Abstract
Single-subunit polymerases are universally encoded in both cellular organisms and viruses. Their three-dimensional structures have the shape of a right-hand with the active site located in the palm region, which has a topology similar to that of the RNA recognition motif (RRM) found in many RNA-binding proteins. Considering that polymerases have well conserved structures, it was surprising that the RNA-dependent RNA polymerases from birnaviruses, a group of dsRNA viruses, have their catalytic motifs arranged in a permuted order in sequence. Here we report the 2.5 Å structure of a birnavirus VP1 in which the polymerase palm subdomain adopts a new active site topology that has not been previously observed in other polymerases. In addition, the polymerase motif C of VP1 has the sequence of -ADN-, a highly unusual feature for RNA-dependent polymerases. Through site-directed mutagenesis, we have shown that changing the VP1 motif C from -ADN- to -GDD- results in a mutant with an increased RNA synthesis activity. Our results indicate that the active site topology of VP1 may represent a newly developed branch in polymerase evolution, and that birnaviruses may have acquired the -ADN- mutation to control their growth rate.
Keywords: evolution, virus, RdRp
Single-subunit polymerases, including RNA/DNA-dependent RNA/DNA polymerases, are universally encoded in cellular organisms and viruses. Sequence analysis shows that RNA-dependent RNA polymerases (RdRps) are a cluster of closely related enzymes that are mostly found in viruses, in which they assume critical functional roles by replicating and transcribing viral genome. The recently reported crystal structures of several RdRps and their functional complexes have provided important insights into the biological functions and catalytic mechanisms of these enzymes (1–10). These structures contain a well conserved core polymerase domain, the shape of which resembles a right hand with fingers, palm, and thumb subdomains. The palm, which is the most conserved region, contains a central, nonvariant, four-stranded β-sheet with five recurring catalytic sequence motifs (from A to E).
In contrast to these conventional features, birnavirus polymerase VP1 exhibits several unusual characteristics (11, 12). Most noticeably, the essential -X(G)DD- sequence, which is often referred to as the polymerase motif C (13, 14), is missing from VP1 (12). This raises the question as to whether VP1 promotes catalysis by the two-metal mechanism like in conventional polymerases, or whether VP1 employs a different mechanism for nucleotidyl transfer. Recent results indicate that birnavirus VP1, as well as the polymerases from some tetraviruses, may belong to a special group of unconventional polymerases with five essential RNA polymerase motifs arranged in the permuted order of C–A–B–D–E (15). In addition, the motif C in birnaviruses may have the sequence -ADN-, resulting in only two aspartate residues in the active site (15).
Like polymerases from other dsRNA viruses, birnavirus VP1 catalyzes both replication and transcription of the bisegmented viral genome. Intact birnavirus particles are transcription-competent, and they are capable of producing viral messengers in a semiconservative fashion (16). In vitro, isolated VP1 proteins have been shown to possess a replicase activity using viral (+)RNA templates (17). Three-dimensional structures of birnaviruses show that the ordered capsid shell is made of VP2, whereas the viral genome, polymerase VP1, and VP3, another major capsid protein, are internally disordered (18–20). The VP2 capsid shell has a T = 13 icosahedral symmetry, but the “T = 2” viral core, which is commonly observed in dsRNA viruses, is missing in birnaviruses.
Birnavirus polymerase VP1 initiates RNA synthesis via protein-priming (21–24). Protein-priming is an important mechanism that many viruses (e.g., adenovirus, picornavirus, bacteriophage φ29) use to initiate genome replication, thereby preventing the loss of terminal sequence information (25). Although the protein primer and the polymerase are usually two separate molecular moieties, the protein priming function in birnaviruses is carried out by the RNA-dependent RNA polymerase VP1 itself. It has been shown that both virion-associated and recombinant birnavirus VP1 have the self-guanylylation activity (12, 17, 22, 26). VP1 self-guanylylation, which does not require viral RNA template, produces two products, VP1-pG and VP1-pGpG (12, 17, 22, 26). It has been proposed that the pGpG moiety in VP1-pGpG binds to the conserved pCpC sequence at the terminal end of the viral RNA template during the initiation of nucleotide polymerization (17, 27). Consequently, the 5′ ends of both genomic and messenger RNAs of birnaviruses are covalently linked to a VP1 molecule.
To elucidate whether birnavirus VP1 is a permuted polymerase and how it catalyzes template-independent protein priming, we have determined the crystal structure of a birnavirus polymerase VP1 from the infectious bursal disease virus (IBDV), a well studied birnavirus that causes severe immunosuppression in avian species. The structure reveals several highly unusual features. First, IBDV VP1 adopts a unique active site topology, which brings the five essential RNA polymerase motifs in the permuted order of C–A–B–D–E to form a conserved catalytic active site. Second, the -GDD- motif strictly conserved in many other polymerases is indeed replaced by -ADN- in IBDV VP1. Converting the sequence motif -ADN- to -GDD- by site-directed mutagenesis resulted in a mutant with an increased polymerase activity. Third, the putative guanlylylation site Ser-166 (17) is located ≈23 Å from the polymerase active site. In addition, RNA modeling on VP1 suggests that terminal protein-priming by VP1 would require large movements of several structural modules.
Results and Discussion
Biochemical Characterization of IBDV VP1.
Isolated IBDV VP1 exists as monomers in solution, as evidenced by gel filtration chromatography and electron microscopy [supporting information (SI) Fig. 5a]. Recombinant IBDV VP1 exhibits a self-guanylylation activity in vitro and becomes radio-labeled in the presence of [α-32P]rGTP (SI Fig. 5b). Guanylylation of IBDV VP1 does not require a viral RNA template (SI Fig. 5b, lane 1). RNA replication activities were observed in the presence of a virus-specific RNA, but not with nonspecific ssDNA molecules (SI Fig. 5b, lanes 2 and 3). These results are similar to the activities previously reported for VP1 from a homologous birnavirus, the infectious pancreatic necrosis virus (26, 28). Using limited proteolysis, we have identified a stable core fragment VP1c that encompasses amino acid residues 19–810. VP1c forms monomers in solution and retains the self-guanylylation and polymerase activities, similar to the full-length protein.
