Abstract
A key question in development is how pluripotent progenitors are progressively restricted to acquire specific cell fates. Here we investigate how embryonic blastomeres in C. elegans develop into foregut (pharynx) cells in response to the selector gene PHA-4/FoxA. When pha-4 is removed from pharyngeal precursors, they exhibit two alternative responses. Before late-gastrulation (8E stage), these cells lose their pharyngeal identity and acquire an alternative fate such as ectoderm (Specification stage). After the Specification stage, mutant cells develop into aberrant pharyngeal cells (Morphogenesis/Differentiation stage). Two lines of evidence suggest that the Specification stage depends on transcriptional repression of ectodermal genes by pha-4. First, pha-4 exhibits strong synthetic phenotypes with the B class synMuv gene tam-1 (Tandam Array expression Modifier 1) and with a mediator of transcriptional repression, the NuRD complex (NUcleosome Remodeling and histone Deacetylase). Second, pha-4 associates with the promoter of the ectodermal regulator lin-26 and is required to repress lin-26 expression. We propose that restriction of early blastomeres to the pharyngeal fate depends on both repression of ectodermal genes and activation of pharyngeal genes by PHA-4.
Keywords: embryogenesis, Mi-2, organogenesis, pharynx, synMuv, selector gene
INTRODUCTION
Embryonic progenitor cells are born pluripotent, but over time their developmental choices become restricted until ultimately they, or their descendants, differentiate into specialized cell types. For example, cells derived from a mammalian blastula can be induced to differentiate into virtually any cell type, whereas only a limited number of cell fate choices are available to fetal cells (Tiedemann et al., 2001). The transition from naïve precursor towards the differentiated state is characterized by sequential waves of gene expression that are determined by regulatory transcription factors. A key question is how transcriptional circuitry dictates developmental competence, cell fate specification and ultimately differentiation. Here we investigate this question by studying development of the C. elegans pharynx and the specification of early blastomeres to a pharyngeal fate.
Two lines of evidence suggest that embryonic cells in C. elegans are pluripotent at the beginning of gastrulation at the 2E stage (equivalent to 16–44 cells; embryos are staged by the number of endodermal or E cells). First, at the 2E stage and earlier, individual blastomeres contribute to multiple cell types, as revealed by the C. elegans cell lineage. For example the MSp blastomere generates both pharyngeal cells and body wall muscles (Sulston et al., 1983). One cell division later (4E stage, 50–100 cells), many cells give rise to descendants that contribute to only a single tissue or organ (Labouesse and Mango, 1999). Second, blastomeres at the 2E stage can adopt alternate fates in response to forced ubiquitous expression of heterologous cell fate regulators (Fukushige and Krause, 2005; Horner et al., 1998; Labouesse and Mango, 1999; Zhu et al., 1998). For example, ectopic expression of the midgut regulator end-1 transforms all early embryonic cells to a midgut fate (Zhu et al., 1998). However, previous studies suggest that some time between the 4–10E stages, early blastomeres can no longer adopt alternate fates when challenged with an ectopic cell fate regulator (Fukushige and Krause, 2005; Horner et al., 1998; Labouesse and Mango, 1999; Zhu et al., 1998). These observations suggest that cells commit to their respective cell fates by the 10E stage.
An appealing candidate to contribute to cell fate restriction is the selector gene pha-4. pha-4 encodes a FoxA transcription factor that is conserved in all metazoans (Mango et al., 1994a; Zaret, 1999). pha-4 is essential to establish pharyngeal cells and in the absence of pha-4, pharyngeal cells develop into non-pharyngeal cell types such as ectoderm (Horner et al., 1998). Strikingly, pha-4 expression initiates at the 2E stage and continues to be expressed throughout the life of the worm. However, the absence of a conditional pha-4 allele made it impossible previously to investigate when pha-4 functions to control cell fate.
Recent studies in other organisms have revealed that selector genes can control cell fates by functioning as both activators and repressors (Mann and Carroll, 2002). For example, the transcription factor GATA-1 both activates and represses target genes to promote the generation of diverse hematopoietic cell types (Ferreira et al., 2005). In C. elegans, pha-4 is known to activate expression of a wide array of pharyngeal genes (Gaudet and Mango, 2002). Similarly, FoxA proteins in other organisms function as transcriptional activators during foregut development (Zaret, 1999). There are two experiments that suggest pha-4 may also restrict cells to the pharyngeal fate by repressing ectodermal development. First, in the absence of pha-4, pharyngeal precursors transfate to ectodermal cell types that express the zinc finger protein LIN-26 (Horner et al., 1998; Mango et al., 1994a). Second, expression of ectopic PHA-4 confers pharyngeal identity to embryonic blastomeres at the expense of LIN-26+ ectoderm (Horner et al., 1998). These observations lead to the intriguing hypothesis that pha-4 might control cell fate by repressing ectodermal gene expression directly. Alternatively, pha-4 could function indirectly, by activating a repressor.
