carrier gas at a linear velocity of 20 cm/sec. The splitless injector temperature was 200°C, the flame ionization detector was held at 240°C, and the oven temperature was 40°C for 4 min and then increased 8°C/min to 230°C and held for 5 min. Detected volatile compounds in each species were identified by matching the obtained spectra with standard mass spectral libraries (NBS 75.K). Many of the major compounds were also identified by comparing obtained spectra with spectra and retention times of authentic standards.
Chemical Variation Within Species.
To examine the degree of chemical variation within species and populations, I calculated the average Pearson correlation of the relative abundances of chemical compounds between pairs of individuals in the same species by using the statistical package JMP (42). For most of the species, there was little variation among individuals in the same population and among populations, confirming previous results that showed high chemical cohesiveness within species (SI Table 3). When correlation coefficients among individuals of same or different populations were high (usually true), individuals in a species were put in one single population. When individuals from different populations had average correlation coefficients of <0.4, the populations were not combined. This happened with some species that have relatively wide geographic ranges, such as B. grandifolia, B. discolor, B. fagaroides, and B. glabrifolia.
Chemical Distance Between Species.
I calculated averages of abundance of all constituents for each species. For the species that had populations that were chemically different, I calculated averages for each population. A matrix of chemical distance between all species and populations considered was constructed on the basis of the relative abundance of 74 chemical compounds by calculating Pearson correlations between species pairs. Each correlation was then subtracted from 1 to convert it to a distance. For species with chemically different populations, average distances for a community included only the populations in that area.
Scaling of Chemical Overdispersion in Bursera Communities.
Testing for coevolution-mediated chemical overdispersion is complicated by the ability of herbivores to fly and potentially colonize plants far from where their presence has been reported. When communities are too small, herbivores are likely to affect larger communities than the actual community tested. Measuring communities that are too large, on the other hand, increases the possibility that rare or isolated plant species can be missed and unaffected by herbivores. A solution to this problem was to measure the same community at increasing scales. The smallest communities measured were at canyon bottoms. Canyon bottoms are the prime habitat for the genus Bursera, overall (35); they are where diversity and overall abundance of the genus are highest. Both Bursera and Blepharida are tropical and highly adapted to the dry and warm conditions that are prevalent at the base of the basins. They do not stand up well to freezing weather, which although infrequent, does occur at the tops of the basins (25). Habitats radiating outward (and upward) from canyon bottoms, though more favorable for some adapted species, are less favorable for Burseras, overall, so that diversity and overall abundance decline. Thus, within the 1° target area, I chose canyon bottoms as central targets and increased the scale by working outward and upward.
Randomizations.
To determine whether community chemical profiles are overdispersed, I determined the probability that the average chemical distance of a community would be as large or larger than that expected by chance alone. For each real community, the observed average chemical distance between species was compared with the distribution of average chemical distances of an equal number of randomly selected species (the null model). The pool from which species were randomly selected was all of the species and populations included in the matrix of chemical distances. These species are all of the species and populations that grow in the regions studied. Thus, I tested whether the particular configurations of species found in communities are more chemically diverse than communities randomly chosen from the same overall set of species. The null distributions of average chemical distance were generated with 10,000 randomizations for each test. P values are for one-tailed tests, i.e., the observed chemical distance was equal to or greater than all but the proportion P of the 10,000 null communities. Randomizations were done with a program written in R (43).
Effect of Specialization on Overdispersion.
I performed a regression analysis to determine the relationship between chemical overdispersion and insect–host specialization. Most Bursera species are attacked by only one species of Blepharida (SI Table 2). I measured the degree of specialization in a particular community as the percentage of Bursera species that are attacked by monophagous or oligophagous Blepharida. For each community, chemical overdispersion was measured as the difference between average chemical distance from the average null expectation in terms of SDs (calculated as Z scores) (36).
Because the communities at different scales from the same location are not independent, I could not rely on the standard regression assumption of independent errors. Using the latitude and longitude coordinate system, I constructed a spatial model with SAS Proc MIXED to explicitly model correlated error structure (44). The initial models permitted spatial correlation among all communities and sites. They indicated significant correlation among errors (P < 0.047), but the error correlations dissipated at <1° latitude or longitude (i.e., parameter estimates for the “range” of correlated errors were less than the distances between the seven study areas). This justified remodeling the correlated error structure as block-diagonal (assuming correlated errors within, but not between, the seven principle areas of study). Akaike's information criterion and Schwartz's Bayesian criterion (44) were used to select an appropriate block diagonal error structure (compound symmetric). Regression coefficients and associated probabilities reported here have all been adjusted for correlated errors by using the above assumptions (local, compound symmetric error covariance).