Overview of the Structure.
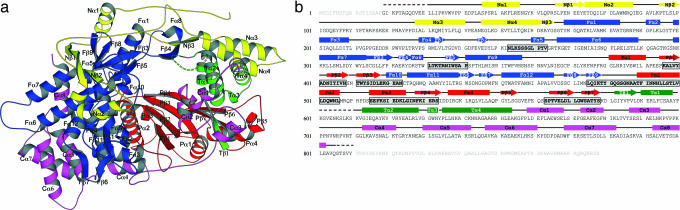
The crystal structure of IBDV VP1c was determined to 2.5Å resolution using multiple isomorphous replacement and anomalous scattering (MIRAS). VP1c is an oval-shaped molecule that is ≈50 × 50 × 60 Å3 in size. The final model contains amino acid residues from 31 to 602 and 612 to 804 (Fig. 1a and SI Fig. 6). The polypeptide can be divided into three functional regions, namely the central polymerase domain (amino acids 168–658), the N-terminal (amino acids 1–167) and the C-terminal (amino acids 659–878) domains (Fig. 1). The central polymerase domain folds into a structure of a right-hand shape (fingers–palm–thumb) with a central canyon running through the whole molecule. Both N- and C-terminal domains have an extended conformation, and they span the canyon at the back and front of the polymerase domain, respectively.
Fig. 1.
IBDV VP1c crystal structure. (a) The ribbon diagram. The N- and C-terminal domains are colored in yellow and magenta, respectively. The central polymerase domain is shown in three different colors with the fingers in blue, the palm in red, and the thumb in green. An internal disordered region (amino acids 603–611) is shown by a dashed line. The molecule is viewed from the downstream end of the active site canyon. (b) Secondary structure assignment of IBDV VP1. Disordered regions are shown by dashed lines, whereas cylinders and arrows represent α-helices and β-strands, respectively. Terminal regions removed by chymotrypsin are not assigned with any symbols. Conserved polymerase motifs are highlighted in gray in the order of G, F, C, A, B, D, and E.
The palm of IBDV VP1 Has an Unusual Active Site Topology.
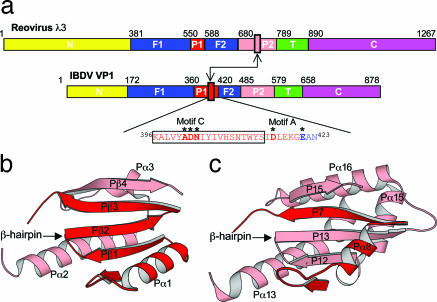
Close inspection of VP1 structure reveals that its palm has adopted a topology that has not been observed in any other RNA or DNA polymerases. The β-hairpin, which is formed by secondary structure elements Pβ1 and Pβ2 and contains the polymerase motif C, is connected to Pα1 and Pβ3 in VP1 (Fig. 2). The VP1 polymerase active site is formed by the motif C in the β-hairpin and motif A from the neighboring β-strand Pβ3. However, in a conventional RNA polymerase, Pα1 and Pβ3 would be directly connected, and the β-hairpin containing motif C would be inserted between Pα2 and Pα3, which are located in the palm II region (Fig. 2). For example, the β-hairpin P12 and P13 from reovirus polymerase λ3, which is equivalent to Pβ1 and Pβ2 in VP1, is inserted between Pα13 and Pα16 that are equivalent to Pα2 and Pα3 in VP1 (Fig. 2). At the amino acid sequence level, it is evident that motif C in VP1 has been relocated from its conserved site, as shown in reovirus λ3, to an upstream position immediately in front of motif A by ≈120 aa residues (Fig. 2). The topology of IBDV VP1 seen in our structure closely matches the prediction made by Gorbalenya et al. (15), who proposed that birnavirus, as well as a small number of tetraviruses, may have a permuted polymerase fold based on sequence analysis.
Fig. 2.
New topology adopted by IBDV VP1 palm. (a) Domain assignment with polypeptide regions colored according to Fig. 1a. F1, fingers region I; P1, palm region I; F2, fingers region II, P2, palm region II; T, thumb. The two palm regions are colored in red and pink, respectively, for clarity. Motif C, highlighted by a small box, is always located in the palm region II in conventional polymerases (e.g., reovirus λ3), but is now observed in the palm region I in IBDV VP1. (b) IBDV VP1 palm. (c) The palm of reovirus λ3. The β-hairpin containing motif C is connected to non-equivalent secondary structure elements in IBDV VP1 and reovirus λ3, resulting in different topologies.
Spatial Positions of The Five Polymerase Motifs Are Conserved in IBDV VP1.
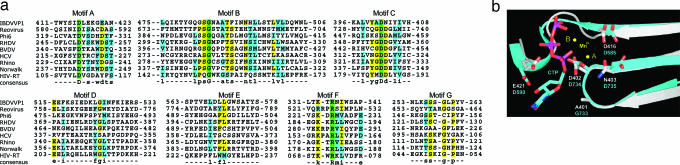
Except for the different connectivity involving the β-hairpin, the rest of the palm resembles that of other viral RNA polymerases with no large-scale deletions or insertions of secondary structure elements. In particular, the four other polymerase motifs, including A, B, D, and E, are arranged in the same sequential order as in conventional polymerases. The polymerase motif B folds into a strand-turn-helix structure at the interface between the fingers II and the palm II regions (Figs. 1–3). In nearly all RdRps, motif B has the consensus sequence of -SGxxxT- and has been proposed to interact with the 2′ OH group on the incoming nucleotide (1, 7). Motif B may also function in guiding the template entry into the active site, as it has been shown to form the base of the template-entry channel (29). The polymerase motif D in IBDV VP1 forms a helix–turn–strand structure (Figs. 1 and 3). The β-strand of this structure forms the last strand in the central four-stranded β-sheet in the palm. The conserved lysine residue K529 is likely to interact with nucleotide substrate to facilitate its entry into the active site (Fig. 4). The last β-hairpin in the palm, consisting of Pβ6 and Pβ7, forms the polymerase motif E (Figs. 1 c and d and 3), which is also an important part of the primer grip, as it has been shown to interact with the primer strand. Amino acid residues from motif E are mostly hydrophobic but often poorly conserved (Fig. 3). As in other RNA-dependent polymerases, the primer grip forms a second β-sheet with the β-strand (Tβ1) at the N terminus of the thumb. Motif E may have an important function in the proper positioning of the thumb relative to the palm.
