Abstract
Purpose
To determine the safety, dosing schedules, pharmacokinetic profile, and biologic effect of orally administered histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) in patients with advanced cancer.
Patients and Methods
Patients with solid and hematologic malignancies were treated with oral SAHA administered once or twice a day on a continuous basis or twice daily for 3 consecutive days per week. Pharmacokinetic profile and bioavailibity of oral SAHA were determined. Western blots and enzyme-linked immunosorbent assays of histones isolated from peripheral-blood mononuclear cells (PBMNCs) pre and post-therapy were performed to evaluate target inhibition.
Results
Seventy-three patients were treated with oral SAHA and major dose-limiting toxicities were anorexia, dehydration, diarrhea, and fatigue. The maximum tolerated dose was 400 mg qd and 200 mg bid for continuous daily dosing and 300 mg bid for 3 consecutive days per week dosing. Oral SAHA had linear pharmacokinetics from 200 to 600 mg, with an apparent half-life ranging from 91 to 127 minutes and 43% oral bioavailability. Histones isolated from PBMNCs showed consistent accumulation of acetylated histones post-therapy, and enzyme-linked immunosorbent assay demonstrated a trend towards a dose-dependent accumulation of acetylated histones from 200 to 600 mg of oral SAHA. There was one complete response, three partial responses, two unconfirmed partial responses, and 22 (30%) patients remained on study for 4 to 37+ months.
Conclusions
Oral SAHA has linear pharmacokinetics and good bioavailability, inhibits histone deacetylase activity in PBMNCs, can be safely administered chronically, and has a broad range of antitumor activity.
INTRODUCTION
Histone deacetylases (HDACs) are enzymes that regulate chromatin structure and function through the catalysis of the removal of the acetyl modification from lysine residues of histones.1 While the base sequence of DNA provides the fundamental code for proteins, post-translational modification of proteins plays a major role in the control of gene transcription. The amino acid tails of the core nucleosomal histones are subject to post-translational modifications by acetylation of lysines, methylation of lysines and arginines, phosphorylation of serines, and ubiquitination of lysines. The most extensively studied post-translational modification of histones is the acetylation of lysine. The opposing activities of HDACs and histone acetyl transferases (HATs) regulate the balance of acetylation of histones. HDACs are also involved in reversible acetylation of nonhistone proteins, such as p53, tubulin, various transcription factors, and other proteins.2-4
HDAC inhibitors have been shown to cause cultured, transformed cells to undergo growth arrest, terminal differentiation, apoptosis, or autophagic cell death.1 These agents act selectively in altering the transcription of relatively few of the expressed genes (generally 2% to 10% of expressed genes are increased or decreased in their rate of transcription).5-8 HDAC inhibitors have been found to be additive and even synergistic with a number of anticancer agents including radiation, anthracyclines, flavopiridol, imatinib, proteasome inhibitors, and all trans-retinoic acid in blocking the proliferation or inducing apoptosis in tumor cells in culture.1
Inhibitors of HDAC represent a new class of targeted anticancer agents. A number of structural classes of HDAC inhibitors have been developed and are in clinical trials, including short chain fatty acids (the benzamides [MS-275]), the cyclic peptide, depsipeptide (FK-228), and suberoylanilide hydroxamic acid (SAHA).1 We have previously reported the results of a phase I clinical trial of the HDAC inhibitor, SAHA, administered intravenously (IV) to patients with solid or hematologic malignancy.9 This trial showed that SAHA caused an accumulation of acetylated histones in normal and malignant cells post-therapy, was well tolerated, and had antitumor activity, indicating the agent was effective in reaching and inhibiting its targets. These data, supported by in vitro studies, suggested that daily administration of SAHA may improve the therapeutic benefit, and an oral formulation was developed to improve the feasibility of daily administration. We now report the results of the phase I clinical study with orally administered SAHA in patients with advanced cancers.
PATIENTS AND METHODS
Patient Eligibility
Adult patients with solid tumors or hematologic malignancies who had failed or relapsed from standard therapy were eligible. All patients were required to have a Karnofsky performance status ≥ 70% and adequate hepatic and renal function. Solid tumor patients were required to have platelet count ≥ 100,000 cells/μL and WBC ≥ 3,500 cells/μL, and patients with hematologic malignancies were required to have an absolute neutrophil count ≥ 500 cells/μL and a platelet count ≥ 25,000 cells/μL. All patients with solid tumors were required to have radiographic evidence of measurable or nonmeasurable metastatic disease. Patients were required to have recovered from the acute toxicities of any prior therapy, and no chemotherapy, radiation therapy, or other investigational anticancer therapy for a minimum of 4 weeks before initiating the protocol. Leukemia patients could have received conventional cytotoxic therapy and lymphoma patients could receive steroids up to 2 weeks before starting therapy.
Patients with clinically significant cardiac or pulmonary disease, active CNS disease, or active infection were not eligible. Pregnant women and lactating females were excluded. The study was approved by the Memorial Sloan-Kettering Cancer Center (New York, NY) institutional review board, and all patients signed an informed consent.
Trial Design and Treatment Plan
Oral SAHA was provided by Aton Pharma, Inc (Tarrytown, NY) as 200-mg capsules and later in the study a 50-mg capsule was available. The gelatin capsules contain SAHA, and standard pharmaceutical excipients (microcrystalline cellulose, sodium croscarmellose, and magnesium stearate). The starting dose was one tenth the maximum tolerated dose (MTD) in nonrodent species (90 mg/m2/d or approximately 200 mg daily). Due to the initial availability of only the 200-mg capsule, and preclinical data predicting poor bioavailability of SAHA, a fixed dosing schedule was used. There were eight cohorts of oral SAHA studied. SAHA was given daily at 200 mg qd, 400 mg qd, 600 mg qd, or 400 mg bid (cohorts 1 to 4). The dose was planned to be escalated to 1,200 mg bid, however, dose-limiting toxicity (DLT) was encountered at 400 mg bid. The protocol was amended to evaluate 200 mg bid and 300 mg bid dose levels (cohorts 5 and 6) and an intermittent dosing schedule of 300 mg bid and 400 mg bid for three consecutive days weekly (cohorts 7 and 8). Dose escalation proceeded independently in patients with solid tumors and hematologic malignancies. To minimize exposing patients to subtherapeutic treatment, hematologic patients were enrolled in cohorts 2 to 5. Patients were instructed to take oral SAHA at home in a fasting state, but later were allowed to take with food. Patients recorded the date and time of the ingestion of the oral SAHA capsule(s), and a pill count was performed to evaluate compliance and accountability of the study drug.