Testing for Resource Competition Within Communities.
To measure phylogenetic overdispersion, I used a previously published molecular phylogeny of Bursera (23, 34), reconstructed by using sequences from the internal transcribed spacer region and the external transcribed spacer. Parsimony and likelihood methods as well as Bayesian techniques were implemented for its reconstruction. Phylogenetic distances between 60 species were calculated as their patristic distances by using PAUP* 4.0b10 (45).
A matrix of phylogenetic distance was constructed for 58 Bursera species, and the average phylogenetic distance was calculated for the same communities that were tested for chemical overdispersion. Randomization procedures to test for phylogenetic overdispersion were the same as those to test for chemical overdispersion. The observed average phylogenetic distance between species was compared with the null model by using a one-tailed test.
Supplementary Material
Acknowledgments
I thank L. Venable, D. Ackerly, M. Singer, and two anonymous reviewers for critical reading of the manuscript; P. Evans for help with chemical analyses of Bursera; and J. Pither for writing the randomization program in R. This work was supported by National Science Foundation CAREER Grant DEB-9815648, a young investigator award from the Beckman Foundation, and a grant from the Vice President for Research and the Colleges of Agriculture and Science of the University of Arizona.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0608253104/DC1.
References
- 1.Losos JB, Leal M, Glor RE, de Queiroz K, Hertz PE, Schettino RL, Lata AC, Jackman TR, Larson A. Nature. 2003;424:542–545. doi: 10.1038/nature01814. [DOI] [PubMed] [Google Scholar]
- 2.Weiher E, Keddy PA. The Search for Assembly Rules in Ecological Communities. Cambridge, UK: Cambridge Univ Press; 1999. [Google Scholar]
- 3.Aizen MA, Vazquez DP. Ecography. 2006;29:357–366. [Google Scholar]
- 4.Armbruster WS, Edwards ME, Debevec EM. Ecology. 1994;75:315–329. [Google Scholar]
- 5.Gilbert LE. In: Coevolution of Animals and Plants. Gilbert LE, Raven PH, editors. Austin: Univ of Texas Press; 1980. [Google Scholar]
- 6.Berenbaum M, Zangerl AR. In: Phytochemical Diversity and Redundancy in Ecological Interactions. Romeo JT, Saunders JA, Barbosa P, editors. New York: Plenum; 1995. pp. 1–24. [Google Scholar]
- 7.Ehrlich PR, Raven PH. Evolution (Lawrence, Kans) 1964;18:586–608. [Google Scholar]
- 8.Becerra JX. Proc Natl Acad Sci USA. 2003;100:12804–12807. doi: 10.1073/pnas.2133013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benderoth M, Textor S, Windsor A, Mitchell-Olds T, Gershenzon J, Kroymann J. Proc Natl Acad Sci USA. 2006;103:9118–9123. doi: 10.1073/pnas.0601738103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berenbaum M, Feeny P. Science. 1981;212:927–929. doi: 10.1126/science.212.4497.927. [DOI] [PubMed] [Google Scholar]
- 11.Berenbaum MY, Zangerl AR. Ecology. 2006;87:3070–3081. doi: 10.1890/0012-9658(2006)87[3070:pwahpa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Lokvam J, Brenes-Arguedas T, Lee JS, Coley PD, Kursar TA. Am J Bot. 2006;98:1109–1115. doi: 10.3732/ajb.93.8.1109. [DOI] [PubMed] [Google Scholar]
- 13.Price PW. Insect Ecology. New York: Wiley; 1997. [Google Scholar]
- 14.Becerra JX. Science. 1997;276:253–256. doi: 10.1126/science.276.5310.253. [DOI] [PubMed] [Google Scholar]