Fig. 3.
IBDV VP1 structure compared with other viral polymerases. (a) Structure-based sequence alignment of the seven polymerase motifs. These polymerases are all RdRps from either dsRNA or ss(+)RNA viruses except for HIV-RT. The complete virus names are provided in Materials and Methods. Green, yellow, and blue highlight strictly conserved, highly conserved, and generally conserved amino acid residues, respectively. (b) Superposition of IBDV VP1 and reovirus λ3 active sites. The substrate CTP and divalent metal ions are from the reovirus λ3 elongation complex (1).
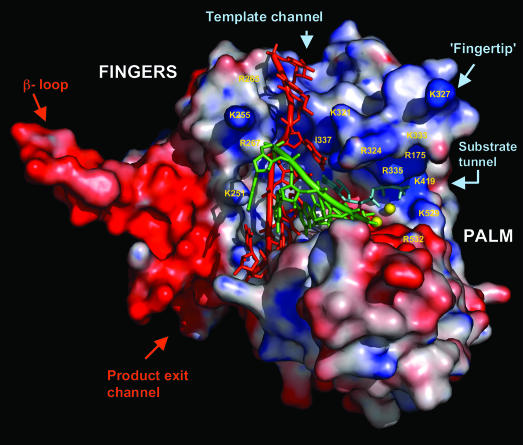
Fig. 4.
RNA modeling onto IBDV VP1. The dsRNA duplex is brought from reovirus elongation complex by superimposing 61 conserved Cα atoms, as described in Table 1. The VP1 model in this figure contains residues 169–579. The thumb, N-terminal, and C-terminal domains have been removed from the molecule to achieve a better view of the polymerase active site. Important amino acid residues are labeled. Molecular surface is colored according to electrostatic potential. In the active site, the template is shown in red; nascent, green; metal ion, yellow spheres; nucleotide substrate, pale blue.
Structure comparison indicates that the five recurring polymerase motifs from IBDV VP1 adopt similar secondary structures and occupy nearly identical spatial locations to those observed in other viral RNA polymerases. Superposition of 61 Cα atoms from the five polymerase motifs resulted in RMS distance deviations of 1.8–3.0 Å (Fig. 3 and Table 1). Among the eight polymerases used for comparison, reovirus λ3, HCV polymerase, and HIV-RT gave deviations <2 Å. The close match of these motifs from VP1 to other polymerases indicates that by adopting the new topology, IBDV VP1 is able to accommodate the dramatic shuffling of catalytic motifs in sequence without changing the overall 3D structure and active site configuration.
Table 1.
Distance rmsd when different viral polymerase pairs are superimposed based on motifs A–E
| rmsd, Å | Reovirus | Phi6 | RHDV | BVDV | HCV | Rhino | Norwalk | HIV-RT |
|---|---|---|---|---|---|---|---|---|
| IBDV | 1.76 | 2.61 | 2.22 | 2.23 | 1.97 | 2.39 | 2.99 | 1.95 |
| Reovirus | 2.25 | 1.43 | 1.67 | 1.56 | 1.80 | 2.56 | 2.17 | |
| Phi6 | 2.13 | 2.29 | 2.24 | 2.41 | 2.89 | 2.68 | ||
| RHDV | 1.51 | 1.36 | 1.34 | 2.07 | 2.20 | |||
| BVDV | 0.98 | 1.71 | 2.50 | 2.00 | ||||
| HCV | 1.73 | 3.03 | 1.92 | |||||
| Rhino | 1.82 | 2.42 | ||||||
| Norwalk | 2.83 |
Numbers are for a total of 61 Cα atoms only.
Fingers, thumb, and the N- and C-Terminal Domains.
The fingers subdomain (formed by residues 168–360 and 420–489) consists of 12 α-helices and nine β-strands. Many secondary structure elements in VP1 can be matched to those found in HCV, reovirus, and φ6 polymerases, although there are significant differences in their size and orientation. A twisted, four-stranded β-sheet (Fβ3, Fβ5, Fβ8, Fβ9) along with a 15-residue loop (residues 321–335) forms the fingertip structure unique to RdRps (30). VP1 fingertip does not interact directly with the thumb. The rNTP binding loop, often referred to as the polymerase motif F (residues 333–339), is located in the fingertip region. Basic residues in the rNTP binding loop have been shown to interact with phosphates on the incoming nucleotide. A newly recognized polymerase motif G (15) (residues 253–363), a consensus sequence of -SX1–2G- flanked by two lysine residues, is also found in the VP1 fingers. Although sequence similarities between VP1 and Ras-type GTP binding motifs had suggested that motif G participated in VP1 self-guanylylation during protein priming (12), the crystal structure of VP1 shows a rather different conformation and therefore does not support such a notion.
The thumb subdomain (residues 580–658) folds into a β-strand followed by four α-helices. The initial β-strand forms a small β-sheet, called the “primer grip,” with two other β-strands from the palm (see previous discussions).
The N-terminal domain (residues 1–168) of IBDV VP1 folds into a mixed α/β structure. Previous results have implicated VP1 N-terminal domain in protein priming as it possesses the putative guanylylation site residue (see discussion below). The L-shaped N-terminal domain interacts with the fingers and thumb of the core polymerase with its short and long arms, respectively, to maintain the VP1 active site in a closed conformation. The C-terminal domain of the VP1 structure (residues 659–804) is mostly α-helical. It starts at the thumb, runs across the canyon in the front of the palm, and then wraps around the fingers subdomain.
