Abstract
The purpose of the present experiment is to assess potential differences in nicotine withdrawal in both adolescent and adult rats. Nicotine dependence was induced via osmotic minipump in adolescent rats (releasing 22.2 mg/kg/day on Postnatal Day 28) and adults (release rate of 18.4 mg/kg/day on Postnatal Day 60); differential initial release rates were used across age to compensate for the more rapid weight gain of adolescence. On Day 7 of nicotine exposure, withdrawal was induced via the administration of a nicotinic antagonist, mecamylamine (1.0 mg/kg i.p.), and withdrawal-induced anxiogenesis assessed on the elevated plus maze. On Days 1 and 4 after pump removal, animals were examined for startle responses and prepulse inhibition in an acoustic startle chamber. Adult animals exhibited a nicotine withdrawal-induced increase in anxiety, while adolescents did not. One day following the removal of minipumps, only nicotine dependent adolescent animals exhibited a disruption in prepulse inhibition. Nicotine withdrawal failed to produce an alteration in acoustic startle response in either group. Together these data suggest that ontogenic differences in nicotine withdrawal are dependent on the withdrawal measure examined, with adolescents being less sensitive than adults to anxiety-like symptoms, while being more sensitive to withdrawal-induced cognitive disruption.
Despite the overall decrease in adolescent tobacco use that has occurred in the past 10 years, the prevalence of use among teens is still quite high, with 50% of 12th graders reporting trying smoking and 23% reporting smoking within the last 30 days in 2005 (Johnston, et al., 2006). This early use of tobacco is particularly alarming considering that initiation of smoking during adolescence is correlated with an increase in severity of tobacco dependence, greater addiction liability, higher daily consumption and reduced probability of quitting (Breslau & Peterson, 1996; Chen & Millar, 1998; Taioli & Wynder, 1991).
Most teens report that their continued use of tobacco is based on the physiological and psychological consequences of these products (i.e., dependence). In fact about 19% of adolescents who are weekly smokers and 66% of daily smokers are estimated to be nicotine dependent, despite relatively low average cigarette exposures (O’Loughlin et al., 2003). Withdrawal effects, the negative consequences of cessation, have been cited as the major reason most attempts to quit by adolescents are unsuccessful (McNeill et al., 1986). While studies of adolescent smokers indicate that adolescents experience withdrawal symptoms similar to those reported by adults, adolescent smokers report withdrawal signs within days of smoking initiation, even before becoming daily smokers (DiFranza et al., 2000; Corrigall et al., 2001), while adult smokers report withdrawal symptoms only after daily intake exceeds 8–12 cigarettes (Kozlowski & Herman, 1984). Additionally, Killen et al. (2001) recently reported that nicotine replacement therapy fails to attenuate withdrawal symptoms in adolescents, findings that contrast with reports of the effectiveness of these treatment approaches in adults (Rose et al., 1985; Fagerstrom et al., 1993).
Although these studies support the suggestion that the aversive effects experienced by adolescents as the result of smoking cessation are an important factor in maintaining smoking behavior, methodological issues in human research limit the conclusions that can be drawn. Animal models of nicotine exposure have therefore been instrumental in systematically examining nicotine dependence in adolescence. Slotkin and colleagues have employed one such animal model of nicotine exposure to demonstrate long-term neurotoxic effects of chronic nicotine administration during adolescence. Effects observed include cell death in the cerebral cortex, midbrain and hippocampus (Trauth et al., 2000b), as well as alterations in the synaptic function of serotonin, cholinergic, and catecholaminergic pathways (Trauth et al., 2000a, 2001; Xu et al., 2001). Adolescent nicotine exposure also results in an upregulation of nicotinic acetylcholine receptors (AChR), that is more persistent and occurs in a different regional pattern than that observed in their adult counterparts (Trauth et al., 1999).
In addition to adolescents’ sensitivity to the neurotoxic effects of nicotine, age differences are also evident in nicotine’s psychoactive properties. Using the conditioned place preference paradigm, Vastola et al. (2002) have demonstrated that adolescent rats are more sensitive to the rewarding and locomotor stimulatory properties of nicotine, whereas they are less sensitive to nicotine-induced activity suppression relative to adults. Age as well as gender differences have also been reported in response to chronic nicotine administration, with adolescent males being more sensitive than adults or female adolescents to nicotine’s locomotor activating effects (Faraday et al., 2001).
Less is known, however, regarding age differences in sensitivity to nicotine withdrawal, although components of the nicotine withdrawal syndrome have been modeled successfully by a variety of approaches in animal studies. For instance, avoidance of affective symptoms of withdrawal (e.g. withdrawal-induced anxiety) has been hypothesized to be an important factor contributing to smoking maintenance in humans (Koob et al., 1993; Watkins et al., 2000a), and has been successfully modeled in rats using the elevated plus maze (EPM) test. When assessed 18–24 hours following nicotine withdrawal, animals chronically treated with nicotine demonstrated an increase in anxiety-like behavior, as indexed by a decrease in time spent in the open arms of the EPM, when compared with saline-exposed control animals (Bhattacharya et al., 1995; Pandey et al., 2001). The acoustic startle response has been used to model altered sensorimotor reactivity during withdrawal, and has proven to be a sensitive tool for the assessment of alterations in sensorimotor reactivity produced by a variety of drugs of abuse, including nicotine (for review see Koch & Schnitzler, 1997; Swerdlow et al., 2001). Using this procedure, withdrawal-induced elevations in startle reactivity have been observed for up to four days following chronic exposure to 3.0 and 6.0 mg/kg/day of nicotine in Long Evans rats (Helton et al., 1993). Prepulse inhibition (PPI) of the acoustic startle response is thought to reflect sensorimotor gating, which is the ability of a weak sensory stimulus to inhibit the processing of a subsequent intense sensory stimulus. Sensorimotor gating is thought to be critical for the cohesiveness of thoughts (Swerdlow 1996); disruption in this “sensory gating” response has been reported in humans undergoing nicotine withdrawal (DellaCasa et al., 1998) as well as in mice studies (Lewis & Gould, 2003). Withdrawal-related disruption in PPI, however, has not been replicated to date in rats, despite the observation that rats exhibit elevated PPI during exposure to chronic nicotine (Acri et al., 1995).
Given the alarmingly high prevalence of adolescent tobacco use and the important role that withdrawal effects play in the maintenance of smoking behavior, additional exploration of nicotine withdrawal in adolescents is critically needed. The current experiments compared nicotine withdrawal in adolescent and adult rats in animals made nicotine dependent by exposing rats to a continuous infusion of nicotine tartrate via osmotic minipumps. Precipitated nicotine withdrawal was induced by administration of mecamylamine and anxiogenic-like effects were assessed using the elevated plus-maze. Spontaneous nicotine withdrawal was then induced via removal of minipumps, with assessment of startle response and PPI conducted on the following day and again 4 days following nicotine cessation to assess nicotine withdrawal-induced alterations in sensorimotor reactivity and sensorimotor gating, respectively. Tests of startle response and PPI following spontaneous withdrawal were used to assess withdrawal duration given that these measures withstand repeated testing and have previously been demonstrated to index intensity and duration of nicotine withdrawal (Helton et al., 1993), unlike EPM, which is notoriously sensitive to retest effects (for review see Carobrez & Bertoglio, 2005). Given adolescents’ augmented sensitivity to the reinforcing and stimulatory effects of nicotine (Vastola et al., 2002), we anticipated that adolescents would be likely to similarly demonstrate an amplified sensitivity to nicotine withdrawal symptoms.