- 15.Berenbaum M. Evolution (Lawrence, Kans) 1983;37:163–179. doi: 10.1111/j.1558-5646.1983.tb05524.x. [DOI] [PubMed] [Google Scholar]
- 16.Feeny P. In: Hervivores: Their Interactions with Secondary Metabolites. Rosenthal GA, Berenbaum MR, editors. San Diego: Academic; 1992. pp. 1–46. [Google Scholar]
- 17.Agrawal AA, Lau JA, Hamback PA. Q Rev Biol. 2006;81:349–376. doi: 10.1086/511529. [DOI] [PubMed] [Google Scholar]
- 18.Strauss SY, Sahli H, Conner JK. New Phytologist. 2005;165:81–90. doi: 10.1111/j.1469-8137.2004.01228.x. [DOI] [PubMed] [Google Scholar]
- 19.Van Zandt PA, Agrawal AA. Oikos. 2004;104:401–409. [Google Scholar]
- 20.Leimu R, Koricheva J. Am Nat. 2006;168:E15–E37. doi: 10.1086/505766. [DOI] [PubMed] [Google Scholar]
- 21.Strauss SY, Irwin RE. Annu Rev Ecol Syst. 2004;35:435–466. [Google Scholar]
- 22.Cavender-Bares J, Ackerly DD, Baum DA, Bazzaz FA. Am Nat. 2004;163:823–843. doi: 10.1086/386375. [DOI] [PubMed] [Google Scholar]
- 23.Becerra JX, Venable DL. Am J Bot. 1999;86:1047–1057. [PubMed] [Google Scholar]
- 24.Becerra JX. Proc Natl Acad Sci USA. 2005;102:10919–10923. doi: 10.1073/pnas.0409127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rzedowski J. Vegetación de México. México D.F., México: Limusa; 1978. [Google Scholar]
- 26.Trejo I. México D.F., México: Universidad Nacional Autónoma de México; 1998. PhD thesis. [Google Scholar]
- 27.Trejo I, Dirzo R. Biodiversity Conserv. 2002;11:2063–2084. [Google Scholar]
- 28.Becerra JX, Venable DL, Evans PH, Bowers WS. Am Zool. 2001;41:865–876. [Google Scholar]
- 29.Evans PH, Becerra JX. Flavour Fragrance J. 2006;21:616–618. [Google Scholar]
- 30.Evans PH, Becerra JX, Venable DL, Bowers WS. J Chem Ecol. 2000;26:745–754. [Google Scholar]
- 31.Becerra JX. Ecology. 1994;75:1991–1996. [Google Scholar]
- 32.Becerra JX. Mol Phylogenet Evol. 2004;30:107–117. doi: 10.1016/s1055-7903(03)00158-1. [DOI] [PubMed] [Google Scholar]
- 33.Becerra JX. Tucson: Univ of Arizona; 1993. PhD thesis. [Google Scholar]
- 34.Becerra JX. Mol Phylogenet Evol. 2003;26:300–309. doi: 10.1016/s1055-7903(02)00256-7. [DOI] [PubMed] [Google Scholar]
- 35.Becerra JX, Venable DL. Proc Natl Acad Sci USA. 1999;96:12626–12631. doi: 10.1073/pnas.96.22.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sokal RR, Rohlf FL. Biometry. New York: Feeman; 1995. [Google Scholar]
- 37.McGarvey DJ, Croteau R. Plant Cell. 1995;7:1015–1026. doi: 10.1105/tpc.7.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dudareva N, Negre F, Nagegowanda DA, Orlova I. Crit Rev Plant Sci. 2006;25:417–440. [Google Scholar]
- 39.Fine PVA, Mesones I, Coley PD. Science. 2004;305:663–665. doi: 10.1126/science.1098982. [DOI] [PubMed] [Google Scholar]
- 40.Coley PD, Barone JA. Annu Rev Ecol Syst. 1996;27:305–335. [Google Scholar]
- 41.Hamilton WD, Brown SP. Proc R Soc London Ser B. 2001;268:1489–1493. doi: 10.1098/rspb.2001.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.SAS Institute. JMP. Cary, NC: SAS Institute; 2005. [Google Scholar]
- 43.R Development Core Team. Berkeley: Univ of California; 2004. R: A Language and Environment for Statistical Computing. Version 2.0.1. [Google Scholar]
- 44.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. Cary, NC: SAS Institute; 1996. [Google Scholar]
- 45.Swofford DL. PAUP*: Phylogenetic Analysis Using Parsimony (*and other methods) Sunderland, MA: Sinauer; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.