Abstract
Members of the Receptor Protein Tyrosine Phosphatase (RPTP) subfamily of cell adhesion molecules (CAMs) mediate neurite outgrowth and growth cone repulsion. PTPμ is a growth permissive substrate for nasal retinal ganglion cell (RGC) neurites and a growth inhibitory substrate for temporal RGCs. In this manuscript, we demonstrate that the distinct PTPμ-dependent phenotypes of nasal outgrowth and temporal repulsion are regulated by Rho GTPases. The role of Rho GTPases in the regulation of nasal outgrowth and temporal repulsion was tested by utilizing dominant negative and constitutively active forms of Rac1, RhoA and Cdc42 in Bonhoeffer stripe assays. Nasal neurite outgrowth on PTPμ was blocked by Cdc42-DN. Temporal repulsion to a PTPμ substrate was substantially reduced by addition of Cdc42-DN. The molecule that regulates the switch between permissive versus repulsive responses to PTPμ is Rac1 for temporal neurons. Inhibition of Rac1 is required for repulsion of temporal neurons. Interestingly, adding Rac1-CA to temporal RGC neurons converted PTPμ-dependent repulsion to a permissive response. In addition, adding exogenous Rac1-DN to nasal neurons induced a phenotype switch from a permissive to repulsive response to PTPμ. Together these data suggest that Cdc42 activity is required for both permissive and repulsive responses to PTPμ. However, the key to PTPμ-dependent repulsion is inhibition of Rac1 activity in temporal RGC neurons.
Keywords: receptor protein tyrosine phosphatase, cell adhesion molecule, retinal ganglion cell, tyrosine phosphorylation, axon guidance, Rho GTPase, actin cytoskeleton
Introduction
Spatiotemporal patterning of the visual system is a structured event in which retinal ganglion cell (RGC) axons from a particular region of the retina migrate and innervate a specific region of the optic tectum (McLaughlin et al., 2003; O'Leary and McLaughlin, 2005; Thanos and Mey, 2001; van Horck et al., 2004). For example, nasal axons innervate the posterior tectum, while temporal neurons innervate the anterior tectum. Studies have suggested that RGC projection to the tectum is partially due to the graded expression of Eph receptor tyrosine kinases and their ephrin ligands (McLaughlin et al., 2003; O'Leary and McLaughlin, 2005). Therefore, tyrosine phosphorylation is key to the development of the visual system but little is known about the function of tyrosine phosphatases in this system. Receptor tyrosine phosphatases (RPTPs), which catalyze the dephosphorylation of tyrosine residues, are expressed in the nervous system and recent evidence suggests that they may be involved in guiding retinal axons to their targets during development (Brady-Kalnay, 2001; Ensslen-Craig and Brady-Kalnay, 2004). A subfamily of RPTPs, including PTPμ, has cell adhesion molecule-like extracellular segments and intracellular domains with tyrosine phosphatase activity (Brady-Kalnay, 2001; Ensslen-Craig and Brady-Kalnay, 2004; Johnson and Van Vactor, 2003).
The PTPμ-like subfamily of RPTPs includes four members: PTPμ, PTPκ, PTPρ and PCP-2 (Brady-Kalnay, 2001). The PTPμ-like subfamily members contain motifs found in CAMs including a MAM domain, an immunoglobulin (Ig) domain and four FNIII repeats in their extracellular segment. The motifs present in PTPμ suggested that it might function in cell-cell adhesion. We demonstrated that expression of PTPμ induced the aggregation of nonadhesive Sf9 insect cells (Brady-Kalnay et al., 1993; Gebbink et al., 1993). PTPκ and PCP-2 have also been shown to mediate aggregation (Cheng et al., 1997; Sap et al., 1994). These studies demonstrated that the binding is homophilic (i.e. the “ligand” for a transmembrane PTP is an identical PTP molecule on an adjacent cell). Both PTPμ-dependent adhesion and neurite outgrowth are mediated by homophilic binding (Brady-Kalnay and Tonks, 1994; Ensslen-Craig and Brady-Kalnay, 2005). The homophilic binding site resides in the immunoglobulin domain (Brady-Kalnay and Tonks, 1994). The MAM domain of PTPμ also plays a role in cis dimerization (Aricescu et al., 2006; Cismasiu et al., 2004) and cell aggregation by sorting cells expressing PTPμ into distinct aggregates from cells expressing related molecules such as PTPκ (Zondag et al., 1995). Together, these studies demonstrate that PTPμ mediates cell-cell adhesion and suggest it may transduce signals in response to adhesion that are required for neurite outgrowth.
The juxtamembrane domain of the PTPμ-like enzymes contains a region of homology to the intracellular domain of the cadherins (Brady-Kalnay, 2001). We demonstrated that PTPμ interacts specifically with N-cadherin, E-cadherin and R-cadherin (Brady-Kalnay et al., 1998). Both N- and R-cadherin are expressed in the retina and control retinal histogenesis (Redies, 1997; Redies and Takeichi, 1996). Importantly, we have demonstrated a functional role for the PTPμ/N-cadherin interaction because we found that PTPμ regulates N-cadherin-dependent neurite outgrowth of retinal ganglion cells (Burden-Gulley and Brady-Kalnay, 1999).
PTPμ is expressed in a gradient in both the retina and the tectum (Burden-Gulley et al., 2002). Since the axons of RGCs form the optic nerve and are the sole output from the retina to the brain, the expression of PTPμ on these cells was consistent with a putative role for PTPμ in axonal migration. The ability of retinal ganglion cell axons to grow out of a retinal explant onto a purified protein substrate has been used to study axonal (neurite) growth (Lemmon et al., 1992). To test whether PTPμ promoted neurite outgrowth, we used purified PTPμ as a substrate and demonstrated that it promoted neurite outgrowth from E8 retinal explants (Burden-Gulley and Brady-Kalnay, 1999). More importantly, PTPμ is a permissive substrate for neurite outgrowth from nasal RGC neurons, while it is inhibitory to temporal RGC neurons (Burden-Gulley et al., 2002). The molecular details of the intracellular signals that regulate axonal growth and guidance are not well defined.
It is intriguing that PTPμ is able to mediate both permissive and repulsive responses in RGC neurons. PTPμ is expressed at low levels in nasal neurons and is permissive for nasal RGC neurite outgrowth (Burden-Gulley et al., 2002). PTPμ is expressed at high levels in temporal RGCs and is repulsive to those neurons (Burden-Gulley et al., 2002). Our recent data suggest that the gradient of PTPμ expression in the retina regulates whether PTPμ is a repulsive or permissive guidance cue (Ensslen-Craig and Brady-Kalnay, 2005). We found that expression of exogenous PTPμ in nasal neurites, which increases PTPμ levels to those found in temporal neurons, resulted in a phenotypic switch from a permissive to a repulsive response to a PTPμ substrate (Ensslen-Craig and Brady-Kalnay, 2005). Catalytic activity of PTPμ is necessary for both permissive and repulsive guidance events downstream of PTPμ binding. Since both expression and catalytic activity of PTPμ are required, this implies that PTPμ homophilic binding results in a tyrosine phosphatase-dependent signal that is necessary for PTPμ-mediated nasal outgrowth and temporal repulsion, adding support to the growing body of evidence that RPTP catalytic activity is important for axon guidance (Garrity et al., 1999; Johnson et al., 2001). Most importantly, these results demonstrate a functional significance for the observed gradients in retinotectal expression of PTPμ. In this manuscript, we investigate the molecular mechanisms by which PTPμ regulates both nasal outgrowth and temporal repulsion.
The Rho subfamily of small G-proteins plays a central role in regulating the actin cytoskeleton (Raftopoulou and Hall, 2004; Ridley, 2004). Activation of Cdc42 results in the formation of filopodia while Rac activation induces lamellipodia formation, and Rho activation results in stress fibers in fibroblasts (Nobes and Hall, 1995). Rho GTPases are molecular switches, cycling between GTP-bound “on” and GDP-bound “off” states. This cycle is controlled by GTPase activating proteins (GAPs), guanine nucleotide exchange factors (GEFs) and GDP-dissociation inhibitors (Bishop and Hall, 2000; Kaibuchi et al., 1999).
The role of Rho GTPase activation in the regulation of nasal outgrowth and temporal repulsion was tested by utilizing dominant negative and constitutively active TAT-tagged forms of Rac1, RhoA and Cdc42, which were directly taken up by cells. We prepared alternating stripes of PTPμ and laminin as substrates for single stripe assays and a combination of PTPμ mixed with laminin versus laminin only lanes for the mixed stripe assays. Single stripes measure permissive responses, i.e. the ability to grow or not on one adhesion molecule. Mixed stripes measure repulsive responses because a repulsive substrate-like PTPμ is mixed with a normally permissive cue, such as laminin. The TAT-Rho GTPase fusion proteins were added to the stripe assays and changes in nasal outgrowth or temporal crossing were observed. Nasal neurite outgrowth and temporal repulsion to a PTPμ substrate were substantially reduced by the addition of Cdc42-DN, while inhibition of Rac1 is required for temporal repulsion to PTPμ response. These data suggest that the key to PTPμ-dependent repulsion is inhibition of Rac1 activity in temporal RGC neurons.
Results
We cultured retinal explants from embryonic day 8 chicks in Bonhoeffer stripe assays (Vielmetter et al., 1990; Walter et al., 1987a; Walter et al., 1987b). The Bonhoeffer stripe assay examines the ability of neurons to grow on two protein substrates presented in alternating lanes. A permissive substrate must be present in a lane to observe any neurite outgrowth on that lane (Lemmon et al., 1992). Very few adhesion molecules promote neurite outgrowth of chick retinal ganglion cell (RGC) neurons (Lemmon et al., 1992). The Receptor Protein Tyrosine Phosphatase PTPμ is able to mediate both permissive and repulsive guidance events (Burden-Gulley et al., 2002). PTPμ is permissive only to ventral nasal RGC neurons (Burden-Gulley and Brady-Kalnay, 1999; Burden-Gulley et al., 2002; Ensslen and Brady-Kalnay, 2004). In Bonhoeffer stripe assay, if a single substrate is plated on a lane and no neurite outgrowth is observed on that lane then it is unclear if that substrate is repulsive (and the neurons are actively avoiding it) or is just not permissive (i.e. no neurite outgrowth is observed in that lane). To distinguish between these two possibilities, a mixed substrate lane assay must be used. Repulsion is measured when neurites avoid a permissive substrate (laminin) that has been mixed with a repulsive substrate (PTPμ) in a single lane. Temporal neurites do not grow on a PTPμ only substrate lane (Burden-Gulley et al., 2002) nor do they cross a mixed laminin/PTPμ substrate, demonstrating that the PTPμ repulsive signal is dominant over the laminin permissive signal (Ensslen and Brady-Kalnay, 2004). The mixed stripe assay allows one to detect a pharmacological or molecular blockade of the PTPμ repulsive signal since the temporal neurites are then able to grow on the laminin component of the mixed lane. Since both nasal and temporal neurites originate from the same retinal explant, the Bonhoeffer stripe assay provides a powerful means to assess the effects of reagents that alter signaling cascades.