The Active Site and Modeling of the Elongation Complex.
Superposition of the VP1 crystal structure with structures of the catalytic complexes of reovirus λ3 (1), the closest structural homolog of VP1 (Fig. 3 and Table 1), suggests that the VP1 active site has a “closed” conformation even in the absence of a bound substrate. To provide insights into VP1 RNA binding and catalysis, we have modeled the reovirus λ3 elongation complex, including a short RNA duplex, an NTP substrate, and two Mn2+ ions, onto the VP1 structure. The RNA duplex and the NTP substrate fit into the VP1 catalytic cavity with very few steric clashes (Figs. 3b and 4).
The most noticeable feature of the VP1 active site is the substitution of a strictly conserved aspartate residue by an asparagine at position 403 (Fig. 3b). Therefore, VP1 has only two aspartates in its active site, as compared with three found in polymerases from nearly all dsRNA and +ssRNA viruses. Nevertheless, the three amino acids D402, N403 and D416 assume nearly identical positions and conformations as the three aspartic acid residues D734, D735, and D585, respectively, from reovirus λ3. Inspection of the VP1 structure shows that there are no other aspartate or glutamate residues within at least 6Å from N403. The -534IDD- sequence, originally identified as motif C in IBDV VP1 (12), is found in the last β-strand of the central four-stranded β-sheet in the palm. Situated at the interface between the palm and the thumb, -534IDD- is far from the active site and should not directly participate in catalysis.
Previous studies have shown that the major functional role of the three highly conserved aspartates is to coordinate a pair of metal ions (31). Two of the aspartates, including the aspartate from motif A (equivalent to Asp-416 in VP1) and the first aspartate from motif C (equivalent to Asp-402 in VP1), are absolutely essential for polymerase function (32–34). The second aspartate from motif C (equivalent to Asn-403 in VP1) is also required for the proper functioning of RNA polymerases. For example, the Asp → Asn mutation in a calicivirus polymerase resulted in a complete loss of activity (32), and the same change in HCV NS5B produced a mutant with low levels of polymerase activity in vitro (33). Additionally, an Asp → Asn mutation would make poliovirus polymerase inactive, although the mutant can be rescued by substituting Mg2+ with Mn2+ for metal cofactors (34).
Birnavirus VP1 of both virion-associated and recombinant forms can use either Mg2+ or Mn2+ as the metal cofactor for catalysis (17, 35), unlike the above-mentioned poliovirus polymerase mutant (34). Therefore, the question arises as to how birnavirus VP1 efficiently binds to Mg2+ ions under physiological conditions with only two aspartic acid residues in the active site. It has been reported that the asparagines in protein molecules can undergo deamidation to generate aspartic acid residues (36). However, deamidation of the asparagine side chain through unregulated catalysis often has a T1/2 of at least 24 h. Considering that IBDV has an infection life cycle of only 24 h, mature VP1 should retain the original asparagine residue. Close inspection of the IBDV VP1 crystal structure reveals that the side chain of Asn-403 is stacked against a Tyr (Tyr-405) side chain, which is strictly conserved in all birnavirus polymerases (SI Figs. 6b and 7). The electron-rich aromatic ring may transfer some electrons to the side-chain carbonyl of Asn-403, therefore enabling VP1 to bind not just Mn2+, but also the weak-chelating metal Mg2+. In other polymerases from dsRNA and +ssRNA viruses, the position of Tyr-405 in VP1 is generally occupied by a hydrophobic residue (Fig. 3). HIV-RT has a Tyr at this position, but its side chain is flipped away from the aspartate side chain in the enzyme–substrate complex (37).
The structure of our model VP1 elongation complex also shows that Glu-421, the second acidic residue from motif A, superimposes onto Asp-590 in λ3 and may be responsible for discriminating rNTP against dNTP (Fig. 3b) (30, 38). The side chain of glutamic acid residue is one carbon bond longer than that of the aspartic acid residue, which is strictly conserved in all other RdRps (Fig. 3a). Therefore, for Glu-421 to properly interact with the 2′-OH of rNTP in VP1, either the nucleotide substrate or the conserved acid residue needs to adopt a slightly different spatial position compared with that in λ3. Ser-484 from motif B may also play an important role in nucleotide selection, as discussed above. The rNTP may access the active site through a positively charged tunnel in the back of the VP1 molecule. The positively charged substrate tunnel is lined by a number of basic amino acid residues from the fingertip, palm, and thumb (Fig. 4). The modeled complex also reveals a template channel on top of the fingers, surrounded by residues from fingers and thumb (Fig. 4). Many basic residues, such as K251, K255, R265, and K267, are located at the outer opening of the channel, forming a positively charged pore leading to the active site. Ile-337 from motif F packs its hydrophobic side chain against the template base at the i + 1 position (Fig. 4). An Ile/Leu/Val is conserved in all RNA polymerases (Fig. 3a) and may be important for maintaining polymerase fidelity by ensuring proper base pairing between template and the nucleotide substrate (3, 37). The base stacking effects of Ile-337 also force the template strand to make a ≈90° turn upon entering the active site. At the downstream end of the product exit channel, the sugar-phosphate backbone of nascent strand faces the second α-helix in the thumb (Tβ2). This α-helix has been shown to interact with the minor groove of the dsRNA product duplex in several polymerases. The end of the VP1 product exit channel is surrounded by negatively charged residues, which may have the effect of making translocation more efficient because of charge repulsion.