General Methods
Subjects
Male Sprague-Dawley rats (Taconic Farms) bred in our colony were used in these experiments. All animals were maintained in a temperature-controlled (22° C) vivarium on a 14 hr/10 hr light cycle (lights on 0700) with ad libitum access to food (Purina Rat Chow, Lowell, MA) and water. On Postnatal Day 1 (P1), litters were culled to 7–10 pups. Animals were weaned on P21 and pair-housed with same-sex littermates in wire-hanging cages until the time of the experiment. No more than one animal from a given litter was used in any experimental group. At all times, rats used in this experiment were maintained and treated in accordance with guidelines for animal care established by the National Institutes of Health (1986).
Nicotine Treatment
Animals were implanted subcutaneously with an osmotic mini-pump (type 2002, Alza Corp., Palo Alto, CA) designed to deliver a continuous infusion of nicotine for 14 days. Given the constant release rates from minipumps, delivered dose decreases as animals gain weight, an effect that would be exacerbated among adolescents due their more rapid weight gain. Consequently, initial dose rates were varied with age to equate the overall nicotine exposure levels (i.e., area under the curve) for the more rapidly growing adolescents with that of adults. Based on preliminary data, chronic infusion rates of 2.2 mg/day for adolescents and 6.35 mg/day for adults were chosen, resulting in an estimated initial dose rate for adolescents of 22.2 mg/kg/day, and for adults 18.4 mg/kg/day, and final estimated dose levels of 15.0 mg/kg/day for adolescents and 18.0 mg/kg/day for adults on Day 7 of the exposure period. Cessation of nicotine exposure following 7 days of exposure within this dose range has been reported to be sufficient to produce somatic withdrawal signs, conditioned place aversion and an elevation in brain-stimulation reward threshold in adult rats (Malin et al., 1992; Suzuki et al., 1996; Watkins et al., 2000b).
To implant the minipumps, subjects were lightly anesthetized with isoflurane. A small area just below the scapulas was shaved, the skin swabbed with Betadine solution, and 1-cm mid-scapular incision made adjacent to the site chosen for pump placement. After making a “pocket” for the minipump by inserting a hemostat into the incision and spreading the subcutaneous tissue, the minipump was inserted and the incision closed with a wound clip. Animals assigned to the control group underwent a sham surgery identical to that of the nicotine exposed group, with the exception that no minipump was implanted. Post-surgery, animals were returned to their home cages and housing partner and allowed to recover under the observation of the experimenter.
Apparatus
The adult elevated plus-maze (EPM) was constructed of wood and consisted of two open arms, 48.26 x 12.7 cm, and two closed arms, 48.26 x 12.7 x 29.21 cm, elevated 50 cm above the floor. Adolescents were tested on a proportionally-sized maze elevated to the same level as the adult maze, with dimensions of 30 x 8.89 cm for the open arms and 30 x 8.89 x 20.32 cm for the closed arms. A small plastic edge (0.6 cm for adolescents and 1.3 cm for adults) was located along each side and end of the open arms. A gap at the junction of the open and closed arm (1.3 cm long for adolescents and 4.0 cm for adults) allowed protected head dips over the sides of the maze.
An auditory startle response system (San Diego Instruments, San Diego, CA) was used to measure the startle reflex. Each test chamber consisted of a cylindrical animal enclosure constructed of clear acrylic, measuring 8.9 cm in diameter. Chamber lengths were adjusted, using removable walls, to 14 cm for adolescent animals and 18 cm for adults. Each chamber was mounted on a platform, which was located within a 35 x 33 x 38.5 cm sound-attenuating box equipped with a 60-dB fan, a house light (3-lux), and a speaker that produced the white noise startle stimulus. Startle response amplitude was indexed by a voltage measurement of the force applied to the platform by the subject in response to the startle stimulus. Starting with the onset of the auditory stimulus, data were sampled at 1-ms intervals and peak voltage (Vmax) inputs were analyzed and recorded by a computer. Chambers were calibrated weekly using a standardization unit from San Diego Instruments.
Procedure
A 2 age (adolescent vs. adult) x 2 chronic treatment (nicotine minipump vs. sham surgery) x 2 withdrawal test condition (mecamylamine vs. saline) factorial design was used, with 8 animals placed in each condition, resulting in a total of 64 animals. Animals were implanted with minipumps as described earlier on P28 for adolescent subjects and P60 for adult subjects. After surgery, rats were pair housed in breeder tubes with a littermate given the same chronic treatment, with each animal in the pair assigned to a different withdrawal test condition.
On Day 7, one of the animals in each housing pair received a subcutaneous injection of the nicotinic antagonist mecamylamine (1.0 mg/kg/cc), whereas the other animal in the pair received an equivalent volume of 0.9% saline solution. This dose of mecamylamine previously has been found to be effective in producing withdrawal symptoms in several animal models of nicotine withdrawal, including animals chronically exposed to nicotine via osmotic minipump (for review see Suzuki et al. 1996; Watkins et al. 1999). Immediately following injection, each animal was isolated in a wire mesh holding cage for 30–35 minutes prior to testing on the plus maze; this pretest isolation has been shown to increase overall activity on the EPM (Pellow et al., 1985). Following the isolation period, each animal was placed on the center square of the elevated plus-maze facing a closed arm, and allowed to move freely about the maze for a 5-minute test period. Order of testing within each pair was noted (although no test order effects emerged in subsequent analyses). Each test session was conducted without the experimenter present and was videotaped by a camera mounted above the apparatus. A dim light (3 lux) was used to illuminate the apparatus for testing, with a white noise generator providing masking noise. Following each trial, the apparatus was cleaned with a 3% hydrogen peroxide solution. Behaviors were later scored from the videotapes by an observer “blind” to the chronic treatment and test condition variables of each animal.
Behaviors scored during the EPM test included: number of open and closed arm entries, time spent in the open and closed arms, protected and unprotected head dips, and protected and unprotected stretched attend postures. An arm entry was recorded when all four paws were placed in the arm, while an animal was considered to have exited the arm when at least two front paws were out of the arm. Head dips were considered protected when the animal dipped its head over the side of the maze while its body was in the closed arm or center platform, while head dips that occurred while the animal’s body was on the open arm were considered unprotected. Protected stretched attend postures were defined as when the animal’s two hind feet remained in closed arm or center platform while the animal elongated its head and shoulders forward, followed by subsequent retraction. An unprotected stretched attend posture was defined similarly, but when the behavior occurred on an open arm. Percent of time spent on open arms and percent of entries into the open arms have been validated as measures of anxiety-like behavior in this test (Lal et al., 1991; Pellow et al., 1985). Recent ethological analyses have suggested that percent protected stretched attend postures and percent protected head dips may be an even more sensitive index of anxiety-like behavior (Espejo, 1997; Rodgers & Dalvi, 1997; Rodgers et al., 1994). Closed arm entries were used as an index of general activity (see Cruz et al., 1994; Rodgers & Dalvi, 1997).