PTPμ is a permissive substrate for ventral nasal RGC neurites, they cross between the PTPμ only and laminin only lanes (Fig. 1). Addition of dominant negative Rac1 (Rac1-DN) and dominant negative Cdc42 (Cdc42-DN) does not affect laminin outgrowth but blocks crossing onto the PTPμ lanes (Fig. 1). Dominant negative RhoA (RhoA-DN) had no effect on RGC crossing onto the PTPμ substrate. Quantitation of the stripe assays was performed using a rating scale as previously described (Ensslen and Brady-Kalnay, 2004; Walter et al., 1987a). Neurites that show no preference for either substrate are assessed at 0, and neurites that grow exclusively on laminin are assessed at 3. An assessment of 2 indicates that most of the neurites grow on the laminin lanes with an occasional neurite crossing over PTPμ lanes, while an assessment of 1 is when there is a significant amount of neurite crossing but a tendency to fasciculate on laminin. Quantitation of the data presented indicates that Rac1 and Cdc42 activity are required for PTPμ to be a permissive growth substrate. However, subsequent experiments demonstrate that the effect of exogenous Rac1-DN addition was to induce nasal repulsion, instead of blocking the permissive response (see Fig. 5). Therefore, we conclude that Cdc42 activation is required for the nasal permissive response to a PTPμ substrate.
Figure 1. Cdc42 activity is required for PTPμ to be a permissive growth substrate for nasal neurons.
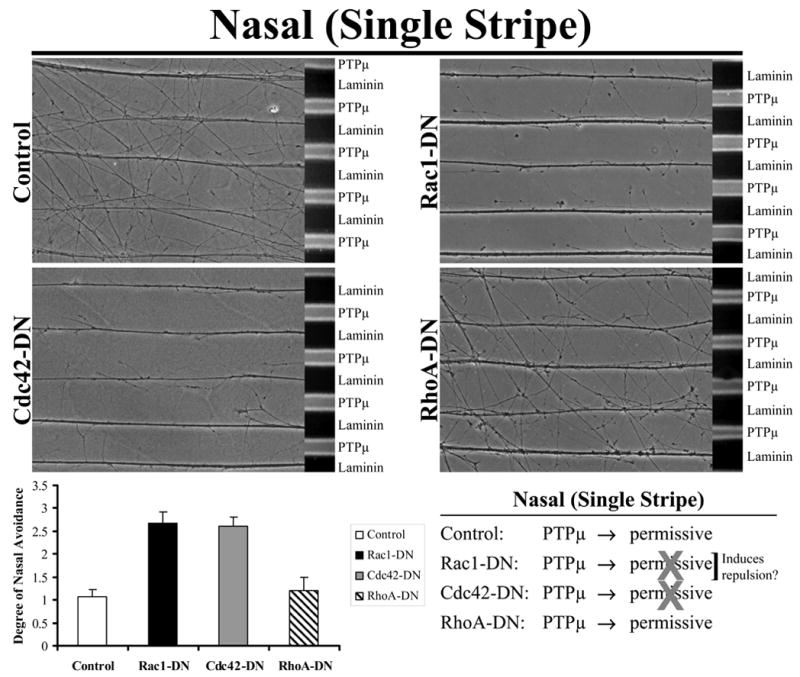
Explants from E8 nasal retina were grown on alternating stripes of PTPμ or laminin only lanes (single stripe assay) and either Rac1, Cdc42 or RhoA dominant negative (DN) mutants were added using protein transduction via their TAT-tag. Control and RhoA-DN transduced nasal neurites cross onto a PTPμ substrate. Following addition of Rac1-DN or Cdc42-DN, however, nasal neurites are no longer able to cross onto PTPμ lanes. These results suggest that activation of Cdc42 is required for a permissive response to a PTPμ substrate. Quantitation of the degree of nasal avoidance of PTPμ lanes is shown following addition of dominant-negative mutants of the Rho GTPases to single stripe assays. The average degree of avoidance (mean +/− STDEV) was plotted for each condition as described in Experimental Methods.
Figure 5. Inhibition of Rac1 converts the PTPμ permissive signal to repulsion in nasal neurites.
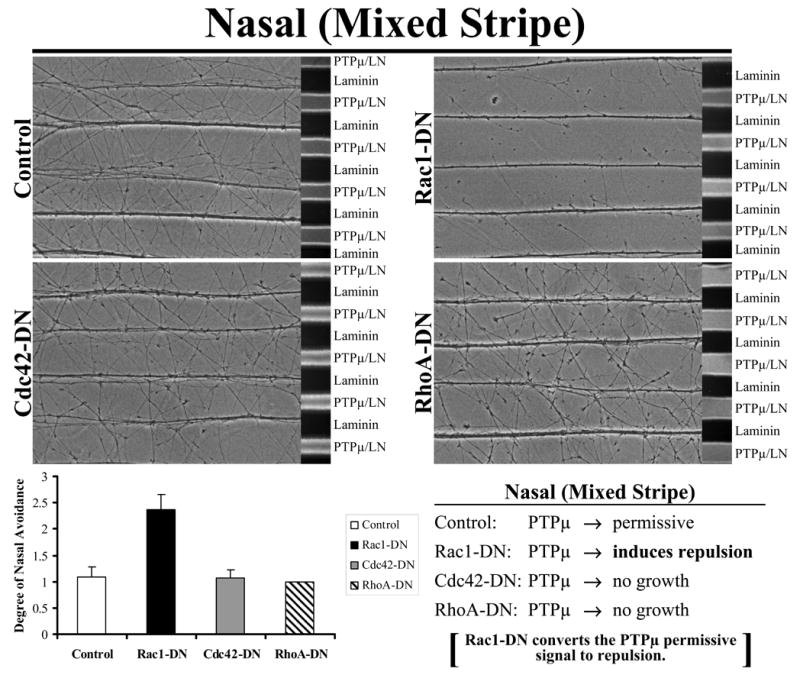
Explants from E8 nasal retina were grown on alternating stripes of PTPμ mixed with laminin (PTPμ/LN) versus laminin only lanes and either Rac1, Cdc42 or RhoA dominant negative mutants were added. Control, RhoA-DN and Cdc42-DN transduced nasal neurites crossed onto the PTPμ mixed substrate lanes because both PTPμ and laminin were permissive substrates for nasal neurons. Following addition of Rac1-DN, nasal neurites avoided the PTPμ-containing lanes. These results suggest that inhibition of Rac1 activity induces a phenotypic switch in the nasal neurons from a permissive to a repulsive response to a PTPμ substrate. Quantitation of the degree of nasal avoidance of PTPμ lanes is shown following addition of dominant-negative mutants of the Rho GTPases to mixed stripe assays. The average degree of avoidance (mean +/− STDEV) was plotted for each condition.
We used a mixed stripe assay to test the effect of dominant negative Rho GTPases on PTPμ-dependent temporal repulsion. Only addition of Cdc42-DN substantially reduced temporal repulsion on a PTPμ substrate (Fig. 2). Neither Rac1-DN nor RhoA-DN altered temporal repulsion to PTPμ (Fig. 2). These data indicate that Cdc42 activity is required for PTPμ-dependent repulsion. We then tested whether the constitutively active (CA) mutants of the Rho GTPases altered temporal repulsion to a PTPμ substrate. Interestingly, Rac1-CA blocks temporal repulsion to PTPμ in the mixed stripe assay (Fig. 3). Neither Cdc42-CA or RhoA-CA alter temporal repulsion to PTPμ (Fig. 3). These data suggest that Rac1 inhibition is required for PTPμ-dependent temporal repulsion.
Figure 2. Cdc42 activity is required for PTPμ-dependent temporal repulsion.
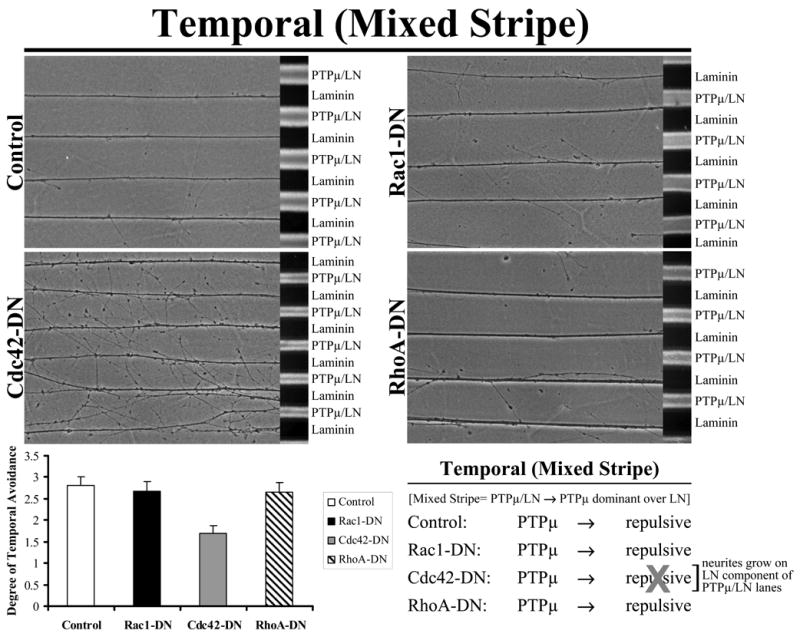
Explants from E8 temporal retina were grown on alternating stripes of PTPμ mixed with laminin (PTPμ/LN) versus laminin only lanes and either Rac1, Cdc42 or RhoA dominant negative (DN) mutants were added. Control, RhoA-DN and Rac1-DN transduced temporal neurites avoided the PTPμ mixed substrate lanes. Following addition of Cdc42-DN, temporal neurites crossed the PTPμ-containing lanes. These results suggest that inhibition of Cdc42 activity affects temporal repulsion to a PTPμ substrate. Quantitation of the degree of temporal avoidance of PTPμ lanes is shown following addition of dominant-negative mutants of the Rho GTPases to mixed stripe assays. The average degree of avoidance (mean +/− STDEV) was plotted for each condition.
Figure 3. Rac1 inhibition is required for PTPμ-dependent temporal repulsion.