In our structure, the product exit channel is partially blocked (Fig. 1a). At the beginning of the C-terminal domain, a 27-residue loop (from residues 658–684), which contains three short α-helices, protrudes into the polymerase active site from the downstream end of the active site canyon. This C-terminal protrusion, or the C-terminal plug, occupies the path of the template strand and would have to be dislodged from its current location as the nascent RNA chain grows. Indeed, the molecular surface area buried by the C-terminal plug is only ≈900 Å, suggesting that it binds weakly to the product exit channel and could be easily displaced. As shown in Fig. 4, graphical deletion of the complete C-terminal domain reveals an unobstructed channel that fits nicely onto the dsRNA product duplex.
During transcription, the viral dsRNA duplex has to be separated so that the negative-strand RNA can be used as a template for VP1. A predominantly positively charged groove is observed next to the template entry channel (SI Fig. 8). This groove, which is formed at the interface between the N-terminal domain and the polymerase thumb subdomain, may function to stabilize the non-template-strand RNA during the initiation of transcription. The non-template-strand RNA becomes the viral messenger, as birnavirus transcription occurs in a semiconservative fashion. A similar positively charge groove is also noted for the polymerase from dsRNA phage φ6 (7).
Functional Significance of the Active Site Asparagine.
With so many RdRp sequences currently available, it is clear that natural occurrences of the Asp → Asn variation are extremely rare (15, 39). When the metal specificity was tested for RNA synthesis using either Mg2+ or Mn2+, the wild-type VP1 was more active in presence of Mn2+ than Mg2+ at 2 mM concentration (SI Fig. 5b, lanes 2 and 4). This result suggests that Mn2+ is indeed more strongly coordinated by the active site Asn in IBDV VP1. To further elucidate the functional significance of Asn-403, the active site asparagine residue, we produced an IBDV VP1 mutant by replacing the -401ADN- motif with the sequence -GDD-. As a result, the mutant active site contains three aspartate residues, like other dsRNA and ssRNA virus polymerases. When the 401GDD mutant was subjected to in vitro polymerase assays in the presence of Mg2+, we found that it was able to replicate virus-specific RNA more efficiently than the wild-type protein (SI Fig. 5b, lanes 7 and 9). The relatively weak polymerase activity of the wild-type VP1 suggests that the initial occurrence of the Asp → Asn change at the 403 position in birnavirus VP1 may enable birnaviruses to slow their growth kinetics, therefore modulating the virulence and facilitating virus spread.
The Implication of VP1 Protein Priming.
In the VP1 crystal structure, the putative guanylylation site residue S166 is present near the junction of the N-terminal domain and the polymerase fingers. The guanylylation site residue in IBDV VP1 has been tentatively mapped to S166 by sequence alignment, as the guanylylation site in the homologous infectious pancreatic necrosis virus VP1 has been determined to be S163 using peptide digestion and site-directed mutagenesis (17). Surprisingly, S166 in IBDV VP1 is at least 23 Å from the polymerase active site in the same molecule. S166 also appears inaccessible for modification by the polymerase active site of a different molecule because it faces the interior of the active site canyon. Therefore, the template-independent guanylylation of IBDV VP1 may be catalyzed by a second active site and not by the polymerase active site, a situation probably different from poliovirus. After guanylylation, significant conformational changes in the N-terminal domain may occur such that Ser-166 and the associated guanylyl group can bind to the polymerase active site for terminal initiation. Indeed, guanylylated VP1 proteins produced crystals with a space group different from that of native VP1 (data not shown), suggesting that some conformational changes may have taken place.
The C-terminal domain of VP1 may function to prevent back-primed RNA synthesis during protein priming. The 27-residue C-terminal plug of VP1 occupies a location similar to the β-flap in HCV polymerase and the C-terminal domain of φ6 polymerase (7, 30). Rhinovirus polymerase, which initiates RNA synthesis via protein priming, also has a C-terminal peptide that protrudes into the active site (9). Site-directed mutagenesis studies on φ6 and HCV polymerases suggest that these elements may serve important roles in RNA synthesis by preventing template back-priming to promote the synthesis of full-length genome (40). Indeed, only very weak back-primed RNA synthesis activity is detected for isolated VP1 in in vitro assays (41).
Evolutionary Relationship Between dsRNA Viruses and ssRNA Viruses.
Birnaviruses are similar to other dsRNA viruses in that they possess a dsRNA genome and a T = 13 capsid structure (20). However, the genome organization of birnaviruses shows several major differences as compared with those of typical dsRNA viruses. For example, most dsRNA viruses contain a linear, segmented genome that is either free or capped at the 5′ end. Birnaviruses, however, have a VPg-linked genome, and initiate transcription/replication by protein priming (SI Fig. 9). In addition, whereas most dsRNA viruses produce mRNA transcripts coding single protein products, birnaviruses encode a polyprotein in gene segment A (SI Fig. 9). Both the protein priming mechanism and the polyprotein coding strategy are common features of +ssRNA viruses. The recently reported crystal structure of birnavirus capsid shows that the major capsid protein adopts a fold similar to those of noda- and tetraviruses, revealing an evolutionary link between birnaviruses and +ssRNA viruses (20). Here, by showing that IBDV VP1 adopts a topology that is also likely to be adopted by polymerases from Thosea asigna virus (TaV) and Euprosterna elaeasa virus (EeV) (15), both +ssRNA viruses in the alphavirus superfamily, our results lend further support for the evolutionary hypothesis. It is important to note, however, that we assume that horizontal gene transfer events have not occurred during birnavirus evolution. Because TaV and EeV possess the conserved -GDD- sequence in motif C, and birnavirus VP1 has the altered -ADN- sequence, it is likely that birnaviruses originated from a TaV/EeV-like ancestor in which the polymerase permutation had already taken place.
Except for birnaviruses, the permuted polymerase fold has not yet been found in other dsRNA viruses. Birnaviruses also differ from other dsRNA viruses by lacking the “T = 2” icosahedral capsid structure. Despite these differences, intact birnavirus particles can readily produce mRNAs, an activity that is often assumed by the “T = 2” core in other dsRNA viruses. How do birnaviruses deliver their messengers to the cytosol? Is birnavirus VP1 (VPg) removed from the 5′ end of viral mRNAs after the mRNAs are synthesized? What functional roles do the capsid proteins have in birnavirus RNA synthesis? Further structural and biochemical studies of birnavirus RNA replication and transcription should provide more insights into how birnaviruses are related to other dsRNA viruses.