Following testing on the EPM, minipumps were removed from those animals assigned to the nicotine treatment group, whereas control animals underwent a similar sham surgery. At the time of pump removal, tail blood was collected for measurement of cotinine, the primary metabolite of nicotine. For the collection of tail blood samples for these cotinine assays, a scalpel was used to make a small incision and 250–300μl blood collected in heparinized tubes. Samples were centrifuged at 2000 rpm and the resulting serum stored at −80 °C for later assessment of cotinine levels using a serum micro-plate ELISA kit (OraSure Technologies, Inc., Bethlehem, PA.).
All animals underwent startle response testing one day and again four days following removal of minipumps (i.e. Day 8 and Day 11). Each test session began with a five-minute acclimation period to the testing chamber in the presence of a 60 dB background noise, followed by 62 startle trials. The 62 trials consisted of: twenty-two 40 ms presentations of a 120 dB sound burst (Pulse-alone trials) to index startle response; ten 20 ms presentations of each prepulse intensity (65, 70, 80 dB) 100 ms prior to a 40 ms presentation of the 120 dB sound burst (Prepulse trials), used to index PPI; and ten trials in which no stimulus was presented (Nostim trials) in order to assess general motor activity. There was a variable intertrial interval of 15 sec between all trials. A block of 5 pulse-alone trials was presented immediately following the acclimation period to establish a stable level of reactivity for the remainder of the session. Another block of 5 pulse-alone trials was presented at the end of the test session to assess startle habituation. The remaining 12 pulse-alone trials were presented in pseudorandom order along with the three different intensity prepulse trials.
Startle-response amplitudes of the middle 12 Pulse-alone trials were used as an index of startle response for each animal. Percent PPI (% PPI) for each of the three prepulse intensities was calculated using the following formula: %PPI = ((startle response for pulse-alone trials – startle response for prepulse trials at that pre-pulse intensity)/(startle response for pulse-alone trials)) x 100.
Statistics
All data were analyzed using analysis of variance (ANOVA), with posthoc comparisons made using Fisher LSD tests (p≤0.05).
Results
Body Weight (see Fig. 1)
Figure 1.
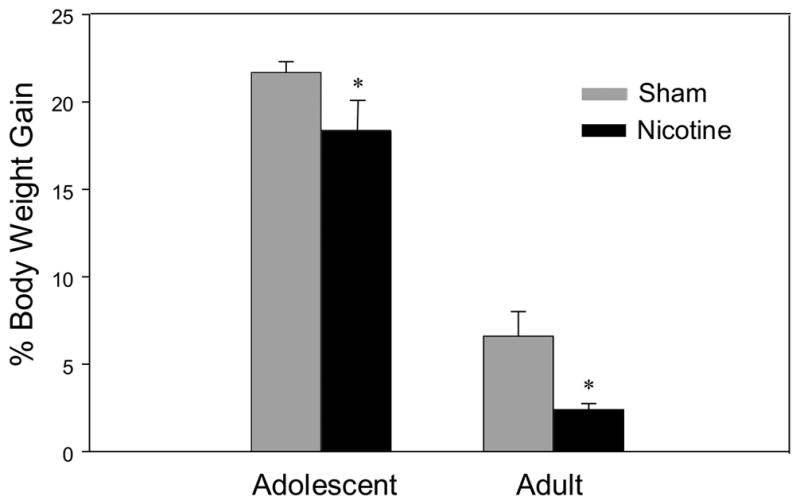
Mean percent body weight gain [(Day 7 weight – Day 1 Weight)/(Day 1 Weight) * 100]. Asterisks (*) indicate significant differences from sham control animals (P<.05). Bars reflect standard error of the mean.
Chronic nicotine exposure reduced percent body weight gain [(Day 7 weight – Day 1 Weight)/(Day 1 Weight) * 100] in both adolescent and adult animals, an anorexic effect that did not interact significantly with age [treatment effect: F(1,56)=12.28, p<0.001]. As expected, adolescent animals gained a greater percentage body weight over the exposure period when compared to adults [age effect: F(1,56)=1146.65, p<0.001].
Based on body weights taken on Day 7 of exposure, adolescents’ nicotine dose was 14.8 mg/kg/day, while adults received 17.8 mg/kg/day. Area under the curve nicotine exposure was approximately equivalent for adolescent and adult rats.
Cotinine
Adolescent animals chronically exposed to nicotine demonstrated significantly higher levels of cotinine (544.0 ng/ml ±17.1) than adults (374.8 ng/ml ±28.5) [t=5.20, p<0.001].
Elevated Plus-Maze
Anxiety measures on the EPM revealed a significant nicotine withdrawal-induced increase in anxiety-like behaviors only in adult animals when indexed by percent open arm time (Fig. 2a) and percent protected stretched attend postures (Fig. 2b); a similar trend was also seen with percent open arm entries (Fig. 2c) [age x treatment x withdrawal condition: F(1,50) = 5.61, p<0.05; F(1,48) = 4.92, p<0.05; F(1, 51) = 2.96, p=0.09 respectively]. Similarly, adult nicotine withdrawal animals were the only group that exhibited a significant reduction in activity as measured by closed arm entries (Fig. 3) [age x treatment x withdrawal condition F(1,51) = 4.33, p<0.05]. Not only did adolescents show no signs of withdrawal using these measures, but sham treated adolescents given mecamylamine prior to being tested on the EPM demonstrated significantly elevated levels of anxiety-like behavior as indexed by percent open arm time (Fig. 2a).
Figure 2.
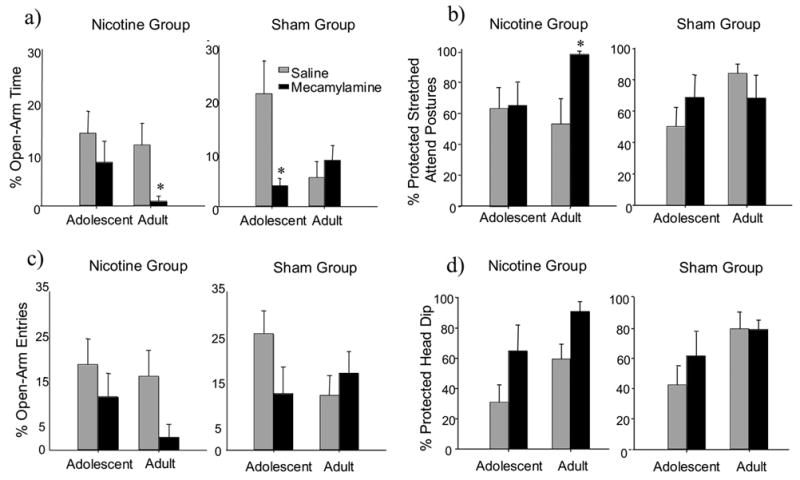
Anxiety measures on the EPM 30 minutes following a mecamylamine (1.0 mg/kg, i.p.) or saline administration. a) Mean percent open-arm time [ time in open arm/(time in open arm + time in closed arm)*100]. b) Mean percent protected stretched attend postures [(protected stretched attend postures)/(protected stretched attend postures + unprotected stretched attend postures)*100]. c) Mean percent of open-arm entries [(open-arm entries)/(open-arms entries + closed-arm entries)*100]. d) Mean percent protected head dip [(protected head dip)/(protected head dip + unprotected head dip)*100]. Asterisks (*) indicate significant differences from saline control animals (P<.05). Bars reflect standard error of the mean.