A treatment cycle was 4 weeks of therapy. DLT was defined as: grade 4 neutropenia or thrombocytopenia; grade 3 neutropenia with fever (solid tumor patients only); and grade 3 or 4 nonhematologic toxicity (solid tumor and hematologic malignancy patients) during the first cycle of therapy. A treatment delay due to toxicity that lasted longer than 1 week was also considered a DLT. At least three patients were entered per cohort and individual cohorts were expanded to six patients after the development of one DLT. MTD was defined as the highest dose with an observed incidence of DLT in no more than one of six patients treated at a dose level. At least six and as many as 20 patients would be treated at the MTD in the solid tumor and hematologic malignancy groups. Toxicities were evaluated by the National Cancer Institute Common Toxicity Criteria (version 2.0).
Patient Evaluation
The pretreatment evaluation included history and physical examination (H&P), Karnofsky performance status, a complete CBC, hepatic and renal function tests, coagulation profile (prothrombin time/partial thromboplastin time), urinalysis, and chest x-ray. A pregnancy test was obtained in women with childbearing potential. Appropriate tumor markers were obtained in patients with prostate or breast cancer. Imaging studies included a chest, abdominal, and pelvic computed tomography (CT) scan, magnetic resonance imaging scan, and positron emission tomography or bone scan as clinically indicated. All patients had a baseline ECG and further cardiac work-up if indicated.
Patients were evaluated weekly with H&P and laboratory tests (CBC, hepatic\renal function, prothrombin time/partial thromboplastin time) and urinalysis during the first 8 weeks of therapy. Tumor markers were repeated every 2 weeks and imaging studies every 8 weeks. An ECG was obtained before every cycle of therapy. If the patient was on study longer than 8 weeks, H&P and laboratory tests were performed every other week for 8 weeks and then monthly thereafter with imaging studies performed every 4 months.
All patients were assessed for toxicity and response if they received any treatment. Inpatients with measurabledisease, standard WHO phase II response criteria10 were utilized and radiographs underwent a blinded review by a radiologist. The Cheson criteria were employed for patients with lymphoma and leukemia.11,12
Pharmacokinetics Studies
Pharmacokinetic (PK) studies were performed during the first cycle of therapy in 44 patients. To assess the absolute bioavailability of oral SAHA, the initial 18 patients received a 2-hour infusion of intravenous SAHA that was equivalent to the assigned oral dose of SAHA on day 1 of therapy. Blood (10 mL) was drawn at time 0, 30, 60, 115, 135, 150, 180, 210, 240, 300, and 360 minutes following the intravenous infusion of SAHA. After 1 week without receiving SAHA, patients started the oral SAHA (day 8). On day 8 (fasting), patients fasted (no food or beverage other than water for 2 hours before the ingestion of SAHA) before the administration of oral SAHA, and on day 9 (fed), all patients received a standardized meal (a bagel with cream cheese or butter, a pint of orange juice, and a cup of coffee with milk and sugar) 30 minutes before the ingestion of SAHA capsule(s). PK studies were performed on days 8 and 9 and on day 15 or 22. Four patients that were on the oral SAHA therapy for more than 6 months repeated the PK study. PK study consisted of drawing 10 mL of heparinized blood at time 0, 15, 30, 45, 60, 90, 120, 150, 180, 240, 300, 360, 420, 480, 540, and 600 minutes following oral SAHA ingestion.
Blood samples were placed on ice and refrigerated until they were centrifuged to separate the plasma. The plasma (3 to 5 mL) was transferred to a labeled conical 15 mL polypropylene screw top tube and stored at -20°C. Determination of SAHA concentrations in plasma samples was conducted in the Pharmacology Analytical Laboratory at the Memorial Sloan-Kettering Cancer Center. A liquid chromatography-mass spectrometry method was employed. A 450-μL aliquot of thawed plasma was mixed with 50 μL of d5-SAHA (internal standard). The plasma was filtered through 0.45-μm cellulose acetate filters (Costar, Corning, NY) by centrifugation at 3,000 × g for 12 minutes. A 200-μL aliquot of filtrate was transferred to an autosampler vial. An injection volume of 30 μl was directly injected through a Prospekt 2 in-line solid phase extraction system (Spark-Holland, Emmen, the Netherlands), which consisted of a high-pressure eluter and an automated cartridge exchanger. After washing the cartridge with water, the mobile phase was changed to methanol: 0.1%:formic acid (1:1, vol:vol) at a flow rate of 0.4 mL/min, and passed over to a Reliance SBC8 4 mm × 80 mm column (Agilent Technologies, Wilmington, DE). The eluant was assayed using a diode array UV/VIS detector (at 240 nm), and an Agilent mass spectrometer detector (Agilent Technologies). The mass spectrometer detector was operated in the atmospheric pressure chemical ionization-positive mode. The masses monitored were 265 for SAHA and 270 for d5-SAHA. The detection limit for SAHA was about 15 ng/mL, and the response was linear from 15 ng/mL to 1,000 ng/mL (r2 > 0.99).
The area under the plasma concentration time curve (AUC) was calculated using the linear trapezoid method. The terminal elimination rate constant, λz, was calculated as the negative of the slope of the terminal log-linear portion of the plasma concentration time curve. Total plasma clearance (CL) and volume of distribution (Vz) were calculated using standard formulas without correcting for bioavailability. The bioavailability after oral administration (F) was calculated for each patient at a given dose on the day of sampling (F = AUCoral/AUCIV × Doseoral/DoseIV). All PK calculations were performed using noncompartmental methods with WinNonLin version 3.1 (Pharsight Corp, Mountain View, CA).
Correlative Studies
Effect of SAHA on histone acetylation in mononuclear cells was assessed by Western blotting and enzyme-linked immunosorbent assay (ELISA). Peripheral blood (10 to 30 mL) was obtained in heparinized tubes at pretreatment, 2 hours postinfusion and between 2 to 10 hours after ingestion of SAHA capsule(s). Histones were isolated from the peripheral-blood mononuclear-cells (PBMNCs) and acetylated histone H3 was evaluated by Western blot analysis as previously described.9
For the ELISA, 50 to 100 ng of histone extract was passively adsorbed to triplicate wells of Immulon microtiter plates (VWR, West Chester, PA) and incubated overnight at 4°C. After washing with phosphate-buffered saline (PBS) containing 0.05% Tween-20 (PBS-Tween), the plates were blocked for 1 hour at room temperature using PBS-Tween containing 5% nonfat milk and 1% goat serum. Total and acetylated histone H3 levels were then quantified by using an antihistone H3 rabbit polyclonal (Abcam, Cambridge, MA) or an antiacetylated histone H3 rabbit polyclonal (Upstate Biotechnology, Lake Placid, NY), respectively. The primary antibodies were diluted in PBS-Tween containing goat serum (0.5%) and added to the appropriate microtiter plates. After 1 hour at room temperature, the plates were washed with PBS-Tween and a horseradish peroxidase-conjugated goat antirabbit secondary antibody (Bio-Rad, Hercules, CA) was added. The plates were incubated at room temperature for 1 hour and then extensively washed with PBS-Tween. Horseradish peroxidase signals were visualized using the TMB peroxidase substrate kit (Bio-Rad) according to manufacturer’s instructions. Normalized histone H3 acetylation levels for each sample were derived by dividing the acetylated histone H3 optical density value by the total histone H3 optical density value derived independently from the same sample. All samples within each patient set were normalized to the level of histone H3 acetylation from the pretreatment sample of that patient, which was assigned a value of 1.