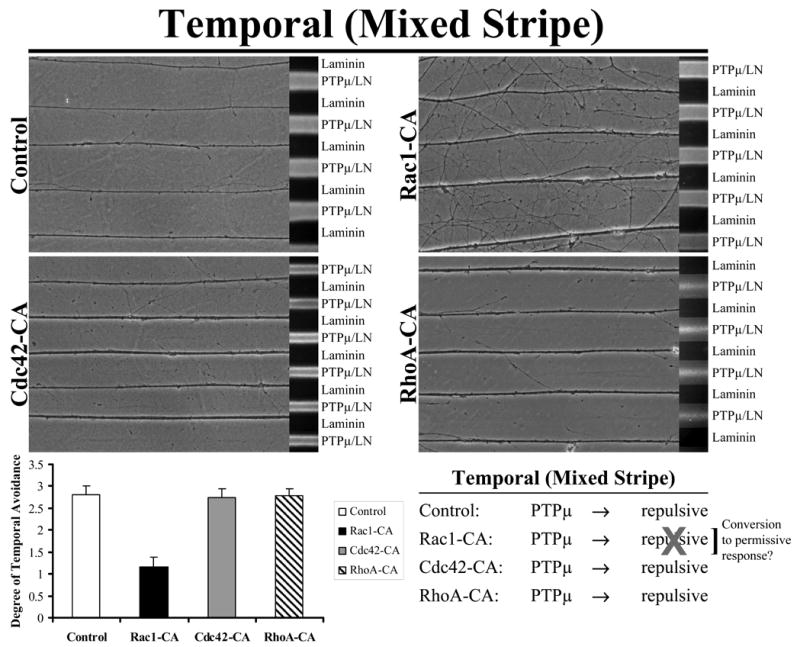
Explants from E8 temporal retina were grown on alternating stripes of PTPμ mixed with laminin (PTPμ/LN) versus laminin only lanes and either Rac1, Cdc42 or RhoA constitutively active mutants were added. Control, Cdc42-CA and RhoA-CA transduced temporal neurites avoided the PTPμ mixed substrate lanes. Following addition of Rac1-CA, however, temporal neurites crossed onto the PTPμ-containing lanes. These results suggest that inhibition of Rac1 activity is required for temporal repulsion by a PTPμ substrate. Quantitation of the degree of temporal neurite avoidance of PTPμ lanes is shown following addition of constitutively active mutants of the Rho GTPases to mixed stripe assays. The average degree of avoidance (mean +/− STDEV) was plotted for each condition.
To test whether Rac1-CA was simply blocking temporal repulsion in a mixed stripe assay or altering the response of temporal neurons to the PTPμ substrate, we performed single stripe assays. The single stripe assay measures the ability of temporal neurons to respond to the presence of PTPμ alone with either a permissive or repulsive response. Rac1-CA caused the temporal neurons to behave as if PTPμ were a permissive substrate (Fig. 4). Neither Cdc42-CA nor RhoA-CA was able to induce this phenotypic switch from repulsion to a permissive response (Fig. 4). Together, our data suggest that Rac1 activation converts PTPμ to a permissive substrate and that Rac1 inhibition is required for PTPμ-mediated temporal repulsion.
Figure 4. Rac1-CA converts PTPμ to a permissive signal in single stripe, PTPμ only lanes.
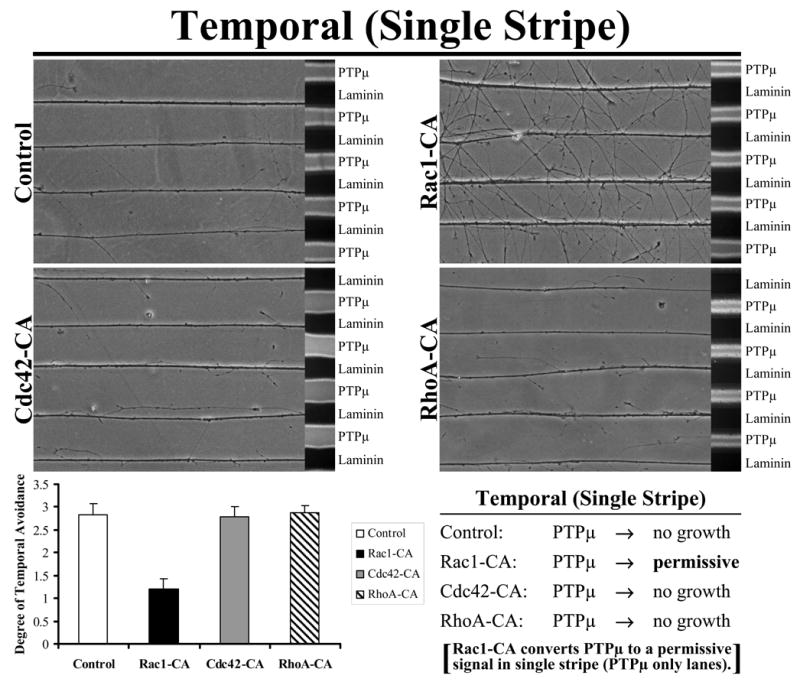
Explants from E8 temporal retina were grown on alternating stripes of PTPμ or laminin only lanes and either Rac1, Cdc42 or RhoA constitutively active mutants were added. Control, Cdc42-CA and RhoA-CA transduced temporal neurites avoided PTPμ. Following addition of Rac1-CA, temporal neurites crossed over onto the PTPμ only lanes. These results suggest that activation of Rac1 converts PTPμ to a permissive substrate for temporal neurons. Quantitation of the degree of temporal neurite avoidance of PTPμ lanes is shown following addition of constitutively active mutants of the Rho GTPases to single stripe assays. The average degree of avoidance (mean +/− STDEV) was plotted for each condition.
PTPμ is expressed in the retina in a gradient at low levels in nasal neurons and is permissive. In contrast, PTPμ is expressed at high levels in temporal RGCs and is repulsive to those neurons (Burden-Gulley et al., 2002; Ensslen-Craig and Brady-Kalnay, 2005). We found that expression of exogenous PTPμ in nasal neurites, which increases PTPμ levels to those found in temporal neurons, results in a phenotypic switch from a permissive to repulsive response to a PTPμ substrate (Ensslen-Craig and Brady-Kalnay, 2005).
The data presented thus far in this study indicate that Rac1 inhibition is required for PTPμ to be a repulsive substrate. If that were the case, artificially inhibiting Rac1 activation (with exogenous Rac1-DN) in nasal neurons might convert a PTPμ-dependent permissive response to repulsion in a mixed stripe assay. Figure 5 shows that addition of Rac1-DN did, in fact, cause a phenotypic switch in nasal neurons from a PTPμ-mediated permissive response to repulsion in mixed stripe assays. Neither Cdc42-DN nor RhoA-DN was able to induce this phenotypic switch in nasal neurons (Fig. 5). Therefore, the data in Figure 1 indicate that exogenous Rac1-DN induced repulsion, instead of blocking the permissive reponse to PTPμ. These data suggest that inhibition of Rac1 activity is required for PTPμ to be a repulsive substrate.
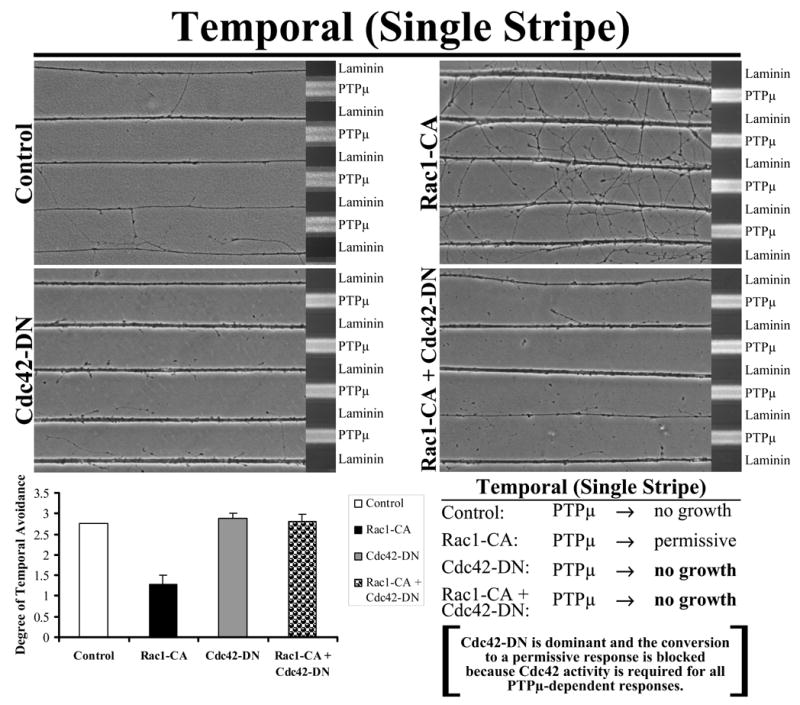
To determine if Rac1 and Cdc42 are in distinct or related pathways, we performed epistasis experiments. Rho GTPase mutants were added singly or in combination. Explants from E8 nasal retina were grown on alternating stripes of PTPμ or laminin only (single stripe assay) and either Rac1-CA, Cdc42-DN or the combination or Rac1-CA + Cdc42-DN were added using protein transduction via their TAT-tag. Control and Rac1-CA transduced nasal neurites cross onto a PTPμ substrate (Fig. 6). Following addition of Cdc42-DN or the combination of Rac1-CA + Cdc42-DN, however, nasal neurites are no longer able to cross onto PTPμ lanes. These results suggest that activation of Cdc42 is required for a permissive response to a PTPμ substrate and that Cdc42 is dominant over Rac1.
Figure 6. Cdc42 is dominant over Rac1 in the permissive response of nasal neurites to PTPμ.
Explants from E8 nasal retina were grown on alternating stripes of PTPμ or laminin only lanes (single stripe assay) and either Rac1-CA, Cdc42-DN or the combination of Rac1-CA + Cdc42-DN mutants were added. Control and Rac1-CA transduced nasal neurites cross onto a PTPμ substrate. Following addition of Cdc42-DN or Rac1-CA + Cdc42-DN, however, nasal neurites are no longer able to cross onto PTPμ lanes. These results suggest that activation of Cdc42 is required for a permissive response to a PTPμ substrate. Quantitation of the degree of nasal avoidance of PTPμ lanes is shown following addition of dominant-negative mutants of the Rho GTPases to single stripe assays. The average degree of avoidance (mean +/− STDEV) was plotted for each condition.