Figure 3.
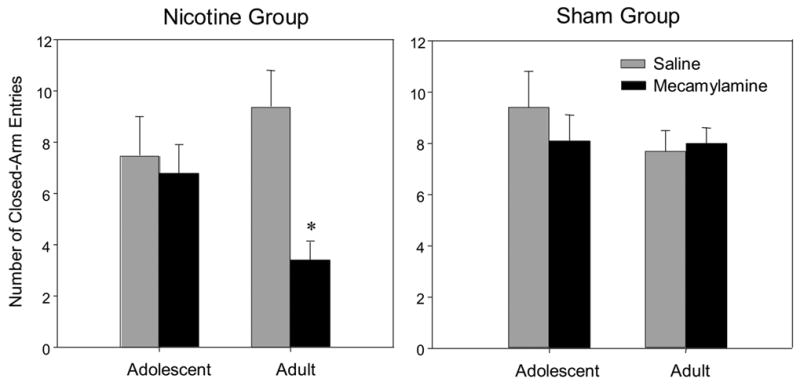
Mean number of closed-arm entries 30 minutes following a mecamylamine (1.0 mg/kg, i.p.) or saline administration. Asterisks (*) indicates significant differences from saline control animals (P<.05). Bars reflect standard error of the mean.
In terms of percent protected head dips (Fig. 2d), no interactions were observed, however there were main effects of age and EPM condition, with adult rats exhibiting higher overall levels of anxiety-like behavior when indexed by this measure than adolescent animals [F(1,46)=10.45, p<0.005] and mecamylamine-treated animals demonstrating higher levels of anxiety-like behavior than saline-injected animals [F(1, 46)=6.21, p<0.01].
Startle Response
In adults, their startle response amplitude on Days 1 and 4 following minipump removal habituated from the first to the last block of five pulse-alone trials, whereas the startle response of adolescents remained consistent across the test sessions (Fig 4) [block x age F(1,47)=31.6, p<0.001]. Prior nicotine exposure failed to influence startle response amplitude in either age group on Day 1 or 4 following nicotine cessation (i.e. Experimental days 8 and 11), as measured by startle reactivity on pulse-alone trials. In the analysis of the pulse-alone trials, adolescent rats demonstrated significantly lower startle response amplitudes when compared to adults (adolescent= 532.5 (±30.9); adult= 1094.4 (±85.9) [age F(1, 47)=11.77, p<0.001].
Figure 4.
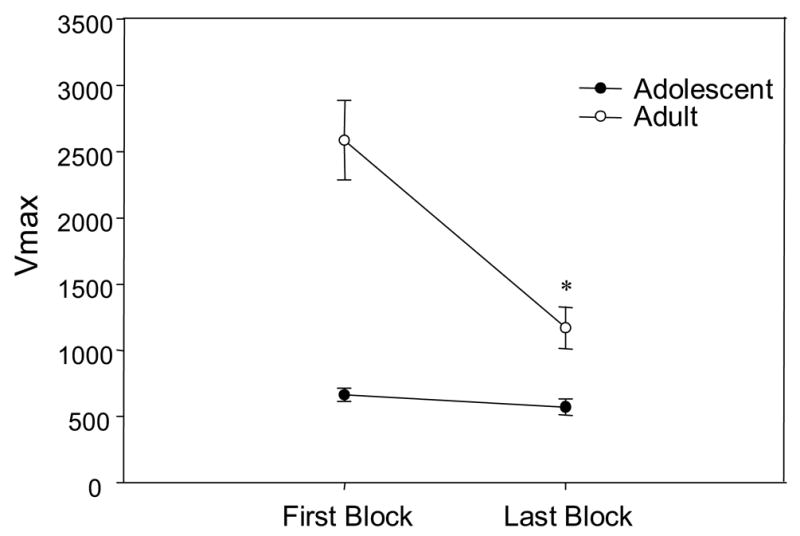
Mean startle response magnitude (Vmax) during pulse-alone trials in blocks 1 and 4, collapsed across withdrawal days 1 and 4. Asterisk (*) indicate significant differences from first block (P<.05). Bars reflect standard error of the mean.
As expected, prepulse intensity exerted a large influence on %PPI on both test days, with higher intensities resulting in greater PPI [dB level F(2, 94)=187.22], an effect that did not interact with age or treatment. An ANOVA of average PPI’s across dB intensities revealed that adolescents generally showed less PPI than adults, although this main effect of age [ F(1,47)=22.58, p<0.05], was tempered by an interaction of age x day x treatment [F(1,47)=5.43, p<0.05]. Separate ANOVAs conducted at each age to explore this interaction revealed that 24 hours following the end of the exposure period, PPI was significantly disrupted in adolescent animals previously exposed to nicotine as compared to sham animals (Fig. 5a) [day x treatment F(1,22)=7.22, p<0.05], whereas no withdrawal effect emerged in the adult data (Fig. 5b). This withdrawal-relative disruption in PPI was no longer evident in adolescent animals 4 days following minipump removal, with the adolescents previously exposed to nicotine demonstrating a significant increase in %PPI on Day 4 relative to Day 1. General activity levels, as indexed by the no-stimulus trials, were similar in both age groups regardless of their previous exposure condition.
Figure 5.

a) Mean percent prepulse inhibition [(startle response for pulse-alone trials – startle response for prepulse trials)/(startle response for pulse-alone trails)) * 100] for adolescent animals. Bars reflect standard error of the mean. b) Mean percent prepulse inhibition [(startle response for pulse-alone trials – startle response for prepulse trials)/(startle response for pulse-alone trails)) * 100] for adult animals. Asterisk (*) indicate significant differences from sham control animals (P<.05). Bars reflect standard error of the mean.
Discussion
Both adolescent and adult rats demonstrated nicotine withdrawal, although ontogenetic differences in sensitivity were found to be dependent on the withdrawal measures examined. In terms of withdrawal-related anxiogenesis, adolescents were found to be resistant to this withdrawal effect that was evident in adult animals in the elevated plus maze. While this lack of an anxiogenic-like effect of nicotine withdrawal could potentially reflect age differences in nicotine levels following the single dose of nicotine chosen for examination, this possibility appears unlikely in that adolescents were conversely more sensitive than adults to the disruptive effects of nicotine withdrawal on sensorimotor gating. The latter finding is particularly surprising in that at the time of these tests, the daily nicotine dose delivered through the minipump to adolescents had dropped significantly below that of adults due the more rapid weight gain of adolescents (Fig. 1).