The cell fate regulators, the synthetic multivulval (synMuv) genes, include components of the Nucleosome Remodeling Deacetylase (NuRD) transcriptional repressor complex. Single mutants in either of the genetically redundant synMuv A and B classes exhibit normal vulval development, whereas double mutants develop ectopic vulva and exhibit a multivulva (Muv) phenotype (Fay and Han, 2000). Many synMuv genes are conserved in vertebrates, including homologs of retinoblastoma (RB)/lin-35, and E2F and DP transcription factors, efl-1 and dpl-1. In addition, three synMuv genes, Mi-2/chd-4, RbAp48/lin-53 and, HDAC/hda-1, are members of the predicted C. elegans NuRD complex, which has histone deacetylase and chromatin remodeling activities (Bowen et al., 2004; Solari and Ahringer, 2000). C. elegans orthologues of NuRD complex components include the SNF2 chromatin remodeling ATPases Mi-2/chd-3 and Mi-2/chd-4, the metastasis-associated proteins MTA/egr-1 and MTA/egl-27, the histone binding protein RbAp48/lin-53, the histone deacetylase HDAC/hda-1, and the zinc-finger protein p66/dpc-66 (Ahringer, 2000). Thus, the synMuv pathway comprises multiple regulators of transcription, including components of the NuRD complex.
In C. elegans, there are two examples where synMuv and NuRD factors restrict the cell fate potential of precursor cells by repressing alternate cell fates. In the vulva, NuRD and synMuv genes inhibit vulval precursor cells from adopting vulval fates (Cui et al., 2006; Solari and Ahringer, 2000; von Zelewsky et al., 2000). As a result, vulval precursor cells differentiate as epidermis and fuse with the surrounding epithelium. synMuv and NuRD genes also promote development of somatic cells by preventing expression of germline genes in the soma (Unhavaithaya et al., 2002; Wang et al., 2005). A critical question is how the synMuv genes distinguish these alternative fates. In particular, what genes are targeted and what transcription factors interact with synMuv genes?
In this study we investigate the molecular mechanisms of pharyngeal cell fate restriction. Our data reveal that pha-4 controls lineage restriction of pharyngeal cells, and is an important regulator of both the Specification stage and the Morphogenesis/Differentiation stage. Furthermore, we show that specification of the pharyngeal precursors is dependent on pha-4 co-operating with the B class synMuv gene tam-1 and other synMuv genes including NuRD components. Finally, we present evidence that PHA-4 directly represses expression of ectodermal genes in pharyngeal cells, arguing that restriction to pharyngeal fate depends on both repression of ectodermal genes and activation of pharyngeal genes by PHA-4. An appealing possibility is that FoxA factors in other organisms may also have dual transcriptional roles as activators and repressors.
MATERIALS AND METHODS
Strains
Strains were maintained according to Brenner (Brenner, 1974) and maintained at 20°C, and were provided by Caenorhabditis Genetics Center, unless stated otherwise. Bristol N2 was used as the wild-type strain. The following mutations were used LGI: unc-13(e1091), lin-35(n745), dpy-5(e61), lin-53(n833), smg-1(cc546), smg-1(r861); LGII: dpl-1(n2994), egl-27(n170), lin-8(n111), lin-38(n751), lin-56(n2738); LGIII: lin-9(n112), lin-13(n770), lin-36(n766), lin-37(n758), lin-52(n771); LGIV: cha-1(p1182), lin-54(n2231); LGV: chd-4(ar113), dpy-11(e224), efl-1(se1), fog-2(q71), egr-1(ku285), pha-4(q490), pha-4(q500), pha-4(zu225), rol-9(sc148), stu-3(q265), tam-1(cc567), unc-46(e177), unc-76(e911); LGX: chd3(eh4), lin-15(n767). Experiments with tam-1(cc567) were carried out at restrictive temp. (25°C) where it behaves as a predicted null (Hsieh et al., 1999), and maintained at permissive temp. (15°C). Temperature sensitive pha-4 strains were maintained at 24°C, restrictive temp. is 15°C; smg-1(cc546ts);pha-4(zu225) [referred to as pha-4(ts)] and smg-1(r861);pha-4(zu225) [referred to as smg-1(0);pha-4] are sensitized to pharynx phenotypes at 24° (Gaudet and Mango, 2002)(Kaltenbach et al., 2005). pha-4 null: lethal progeny from fog-2(q71) pha-4(q490)/stu-3(q265). elt-3::gfp (JG5) was a gift from Jim McGhee (Gilleard and McGhee, 2001).