RESULTS
Patient Characteristics and Treatment Administration
Seventy-six patients were enrolled onto the study and 73 received at least one dose of oral SAHA. Three patients did not receive the study drug due to the development of brain metastasis in one and rapid disease progression in two patients. The most common tumors were mesothelioma (n = 13) and non-Hodgkin’s lymphoma (n = 12). Patient characteristics are described in Table 1. Seventy-eight percent of all patients had received two or more prior systemic therapies. The majority of hematologic malignancy patients had received three or more prior systemic therapies (n = 18; 78%). Seventy-three patients received a total of 416 treatment cycles. The median number of treatment cycles was two (range, one to 37+ cycles), which was the same in patients with solid tumor or hematologic malignancies. Twenty-two patients (solid tumor, n = 16; hematologic malignancy, n = 6) completed four or more treatment cycles. Eight completed 12 or more treatment cycles and four patients are still on study with the longest treatment duration exceeding 37 cycles in two patients.
Table 1.
Patient Characteristics (N = 73)
| Characteristic | Solid Tumors (n = 50) | Hematologic Malignancies (n = 23) |
|---|---|---|
| Age, years | ||
| Median | 60 | 59 |
| Range | 25-78 | 20-79 |
| Sex | ||
| Male | 34 | 16 |
| Female | 16 | 7 |
| Primary tumor type | ||
| Mesothelioma | 13 | |
| Prostate | 7 | |
| Urothelial | 7 | |
| Thyroid | 6 | |
| Renal | 6 | |
| Breast | 2 | |
| Lung | 2 | |
| Adrenal cortical | 1 | |
| Germ cell | 1 | |
| Laryngeal | 1 | |
| Melanoma | 1 | |
| Paraganglioma | 1 | |
| Skin | 1 | |
| Cervical | 1 | |
| Hodgkin’s lymphoma | 7 | |
| Non-Hodgkin’s lymphoma | ||
| Diffuse large B-cell | 7 | |
| Small lymphocytic | 1 | |
| Mantle cell | 2 | |
| Cutaneous T-cell | 1 | |
| Peripheral T-cell | 1 | |
| Myeloma | 2 | |
| Acute myeloid leukemia | 1 | |
| Myelodysplastic syndrome | 1 | |
| No. of prior systemic therapies: chemotherapy, or biologic therapy, or both | ||
| None | 1 | 0 |
| One | 14 | 1 |
| Two | 11 | 4 |
| Three or more | 24 | 18 |
Fifty-six patients (77%) were discontinued from the study because of progressive disease (solid tumor, n = 40; hematologic malignancy, n = 16). Ten patients (14%) were discontinued because of adverse event (solid tumor n = 7; hematologic malignancy n = 3), including one patient with widely metastatic mesothelioma who died of infection without neutropenia during the second week of treatment. The death was considered unlikely to be caused by the study drug. Three patients with hematologic malignancies were removed from the study for protocol violation, noncompliance, and patient withdrawal of consent.
DLT and MTD
The number of patients and DLT for each dose level for the solid tumor and hematologic patients are listed in Table 2. Most of the patients (n = 67, 92%) were treated at the MTD or above. The DLTs were predominantly anorexia, dehydration, diarrhea, and fatigue. The MTD for continuous daily dosing for hematologic and solid tumors was 400 mg qd or 200 mg bid, and for solid tumors 300 mg bid × 3 consecutive days per week. The dosing schedule did not appear to have a major effect on the pattern of DLTs and at the doses that exceeded the MTD; the frequency of DLT increased but the pattern and severity remained the same. At the 400 mg bid dose level, six of nine patients (hematologic and solid tumors) developed DLT in the first cycle of therapy and four of six DLTs occurred in 14 days or less after starting the oral SAHA. The median time to resolution of the DLTs was 7 days (range, 3 to 10 days).
Table 2.
DLT and Dose Escalation
| Solid Tumor (N = 50) |
Hematologic Malignancy (N = 23) |
||||
|---|---|---|---|---|---|
| Dose Level | Dosing Regimen | No. of Patients | DLT | No. of Patients | DLT |
| 1 | 200 mg qd | 6* | None | — | — |
| 2 | 400 mg qd | 5† | None | 11‡ | Dehydration/diarrhea (n = 1) |
| Dehydration/diarrhea/fatigue (n = 1) | |||||
| 3 | 400 mg bid | 6 | Dehydration/diarrhea (n = 1) | 3 | Anorexia (n = 1) |
| Fatigue (n = 1) | Dehydration (n = 1) | ||||
| Thrombocytopenia (n = 1) | Anorexia/dehydration (n = 1) | ||||
| 4 | 600 mg qd | 4 | Anorexia/dehydration/fatigue/ nausea (n = 1) | 3 | Dehydration/diarrhea (n = 1) |
| Diarrhea (n = 1) | |||||
| 5 | 200 mg bid | 4 | None | 6 | Anorexia (n = 1) |
| 6 | 300 mg bid | 6 | Elevated ALT/AST (n = 1) | — | — |
| Anorexia/fatigue (n = 1) | |||||
| Fatigue (n = 1) | |||||
| 7 | 300 mg bid × 3 days/week | 13§ | None | — | — |
| 8 | 400 mg bid × 3 days/week | 6 | Fatigue (n = 2) | — | — |
| Dehydration/nausea/vomiting (n = 1) | |||||
Abbreviations: DLT, dose-limiting toxicity; qd, every day; bid, twice a day.
Three patients removed for early progression of disease (POD) during first 4 weeks.
One patient removed for early POD, and one additional patient treated at this dose level.
No DLTs observed in the first five patients enrolled; six additional patients enrolled at the maximum tolerated dose (MTD), and two developed DLTs.