Adult rats demonstrated elevated levels of anxiety-like behaviors during mecamylamine-elicited nicotine withdrawal compared to saline controls, findings consistent with previous work (e.g. Irvine et al., 2001; Bhattacharya et al., 1995; Pandey et al., 2001). Adolescent rats, however failed to display a nicotine withdrawal-induced increase in anxiety-like behaviors. It is possible that this ontogenetic difference in withdrawal precipitated by mecamylamine could reflect an insensitivity of adolescents to the nicotine receptor antagonist effect of mecamylamine. Although this possibility can not be completely precluded, it seems unlikely that there are marked ontogenetic differences in mecamylamine sensitivity, given that in pilot work in our laboratory a 2.0 mg/kg dose of mecamylamine was found to suppress activity in both adolescent and adult animals (Wilmouth & Spear, unpublished results). Moreover, evidence for attenuated withdrawal from nicotine during adolescence has also been reported when examining another measure of withdrawal, with nicotine-dependent adult rats demonstrating an increase in somatic withdrawal signs during precipitated withdrawal, whereas adolescents did not differ from age-matched saline control animals (O’Dell et al., 2004). The apparent insensitivity of adolescents to anxiogenic-like and somatic consequences of nicotine withdrawal is reminiscent of recent reports that adolescents are likewise less sensitive than adults to the anxiogenic-like effects of alcohol withdrawal (Doremus et al., 2003, Varlinskaya & Spear, 2004).
Mecamylamine administration surprisingly produced an anxiogenic-like effect in adolescent sham animals, while adult sham animals demonstrated no mecamylamine-induced change in anxiety-like behavior. In adult rats, mecamylamine generally has been reported to have anxiolytic-like effects at low doses (0.1, 0.3, & 1.0 mg/kg), with no effects on anxiety-like behavior at a higher dose (3.0 mg/kg) (Newman et al., 2002; Newman et al., 2001). Perhaps the 1.0 mg/kg dose of mecamylamine used in the present study was not anxiolytic in adult sham animals due to the surgical manipulation necessary to implant the minipumps, given that the elevated plus maze has been shown to be quite sensitive to prior perturbation (Doremus et al., 2004). Non-surgically manipulated animals were not included in the design of this study nor were surgical controls implanted with inert pumps, hence age or group-related differences in the magnitude of (or responsivity to) the surgical disruption could have contributed to the age difference in mecamylamine–induced anxiogenesis observed in the sham control animals. To our knowledge, the present study was the first to examine mecamylamine’s modulating effects on anxiety-like behavior in adolescent animals. To the extent that this effect is reproducible, ontogenic differences in presynaptic nicotinic autoreceptors function may be responsible. Although the neural substrates through which mecamylamine produces its anxiety modulating effects are not known at this time, Newman and colleagues (2001) have hypothesized that low doses of mecamylamine selectively inhibit presynaptic nicotinic receptors on cells involved in mediating activation of the HPA stress response. Alternatively, mecamylamine may bind to receptors in addition to AChR, for which there may be an ontogenic difference in numbers or function.
Nicotine withdrawal was not found to alter the acoustic startle response at either age, findings consistent with a previous study using adult Sprague-Dawley rats (Acri et al., 1991), but contrasting with several studies reporting nicotine withdrawal-related increases in the startle response in nicotine-dependent Long-Evans rats (Rasmussen et al., 1996, 1997; Helton et al., 1993). Although strain differences in the startle response following nicotine withdrawal have not been experimentally examined within the same study, strain differences in startle are apparent during a chronic nicotine exposure period, with Sprague-Dawley rats demonstrating an increase in startle response and Long-Evans rats showing a reduction (Faraday et al., 1999).
Age-related differences in both the startle response and PPI were observed. Adolescents demonstrated significantly lower startle responses than adult animals, findings similar to those of Acri and colleagues (1995) where late adolescent rats (P40) were found to exhibit a consistently lower startle response than adults (P70). Because age and weight are correlated during development, age differences in the startle response found in rat models are likely at least in part attributable to differences in body weight. Yet, adolescent animals also exhibited significantly less PPI than adults, data that cannot be attributable simply to age-related changes in body size. This finding is consistent with previous reports that PPI increases across development, with preweanling (P16) rats demonstrating less PPI than adolescents (P44) (Martinez et al., 2000), and adolescent (P40) rats demonstrating less PPI than adults (P70) (Acri et al. 1995; however also see Bell et al., 2003). Such, age-related differences in PPI might be due to developmental alterations in regions critical for the PPI production. Neural systems critical for modulation of PPI include glutamate and dopamine interactions in the nucleus accumbens (NAc), along with input from the medial prefrontal cortex, ventral tegmental area, hippocampus, and amygdala (for review see Koch & Schnitzler, 1997). These brain regions are among those undergoing considerable remodeling during the adolescent period (for review see Spear, 2000).
When disruption in PPI was used as an index of withdrawal-related cognitive disruption, adolescent animals showed evidence of withdrawal 24 hr following nicotine cessation, whereas adult animals did not. Adult rats have previously been found to be insensitive to the PPI disruptive effects of nicotine withdrawal (Faraday et al., 1999; Acri et al. 1991), despite reports of this withdrawal effect in studies conducted in mice as well as humans (Lewis & Gould, 2003; Della Casa et al. 1998). Mesolimbic regions thought to modulate PPI (e.g. Koch & Schnitzler, 1997) undergo developmental changes during adolescence (see Spear, 2000) and overlap with circuitry regulating the reinforcing properties and withdrawal effects of nicotine and other drugs of abuse. For instance reduced dopamine levels within the NAc are thought to play a major role in mediating nicotine withdrawal (Hildebrand, et al., 1999; Rada et al., 2001; Carboni et al., 2000). Reduced PPI during adolescence could reflect ontogenetic immaturity in systems involved in inhibiting irrelevant sensory and cognitive information and in turn increasing distractibility, with this developmental reduction in stimulus filtering exacerbated further during withdrawal in nicotine-dependent adolescents.
The measure-specific nature of the ontogenetic differences in withdrawal observed in the present study may be related to the different neural systems underlying these components of withdrawal and their differential ontogeny (e.g. see Spear 2000). Withdrawal-associated PPI may be related to dopamine and glutamate interactions in the nucleus accumbens, whereas nicotine withdrawal-induced anxiogenesis is thought to be partially mediated through serotonergic neurons of the dorsal raphe nucleus (DRN) (Cheeta et al., 2001). Different aspects of nicotine withdrawal may also be influenced by patterns of expression of specific nAChR subtypes. Although few studies have examined ontogenetic patterns in expression of specific nAChR subunits during the adolescent period, there is evidence for a regionally specific developmental decline in overall nAChR in both the cortex and hippocampus, but not the midbrain (Trauth et al., 1999; however see Zhang et al., 1998), as well as for a developmental increase in inhibitory nicotinic autoreceptors, with younger animals (P21) demonstrating a 4 fold lower level of function than adult animals (Won et al., 2001). It is also possible that the differing ontogenetic patterns of these two measures of nicotine withdrawal might reflect age differences in sensitivity to spontaneous versus precipitated withdrawal. Indeed, in the opioid literature, somewhat different withdrawal symptoms are produced with the more gradual decline in agonist stimulation associated with spontaneous withdrawal than with more abrupt antagonist precipitated withdrawal (Linseman, 1977; Mucha, et al., 1979; Ruiz et al., 1996). In contrast however, generally similar nicotine withdrawal symptoms have been reported with both measures of precipitated verses spontaneous withdrawal (Malin et. al., 1992, 1994), perhaps in part due to the relatively short half-life of nicotine.