DNA constructs
pha-4::yfp (SEM692) was made from a pha-4 genomic clone (Azzaria et al., 1996) encompassing 7076 nucleotides upstream of the first ATG to 1062 nucleotides 3’ of the stop site. An additional 1907 nucleotides 3’ were PCR amplified from N2 genomic DNA and added to create a clone spanning to 2969 nucleotides 3’ of the stop site. The resulting ~16.7kb genomic sequence has previously been shown to rescue pha-4 null mutants (Horner et al., 1998). The pha-4 stop site was PCR mutagenized, and SnaB1 and Avr2 restriction sites were introduced in its place. YFP was PCR amplified from pPD136.64 (a gift from A. Fire) with SnaB1 and Avr2 sites added to the ends. SnaB1/Avr2 YFP was inserted into the mutagenized pha-4 clone. The pha-4 RNAi plasmid (bSEM 865) consists of a 1.5kb pha-4 fragment cloned from N2 cDNA using the following primers: pha-4 AttB1 fw:5’-ggggacaagtttgtacaaaaaagcaggctttatgacatcgccatccagtga-3’, pha-4 AttB2 rv: 5’-ggggaccactttgtacaagaaagctgggttttataggttggcggccgagtt-3’. The PCR product was inserted into pDONORdT7 (Reddien et al., 2005) using a BP reaction (Invitrogen) to create RNAi entry clones. The clones were transformed into E. coli strain HT115. The nuclear spot target was CISg from the lin-26 promoter (Landmann et al., 2004). The 0.9kb fragment was PCR amplified from genomic N2 DNA using the following primers CISg fw:5’-ggttgcatgcccttgactcttgat-3’, CISg rv: 5’-aatggcgacgattgatgggattgg-3’. A second target was made utilizing a PCR fusion based approach (Hobert, 2002), joining together PCR fragments with mutagenized FoxA sites (TRTTKRY, mutagenized sites underlined). Primers: FoxA proximal fw:5’-attcacggatactgatcaatttca-3’, FoxA mid fw:5’-gttcacgagcacagcgccccctag-3’, FoxA distal fw:5’-tacccgtctgacggttgtcgctcc-3’. The PCR products were cloned into pCR 2.1-TOPO (Invitrogen) and sequenced to verify that only FoxA sites were mutagenized.
Immunostaining
In situ antibody staining for α-PHA-4 (pharynx, midgut, hindgut) (Kaltenbach et al., 2005), α-LIN-26 (early ectoderm) (Labouesse et al., 1996), α-intermediate filament (IF, marginal cells) (Pruss et al., 1981), was performed as described (Mango et al., 1994a; Mango et al., 1994b) with the following changes. Embryos were fixed with 2% paraformaldehyde, permeabilized by freeze-crack, then submersed in ice-cold methanol for 5 min. Antibody staining for MH27 (adherens junctions) (Francis and Waterston, 1991), 3NB12 (early pharyngeal muscle) (Priess and Thomson, 1987), 9.2.1 (late pharyngeal muscle) (Miller et al., 1983), was performed as described (Portereiko and Mango, 2001). Briefly, following freeze-crack, embryos were fixed with ice-cold methanol, then ice-cold acetone for 5 min., and rehydrated for 30 s. each in 90%, 60%, 30%, and 10% acetone in water. Mounting medium consisted of 50% glycerol in PBS with DAPI and p-phenylenediamine. Samples were analyzed with a Zeiss LSM510 confocal microscope equipped with LSM software.
Embryo manipulation
Temperature shifts
2 and 4-cell embryos were dissected from gravid smg-1(ts);pha-4(q500) hermaphrodites into M9 (Brenner, 1974) and transferred quickly to 100μl M9 in a PCR tube. Permissive to restrictive temperature shift: Embryos were incubated at 24°C for 60 min. (2E), 110 min. (4E), 3h. (8E), 4.5h. (16E) (at least 90% of embryos were at the appropriate stage at the indicated time), the temperature was ramped at 0.1°C/sec. to 15°C and embryos incubated from 8h. to overnight until they reached 2- to 3-fold stages. Restrictive to permissive temperature shift: Embryos were incubated at 15°C for 2h. (2E stage), 3h. (4E), 5h. (8E), 8h. (16E), the temperature was ramped at 0.1°C/sec. to 24°C and embryos incubated 8–12 h. until they reached the 2- to 3-fold stages. Shifting pha-4(ts) to restrictive temperature reduces PHA-4 expression (Kaltenbach et al., 2005).