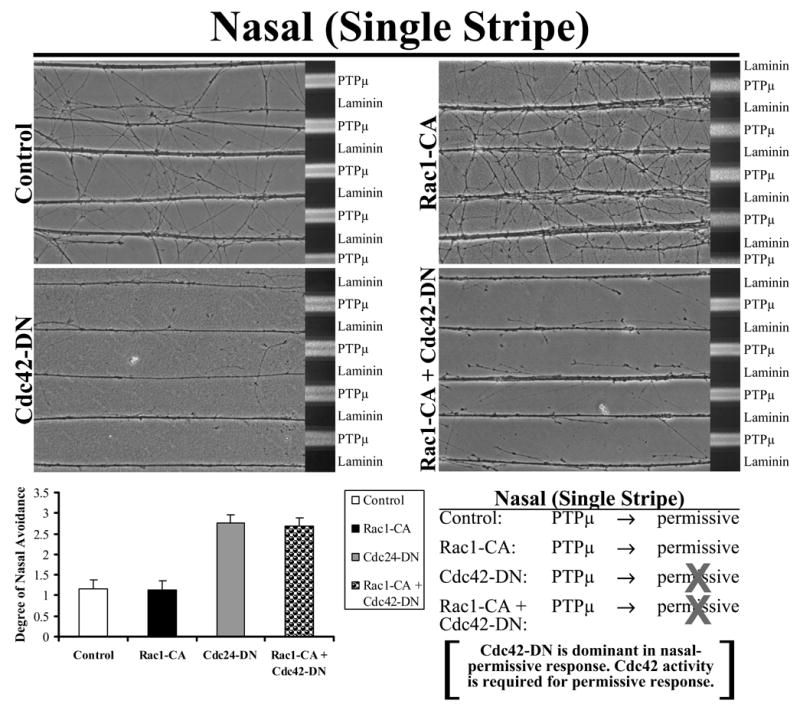
To confirm that Cdc42 is required for PTPμ to be a permissive substrate, E8 temporal retina explants were grown on alternating stripes of PTPμ or laminin only (single stripe assay), and either Rac1-CA, Cdc42-DN or the combination of Rac1-CA + Cdc42-DN were added by protein transduction. Control and Cdc42-DN transduced temporal neurites avoided PTPμ (Fig. 7). Following addition of Rac1-CA, temporal neurites crossed over onto the PTPμ lanes. Addition of Cdc42-DN to Rac1-CA blocked the conversion of PTPμ to a permissive signal for temporal neurons. These experiments confirm that Cdc42 activity is required for the permissive response to PTPμ, and altering Rac1 is not enough to elicit a permissive response. Rac1-CA is sufficient to reduce repulsion to PTPμ, indicating that it is capable of effectively converting the high PTPμ in temporal neurons to a low PTPμ signal found in nasal neurons.
Figure 7. Cdc42 is required for PTPμ to be a permissive substrate.
Explants from E8 temporal retina were grown on alternating single stripes of PTPμ or laminin only lanes and either Rac1-CA, Cdc42-DN or the combination of Rac1-CA + Cdc42-DN were added. Control and Cdc42-DN transduced temporal neurites avoided PTPμ. Following addition of Rac1-CA, temporal neurites crossed over onto the PTPμ only lanes. These results suggest that activation of Rac1 converts PTPμ to a permissive substrate for temporal neurons. Quantitation of the average degree of avoidance (mean +/− STDEV) was plotted for each condition. The addition of Cdc42-DN to Rac1-CA blocked the permissive response. These results confirm that activation of Cdc42 is required for a permissive response to PTPμ.
Figure 5 demonstrates that exogenous Rac1 inhibition converts the permissive to a repulsive response to PTPμ in nasal neurites. On alternating stripes of PTPμ mixed with laminin (PTPμ/LN) versus laminin only lanes, explants from E8 nasal retina were grown with the addition of either Rac1-DN, Cdc42-CA or the combination of Rac1-DN + Cdc42-CA. Control and Cdc42-CA transduced nasal neurites crossed onto the PTPμ mixed substrate lanes because both PTPμ and laminin are permissive substrates for nasal neurons (Fig. 8). Following addition of exogenous Rac1-DN, nasal neurites now avoid the PTPμ-containing lanes. These results suggest that inhibition of Rac1 activity induces a phenotypic switch in the nasal neurons from a permissive to a repulsive response to a PTPμ substrate. The combination of Rac1-DN + Cdc42-CA produced the same result as Rac1-DN alone. These data suggest inhibition of Rac1 is key to repulsion in response to PTPμ, and that exogenous Cdc42 activation cannot overcome this Rac1 inhibiting signal.
Figure 8. Exogenous Rac1 inhibition converts the permissive to a repulsive response to PTPμ by nasal neurites, and Cdc42 activation cannot overcome this response.
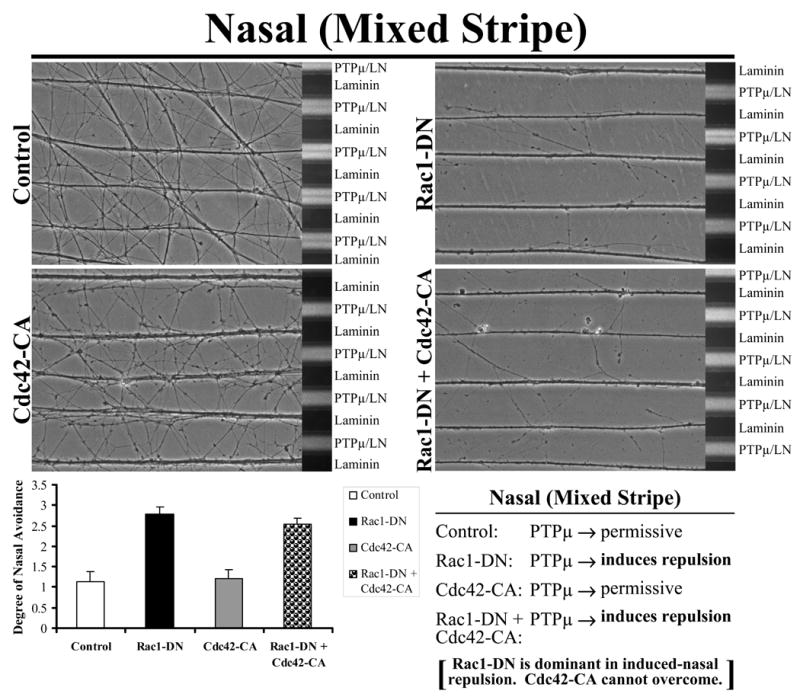
Explants from E8 nasal retina were grown on alternating stripes of PTPμ mixed with laminin (PTPμ/LN) versus laminin only lanes and either Rac1-DN, Cdc42-CA or the combination of Rac1-DN + Cdc42-CA mutants were added. Control and Cdc42-CA transduced nasal neurites crossed onto the PTPμ mixed substrate lanes because both PTPμ and laminin were permissive substrates for nasal neurons. Following addition of Rac1-DN or Rac1-DN + Cdc42-CA, nasal neurites avoided the PTPμ-containing lanes. These results suggest that inhibition of Rac1 activity induces a phenotypic switch in the nasal neurons from a permissive to a repulsive response to a PTPμ substrate. In addition, Rac1-DN is dominant in the induced nasal repulsion. Quantitation of the average degree of nasal avoidance (mean +/− STDEV) was plotted for each condition.
To test whether Cdc42 activity is absolutely required for temporal repulsion, explants from E8 temporal retina were grown on alternating stripes of PTPμ mixed with laminin (PTPμ/LN) versus laminin only lanes and either Rac1-DN, Cdc42-DN or the combination of Rac1-DN + Cdc42-DN dominant negative (DN) mutants were added. Control and Rac1-DN transduced temporal neurites avoided the PTPμ mixed substrate lanes (Fig. 9). Following addition of Cdc42-DN or the combination of Rac1-DN + Cdc42-DN, temporal neurites crossed the PTPμ-containing lanes. These results suggest that inhibition of Cdc42 activity affects temporal repulsion to a PTPμ substrate. Exogenous Rac1 inhibition in temporal neurons is not sufficient to overcome the requirement for Cdc42 activity in temporal neurons. Together, the combination experiments confirm that Cdc42 activity is required for both permissive and repulsive responses, and that Rac1 inhibition is required for PTPμ to be repulsive. Rac1 and Cdc42 do not appear to influence one another in the combination experiments. These results may suggest that Rac1 and Cdc42 are in distinct pathways. Our combination experiments also do not suggest that one molecule is either upstream or downstream of one another.
Figure 9. Exogenous Rac1 inhibition in temporal neurons is not sufficient to overcome the requirement for Cdc42 activity in temporal neurons.
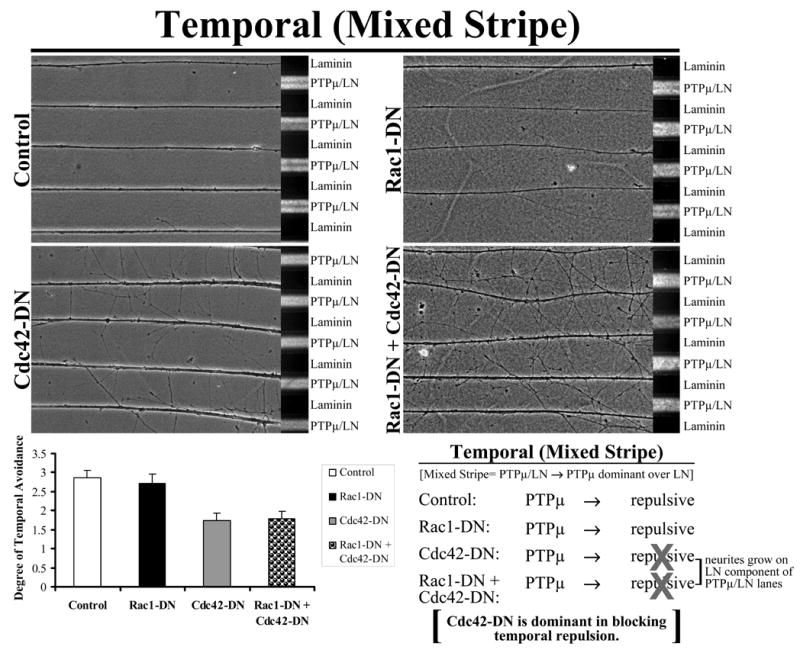
Explants from E8 temporal retina were grown on alternating stripes of PTPμ mixed with laminin (PTPμ/LN) versus laminin only lanes and either Rac1-DN, Cdc42-DN or the combination of Rac1-DN + Cdc42-DN mutants were added. Control and Rac1-DN transduced temporal neurites avoided the PTPμ mixed substrate lanes. Following addition of Cdc42-DN or Rac1-DN + Cdc42-DN, temporal neurites crossed the PTPμ-containing lanes. These results suggest that Cdc42 activity is required for temporal repulsion to a PTPμ substrate. Quantitation of the average degree of temporal avoidance (mean +/− STDEV) was plotted for each condition.