Ten additional patients treated at the MTD.
Safety and Tolerability
The most common drug-related adverse events (Table 3) were constitutional (fatigue), gastrointestinal (anorexia, nausea, diarrhea, and vomiting), metabolic (hyperglycemia, and hypocalcemia), and hematologic (anemia and thrombocytopenia). Grade 4 events occurred in nine patients (solid tumor, three patients; hematologic malignancy, six patients), most of which were hematologic: anemia (n = 4), neutropenia (n = 1), thrombocytopenia (n = 1), hyponatremia (n = 1), elevation in creatine phosphokinase (CPK) (n = 1), and infection (n = 1). Overall, a higher incidence of grade 3 or 4 thrombocytopenia (21% v 36%) and grade 3 dehydration (21% v 36%) was observed in the twice-a-day continuous dosing schedule, more so in hematologic patients than in solid tumor patients. There were no grade 3 or 4 hematologic toxicities seen in the twice-a-day × 3 days-per-week-treated solid tumor patients, but an increase in the incidence of severe fatigue was noticed when compared with continuous twice daily and the once daily regimens (37% v 28% v 17%, respectively). More patients with hematologic malignancies experienced thrombocytopenia (87%) than solid tumor patients (44%), and thrombocytopenia was more severe in those patients. Grade 3 thrombocytopenia occurred in six solid tumor patients (12%) and eight patients with hematologic malignancies (35%), most of which (79%) resolved to grade 2 within 7 days. Bone marrow biopsies in two patients with grade 3 or 4 thrombocytopenia suggested that the most probable cause of thrombocytopenia was maturation arrest. Grade 3 neutropenia occurred in three hematologic patients and grade 4 in one, all of which resolved to grade 2 or less without intervention. No patients had neutropenic fever or discontinued therapy because of neutropenia. Fatigue was more common at the higher dose levels on the twice-a-day dosing regimens but was reversible within 3 to 7 days. Mild to moderate GI symptoms of anorexia, diarrhea, nausea, and vomiting were common and antiemetics and antidiarrheal medications were able to control symptoms. Anorexia was associated with gustatory changes in 8% of the patients.
Table 3.
Ten Most Common Drug-Related Toxicities in 73 Patients (all cycles, highest grade per event per patient)
| Solid Tumors (n = 50) |
Hematologic Malignancy (n = 23) |
|||
|---|---|---|---|---|
| Grade 1/2 | Grade 3/4 | Grade 1/2 | Grade 3/4 | |
| Hematologic | ||||
| Anemia | 36 | 5 | 10 | 7 |
| Thrombocytopenia | 16 | 6 | 11 | 9 |
| Nonhematologic | ||||
| Anorexia | 26 | 4 | 10 | 4 |
| Diarrhea | 19 | 2 | 12 | 7 |
| Elevated serum creatinine | 28 | 1 | 19 | 0 |
| Fatigue | 32 | 14 | 16 | 5 |
| Hyperglycemia | 37 | 6 | 17 | 3 |
| Hypocalcemia | 20 | 2 | 12 | 1 |
| Nausea | 36 | 3 | 15 | 0 |
| Vomiting | 23 | 2 | 7 | 1 |
Twenty-five patients (34%) reported mild to moderate dyspnea without other associated cardiopulmonary symptoms or new abnormalities on the chest x-ray or ECGs. Serial ECGs showed nonspecific ST and QT changes but no consistent patterns were identified. No patients were found to have cardiac arrhythmias, new onset angina, or other cardiac toxicities.
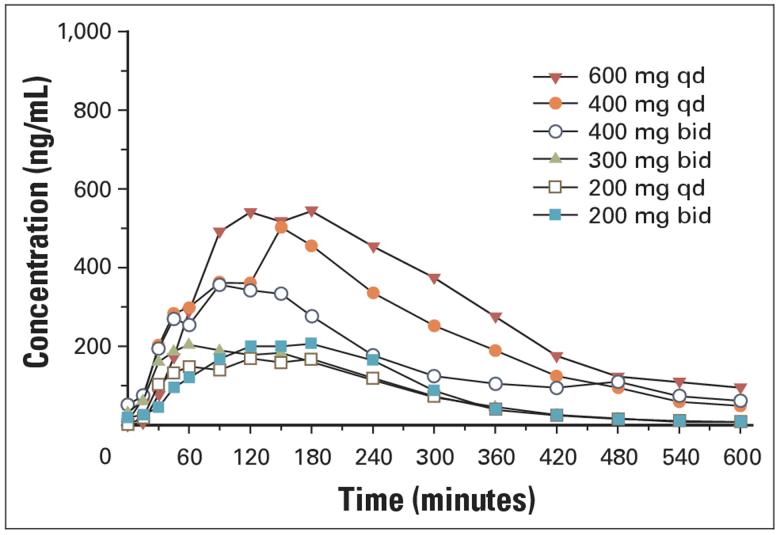
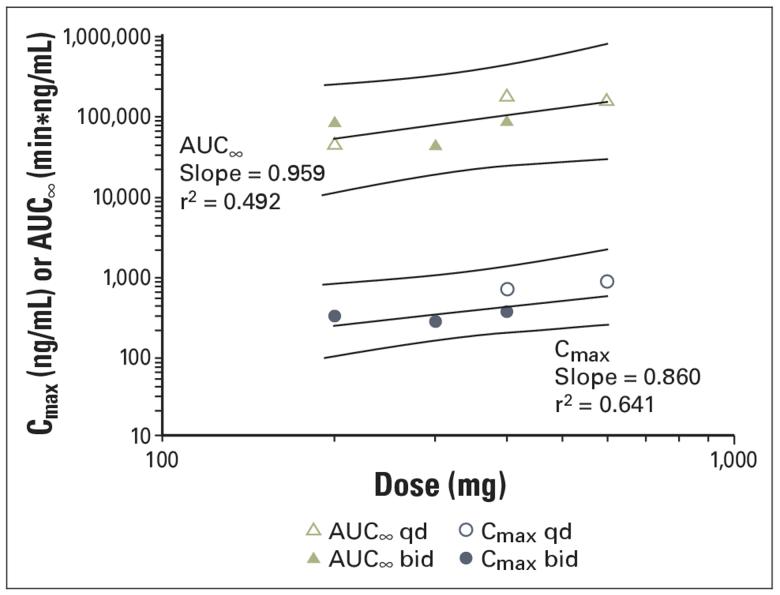
Pharmacokinetics
PK parameters and mean plasma concentration time curves from patients treated with 200, 400, and 600 mg of SAHA are presented in Table 4 and Figure 1. The pharmacokinetics of SAHA after oral administration of a single dose of SAHA are linear from 200 to 600 mg (Fig 2). The mean apparent half-life (t½) following oral administration (range, 91.6 to 127 minutes) was longer than the mean apparent t½ following intravenous administration of the oral equivalent doses (range, 34.7 to 42.4 minutes). The estimated bioavailability of SAHA at doses of 200 and 400 mg administered during the fasting state was 43%. Exploratory studies in the fasting and nonfasting (fed) state suggest that oral administration of SAHA with food does not appear to substantially alter the rate or extent of absorption. PK parameters obtained in four patients after 6 months or more of therapy were similar to baseline values.