Age differences in withdrawal effects in the present study need to be tempered by the finding that cotinine levels in adolescent animals obtained on Exposure Day 7 were significantly higher than those of adults, despite lower calculated levels of nicotine exposure at that time due the more rapid weight gain of adolescents. Adolescent’s higher levels of cotinine could be indicative of an ontogenetic difference in nicotine metabolism, although such a difference has not previously been reported. Developmental differences in the rate of nicotine metabolism and in the production of cotinine may well have behavioral consequences, given that cotinine is psychoactive and has some effects that are similar to nicotine (Yeh et al. 1989; Patterson et al. 1990; Erenmemisoglu & Tekol 1994). Cotinine, however, has been reported to block nicotine’s ability to reduce withdrawal symptoms (Hatsukami et al., 1998) and to increase anxiety in abstinent smokers (Keenan et al., 1994). It seems unlikely, however, that elevated cotinine levels would contribute to both the attenuated anxiolytic-like effect and exacerbated cognitive component of withdrawal characteristics observed among adolescents during nicotine withdrawal in the present study. Moreover, it remains to be seen whether the elevated cotinine levels during withdrawal in the adolescent rats in the present experiment would also be seen in human adolescents. To the extent that elevated cotinine is also evident during withdrawal in human adolescents, however, this could contribute to the general failure of nicotine replacement therapy to reduce nicotine withdrawal symptoms in adolescent smokers (Killen et al., 2001).
These present findings contrast to some extent with the limited human literature suggesting that nicotine withdrawal symptoms experienced by adolescents are similar to those of adults (DiFranza et al., 2000; Corrigall et al., 2001) For instance, in the present -study adolescents were found to be insensitive to withdrawal-related anxiogenesis, whereas adolescent smokers (DiFranza et al., 2000), like their adult counterparts (Hughes & Hatsukami, 1984), report anxiety as one of the most prevalent withdrawal symptoms experienced, second to craving. Likewise, to the extent that affective withdrawal symptoms (i.e. anxiety) are important in the development of nicotine addiction as has been hypothesized (Koob et al., 1993; Watkins et al., 2000a), the present data would suggest that adolescents would be less likely to become dependent, findings that again contrast with survey data that adolescent smokers report signs of withdrawal following more moderate exposure to cigarettes (DiFranza et al., 2000) than do adults (Kozlowski & Herman, 1984). Yet, in the present study, adolescent animals were found to be more sensitive to nicotine withdrawal when assessed via withdrawal-related disruption in PPI, raising the possibly that this cognitive disruption could contribute to the rapid emergence of dependence during adolescence, despite the relatively low daily exposure levels of human adolescents to nicotine (DiFranza et al., 2000).
More work is needed to examine the ontogeny of different components of nicotine withdrawal, and possible across-species variation in these effects. It is possible that developmental exposure to nicotine via osmotic minipumps as in the present study may not adequately model certain aspects of nicotine addiction that might only be expressed following episodic exposures (e.g. sensitization-related phenomenon; Segal & Mandell, 1974).
Work using laboratory animals to model adolescent nicotine exposure are important, however, given the limitations associated with studies conducted using adolescent smokers, where findings largely have been based on self-report, few have verified intake with biological measures, and comparisons with adults generally have been restricted to contrasting findings across different studies and often different laboratories. Additional research using animal models of adolescence in combination with ontogenetic studies of human smokers will likely prove necessary to characterize age differences in the prevalence and intensity of nicotine withdrawal, their underlying mechanisms, and their implications for nicotine dependence phenomenon across ontogeny.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acri JB, Brown KJ, Saah MI, Grunberg NE. Strain and age differences in acoustic startle response and effects of nicotine in rats. Pharmacol Biochem Behav. 1995;50:191–198. doi: 10.1016/0091-3057(94)00285-q. [DOI] [PubMed] [Google Scholar]
- Acri JB, Grunberg NE, Morse DE. Effects of nicotine on the acoustic startle reflex amplitude in rats. Psychopharmacology. 1991;104:244–248. doi: 10.1007/BF02244186. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Dumont NL, Teicher MH. Developmental differences in dopamine synthesis inhibition by (±)-7-OH-DPAT. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:173–181. doi: 10.1007/pl00005038. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Hsu CC, Lumeng L, Murphy JM, McBride WJ. Amphetamine-modified acoustic startle responding and prepulse inhibition in adult and adolescent alcohol-preferring and -nonpreferring rats. Pharmacol Biochem Behav. 2003;75:163–171. doi: 10.1016/s0091-3057(03)00069-8. [DOI] [PubMed] [Google Scholar]
- Bhattacharya SK, Chakrabarti A, Sandler M, Glover V. Rat brain monoamine oxidase A and B inhibitory (tribulin) activity during withdrawal anxiety. Neurosci Lett. 1995;199:103–106. doi: 10.1016/0304-3940(95)12032-y. [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson EL. Smoking cessation in young adults: Age at initiation of cigarette smoking and other suspected influences. Am J Public Health. 1996;86:214–220. doi: 10.2105/ajph.86.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: The elevated plus-maze model 20 years on. Neuroscience and Biobehavioral Reviews. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Carboni E, Bortone L, Giua C, Di Chiara G. Dissociation of physical abstinence signs from changes in extracellular dopamine in the nucleus accumbens and in the prefrontal cortex of nicotine dependent rats. Drug Alcohol Depend. 2000;58:93–102. doi: 10.1016/s0376-8716(99)00064-2. [DOI] [PubMed] [Google Scholar]
- Cheeta S, Irvine EE, Kenny PJ, File SE. The dorsal raphe nucleus is a crucial structure mediating nicotine’s anxiolytic effects and the development of tolerance and withdrawal responses. Psychopharmacology. 2001;155:78–85. doi: 10.1007/s002130100681. [DOI] [PubMed] [Google Scholar]
- Chen J, Millar WJ. Age of smoking initiation: Implications for quitting. Health Rep. 1998;9:39–46. [PubMed] [Google Scholar]