Discussion
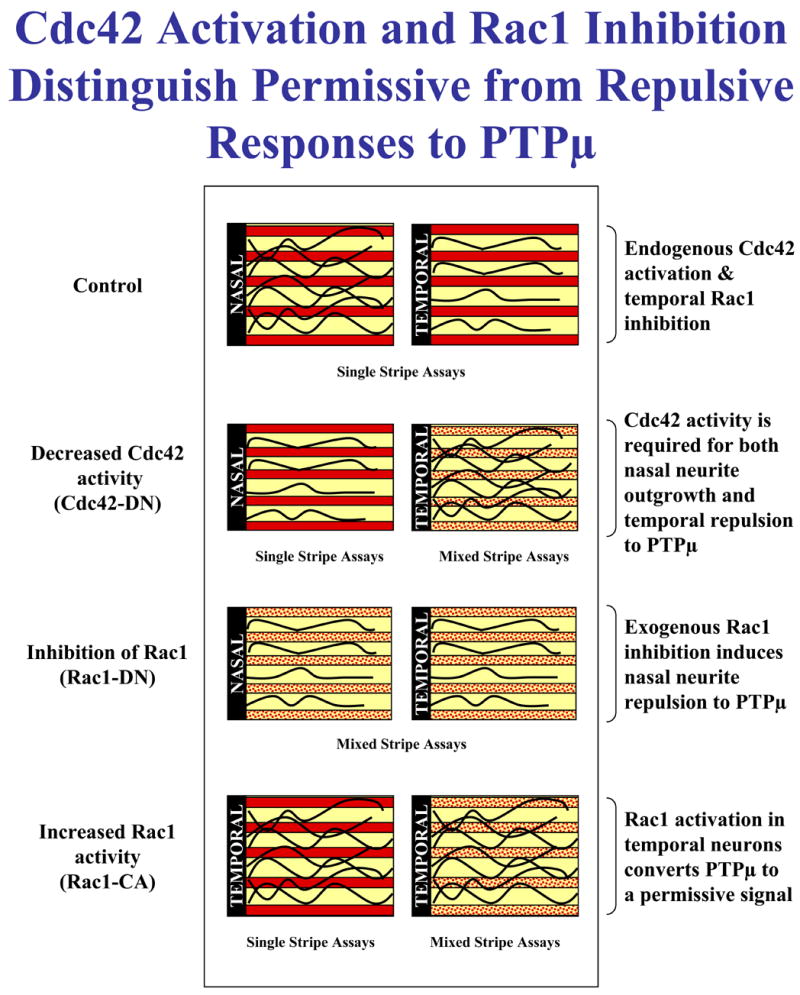
In this manuscript, we tested whether the Rho GTPases regulate PTPμ-dependent nasal outgrowth and temporal repulsion. The data presented here indicate that Cdc42 activity is required for both nasal outgrowth and temporal repulsion (Fig. 10), which is consistent with our previous study that demonstrated a requirement of Cdc42 activity for PTPμ-dependent growth cone rearrangement (Rosdahl et al., 2003). In the previous study, the stimulation of E6 retinal cultures with purified PTPμ resulted in a morphological change in the growth cone, which became dominated by filopodia within five minutes of PTPμ addition. We perturbed GTPase signaling, and found that PTPμ-induced growth cone rearrangement required Cdc42 but not Rac1 or Rho. Dominant-negative forms of Rac1 had no effect on PTPμ-dependent growth cone rearrangement (Rosdahl et al., 2003) whereas a dominant-negative mutant of Cdc42 blocked PTPμ-induced rearrangement (Rosdahl et al., 2003). Importantly, we observed that PTPμ signaling in growth cones resulted in both lamellipodial loss and filopodial extension, which is a phenotype very similar to that observed in static images of nasal outgrowth on a PTPμ substrate (Burden-Gulley and Brady-Kalnay, 1999). It is interesting to note that both lamellipodial loss and filopodial extension are hallmarks of Rac inhibition and Cdc42 activation, respectively (Rosdahl et al., 2003).
Figure 10. Summary diagram of the results from perturbation of RhoGTPase activity on PTPμ-dependent nasal crossing and temporal repulsion.
Cdc42 activation and Rac1 inhibition distinguish permissive from repulsive responses to PTPμ.
In order to examine the differential behavior of nasal or temporal RGCs in response to a PTPμ substrate, we utilized the Bonhoeffer stripe assay. The stripe assay allows the assessment of neurite outgrowth on alternating substrate lanes. In this manuscript, Bonhoeffer stripe assays with nasal and temporal neurons, where distinct signals are perturbed, has yielded information about which signals PTPμ utilizes to induce nasal permissive responses and temporal repulsive responses. The data presented in this manuscript indicate that Cdc42 activation is required for both nasal outgrowth and temporal repulsion on a PTPμ substrate. In addition, we demonstrated that PTPμ-dependent temporal repulsion requires inhibition of Rac1 activity.
We hypothesize that the activation of Cdc42 by PTPμ likely involves IQGAP1 (Fig. 11). PTPμ binds IQGAP1 (Phillips-Mason et al., 2006), which interacts with the Rho GTPases Rac1 and Cdc42 in their GTP-bound state (Briggs and Sacks, 2003). PTPμ binds IQGAP1, which was originally identified as a putative RasGAP due to the fact that it contains a region with significant homology to the catalytic domain of RasGAPs (Weissbach et al., 1994). IQGAP1 neither exhibits RasGAP activity nor binds to Ras, but specifically interacts with the Rho GTPases Rac1 and Cdc42 in their GTP-bound state (Briggs and Sacks, 2003). IQGAP1 has no GAP activity towards Cdc42 or Rac1 (Erickson et al., 1997). Rather, IQGAP1 stabilizes Cdc42 in its GTP-bound state (Hart et al., 1996; Swart-Mataraza et al., 2002; Zhang et al., 1997). Both in vitro and in vivo IQGAP1 appears to have a higher affinity for Cdc42 over Rac1 (Erickson et al., 1997; Hart et al., 1996; Kuroda et al., 1996). Interestingly, IQGAP1 binds directly to PTPμ and their binding is increased by activated Cdc42 (Phillips-Mason et al., 2006). We hypothesize that the requirement for Cdc42 in both nasal outgrowth and temporal repulsion is dependent upon IQGAP1–PTPμ interaction.
Figure 11.
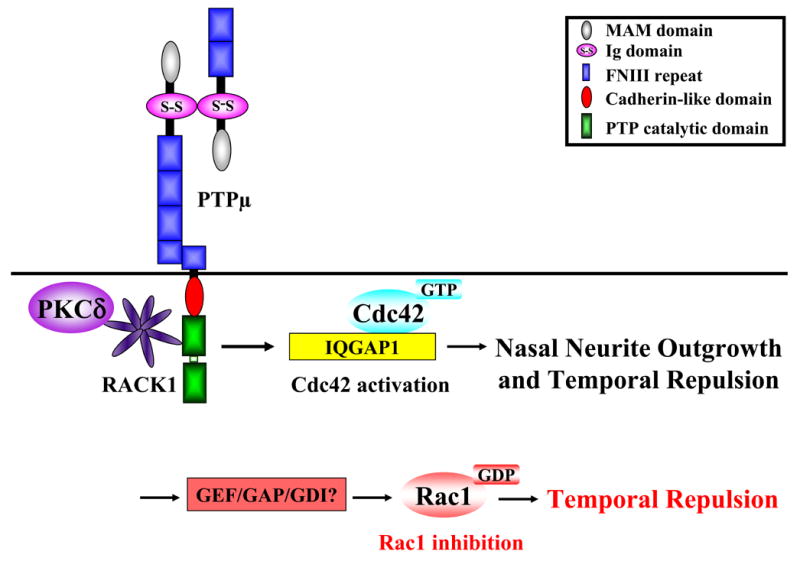
Diagram of the possible molecular mechanisms involved in PTPμ-dependent regulation of Cdc42 and Rac1 activity.
IQGAP1 was recently shown to promote neurite outgrowth although the molecular mechanisms are unclear (Li et al., 2005). It is widely known that the Rho GTPases including Cdc42 and Rac1 regulate the cytoskeleton and neurite outgrowth (Govek et al., 2005; Nikolic, 2002). IQGAP1 is an intriguing molecule because it can link several proteins that have the potential to regulate both the actin cytoskeleton and microtubules, hence neurite outgrowth. IQGAP1 functions as a scaffolding protein and interacts with E-Cadherin (Kuroda et al., 1998; Li et al., 1999), β-catenin (Briggs et al., 2002; Kuroda et al., 1998), CLIP-170 (Fukata et al., 2002), actin (Erickson et al., 1997; Mateer et al., 2002) and the ERK2 mitogen-activated protein kinase (MAPK) (Roy et al., 2004). We have recently demonstrated that PTPμ associates with an IQGAP1 complex that also contains ERK2 (Phillips-Mason et al., 2006) (Fig. 11). Furthermore, ERK2 is known to regulate neurite outgrowth in response to neurotrophins and CAMs (Dimitropoulou and Bixby, 2000; Doherty et al., 2000; Yamada et al., 1997). Interestingly, MAPK activation is required for PTPκ, a PTPμ-subfamily member, to promote neurite outgrowth (Drosopoulos et al., 1999).
Our results also suggest that a key difference between the two phenotypic responses to a PTPμ substrate, nasal crossing and temporal repulsion, is the activity of the Rac1 GTPase (Fig. 10). We have found that Rac1 inhibition is required for temporal repulsion to a PTPμ substrate. If constitutively active Rac1 (Rac1-CA) is added to temporal neurons, a phenotypic switch occurs which converts PTPμ to a permissive substrate. Neither Cdc42-CA nor Rho-CA is able to induce this phenotypic switch. Consistent with this hypothesis, addition of dominant-negative Rac1 to nasal neurons in a single stripe assay and a mixed substrate assay (used to measure repulsion) induces repulsion of nasal neurons on a PTPμ substrate. It is interesting that inhibition of Rac1 includes repulsion. Most studies link RhoA activation to growth cone collapse and repulsion. Our study found no effect of RhoA on PTPμ-dependent neurite outgrowth or repulsion. The molecular mechanism by which PTPμ inhibits Rac1 is currently unknown and is the subject of future studies.
PTPμ also interacts with RACK1, a WD-40 repeat protein, that was originally identified as a protein that binds to activated protein kinase C (PKC) (Ron et al., 1994) (Fig. 11). Interestingly PKCδ, a RACK1 binding protein, also interacts with PTPμ in a signaling complex in the retina (Rosdahl et al., 2002). We recently found that PKCδ activity is required for both permissive and inhibitory responses of RGCs to PTPμ, with higher levels of PKCδ activation associated with temporal growth cone collapse and repulsion (Ensslen and Brady-Kalnay, 2004). A potential mechanism for differential PKCδ activation is due to the gradient of PTPμ expression in the retina. A lower level of PKCδ activity is required for nasal outgrowth on a PTPμ substrate (Rosdahl et al., 2002).