Table 4.
Pharmacokinetic Parameter for SAHA After Oral Administration
| Day | Parameter* | 200 mg qd | 200 mg bid | 300 mg bid | 400 mg qd | 400 mg bid | 600 mg qd |
|---|---|---|---|---|---|---|---|
| 1(intravenous) | No. of patients | 6 | NA | NA | 8 | 6 | NA |
| Cmax, ng/mL | 1,088 ± 567 | 2,306 ± 1,099 | 2,184 ± 1,253 | ||||
| Tmax, min | 60 | 60 | 60 | ||||
| AUC0-t, min-ng/mL | 105,300 ± 64,224 | 214,132 ± 133,211 | 201,020 ± 105,654 | ||||
| AUC∞, min-ng/mL | 124,518 ± 69,943 | 163,202 ± 27,794 | 219,588 ± 107,344 | ||||
| T1/2, min | 34.7 ± 13.4 | 42.4 ± 15.5 | 38.4 ± 9.2 | ||||
| CL/F, mL/min | 2,134 ± 1,283 | 2,513 ± 439 | 2,231 ± 1,153 | ||||
| Vz/F, L | 89.2 ± 23.1 | 150 ± 51.5 | 117 ± 51.8 | ||||
| 8 (fasted) | No. of patients | 6 | 10 | 6 | 10 | 6 | 6 |
| Cmax, ng/mL | 304 ± 150 | 301 ± 152 | 263 ± 100 | 658 ± 439 | 349 ± 127 | 804 ± 397 | |
| Tmax, min | 135 | 120 | 53 | 106 | 150 | 90 | |
| AUC0-t, min-ng/mL | 43,426 ± 28,400 | 49,466 ± 38,766 | 41,489 ± 22,099 | 101,854 ± 105,570 | 66,439 ± 15,087 | 166,555 ± 86,736 | |
| AUC∞, min-ng/mL | 40,393 ± 23,046 | 74,374 ± 74,914 | 39,730 ± 23,694 | 161,443 ± 169,849 | 77,334 | 139,370 ± 71,002 | |
| T1/2, min | 91.6 ± 27.2 | 122 ± 33.8 | 93.5 ± 25.2 | 88.9 ± 20.5 | 100 | 127 ± 64.2 | |
| CL/F,mL/min | 5,987 ± 2,790 | 4,699 ± 2,994 | 11,006 ± 7,994 | 4,409 ± 2,682 | 5,172 | 5,418 ± 3,387 | |
| Vz/F, L | 853 ± 607 | 834 ± 617 | 1,528 ± 1,147 | 531 ± 346 | 748 | 889 ± 444 | |
| 9 (fed) | No. of patients | 6 | 10 | 6 | 9 | 6 | 6 |
| Cmax, ng/mL | 279 ± 151 | 295 ± 160 | 297 ± 111 | 667 ± 696 | 455 ± 158 | 685 ± 277 | |
| Tmax, min | 120 | 150 | 105 | 90 | 105 | 150 | |
| AUC0-t, min-ng/mL | 41,686 ± 24,516 | 52,074 ± 34,025 | 51,572 ± 18,183 | 134,292 ± 181,574 | 99,293 ± 60,407 | 174,322 ± 72,414 | |
| AUC∞, min-ng/mL | 56,001 ± 37,054 | 48,120 ± 17,518 | 48,868 ± 21,839 | 199,874 ± 252,665 | 121,970 ± 99,787 | 236,094 ± 150,007 | |
| T1/2, min | 70.5 ± 12.9 | 91.9 ± 39.3 | 97.8 ± 38.2 | 135 ± 109 | 100 ± 34.4 | 111 ± 58.2 | |
| CL/F, mL/min | 5,776 ± 5,256 | 4,518 ± 1,243 | 7,410 ± 4,069 | 3,945 ± 2,086 | 5,096 ± 3,657 | 3,184 ± 2,023 | |
| Vz/F, L | 522 ± 373 | 621 ± 359 | 1,016 ± 476 | 885 ± 1,138 | 616 ± 265 | 424 ± 56 | |
| 22-30 | No. of patients | 3 | 7 | 5 | 7 | 2 | 3 |
| Cmax, ng/mL | 233 ± 88.8 | 263 ± 76.3 | 263 ± 89.9 | 446 ± 105 | 268 ± 78.4 | 334 ± 160 | |
| Tmax, min | 45 | 45 | 60 | 90 | 195 | 120 | |
| AUC0-t, min-ng/mL | 33,333 ± 23,678 | 39,634 ± 13,170 | 32,658 ± 9,714 | 65,324 ± 19,435 | 65,740 ± 29,677 | 49,602 ± 1,945 | |
| AUC∞, min-ng/mL | † | 43,511 ± 14,009 | 30,759 ± 8,942 | 92,625 ± 8,461 | 88,106 | 75,489 | |
| T1/2, min | † | 78 ± 46.9 | 57.6 ± 44.8 | 98 ± 31.3 | 43.4 | 685 | |
| CL/F, mL/min | † | 5,129 ± 2,149 | 10,400 ± 3,008 | 4,337 ± 396 | 4,540 | 7,948 | |
| Vz/F, L | † | 513 ± 207 | 963 ± 1,016 | 604 ± 140 | 284 | 7,851 | |
| > 6 months | No. of patients | NA | 1 | NA | NA | 3‡ | NA |
| Cmax, ng/mL | 247 | 358 ± 67 | |||||
| Tmax, min | 127 | 80 | |||||
| AUC0-t, min-ng/mL | 33,949 | 47,883 ± 9,185 | |||||
| AUC∞, min-ng/mL | 34,640 | 47,904 ± 9,175 | |||||
| T1/2, min | 76 | 79 ± 30 | |||||
| CL/F, mL/min | 5,890 | 8,540 ± 1,520 | |||||
| Vz/F, L | 650 | 1,000 ± 450 |
Abbreviations: SAHA, suberoylanilide hydroxamic acid; qd, every day; bid, twice a day; NA, not applicable; Cmax, maximum concentration; Tmax, time to maximum concentration; AUC, area under the curve; T1/2, half-life; CL/F, clearance; Vz/F, volume of distribution.