Materials and Methods
Cloning, Protein Expression, and Purification.
The IBDV (strain D78) VP1 coding region was cloned into pFastBacHTa (Invitrogen). The recombinant baculovirus produces a VP1 protein with six histidine residues followed by a TEV recognition site at the N terminus. IBDV VP1 protein was expressed by infecting Sf21 insect cells at the multiplicity of infection (MOI) of 2. Infected cell pellet was resuspended and sonicated in lysis buffer containing 50 mM Tris-HCl pH7.5, 300 mM NaCl, 5 mM imidazole, 10% glycerol, 17 μg/ml PMSF, 1 μg/ml leupeptin, 1 μg/ml pepstatin, and 5 mM β-mercaptoethanol, and the lysate was clarified by centrifugation at 20,000 × g for 20 min. Recombinant VP1 was purified by chromatography using Ni-NTA affinity, a HiTrap Heparin–Sepharose column, a Superdex-200 column, and a Mono Q column (Amersham). To obtain VP1c, full-length VP1 samples collected from the Heparin column were subjected to gentle chymotrypsin digestion at 1:20 protease-to-protein molar ratio at 37°C for 10 min. The reaction was stopped by adding PMSF to a final concentration of 100 μg/ml before the next Superdex-200 chromatography step. The purified protein was at least 95% pure, as judged by SDS/PAGE stained with Coomassie blue.
N-terminal sequencing of VP1c indicated that a cleavage occurred after Tyr-18 at the N terminus. A mass spectrometry experiment (ESI-MS) performed in parallel showed that the molecular mass of the truncated fragment was 88.423 kDs, suggesting that 68 amino acids had been removed from the C-terminal end after Phe-810. The calculated molecular mass of VP1 polypeptide from residue 19 to 810 was 88.299 kDa. The ≈124-Da difference falls within experimental error for proteins of this mass. Both mass spectroscopy and N-terminal sequencing experiments were performed at the Protein Chemistry Core Laboratory at the Baylor College of Medicine (Houston, TX).
Crystallization and Data Collection.
Crystals of VP1c in the space group P6122 (Table 2) were obtained by the hanging-drop vapor diffusion method. The crystallization drop contained 2 μl of recombinant protein and 0.8 μl of well solution containing 100 mM Hepes (pH 7.5) and 1.3 M sodium malonate. The best crystals had the shape of hexagonal plates and could grow to 0.4 × 0.4 × 0.2 mm3 in size at 20°C in 3 days. The addition of 10 mM sodium iodide to the crystallization drop was critical, as it effectively reduced the number of nucleation sites. Each data set was collected from a single frozen crystal that had been transferred in small incremental steps to the cryo solution (containing 50 mM Hepes, pH 7.5, 1.3 M sodium malonate, 30% glycerol, 5 mM DTT) before flash freezing in a boiling nitrogen stream at 100K. Heavy atom derivatives were prepared by soaking crystals in cryo solution containing heavy atom compounds at various concentrations (Table 2). All diffraction data were collected by using the Rigaku RU-H3R X-Ray diffraction system equipped with the R-AXIS IV++ Image plates. Diffraction data reduction and merging were performed by using DENZO and SCALEPACK (42).
Table 2.
IBDV VP1 data collection and refinement statistics
| Data set | Native | EMTS | K2PtCl4 |
|---|---|---|---|
| Data collection | |||
| Unit cell dimensions, Å of space group P6122 Resolution, Å | a = b = 122.1 | a = b = 122.1 | a = b = 121.9 |
| c = 359.0 | c = 358.4 | c = 358.3 | |
| 30–2.5 | 30–2.5 | 30–2.5 | |
| No. of reflections* | 584,198 (55,871) | 863,414 (55,794) | 1,325,449 (55,717) |
| Completeness, %† | 96.4 (72.7) | 97.0 (77.1) | 99.6 (98.4) |
| Rmerge, %† | 6.5 (33.8) | 8.6 (50.1) | 7.8 (39.0) |
| Riso, %++ (15.0–3.0Å) | - | 18.8 (24.0) | 17.1 (20.0) |
| Rano, %† | - | 4.6 (23.3) | 4.2 (19.4) |
| Rcullis† (centric/acentric) | - | 0.85/0.93 (MIR) | 0.89/0.95 (MIR) |
| Phasing power‡ (centric/acentric) | - | 1.12/0.92 (MIR) | 0.95/0.87 (MIR) |
| Figure of merit | - | 0.4509 (MIR-AS) | |
| Refinement statistics | |||
| Resolution range, Å | 30–2.5 | ||
| Rwork, % | 21.6 | ||
| Rfree, % | 25.4 | ||
| RMS of bond lengths and angles | 0.006 Å, 1.3° |
*Numbers in parenthesis are the number of unique reflections.
†Numbers in parenthesis are for the highest resolution bins.
‡Phasing power = rms(FH/E), where E is the lack of closure error.
Structure Determination.
A combination of multiple isomorphous replacement (MIR) and anomalous scattering (AS) from two heavy atom derivatives was used to solve the phase problem (Table 2). The figure of merit was 0.4509 for data from 30- to 2.5-Å resolution. Experimental phases were further improved by solvent flipping and histogram matching using the program SHARP (43). The program O (44) was used for model building. CNS (45) was used for crystallographic refinement with the idealized amino acid parameters (46). A total of 492 water molecules were placed automatically in CNS. The final protein model had an Rfree = 25% and an Rwork = 22%. According to the program PROCHECK (47), >87% of the residues were in the most favored regions of a Ramachandran plot, and two were in disallowed regions. The final model contains 774 amino acid residues out of a total of 878 present in VP1. Those absent are the first 30 residues at the N terminus, of which the first 18 have been eliminated by proteolysis, and 74 residues from the C terminus, of which the last 68 have been proteolytically removed. Moreover, an internal region from residues 603 to 611 is disordered.