- Corrigall WA, Zack M, Eissenberg T, Belsito L, Scher R. Acute subjective and physiological responses to smoking in adolescents. Addiction. 2001;96:1409–1417. doi: 10.1046/j.1360-0443.2001.961014095.x. [DOI] [PubMed] [Google Scholar]
- Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus maze. Pharmacol Biochem Behav. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- Della Casa V, Hofer I, Weiner I, Feldon J. The effects of smoking on acoustic prepulse inhibition in healthy men and women. Psychopharmacology. 1998;137:362–368. doi: 10.1007/s002130050631. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Rigotti NA, McNeill AD, Ockene JK, Savageau JA, St Cyr D, Coleman M. Initial symptoms of nicotine dependence in adolescents. Tob Control. 2000;9:313–319. doi: 10.1136/tc.9.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Varlinskaya EI, Spear LP. Age-related differences in elevated plus maze behavior between adolescent and adult rats. Ann N Y Acad Sci. 2004;1021:427–430. doi: 10.1196/annals.1308.057. [DOI] [PubMed] [Google Scholar]
- Erenmemisoglu A, Tekol Y. Do nicotine metabolites have an effect on pain perception? Antinociceptive effect of cotinine in mice. Die Pharmazie. 1994;49:374–375. [PubMed] [Google Scholar]
- Ershler J, Leventhal H, Fleming R, Glynn K. The quitting experience for smokers in sixth through twelfth grades. Addict Behav. 1989;14:365–378. doi: 10.1016/0306-4603(89)90024-5. [DOI] [PubMed] [Google Scholar]
- Espejo EF. Selective dopamine depletion within the medial prefrontal cortex induces anxiogenic-like effects in rats placed on the elevated plus maze. Brain Res. 1997;762:281–284. doi: 10.1016/s0006-8993(97)00593-3. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO, Schneider NG, Lunell E. Effectiveness of nicotine patch and nicotine gum as individual versus combined treatments of tobacco withdrawal symptoms. Psychopharmacology. 1993;111:271–277. doi: 10.1007/BF02244941. [DOI] [PubMed] [Google Scholar]
- Faraday MM, Elliott BM, Grunberg NE. Adult vs. adolescent rats differ in biobehavioral responses to chronic nicotine administration. Pharmacol Biochem Behav. 2001;70:475–489. doi: 10.1016/s0091-3057(01)00642-6. [DOI] [PubMed] [Google Scholar]
- Faraday MM, O’Donoghue VA, Grunberg NE. Effects of nicotine and stress on startle amplitude and sensory gating depend on rat strain and sex. Pharmacol Biochem Behav. 1999;62:273–284. doi: 10.1016/s0091-3057(98)00159-2. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Pentel PR, Jensen J, Nelson D, Allen SS, Goldman A, Rafael D. Cotinine: Effects with and without nicotine. Psychopharmacology. 1998;135:141–150. doi: 10.1007/s002130050495. [DOI] [PubMed] [Google Scholar]
- Helton DR, Modlin DL, Tizzano JP, Rasmussen K. Nicotine withdrawal: A behavioral assessment using schedule controlled responding, locomotor activity, and sensorimotor reactivity. Psychopharmacology. 1993;113:205–210. doi: 10.1007/BF02245698. [DOI] [PubMed] [Google Scholar]
- Hildebrand BE, Panagis G, Svensson TH, Nomikos GG. Behavioral and biochemical manifestations of mecamylamine-precipitated nicotine withdrawal in the rat: Role of nicotinic receptors in the ventral tegmental area. Neuropsychopharmacology. 1999;21:560–574. doi: 10.1016/S0893-133X(99)00055-X. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1984;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Irvine EE, Cheeta S, File SE. Development of tolerance to nicotine’s anxiogenic effect in the social interaction test. Brain Res. 2001;894:95–100. doi: 10.1016/s0006-8993(01)01984-9. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2005 (NIH Publication No. 06–5882) Bethesda, MD: National Institute on Drug Abuse; 2006. [Google Scholar]
- Keenan RM, Hatsukami DK, Pental PR, Thompson TN, Grillo MA. Pharmacodynamic effects of cotinine in abstinent cigarette smokers. Clin Pharmacol Ther. 1994;55:581–590. doi: 10.1038/clpt.1994.72. [DOI] [PubMed] [Google Scholar]
- Killen JD, Ammerman S, Rojas N, Varady J, Haydel F, Robinson TN. Do adolescent smokers experience withdrawal effects when deprived of nicotine? Exp. Clin Psychopharmacol. 2001;9:176–182. doi: 10.1037//1064-1297.9.2.176. [DOI] [PubMed] [Google Scholar]
- Koch M, Schnitzler HU. The acoustic startle response in rats—circuits mediating evocation, inhibition and potentiation. Behav Brain Res. 1997;89:35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC, Pich EM, Menzaghi F, Baldwin H, Miczek K, Britton KT. The role of corticotropin-releasing factor in behavioral responses to stress. Ciba Found Symp. 1993;172:277–289. doi: 10.1002/9780470514368.ch14. [DOI] [PubMed] [Google Scholar]
- Kozlowski L, Herman C. The interaction of psychosocial and biological determinants of tobacco use: More on the boundary model. J Appl Soc Psychol. 1984;14:244–256. [Google Scholar]
- Lal H, Prather PL, Rezazadeh SM. Anxiogenic behavior in rats during acute and protracted ethanol withdrawal: Reversal by buspirone. Alcohol. 1991;8:467–471. doi: 10.1016/s0741-8329(91)90153-n. [DOI] [PubMed] [Google Scholar]
- Lewis MC, Gould TJ. Nicotine and ethanol enhancements of acoustic startle reflex are mediated in part by dopamine in C57BL/6J mice. Pharmacol Biochem Behav. 2003;76:179–186. doi: 10.1016/s0091-3057(03)00213-2. [DOI] [PubMed] [Google Scholar]
- Linseman MA. Naloxone-precipitated withdrawal as a function of the morphine–naloxone interval. Psychopharmacology. 1977;54:159–164. doi: 10.1007/BF00426773. [DOI] [PubMed] [Google Scholar]
- Ludewig K, Ludewig S, Seitz A, Obrist M, Geyer MA, Vollenweider FX. The acoustic startle reflex and its modulation: Effects of age and gender in humans. Biol Psychol. 2003;63:311–323. doi: 10.1016/s0301-0511(03)00074-7. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Carter VA, Cunningham JS, Wilson OB. The nicotine antagonist mecamylamine precipitates nicotine abstinence syndrome in the rat. Psychopharmacology (Berlin) 1994;115:180–184. doi: 10.1007/BF02244770. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Newlin-Maultsby P, Roberts LK, Lanier JG, Carter VA, Cunningham JS, Wilson OB. A rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav. 1992;43:779–784. doi: 10.1016/0091-3057(92)90408-8. [DOI] [PubMed] [Google Scholar]
- Martinez ZA, Halim ND, Oostwegel JL, Geyer MA, Swerdlow NR. Ontogeny of phencyclidine and apomorphine-induced startle gating deficits in rats. Pharmacol Biochem Behav. 2000;65:449–457. doi: 10.1016/s0091-3057(99)00217-8. [DOI] [PubMed] [Google Scholar]
- McNeill AD, West RJ, Jarvis M, Jackson P, Bryant A. Cigarette withdrawal symptoms in adolescent smokers. Psychopharmacology. 1986;90:533–536. doi: 10.1007/BF00174074. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Kalant H, Linseman MA. Quantitative relationships among measures of morphine tolerance and physical dependence in the rat. Pharmacol Biochem Behav. 1979;10:397–405. doi: 10.1016/0091-3057(79)90204-1. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Guide for the care and use of laboratory animals. Washington, DC: U.S. Government Printing Office; 1986. (DHEW Publication No. 86–23) [Google Scholar]