Mean ± SD except for Tmax, for which the median is reported. Individual patients are reported if n ≤ 2.
Parameter could not be calculated
At the time of pharmacokinetic studies, one patient was on 400 mg bid and two patients were dose reduced to 400 mg daily.
Fig 1.
Mean plasma concentrations of suberoylanilide hydroxamic acid on cycle 1/day 9 after oral administration of 200 mg every day (qd) or twice a day (bid), 300 mg bid, 400 mg qd or bid, or 600 mg qd under fed conditions.
Fig 2.
Relationship between area under the curve to infinity (AUCN) and maximum concentration (Cmax) and dose on cycle 1/day 8 after administration of suberoylanilide hydroxamic acid under fasting conditions. qd, every day; bid, twice a day.
Correlative Studies
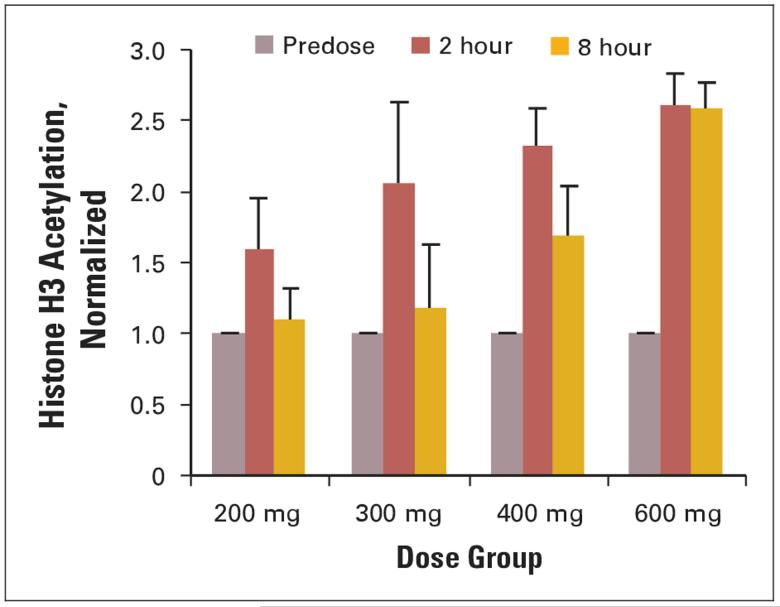
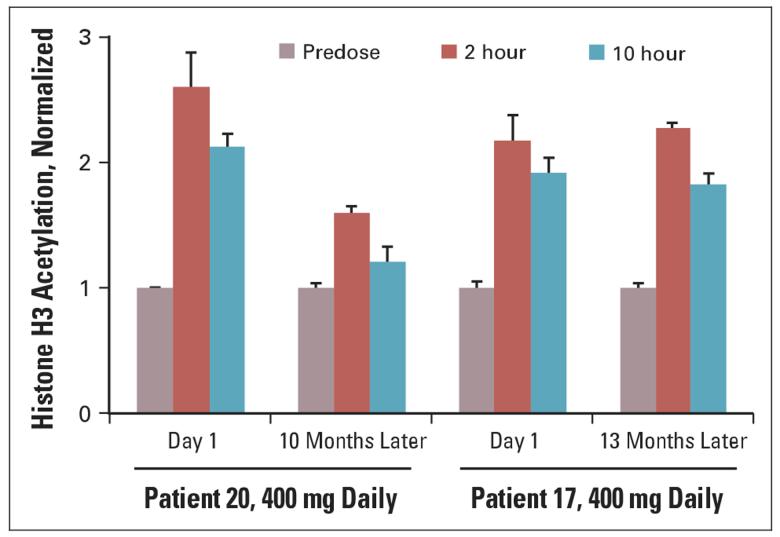
Histone acetylation was evaluated by Western blot or ELISA on histones isolated from PBMNCs. Histone samples were isolated from PBMNCs from 50 patients, and accumulation of acetylated histone H3 (AcH3) was observed at 2 hours after oral ingestion of SAHA consistently in all dose cohorts (Fig 3). As the dose of oral SAHA was increased from 200 to 600 mg, the duration that an accumulation of AcH3 was observed increased from 4 to 10 hours. An accumulation of AcH3 in PBMNCs was consistently observed at all dose levels after 3 weeks of oral SAHA. Two patients that remained on study > 6 months had repeat analysis of the AcH3 (Fig 4). An increase in accumulation of AcH3 was observed in patients on prolonged treatment with oral SAHA.
Fig 3.
Average histone H3 acetylation by dose group. Histones were isolated at the times indicated following ingestion of suberoylanilide hydroxamic acid and enzyme-linked immunosorbent assays performed as described in Patients and Methods. The number of patients analyzed in the 200-, 300-, 400-, and 600-mg groups was five, three, seven, and three, respectively. Each determination was performed in triplicate and the error bars represent the standard error of the mean.
Fig 4.
Long-term evaluation of peripheral-blood mononuclear cells for histone H3 acetylation in patients.
Antitumor Activity
Twenty-two patients (30%) remained on study for 4 to 37+ months (Table 5). Of these 22 patients, there was one complete response (CR) in a patient with transformed diffuse large B-cell lymphoma with normalization of the positron emission tomography scan and resolution of the bone marrow involvement for 17 months, and three partial responses (PRs) were noted in the following patients: de novo diffuse large B-cell lymphoma, laryngeal cancer (n = 1), and papillary thyroid cancer (n = 1). Two unconfirmed partial responses were observed in patients with metastatic mesothelioma. Stable disease was seen at all dose levels but confirmed CRs and PRs were only seen at 400 mg bid and 600 mg daily dose levels. An improvement in tumor-related pain and dyspnea was observed in patients with laryngeal cancer and mesothelioma who had tumor regression. Prolonged disease stabilization was also seen in patients with renal cell carcinoma and thyroid cancer with minor objective tumor regression. There were six patients with metastatic thyroid cancer (four poorly differentiated papillary, one Hürthle cell, and one medullary) maintained on oral SAHA for a median of 27 months (range, 12 to 37+ months). Three papillary thyroid patients had radioactive iodine (RAI) scans performed post-therapy and one had an improvement in the RAI scan post-therapy with oral SAHA.
Table 5.