Structure Superposition and RNA Modeling.
Structure superposition was performed in O using the coordinates of the reovirus λ3 elongation complex (PDB ID code 1N35), φ6 polymerase initiation complex (PDB ID code 1HHT), RHDV polymerase (PDB ID code 1KHW), BVDV polymerase (PDB ID code 1S49), Rhinovirus polymerase (PDB ID code 1TP7), HCV initiation complex (PDB ID code 1GX5), Norwalk virus polymerase (PDB ID code 1SH3), and the HIV-1 RT catalytic complex (PDB ID code 1RTD). The transformation matrices were calculated by aligning 61 Cα atoms from the five polymerase motifs (from A to E) in the palm subdomain (Fig. 3).
In Vitro Self-Guanylylation and Polymerase Activity Assays.
In vitro self-guanylylation assays were performed by using procedures similar to those described by Dobos (26). In short, pure protein samples (10 μg) were incubated with 50μCi [α-32P]rGTP (3,000Ci/mmol) (0.02 mM) in 10 μl reaction buffer containing 50 mM Tris-HCl (pH7.5), 100 mM NaCl, 5% glycerol, 2 mM MgCl2, and 5 mM DTT at 37°C for 15 min. For replication assays, VP1 proteins (10 μg), rNTPs (0.5 mM ATP, CTP, UTP, and 0.01 mM GTP), RNasin (4 units), RNA template (20 ng), and 10 μCi [α-32P]GTP (3,000 Ci/mmol) were mixed in 20 μl of reaction buffer [40 mM Tris, (pH 8.0), 125 mM NaCl, 2 mM MgCl2 or MnCl2, 0.01 mM EGTA, 5 mM DTT, 0.01% Triton X-100], and incubated for 4 h at 37°C. Template was either the (+)RNA of segment A or a ssDNA from φx174. Reaction products were then mixed with SDS sample buffer, incubated at 70°C for 5 min, and loaded onto a 3–10% SDS/PAGE gel. Electrophoresis was carried out at 150 V until a prestained 70-kDa band ran off the gel (≈3 h).
Preparation of Figures.
Secondary structure elements were assigned using the program DSSP (48). Figures were prepared by using Molscript (49), PyMOL (W. L. Delano, www.pymol.org) and Spock (J. A. Christopher, http://quorum.tamu.edu/spock).
Supplementary Material
Acknowledgments
We thank John Bruning, Douglas Mata, Max Nibert, Yousif Shamoo, and B. V. Venkataram Prasad for critical reading of the manuscript. This work is supported by grants from the Welch Foundation (C-1565), National Science Foundation (EEC0118007), and National Institutes of Health (AI065733).
Abbreviation
- IBDV
infectious bursal disease virus.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 2PGG).
This article contains supporting information online at www.pnas.org/cgi/content/full/0611599104/DC1.
References
- 1.Tao Y, Farsetta DL, Nibert ML, Harrison SC. Cell. 2002;111:733–745. doi: 10.1016/s0092-8674(02)01110-8. [DOI] [PubMed] [Google Scholar]
- 2.Hansen JL, Long AM, Schultz SC. Structure. 1997;5:1109–1122. doi: 10.1016/s0969-2126(97)00261-x. [DOI] [PubMed] [Google Scholar]
- 3.Lesburg CA, Cable MB, Ferrari E, Hong Z, Mannarino AF, Weber PC. Nat Struct Biol. 1999;6:937–943. doi: 10.1038/13305. [DOI] [PubMed] [Google Scholar]
- 4.Love RA, Maegley KA, Yu X, Ferre RA, Lingardo LK, Diehl W, Parge HE, Dragovich PS, Fuhrman SA. Structure (Cambridge) 2004;12:1533–1544. doi: 10.1016/j.str.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Ng KK, Pendas-Franco N, Rojo J, Boga JA, Machin A, Alonso JM, Parra F. J Biol Chem. 2004;279:16638–45. doi: 10.1074/jbc.M400584200. [DOI] [PubMed] [Google Scholar]
- 6.Ng KK, Cherney MM, Vazquez AL, Machin A, Alonso JM, Parra F, James MN. J Biol Chem. 2002;277:1381–1387. doi: 10.1074/jbc.M109261200. [DOI] [PubMed] [Google Scholar]
- 7.Butcher SJ, Grimes JM, Makeyev EV, Bamford DH, Stuart DI. Nature. 2001;410:235–240. doi: 10.1038/35065653. [DOI] [PubMed] [Google Scholar]
- 8.Choi KH, Groarke JM, Young DC, Kuhn RJ, Smith JL, Pevear DC, Rossmann MG. Proc Natl Acad Sci USA. 2004;101:4425–4430. doi: 10.1073/pnas.0400660101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appleby TC, Luecke H, Shim JH, Wu JZ, Cheney IW, Zhong W, Vogeley L, Hong Z, Yao N. J Virol. 2005;79:277–288. doi: 10.1128/JVI.79.1.277-288.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bressanelli S, Tomei L, Rey FA, De Francesco R. J Virol. 2002;76:3482–3492. doi: 10.1128/JVI.76.7.3482-3492.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan R, Mason CL, Nagy E, Leong JA, Dobos P. Virology. 1991;181:541–552. doi: 10.1016/0042-6822(91)90887-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shwed PS, Dobos P, Cameron LA, Vakharia VN, Duncan R. Virology. 2002;296:241–250. doi: 10.1006/viro.2001.1334. [DOI] [PubMed] [Google Scholar]
- 13.Poch O, Sauvaget I, Delarue M, Tordo N. EMBO J. 1989;8:3867–3874. doi: 10.1002/j.1460-2075.1989.tb08565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koonin EV. J Gen Virol. 1991;72:2197–2206. doi: 10.1099/0022-1317-72-9-2197. [DOI] [PubMed] [Google Scholar]