The same signaling molecule can regulate both permissive and repulsive responses. For example, Protein Kinase C mediates signals downstream of both growth permissive (Bixby and Jhabvala, 1990; Kabir et al., 2001; Kolkova et al., 2000) and inhibitory cues (Mikule et al., 2003; Powell et al., 2001; Theodore et al., 1995; Xiang et al., 2002). The Rho family of GTPases (Giniger, 2002), cyclic nucleotides (Song et al., 1998; Song et al., 1997), MAPK kinases (Dimitropoulou and Bixby, 2000; Perron and Bixby, 1999; Tong et al., 2003) and calcium (Nishiyama et al., 2003; Zheng, 2000), mediate both repulsive and attractive behaviors in neurons (Skaper et al., 2001). The magnitude of second messenger signal in the growth cone controls the permissive versus repulsive response. It is intriguing that PTPμ signaling involves regulation of some of these enzymes that mediate both permissive responses and repulsion (Fig. 11). Our combined studies suggest that the magnitude of PTPμ-dependent signaling controls the switch from a permissive response to repulsion (Ensslen-Craig and Brady-Kalnay, 2005; Ensslen and Brady-Kalnay, 2004), and that Cdc42 is a likely candidate molecule in the PTPμ signaling cascade.
Tyrosine phosphatases have been implicated in the control of neurite outgrowth (Bixby and Jhabvala, 1992, 1993; Burden-Gulley and Brady-Kalnay, 1999). Some CAM-like RPTPs have been shown to promote neurite outgrowth and act as attractive or repulsive cues. PTPκ has been shown to promote neurite outgrowth of cerebellar neurons (Drosopoulos et al., 1999). PTPδ promotes neurite outgrowth of forebrain and cerebellar neurons (Wang and Bixby, 1999) and a local gradient of soluble PTPδ acts as a chemoattractant, which depends upon PTP activity in forebrain neurons (Sun et al., 2000). The RPTP CRYPα regulates interactions between neurons and glial cells in the retina as well as retinal axon growth (Haj et al., 1999; Ledig et al., 1999). Phosphacan, the soluble splice variant of RPTPζ/β, can promote or inhibit neurite outgrowth depending upon the context (Maeda and Noda, 1996). The Drosophila RPTPs, DLAR, DPTP69D and DPTP99A were shown to be crucial for certain neurons to recognize guidance cues that help them innervate appropriate target muscles (Desai et al., 1996; Desai et al., 1997; Krueger et al., 1996). Perturbation of the leech LAR homolog (HmLAR2) results in abnormal neuronal projections (Gershon et al., 1998). Recent studies suggest that HmLAR2 may regulate mutual repulsion via a homophilic binding mechanism (Baker and Macagno, 2000a, b; Baker et al., 2000). The regulation of tyrosine phosphorylation by RPTPs may affect neurite outgrowth by “steering” growth cones along the appropriate pathway to establish a spatiotemporal pattern of neuronal connections, via both permissive and repulsive responses.
Experimental Methods
Culturing of Chick Retinal Explants
Chick embryonic day 8 embryos were staged according to Hamburger and Hamilton (Hamburger, 1951). Retinal explants were prepared as described (Drazba and Lemmon, 1990; Halfter et al., 1983). E8 (stage 32) neural retinas were dissected, flattened on concanavalin-coated nitrocellulose filters, and cut into 350 μm wide explants. Explants were placed retinal ganglion side down on substrate-coated dishes and grown in 10% fetal bovine serum (Hyclone, Logan, UT), 2% chick serum (Sigma, St. Louis, MO), 2mM L-glutamine (Invitrogen, Carlsbad, CA), and 2 units/ml penicillin, 2 μg/ml streptomycin, 5ng/ml amphotericin (Sigma) in RPMI-1640 (Invitrogen) for 48 hours for stripe assays.
Bonhoeffer Stripe Assay
The substrate lane assay used was a slightly modified version of the Bonhoeffer method (Vielmetter et al., 1990), as described (Burden-Gulley et al., 2002). Tissue culture dishes were coated with nitrocellulose (Lagenaur and Lemmon, 1987) and dried before applying the silicon lane matrix to the dish surface. The PTPμ Fc chimera has been described (Rosdahl et al., 2003). Laminin was purchased from BTI (Stoughton, MA). 80ng of PTPμ-Fc chimera containing a small amount of Texas-Red conjugated BSA (for visualization of the lanes, Molecular Probes, Eugene, OR) was injected into the channels of the matrix, incubated for 10 minutes, aspirated then replaced with a fresh aliquot of the same substrate. All remaining binding sites within the lanes were blocked with bovine serum albumin (BSA; fraction V; Sigma) and rinsed with calcium-magnesium free phosphate buffer (CMF). The matrix was removed and 1.75μg of laminin was spread across the lane area and incubated for 20 minutes. The mixed substrate assays were performed as described (Ensslen and Brady-Kalnay, 2004). For mixed substrate lanes, 160ng of PTPμ-Fc chimera was mixed with 0.9μg laminin containing Texas-Red BSA and was used as the first substrate as described above. For the second substrate 1.75μg of laminin was spread over the lane area. The entire dish was blocked with BSA and rinsed with RPMI. Explants were cultured for 48 hours prior to imaging. For Rho GTPase perturbations, 6.5μg of TAT-tagged mutant protein was added at the time of plating explants. Representative images from a minimum of three separate experiments are shown. Quantitation of the stripe assays was performed using a rating scale as described (Ensslen and Brady-Kalnay, 2004; Walter et al., 1987a). Neurites that show no preference for either substrate are assessed at 0, and neurites that grow exclusively on one substrate are assessed at 3. An assessment of 2 indicates that most of the neurites grow on the laminin lanes with an occasional neurite crossing over PTPμ lanes, while an assessment of 1 is when there is a significant amount of neurite crossing but a tendency to fasciculate on laminin. Data from a minimum of three experiments were combined (with a minimum sample size of 9 explants per condition) to determine the average degree of avoidance for each condition, then plotted using Microsoft Excel.
TAT Protein Expression and Purification
Expression plasmids for constitutively active (CA) and dominant negative (DN) Rac1, RhoA and Cdc42 were kindly provided by Dr. Steven Dowdy (UCSD, CA) (Soga et al., 2001). The expression plasmids were transformed into BL21 (DE3) pLysS competent cells (Novagen). Protein expression was induced with 200μM IPTG (final concentration).
Cells were resuspended in 1X PBS containing 2μL/mL PIC (Sigma) and 1μL/mL 1M benzamidine, sonicated on ice three times for 30 seconds, and centrifuged at 4200rpm for 25min. at 4°C. The supernatant was applied to 1mL packed Talon Metal Affinity Resin (BD Biosciences, Clontech) to bind to the His-tag of the Rho GTPase fusion proteins and rocked overnight at 4°C. Beads were pelleted (1500rpm, 3min, 4°C) and washed three times with 10mL 1XPBS/PIC/benzamidine followed by 3 additional washes with 10mL 1X PBS. Protein was eluted from beads with 1XPBS containing 200mM imidazole.
Acknowledgments
This research was supported by a grant from the National Institutes of Health Grant R01-EY12251 (S.B.K). Additional support was obtained from the Visual Sciences Research Center Core Grant PO-EY11373 from the National Eye Institute. We thank Dr. Steven Dowdy for providing the TAT-Rho GTPase fusion proteins. We thank Carol Luckey for technical support including preparation and purification of the TAT-fusion Rho GTPase proteins and members of the Brady-Kalnay lab for insightful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aricescu AR, Hon WC, Siebold C, Lu W, van der Merwe PA, Jones EY. Molecular analysis of receptor protein tyrosine phosphatase mu-mediated cell adhesion. Embo J. 2006;25:701–712. doi: 10.1038/sj.emboj.7600974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MW, Macagno ER. RNAi of the receptor tyrosine phosphatase HmLAR2 in a single cell of an intact leech embryo leads to growth-cone collapse. Curr Biol. 2000a;10:1071–1074. doi: 10.1016/s0960-9822(00)00674-6. [DOI] [PubMed] [Google Scholar]
- Baker MW, Macagno ER. The role of a LAR-like receptor tyrosine phosphatase in growth cone collapse and mutual-avoidance by sibling processes. J Neurobiol. 2000b;44:194–203. doi: 10.1002/1097-4695(200008)44:2<194::aid-neu9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Baker MW, Rauth SJ, Macagno ER. Possible role of the receptor protein tyrosine phosphatase HmLAR2 in interbranch repulsion in a leech embryonic cell. J Neurobiol. 2000;45:47–60. doi: 10.1002/1097-4695(200010)45:1<47::aid-neu5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J 348 Pt. 2000;2:241–255. [PMC free article] [PubMed] [Google Scholar]
- Bixby JL, Jhabvala P. Extracellular matrix molecules and cell adhesion molecules induce neurites through different mechanisms. J Cell Biol. 1990;111:2725–2732. doi: 10.1083/jcb.111.6.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixby JL, Jhabvala P. Inhibition of tyrosine phosphorylation potentiates substrate-induced neurite growth. Journal of Neurobiology. 1992;23:468–480. doi: 10.1002/neu.480230503. [DOI] [PubMed] [Google Scholar]
- Bixby JL, Jhabvala P. Tyrosine phosphorylation in early embryonic growth cones. The Journal of Neuroscience. 1993;13:3421–3432. doi: 10.1523/JNEUROSCI.13-08-03421.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady-Kalnay SM. Protein tyrosine phosphatases. Oxford University Press; Oxford, UK: 2001. [Google Scholar]