Patients on Study ≥ 4 Months
| Tumor | SAHA dose (mg) | No. of Prior Systemic Therapy | Best Response | On-Study Duration (months) |
|---|---|---|---|---|
| Solid tumor | ||||
| Thyroid | 200 qd | 1 | SD | 22 |
| Renal cell* | 400 qd | 3 | SD | 4 |
| Urothelial | 200 bid | 3 | SD | 10 |
| Thyroid | 200 bid | 1 | SD | 28 |
| Thyroid | 300 bid | 1 | SD | 28+ |
| Mesothelioma | 300 bid × 3 days/week | 1 | SD | 8+ |
| Mesothelioma | 300 bid × 3 days/week | 5 | PR†† | 8 |
| Mesothelioma† | 300 bid × 3 days/week | 2 | SD | 5 |
| Mesothelioma | 300 bid × 3 days/week | 0 | SD | 5 |
| Mesothelioma | 300 bid × 3 days/week | 1 | PR†† | 6 |
| Mesothelioma‡ | 400 bid × 3 days/week | 1 | SD | 10 |
| Thyroid | 400 bid × 3 days/week | 1 | SD | 12 |
| Laryngeal§ | 400 bid | 1 | PR | 10 |
| Renal cell¶ | 400 bid | 4 | SD | 37+ |
| Thyroid∥ | 400 bid | 1 | PR | 34 |
| Thyroid** | 400 bid | 2 | SD | 37+ |
| Hematologic malignancy | ||||
| Hodgkin’s lymphoma | 400 qd | 2 | SD | 10 |
| Hodgkin’s lymphoma | 400 qd | 8 | SD | 5 |
| DLBCL (de novo) | 600 qd | 4 | PR | 5 |
| DLBCL (transformed) | 200 bid | 3 | SD | 8 |
| Cutaneous T-cell | 200 bid | 5 | SD | 4 |
| DLBCL (transformed) | 400 bid | 8 | CR | 13 |
Abbreviations: SAHA, suberoylanilide hydroxamic acid; qd, every day; SD, stable disease; bid, twice a day; PR, partial response; DLBCL, diffuse large B-cell lymphoma; CR, complete response.
400 qd for 7 weeks; 200 qd for 9 weeks.
300 bid × 3 days/week for 4 weeks; 200 bid × 3 days/week for 14 weeks.
400 bid × 3 days/week for 33 weeks; 300 bid for 7 weeks.
400 bid for 2 weeks; 400 qd for 36 weeks.
400 bid for 12 weeks; 400 qd for 136+ weeks.
400 bid for 4 weeks; 400 qd for 132 weeks.
400 bid for 93 weeks; 400 qd for 53+ weeks
Unconfirmed.
DISCUSSION
This study demonstrates that oral SAHA can be administered safely for prolonged durations at doses that inhibit HDAC activity, has linear pharmacokinetics with good bio-availability, and has a broad range of antitumor activity. This study defined a once daily (400 mg qd), twice daily (200 mg bid), and a twice daily for 3 consecutive days every week (300 mg bid) dosing schedule that could be used safely in future studies. Fatigue, anorexia, dehydration, and diarrhea were the DLTs observed across the three dosing schedules. The DLTs and the doses at which DLTs occurred were similar between patients with hematologic malignancies and solid tumor patients. This is in contrast to the intravenous study of SAHA that showed that myelosuppression limited the dose escalation in patients with hematologic malignancies and there was a three-fold difference in the MTD between solid tumor and hematologic patients.9 The more extensive prior therapy hematologic patients received may account for these differences. The DLTs with oral SAHA were not related to prior therapy or the type of underlying malignancies but remained relatively unpredictable within treatment cohorts. The fatigue could occur rapidly and was associated with anorexia, dehydration, diarrhea, and a feeling of dyskinesia. Once the oral SAHA was discontinued, these toxicities resolved quickly within 4 to 7 days. The etiology of the fatigue is not known, but their rapid resolution after withdrawing the SAHA suggests a readily reversible metabolic process.
In this study, the majority of the patients were treated at MTD or above and once on a tolerable dose, patients could be treated for prolonged periods of time, in some cases for over 2 years without loss of the biologic effect. Chronic adverse effects (fatigue, renal insufficiency, and weight loss) seen in long-term treated patients were generally mild to moderate and were reversible on discontinuation of the study drug, suggesting that chronic administration of SAHA is feasible and safe.
The altered PK profile of oral SAHA as compared with IV SAHA is likely to have contributed to the differences in toxicity, prolonged biologic effect, and the clinical outcomes in patients. As with the IV formulation of SAHA, the extent of exposure after oral SAHA administration was linear in dose ranges from 200 to 600 mg.9 Peak concentrations were substantially lower after oral administration, but there was a two- to three-fold increase in the apparent half-life when compared to the IV administration. Oral SAHA plasma concentrations could be detected at 10 hours postingestion at the higher dose levels while the plasma concentration of IV SAHA at similar doses were not detectable after 4 to 6 hours. This would suggest an absorption-rate-limited drug disposition in the GI tract and possibly hepatic recirculation to the GI tract.
As previously shown with intravenous SAHA using Western blot analysis, an increase in histone acetylation in PBMNCs was observed 2 hours post-therapy consistently in all patients evaluated and could persist for up to 10 hours after a single 400 mg or higher dose. This biologic effect paralleled the prolonged plasma concentrations of oral SAHA. An increase in acetylated histones was detected in patients who were on study for 6 months or longer, suggesting there is a sustained biologic effect over time.
Tumor regression and stable disease were observed in a wide range of patients with solid tumors and lymphomas. Four patients had confirmed CR and PR that occurred at the 400 mg bid and 600 mg qd dosing schedules and three of the 4 patients were treated on a twice daily regimen. This suggests that higher doses of SAHA with more prolonged daily exposure may be required for tumor regression. However, prolonged stable disease with minor tumor regression was seen at all dose levels and dosing schedules. Of particular interest was the clinical activity observed in patients with lymphoma and malignant mesothelioma. One CR and one PR were seen in patients with diffuse large B-cell lymphoma and two unconfirmed partial responses in patients with mesothelioma. Preclinical data suggest that HDACs play a critical role in the malignant transformation and cell differentiation13-16 in these tumors and provides a rationale for developing HDAC inhibitors in these diseases. Of note in this study, 30% of the heavily pretreated patients had stable disease for 4 or more months and five patients remained on therapy for more than 2 years. Four of the five of these long-term patients had metastatic thyroid cancer which may have a more indolent course; however, all had objective disease progression before study entry. Thyroid cancer patients were initially accrued to this trial based on the data from Kitazono et al, showing that the HDAC inhibitor, depsipeptide, led to an increase in the expression to the Na+/iodine symporter that could result in an increase 125I uptake in thyroid cells.17 This could possibly lead to resensitizing RAI-refractory patients to RAI.17,18 In this study, one patient did have an increase in the RAI scan post-therapy. Other plausible mechanisms for disease stabilization need to be investigated in thyroid cancer, since one patient with medullary thyroid cancer was also maintained on therapy for over 2 years.