- 15.Gorbalenya AE, Pringle FM, Zeddam JL, Luke BT, Cameron CE, Kalmakoff J, Hanzlik TN, Gordon KH, Ward VK. J Mol Biol. 2002;324:47–62. doi: 10.1016/S0022-2836(02)01033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spies U, Muller H, Becht H. Virus Res. 1987;8:127–140. doi: 10.1016/0168-1702(87)90024-4. [DOI] [PubMed] [Google Scholar]
- 17.Xu HT, Si WD, Dobos P. Virology. 2004;322:199–210. doi: 10.1016/j.virol.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 18.Bottcher B, Kiselev NA, Stel'Mashchuk VY, Perevozchikova NA, Borisov AV, Crowther RA. J Virol. 1997;71:325–330. doi: 10.1128/jvi.71.1.325-330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caston JR, Martinez-Torrecuadrada JL, Maraver A, Lombardo E, Rodriguez JF, Casal JI, Carrascosa JL. J Virol. 2001;75:10815–10828. doi: 10.1128/JVI.75.22.10815-10828.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coulibaly F, Chevalier C, Gutsche I, Pous J, Navaza J, Bressanelli S, Delmas B, Rey FA. Cell. 2005;120:761–772. doi: 10.1016/j.cell.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Revet B, Delain E. Virology. 1982;123:29–44. doi: 10.1016/0042-6822(82)90292-6. [DOI] [PubMed] [Google Scholar]
- 22.Spies U, Muller H. J Gen Virol. 1990;71:977–981. doi: 10.1099/0022-1317-71-4-977. [DOI] [PubMed] [Google Scholar]
- 23.Calvert JG, Nagy E, Soler M, Dobos P. J Gen Virol. 1991;72:2563–2567. doi: 10.1099/0022-1317-72-10-2563. [DOI] [PubMed] [Google Scholar]
- 24.Muller H, Nitschke R. Virology. 1987;159:174–177. doi: 10.1016/0042-6822(87)90363-1. [DOI] [PubMed] [Google Scholar]
- 25.Salas M. Annu Rev Biochem. 1991;60:39–71. doi: 10.1146/annurev.bi.60.070191.000351. [DOI] [PubMed] [Google Scholar]
- 26.Dobos P. Virology. 1993;193:403–413. doi: 10.1006/viro.1993.1137. [DOI] [PubMed] [Google Scholar]
- 27.Magyar G, Chung HK, Dobos P. Virology. 1998;245:142–150. doi: 10.1006/viro.1998.9137. [DOI] [PubMed] [Google Scholar]
- 28.Dobos P. Virology. 1995;208:19–25. doi: 10.1006/viro.1995.1125. [DOI] [PubMed] [Google Scholar]
- 29.Bruenn JA. Nucleic Acids Res. 2003;31:1821–1829. doi: 10.1093/nar/gkg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bressanelli S, Tomei L, Roussel A, Incitti I, Vitale RL, Mathieu M, De Francesco R, Rey FA. Proc Natl Acad Sci USA. 1999;96:13034–13039. doi: 10.1073/pnas.96.23.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joyce CM, Steitz TA. Annu Rev Biochem. 1994;63:777–822. doi: 10.1146/annurev.bi.63.070194.004021. [DOI] [PubMed] [Google Scholar]
- 32.Vazquez AL, Alonso JM, Parra F. J Virol. 2000;74:3888–3891. doi: 10.1128/jvi.74.8.3888-3891.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lohmann V, Korner F, Herian U, Bartenschlager R. J Virol. 1997;71:8416–8428. doi: 10.1128/jvi.71.11.8416-8428.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jablonski SA, Morrow CD. J Virol. 1995;69:1532–1539. doi: 10.1128/jvi.69.3.1532-1539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen J. Biochem Biophys Res Commun. 1975;62:689–695. doi: 10.1016/0006-291x(75)90454-4. [DOI] [PubMed] [Google Scholar]
- 36.Wright HT. Protein Eng. 1991;4:283–294. doi: 10.1093/protein/4.3.283. [DOI] [PubMed] [Google Scholar]
- 37.Huang H, Chopra R, Verdine GL, Harrison SC. Science. 1998;282:1669–1675. doi: 10.1126/science.282.5394.1669. [DOI] [PubMed] [Google Scholar]
- 38.Gao G, Orlova M, Georgiadis MM, Hendrickson WA, Goff SP. Proc Natl Acad Sci USA. 1997;94:407–411. doi: 10.1073/pnas.94.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruenn JA. Nucleic Acids Res. 1991;19:217–226. doi: 10.1093/nar/19.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laurila MR, Salgado PS, Stuart DI, Grimes JM, Bamford DH. J Gen Virol. 2005;86:521–526. doi: 10.1099/vir.0.80492-0. [DOI] [PubMed] [Google Scholar]
- 41.von Einem UI, Gorbalenya AE, Schirrmeier H, Behrens SE, Letzel T, Mundt E. J Gen Virol. 2004;85:2221–2229. doi: 10.1099/vir.0.19772-0. [DOI] [PubMed] [Google Scholar]
- 42.Otwinowski Z, Minor W. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 43.Bricogne G, Vonrhein C, Flensburg C, Schiltz M, Paciorek W. Acta Crystallogr D. 2003;59:2023–2030. doi: 10.1107/s0907444903017694. [DOI] [PubMed] [Google Scholar]
- 44.Jones TA, Zou JY, Cowan SW, Kjeldgaard Acta Crystallogr A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 45.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, et al. Acta Crystallogr D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 46.Engh RA, Huber R. Acta Crystallogr A. 1991;47:392–400. [Google Scholar]
- 47.Laskowski RA, MacArthur MW, Moss DS, Thomton JM. J Appl Crystallogr. 1993;26:283–291. [Google Scholar]
- 48.Kabsch W, Sander C. Biopolymers. 1983;22:2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- 49.Kraulis P. J Appl Crystallogr. 1991;24:946–950. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.