- Brady-Kalnay SM, Boghaert ER, Zimmer S, Brackenbury R. Increasing N-CAM-mediated cell-cell adhesion does not reduce invasion of RSV-transformed WC5 rat cerebellar cells. Clin Exp Metastasis. 1993;11:313–324. doi: 10.1007/BF00058051. [DOI] [PubMed] [Google Scholar]
- Brady-Kalnay SM, Mourton T, Nixon JP, Kinch M, Chen H, Brackenbury R, Rimm DL, Del Vecchio RL, Tonks NK. Dynamic interaction of PTPμ with multiple cadherins in vivo. J Cell Biol. 1998;141:287–296. doi: 10.1083/jcb.141.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady-Kalnay SM, Tonks NK. Identification of the homophilic binding site of the receptor protein tyrosine phosphatase PTP mu. J Biol Chem. 1994;269:28472–28477. [PubMed] [Google Scholar]
- Briggs MW, Li Z, Sacks DB. IQGAP1-mediated stimulation of transcriptional co-activation by beta-catenin is modulated by calmodulin. J Biol Chem. 2002;277:7453–7465. doi: 10.1074/jbc.M104315200. [DOI] [PubMed] [Google Scholar]
- Briggs MW, Sacks DB. IQGAP proteins are integral components of cytoskeletal regulation. EMBO Rep. 2003;4:571–574. doi: 10.1038/sj.embor.embor867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden-Gulley SM, Brady-Kalnay SM. PTPμ Regulates N-Cadherin-dependent Neurite Outgrowth. J Cell Biol. 1999;144:1323–1336. doi: 10.1083/jcb.144.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden-Gulley SM, Ensslen SE, Brady-Kalnay SM. Protein tyrosine phosphatase-mu differentially regulates neurite outgrowth of nasal and temporal neurons in the retina. J Neurosci. 2002;22:3615–3627. doi: 10.1523/JNEUROSCI.22-09-03615.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Wu K, Armanini M, O'Rourke N, Dowbenko D, Lasky LA. A novel protein-tyrosine phosphatase related to the homotypically adhering κ and μ receptors. J Biol Chem. 1997;272:7264–7277. doi: 10.1074/jbc.272.11.7264. [DOI] [PubMed] [Google Scholar]
- Cismasiu VB, Denes SA, Reilander H, Michel H, Szedlacsek SE. The MAM (meprin/A5-protein/PTPmu) domain is a homophilic binding site promoting the lateral dimerization of receptor-like protein-tyrosine phosphatase mu. J Biol Chem. 2004;279:26922–26931. doi: 10.1074/jbc.M313115200. [DOI] [PubMed] [Google Scholar]
- Desai C, Gindhart JG, Goldstein LSB, Zinn K. Receptor tyrosine phosphatases are required for motor axon guidance in the Drosophila embryo. Cell. 1996;84:599–609. doi: 10.1016/s0092-8674(00)81035-1. [DOI] [PubMed] [Google Scholar]
- Desai CJ, Sun Q, Zinn K. Tyrosine phosphorylation and axon guidance: of mice and flies. Curr Opin Neuro. 1997;7:70–74. doi: 10.1016/s0959-4388(97)80122-5. [DOI] [PubMed] [Google Scholar]
- Dimitropoulou A, Bixby JL. Regulation of retinal neurite growth by alterations in MAPK/ERK kinase (MEK) activity. Brain Res. 2000;858:205–214. doi: 10.1016/s0006-8993(00)01946-6. [DOI] [PubMed] [Google Scholar]
- Doherty P, Williams G, Williams EJ. CAMs and axonal growth: a critical evaluation of the role of calcium and the MAPK cascade. Mol Cell Neurosci. 2000;16:283–295. doi: 10.1006/mcne.2000.0907. [DOI] [PubMed] [Google Scholar]
- Drazba J, Lemmon V. The role of cell adhesion molecules in neurite outgrowth on Müller cells. Dev Biol. 1990;138:82–93. doi: 10.1016/0012-1606(90)90178-l. [DOI] [PubMed] [Google Scholar]
- Drosopoulos NE, Walsh FS, Doherty P. A soluble version of the receptor-like protein tyrosine phosphatase kappa stimulates neurite outgrowth via a Grb2/MEK1-dependent signaling cascade. Mol Cell Neurosci. 1999;13:441–449. doi: 10.1006/mcne.1999.0758. [DOI] [PubMed] [Google Scholar]
- Ensslen-Craig SE, Brady-Kalnay SM. Receptor protein tyrosine phosphatases regulate neural development and axon guidance. Dev Biol. 2004;275:12–22. doi: 10.1016/j.ydbio.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Ensslen-Craig SE, Brady-Kalnay SM. PTPmu expression and catalytic activity are required for PTPmu-mediated neurite outgrowth and repulsion. Mol Cell Neurosci. 2005;28:177–188. doi: 10.1016/j.mcn.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Ensslen SE, Brady-Kalnay SM. PTPmu signaling via PKCdelta is instructive for retinal ganglion cell guidance. Mol Cell Neurosci. 2004;25:558–571. doi: 10.1016/j.mcn.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Erickson JW, Cerione RA, Hart MJ. Identification of an actin cytoskeletal complex that includes IQGAP and the Cdc42 GTPase. J Biol Chem. 1997;272:24443–24447. doi: 10.1074/jbc.272.39.24443. [DOI] [PubMed] [Google Scholar]
- Fukata M, Watanabe T, Noritake J, Nakagawa M, Yamaga M, Kuroda S, Matsuura Y, Iwamatsu A, Perez F, Kaibuchi K. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell. 2002;109:873–885. doi: 10.1016/s0092-8674(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Garrity PA, Lee CH, Salecker I, Robertson HC, Desai CJ, Zinn K, Zipursky SL. Retinal axon target selection in Drosophila is regulated by a receptor protein tyrosine phosphatase. Neuron. 1999;22:707–717. doi: 10.1016/s0896-6273(00)80730-8. [DOI] [PubMed] [Google Scholar]
- Gebbink MFBG, Zondag GCM, Wubbolts RW, Beijersbergen RL, van Etten I, Moolenaar WH. Cell-cell adhesion mediated by a receptor-like protein tyrosine phosphatase. J Biol Chem. 1993;268:16101–16104. [PubMed] [Google Scholar]
- Gershon TR, Baker MW, Nitabach M, Macagno ER. The leech receptor protein tyrosine phosphatase HmLAR2 is concentrated in growth cones and is involved in process outgrowth. Dev. 1998;125:1183–1190. doi: 10.1242/dev.125.7.1183. [DOI] [PubMed] [Google Scholar]
- Giniger E. How do Rho family GTPases direct axon growth and guidance? A proposal relating signaling pathways to growth cone mechanics. Differentiation. 2002;70:385–396. doi: 10.1046/j.1432-0436.2002.700801.x. [DOI] [PubMed] [Google Scholar]
- Govek EE, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19:1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- Haj F, McKinnell I, Stoker A. Retinotectal ligands for the receptor tyrosine phosphatase CRYPalpha. Mol Cell Neurosci. 1999;14:225–240. doi: 10.1006/mcne.1999.0785. [DOI] [PubMed] [Google Scholar]
- Halfter W, Newgreen DF, Sauter J, Schwarz U. Oriented axon outgrowth from avian embryonic retinae in culture. Dev Biol. 1983;95:56–64. doi: 10.1016/0012-1606(83)90006-4. [DOI] [PubMed] [Google Scholar]
- Hamburger H, Hamilton H. A series of normal stages in the development of the chick embryo. Journal of Morphology. 1951;88:49–92. [PubMed] [Google Scholar]
- Hart MJ, Callow MG, Souza B, Polakis P. IQGAP1, a calmodulin-binding protein with a rasGAP-related domain, is a potential effector for cdc42Hs. Embo J. 1996;15:2997–3005. [PMC free article] [PubMed] [Google Scholar]
- Johnson KG, McKinnell IW, Stoker AW, Holt CE. Receptor protein tyrosine phosphatases regulate retinal ganglion cell axon outgrowth in the developing Xenopus visual system. J Neurobiol. 2001;49:99–117. doi: 10.1002/neu.1068. [DOI] [PubMed] [Google Scholar]
- Johnson KG, Van Vactor D. Receptor protein tyrosine phosphatases in nervous system development. Physiol Rev. 2003;83:1–24. doi: 10.1152/physrev.00016.2002. [DOI] [PubMed] [Google Scholar]
- Kabir N, Schaefer AW, Nakhost A, Sossin WS, Forscher P. Protein kinase C activation promotes microtubule advance in neuronal growth cones by increasing average microtubule growth lifetimes. J Cell Biol. 2001;152:1033–1044. doi: 10.1083/jcb.152.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibuchi K, Kuroda S, Fukata M, Nakagawa M. Regulation of cadherin-mediated cell-cell adhesion by the Rho family GTPases. Curr Opin Cell Biol. 1999;11:591–596. doi: 10.1016/s0955-0674(99)00014-9. [DOI] [PubMed] [Google Scholar]
- Kolkova K, Novitskaya V, Pedersen N, Berezin V, Bock E. Neural cell adhesion molecule-stimulated neurite outgrowth depends on activation of protein kinase C and the Ras-mitogen-activated protein kinase pathway. J Neurosci. 2000;20:2238–2246. doi: 10.1523/JNEUROSCI.20-06-02238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger NX, Van Vactor D, Wan HI, Gelbart WM, Goodman CS, Saito H. The transmembrane tyrosine phosphatase DLAR controls motor axon guidance in Drosophila. Cell. 1996;84:611–622. doi: 10.1016/s0092-8674(00)81036-3. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Kobayashi K, Nakafuku M, Nomura N, Iwamatsu A, Kaibuchi K. Identification of IQGAP as a putative target for the small GTPases, Cdc42 and Rac1. J Biol Chem. 1996;271:23363–23367. doi: 10.1074/jbc.271.38.23363. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Nakagawa M, Fujii K, Nakamura T, Ookubo T, Izawa I, Nagase T, Nomura N, Tani H, Shoji I, Matsuura Y, Yonehara S, Kaibuchi K. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin- mediated cell-cell adhesion. Science. 1998;281:832–835. doi: 10.1126/science.281.5378.832. [DOI] [PubMed] [Google Scholar]
- Lagenaur C, Lemmon V. An L1-like molecule, the 8D9 antigen, is a potent substrate for neurite extension. Proc Natl Acad Sci U S A. 1987;84:7753–7757. doi: 10.1073/pnas.84.21.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledig MM, Haj F, Bixby JL, Stoker AW, Mueller BK. The receptor tyrosine phosphatase CRYPalpha promotes intraretinal axon growth. J Cell Biol. 1999;147:375–388. doi: 10.1083/jcb.147.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon V, Burden SM, Payne HR, Elmslie GJ, Hlavin ML. Neurite growth on different substrates: Permissive versus instructive influences and the role of adhesive strength. The Journal of Neuroscience. 1992;12:818–826. doi: 10.1523/JNEUROSCI.12-03-00818.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Kim SH, Higgins JM, Brenner MB, Sacks DB. IQGAP1 and calmodulin modulate E-cadherin function. J Biol Chem. 1999;274:37885–37892. doi: 10.1074/jbc.274.53.37885. [DOI] [PubMed] [Google Scholar]