In summary, this first study of oral SAHA demonstrated that SAHA could be administered safely for prolonged periods of time while maintaining the biologic effect of the drug and exhibiting a broad range of antitumor activity at multiple dose levels and dosing schedules. Future studies need to define the optimal dosing schedule and elucidate the biologic consequences of HDAC inhibition in patients. Currently, there are multiple phase II studies in patients with hematologic and solid tumor malignancies that are exploring the efficacy of daily and twice daily schedules that will help to determine the most optimal dosing regimen.
Footnotes
Supported by the National Institutes of Health: CA96228-0 (W.K.K.); CaPCURE, PepsiCo Foundation, The DeWitt Wallace Fund for Memorial Sloan-Kettering Cancer Center, The Kleberg Foundation, David Koch Foundation and Aton Pharma, Inc, a wholly owned subsidiary of Merck & Co Inc. O.A.O. is the recipient of the Research Scholar Award from the Leukemia and Lymphoma Society.
Authors′ Disclosures of Potential Conflicts of Interest
The following authors or their immediate family members have indicated a financial interest. No conflict exists for drugs or devices used in a study if they are not being evaluated as part of the investigation. Employment: Judy H. Chiao, Aton Pharma; Paul Secrist, Aton Pharma; Victoria M. Richon, Merck & Company. Leadership Position: Paul A. Marks, Aton Pharma. Consultant: William Kevin Kelly, Merck; Lee M. Krug, Merck. Stock Ownership: Judy H. Chiao, Aton Pharma, Merck; Paul A. Marks, Aton Pharma; Victoria M. Richon, Merck, Aton Pharma. Honoraria: William Kevin Kelly, Aton Pharma. Research Funding: William Kevin Kelly, Aton Pharma; Mark Heaney, Aton Pharma. For a detailed description of these categories, or for more information about ASCO’s conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section of Information for Contributors found in the front of every issue.
REFERENCES
- 1.Marks PA, Richon VM, Miller T, et al. Histone deacetylase inhibitors. Adv Cancer Res. 2004;91:137–168. doi: 10.1016/S0065-230X(04)91004-4. [DOI] [PubMed] [Google Scholar]
- 2.Freiman RN, Tjian R. Regulating the regulators: Lysine modifications make their mark. Cell. 2003;112:11–17. doi: 10.1016/s0092-8674(02)01278-3. [DOI] [PubMed] [Google Scholar]
- 3.Kouzarides T. Histone methylation in transcriptional control. Curr Opin Genet Dev. 2002;12:198–209. doi: 10.1016/s0959-437x(02)00287-3. [DOI] [PubMed] [Google Scholar]
- 4.Polevoda B, Sherman F. The diversity of acetylated proteins. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-5-reviews0006. Reviews 0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler LM, Zhou X, Xu WS, et al. The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin. Proc Natl Acad Sci U S A. 2002;99:11700–11705. doi: 10.1073/pnas.182372299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Della Ragione F, Criniti V, Della Pietra V. Genes modulated by histone acetylation as new effectors of butyrate activity. FEBS Lett. 2001;499:199–204. doi: 10.1016/s0014-5793(01)02539-x. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki H, Gabrielson E, Chen W, et al. A genomic screen for genes unregulated by demethylation and histone deacetylase inhibition in human colorectal cancer. Nat Genet. 2002;31:141–149. doi: 10.1038/ng892. [DOI] [PubMed] [Google Scholar]
- 8.Van Lint C, Emiliani S, Verdin E. The expression of a small fraction of cellular gene is changes in response to histone hyperacetylation. Gene Expr. 1996;5:245–254. [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly WK, Richon VM, O′Connor O, et al. Phase I clinical trial of histone deacetylase inhibitor: Suberoylanilide hydroxamic acid administered intravenously. Clin Cancer Res. 2003;9:3578–3588. [PubMed] [Google Scholar]
- 10.Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Cheson B, Horning S, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin′s lymphomas: NCI sponsored International Working Group. J Clin Oncol. 17:1244–1999. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 12.Cheson BD, Cassileth PA, Head DR, et al. Report of the National Cancer Institute-sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J Clin Oncol. 1990;8:813–819. doi: 10.1200/JCO.1990.8.5.813. [DOI] [PubMed] [Google Scholar]
- 13.Pasqualucci L, Migliazza A, Basso K, et al. Mutations of the BCL6 proto-oncogene disrupt its negative autoregulation in diffuse large B-cell lymphoma. Blood. 2003;101:2914–2923. doi: 10.1182/blood-2002-11-3387. [DOI] [PubMed] [Google Scholar]
- 14.Bereshchenko O, Gu W, Dalla-Favera R. Acetylation inactivates the transcriptional BCL6. Nat Genet. 2002;32:606–613. doi: 10.1038/ng1018. [DOI] [PubMed] [Google Scholar]
- 15.Baron B, Anastasi J, Thirman M, et al. The human programed cell death-2 (PDCD2) gene is a target of BCL6 repression: Implications for a role of BCL6 in the down-regulation of apoptosis. Proc Natl Acad Sci U S A. 2002;99:2860–2865. doi: 10.1073/pnas.042702599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao X, Mohuiddin I, Ece F, et al. Histone deacetylase inhibitor down-regulation of bcl-xl gene expression leads to apoptotic cell death in mesothelioma. Am J Respir Cell Mol Biol. 2001;25:562–568. doi: 10.1165/ajrcmb.25.5.4539. [DOI] [PubMed] [Google Scholar]
- 17.Kitazono M, Robey R, Zhan Z, et al. Low concentrations of the histone deacetylase inhibitor, depsipeptide ( FR901228), increase expression of the Na+/I-symporter and iodine accumulation in poorly differentiated thyroid carcinoma cells. J Clin Endocrinol Metab. 2001;86:3430–3435. doi: 10.1210/jcem.86.7.7621. [DOI] [PubMed] [Google Scholar]
- 18.Furuya F, Shimura H, Suzuki H, et al. Histone deacetylase inhibitors restore radioiodide uptake and retention in poorly differentiated and anaplastic thyroid cancer cells by expression of the sodium/iodide symporter thyroperoxidase and thyroidglobulin. Endocrinology. 2004;145:2865–2875. doi: 10.1210/en.2003-1258. [DOI] [PubMed] [Google Scholar]