- Newman MB, Manresa JJ, Sanberg PR, Shytle RD. Anxiolytic effects of mecamylamine in two animal models of anxiety. Exp Clin Psychopharmacol. 2002;10:18–25. doi: 10.1037//1064-1297.10.1.18. [DOI] [PubMed] [Google Scholar]
- Newman MB, Nazian SJ, Sanberg PR, Diamond DM, Shytle RD. Corticasterone-attenuating and anxiolytic properties of mecamylamine in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:609–620. doi: 10.1016/s0278-5846(00)00178-0. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Bruijnzeel AW, Ghozland S, Markou A, Koob GF. Nicotine withdrawal in adolescent and adult rats. Ann N Y Acad Sci. 2004;1021:167–174. doi: 10.1196/annals.1308.022. [DOI] [PubMed] [Google Scholar]
- O’Loughlin J, DiFranza J, Tyndale R, Meshefedjian G, McMillan-Davey E, Clarke P, Hanley J, Paradis G. Nicotine-Dependence symptoms are associated with smoking frequency in adolescents. Am J Prev Med. 2003;25:219–225. doi: 10.1016/s0749-3797(03)00198-3. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Xu T, Mittal N. Effects of protracted nicotine exposure and withdrawal on the expression and phosphorylation of the CREB gene transcription factor in rat brain. J Neurochem. 2001;77:943–952. doi: 10.1046/j.1471-4159.2001.00309.x. [DOI] [PubMed] [Google Scholar]
- Patterson TR, Stringham JD, Meikle AW. Nicotine and cotinine inhibit steroidogenesis in mouse leydig cells. Life Sci. 1990;46:265–272. doi: 10.1016/0024-3205(90)90032-m. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Rada P, Jensen K, Hoebel BG. Effects of nicotine and mecamylamine-induced withdrawal on extracellular dopamine and acetylcholine in the rat nucleus accumbens. Psychopharmacology. 2001;157:105–110. doi: 10.1007/s002130100781. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Czachura JF, Kallman MJ, Helton DR. The CCK-B antagonist LY288513 blocks the effects of nicotine withdrawal on auditory startle. Neuroreport. 1996;7:1050–1052. doi: 10.1097/00001756-199604100-00019. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Kallman MJ, Helton DR. Serotonin1A - antagonist attenuate the effects of nicotine withdrawal on the auditory startle response. Synapse. 1997;27:145–152. doi: 10.1002/(SICI)1098-2396(199710)27:2<145::AID-SYN5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Dalvi A. Anxiety, defense and the elevated plus-maze. Neurosci Biobehav Rev. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Cole JC, Harrison-Phillips DJ. “Cohort removal” induces hyperthermia but fails to influence plus-maze behaviour in male mice. Physiol Behav. 1994;55:189–192. doi: 10.1016/0031-9384(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Rose JE, Herskovic JE, Trilling Y, Jarvik ME. Transdermal nicotine reduces cigarette craving and nicotine preference. Clin Pharmacol Ther. 1985;384:450–456. doi: 10.1038/clpt.1985.203. [DOI] [PubMed] [Google Scholar]
- Ruiz F, Fournie-Zaluski MC, Roques BP, Maldonado R. Similar decrease in spontaneous morphine abstinence by methadone and RB 101, an inhibitor of enkephalin catabolism. Br J Pharmacol. 1996;119:174–82. doi: 10.1111/j.1476-5381.1996.tb15691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal DS, Mandell AJ. Long-term administration of d-amphetamine: progressive augmentation of motor activity and stereotypy. Pharmacol Biochem Behav. 1974;2:249–255. doi: 10.1016/0091-3057(74)90060-4. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Ise Y, Tsuda M, Maeda J, Misawa M. Mecamylamine-precipitated nicotine-withdrawal aversion in rats. Eur J Pharmacol. 1996;314:281–284. doi: 10.1016/s0014-2999(96)00723-6. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR. Cortico-striatal substrates of cognitive, motor and sensory gating speculations and implications for psychological function and dysfunction. In: Panksepp J, editor. Advances in biological psychiatry. Vol. 2. JAI Press; Greenwich, CT: 1996. pp. 179–208. [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: Current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Taioli E, Wynder EL. Effects of the age at which smoking begins on frequency of smoking in adulthood. N Engl J Med. 1991;325:968–969. doi: 10.1056/NEJM199109263251318. [DOI] [PubMed] [Google Scholar]
- Trauth JA, McCook EC, Seidler FJ, Slotkin TA. Modeling adolescent nicotine exposure: Effects on cholinergic systems in rat brain regions. Brain Res. 2000a;873:18–25. doi: 10.1016/s0006-8993(00)02465-3. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Ali SF, Slotkin TA. Adolescent nicotine exposure produces immediate and long-term changes in CNS noradrenergic and dopaminergic function. Brain Res. 2001;892:269–280. doi: 10.1016/s0006-8993(00)03227-3. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 1999;851:9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Slotkin TA. An animal model of adolescent nicotine exposure: Effects on gene expression and macromolecular constituents in rat brain regions. Brain Res. 2000b;867:29–39. doi: 10.1016/s0006-8993(00)02208-3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague Dawley rats. Alcohol Clin Exp Res. 2004;28:40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62:743–751. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Koob GF, Markou A. Neural mechanism underlying nicotine addiction: Acute positive reinforcement and withdrawal. Nicotine Tob Res. 2000a;2:19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Stinus L, Koob GF, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: Centrally and peripherally mediated effects. J Pharmacol Exp Ther. 2000b;292:1053–1064. [PubMed] [Google Scholar]
- Won YK, Liu J, Olivier K, Zheng Q, Pope CN. Age-related effects of chlorpyrifos on acetylcholine release in rat brain. Neurotoxicology. 2001;22:39–48. doi: 10.1016/s0161-813x(00)00009-7. [DOI] [PubMed] [Google Scholar]
- Xu Z, Seidler FJ, Ali SF, Slikker W, Slotkin TA. Fetal and adolescent nicotine administration: Effects on CNS serotonergic systems. Brain Res. 2001;914:166–178. doi: 10.1016/s0006-8993(01)02797-4. [DOI] [PubMed] [Google Scholar]
- Yeh J, Barbieri RL, Friedman AJ. Nicotine and cotinine inhibit rat testis androgen biosynthesis in vitro. J Steroid Biochem. 1989;33:627–630. doi: 10.1016/0022-4731(89)90051-4. [DOI] [PubMed] [Google Scholar]
- Zhang X, Liu C, Miao H, Gong ZH, Nordberg A. Postnatal changes of nicotinic acetylcholine receptor α2, α3, α4, α7, and β2 subunits genes expression in rat brain. Int J Dev Neurosci. 1998;16:507–518. doi: 10.1016/s0736-5748(98)00044-6. [DOI] [PubMed] [Google Scholar]