- Li Z, McNulty DE, Marler KJ, Lim L, Hall C, Annan RS, Sacks DB. IQGAP1 promotes neurite outgrowth in a phosphorylation-dependent manner. J Biol Chem. 2005 doi: 10.1074/jbc.M413482200. [DOI] [PubMed] [Google Scholar]
- Maeda N, Noda M. 6B4 proteoglycan/phosphacan is a repulsive substratum but promotes morphological differentiation of cortical neurons. Development. 1996;122:647–658. doi: 10.1242/dev.122.2.647. [DOI] [PubMed] [Google Scholar]
- Mateer SC, McDaniel AE, Nicolas V, Habermacher GM, Lin MJ, Cromer DA, King ME, Bloom GS. The mechanism for regulation of the F-actin binding activity of IQGAP1 by calcium/calmodulin. J Biol Chem. 2002;277:12324–12333. doi: 10.1074/jbc.M109535200. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Hindges R, O'Leary DD. Regulation of axial patterning of the retina and its topographic mapping in the brain. Curr Opin Neurobiol. 2003;13:57–69. doi: 10.1016/s0959-4388(03)00014-x. [DOI] [PubMed] [Google Scholar]
- Mikule K, Sunpaweravong S, Gatlin JC, Pfenninger KH. Eicosanoid activation of protein kinase C epsilon: involvement in growth cone repellent signaling. J Biol Chem. 2003;278:21168–21177. doi: 10.1074/jbc.M211828200. [DOI] [PubMed] [Google Scholar]
- Nikolic M. The role of Rho GTPases and associated kinases in regulating neurite outgrowth. Int J Biochem Cell Biol. 2002;34:731–745. doi: 10.1016/s1357-2725(01)00167-4. [DOI] [PubMed] [Google Scholar]
- Nishiyama M, Hoshino A, Tsai L, Henley JR, Goshima Y, Tessier-Lavigne M, Poo MM, Hong K. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature. 2003;424:990–995. doi: 10.1038/nature01751. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac and cdc42 GTPases: regulators of actin strucutres, cell adhesion and motility. Biochem Soc Trans. 1995;23:456–459. doi: 10.1042/bst0230456. [DOI] [PubMed] [Google Scholar]
- O'Leary DD, McLaughlin T. Mechanisms of retinotopic map development: Ephs, ephrins, and spontaneous correlated retinal activity. Prog Brain Res. 2005;147:43–65. doi: 10.1016/S0079-6123(04)47005-8. [DOI] [PubMed] [Google Scholar]
- Perron JC, Bixby JL. Distinct neurite outgrowth signaling pathways converge on ERK activation. Mol Cell Neurosci. 1999;13:362–378. doi: 10.1006/mcne.1999.0753. [DOI] [PubMed] [Google Scholar]
- Phillips-Mason PJ, Gates TJ, Sacks DB, Brady-Kalnay SM. The receptor protein tyrosine phosphatase PTPμ interacts with IQGAP1. J Biol Chem. 2006;281:4903–4910. doi: 10.1074/jbc.M506414200. [DOI] [PubMed] [Google Scholar]
- Powell EM, Mercado ML, Calle-Patino Y, Geller HM. Protein kinase C mediates neurite guidance at an astrocyte boundary. Glia. 2001;33:288–297. doi: 10.1002/1098-1136(20010315)33:4<288::aid-glia1027>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Redies C. Cadherins and the formation of neural circuitry in the vertebrate CNS. Cell Tissue Res. 1997;290:405–413. doi: 10.1007/s004410050947. [DOI] [PubMed] [Google Scholar]
- Redies C, Takeichi M. Cadherins in the developing central nervous system: an adhesive code for segmental and functional subdivisions. Dev Biol. 1996;180:413–423. doi: 10.1006/dbio.1996.0315. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Rho proteins and cancer. Breast Cancer Res Treat. 2004;84:13–19. doi: 10.1023/B:BREA.0000018423.47497.c6. [DOI] [PubMed] [Google Scholar]
- Ron D, Chen CH, Caldwell J, Jamieson L, Orr E, Mochly-Rosen D. Cloning of an intracellular receptor for protein kinase C: a homolog of the beta subunit of G proteins [published erratum appears in Proc Natl Acad Sci U S A 1995 Feb 28;92(5):2016] Proc Natl Acad Sci U S A. 1994;91:839–843. doi: 10.1073/pnas.91.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosdahl JA, Ensslen SE, Niedenthal JA, Brady-Kalnay SM. PTP mu-dependent growth cone rearrangement is regulated by Cdc42. J Neurobiol. 2003;56:199–208. doi: 10.1002/neu.10231. [DOI] [PubMed] [Google Scholar]
- Rosdahl JA, Mourton TL, Brady-Kalnay SM. Protein kinase C delta (PKCdelta) is required for protein tyrosine phosphatase mu (PTPmu)-dependent neurite outgrowth. Mol Cell Neurosci. 2002;19:292–306. doi: 10.1006/mcne.2001.1071. [DOI] [PubMed] [Google Scholar]
- Roy M, Li Z, Sacks DB. IQGAP1 binds ERK2 and modulates its activity. J Biol Chem. 2004;279:17329–17337. doi: 10.1074/jbc.M308405200. [DOI] [PubMed] [Google Scholar]
- Sap J, Jiang YP, Friedlander D, Grumet M, Schlessinger J. Receptor tyrosine phosphatase R-PTP-κ mediates homophilic binding. Mol Cell Biol. 1994;14:1–9. doi: 10.1128/mcb.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper SD, Moore SE, Walsh FS. Cell signalling cascades regulating neuronal growth-promoting and inhibitory cues. Prog Neurobiol. 2001;65:593–608. doi: 10.1016/s0301-0082(01)00017-x. [DOI] [PubMed] [Google Scholar]
- Soga N, Namba N, McAllister S, Cornelius L, Teitelbaum SL, Dowdy SF, Kawamura J, Hruska KA. Rho family GTPases regulate VEGF-stimulated endothelial cell motility. Exp Cell Res. 2001;269:73–87. doi: 10.1006/excr.2001.5295. [DOI] [PubMed] [Google Scholar]
- Song HJ, Ming GL, He Z, Lehman M, McKerracher L, Tessier-Lavigne M, Poo M. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- Song HJ, Ming GL, Poo MM. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- Sun QL, Wang J, Bookman RJ, Bixby JL. Growth cone steering by receptor tyrosine phosphatase delta defines a distinct class of guidance cue. Mol Cell Neurosci. 2000;16:686–695. doi: 10.1006/mcne.2000.0893. [DOI] [PubMed] [Google Scholar]
- Swart-Mataraza JM, Li Z, Sacks DB. IQGAP1 is a component of Cdc42 signaling to the cytoskeleton. J Biol Chem. 2002;277:24753–24763. doi: 10.1074/jbc.M111165200. [DOI] [PubMed] [Google Scholar]
- Thanos S, Mey J. Development of the visual system of the chick. II. Mechanisms of axonal guidance. Brain Res Brain Res Rev. 2001;35:205–245. doi: 10.1016/s0165-0173(01)00049-2. [DOI] [PubMed] [Google Scholar]
- Theodore L, Derossi D, Chassaing G, Llirbat B, Kubes M, Jordan P, Chneiweiss H, Godement P, Prochiantz A. Intraneuronal delivery of protein kinase C pseudosubstrate leads to growth cone collapse. J Neurosci. 1995;15:7158–7167. doi: 10.1523/JNEUROSCI.15-11-07158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Elowe S, Nash P, Pawson T. Manipulation of EphB2 regulatory motifs and SH2 binding sites switches MAPK signaling and biological activity. J Biol Chem. 2003;278:6111–6119. doi: 10.1074/jbc.M208972200. [DOI] [PubMed] [Google Scholar]
- van Horck FP, Weinl C, Holt CE. Retinal axon guidance: novel mechanisms for steering. Curr Opin Neurobiol. 2004;14:61–66. doi: 10.1016/j.conb.2004.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielmetter J, Stolze B, Bonhoeffer F, Stuermer CA. In vitro assay to test differential substrate affinities of growing axons and migratory cells. Exp Brain Res. 1990;81:283–287. doi: 10.1007/BF00228117. [DOI] [PubMed] [Google Scholar]
- Walter J, Henke-Fahle S, Bonhoeffer F. Avoidance of posterior tectal membranes by temporal retinal axons. Development. 1987a;101:909–913. doi: 10.1242/dev.101.4.909. [DOI] [PubMed] [Google Scholar]
- Walter J, Kern-Veits B, Huf J, Stolze B, Bonhoeffer F. Recognition of position-specific properties of tectal cell membranes by retinal axons in vitro. Development. 1987b;101:685–696. doi: 10.1242/dev.101.4.685. [DOI] [PubMed] [Google Scholar]
- Wang J, Bixby JL. Receptor Tyrosine Phosphatase-delta Is a Homophilic, Neurite-Promoting Cell Adhesion Molecule for CNS Neurons. Mol Cell Neurosci. 1999;14:370–384. doi: 10.1006/mcne.1999.0789. [DOI] [PubMed] [Google Scholar]
- Weissbach L, Settleman J, Kalady MF, Snijders AJ, Murthy AE, Yan YX, Bernards A. Identification of a human rasGAP-related protein containing calmodulin-binding motifs. J Biol Chem. 1994;269:20517–20521. [PubMed] [Google Scholar]
- Xiang Y, Li Y, Zhang Z, Cui K, Wang S, Yuan XB, Wu CP, Poo MM, Duan S. Nerve growth cone guidance mediated by G protein-coupled receptors. Nat Neurosci. 2002;5:843–848. doi: 10.1038/nn899. [DOI] [PubMed] [Google Scholar]
- Yamada M, Ikeuchi T, Hatanaka H. The neurotrophic action and signalling of epidermal growth factor. Prog Neurobiol. 1997;51:19–37. doi: 10.1016/s0301-0082(96)00046-9. [DOI] [PubMed] [Google Scholar]
- Zhang B, Wang ZX, Zheng Y. Characterization of the interactions between the small GTPase Cdc42 and its GTPase-activating proteins and putative effectors. Comparison of kinetic properties of Cdc42 binding to the Cdc42-interactive domains. J Biol Chem. 1997;272:21999–22007. doi: 10.1074/jbc.272.35.21999. [DOI] [PubMed] [Google Scholar]
- Zheng JQ. Turning of nerve growth cones induced by localized increases in intracellular calcium ions. Nature. 2000;403:89–93. doi: 10.1038/47501. [DOI] [PubMed] [Google Scholar]
- Zondag G, Koningstein G, Jiang YP, Sap J, Moolenaar WH, Gebbink M. Homophilic interactions mediated by receptor tyrosine phosphatases μ and κ. J Biol Chem. 1995;270:14247–14250. doi: 10.1074/jbc.270.24.14247. [DOI] [PubMed] [Google Scholar]