Heat shock
2 and 4-cell embryos were dissected from gravid hermaphrodites into M9 and transferred quickly to 100μl M9 in a PCR tube. Embryos were incubated at 20°C for 75 min. (2E stage), 2h. 15 min. (4E), 3h. 45 min. (8E), 5h. (16E), the temperature was then ramped at 0.1°C/sec. up to 33°C, and the embryos incubated at 33°C for 30 min., temperature was ramped down to 20°C, and embryos incubated from 8h. to overnight until wild-type animals reach the 3-fold stage.
RNA interference
RNAi by microinjection
RNAi by microinjection of PHA-4 interacting proteins was performed as previously described (Fire et al., 1998) with the following modifications. RNAi clones were obtained in the pL4440-dest-RNAi vector (Rual et al., 2004) and in vitro transcribed with T7 polymerase using Ampliscribe RNA transcription kit (Epicentre). RNA transcriptions were purified on RNeasy columns (Qiagen), diluted to 0.5 to 1 ng/ul, and injected into smg-1(r861); pha-4(zu225) young adults. After 12 hours, injected worms were moved to fresh plates; progeny were scored for phenotypes at intervals 24–48 hours post-injection.
Feeding RNAi
Bacterial feeding RNAi clones, except gfp, and pha-4, were from the Ahringer/Geneservice Ltd. library of 16,757 clones (Fraser et al., 2000; Kamath et al., 2003). Clones were confirmed by restriction enzyme digest. Feeding RNAi was performed as described previously (Kamath et al., 2001) with the following modifications: Bacteria were grown 12–16h. in LB with 50μg/ml ampicillin (Sigma) at 37°C, 5ml cultures were spun at 2,000 rpm for 7 min., 4.5 ml of supernatant was removed, the remaining pellet and supernatant were resuspended, and 50μl plated onto 6cm NGM plates with 5mM IPTG (Fisher Scientific) and 25μg/μl Carbenicillin (Sigma). Plates dried at 37°C for 4–6 h. 4–6 L4 hermaphrodites were placed onto cooled plates (20°C, or appropriate temp.), moved to a new RNAi plate after 1 day, and moved again after laying eggs for 1 day. Embryos developed for 1–2 days before dead embryos and L1s were scored. Effectiveness of synMuvA, and synMuvB RNAi were verified by ability to induce Muv phenotypes in lin-37(n758) or lin-15A(n767) strains, respectively.
Weak pha-4(RNAi)
Bacteria expressing pha-4 (bSEM 865) or gfp (pPD128.110, a gift from A. Fire) RNAi cultures were grown overnight as described above, except that pha-4 clones were grown in LB with 50ng/μl kanamycin. pha-4 and gfp-expressing cultures were mixed at a ratio of 1:10–1:20 prior to spinning at 2,000 rpm. Resuspended bacteria were plated onto NGM plates with 1mM IPTG. Ratios that generated 10–51% lethality of N2 worms were used for the enhancement assays.
Enhancement assays
Candidate PHA-4 interactor(RNAi)
Double-stranded RNA was injected into 10 smg-1(r861);pha-4(zu225) [smg-1(0);pha-4] young adults, and progeny were examined for phenotypes. Progeny were scored for lethality, sterility, and slow growth. Results were compared to published data in wild-type (N2 or rrf-3) worms (Kamath et al., 2003; Simmer et al., 2003).
Weak pha-4(RNAi) on test worm strains
Diluted pha-4(RNAi) was fed to WT or test (synMuv, NuRD mutants) strains and percent lethal progeny (eggs, L1s) was scored. At least 50 animals were scored for each of the 3 trials performed for each test strain. The fold difference was calculated by dividing % lethal in the test strain by % lethal in controls. A 2x2 Fisher’s exact test (Agresti, 2002) was used to identify trials with significant enhancement (p<0.05). A test strain was characterized as an enhancer if calculated p-value was <0.05 and the fold-enhancement was >1 for each trial. Each strain displayed a low “background” lethality (≤5%) when fed control (gfp) double-stranded RNA, except egr-1(ku285) which had a background lethality of 20% (n>50, 3 trials). In the case of egr-1(ku285), 20% was subtracted from “test strain weak pha-4(RNAi)” lethality value before calculating statistical significance and fold-enhancement (Supplementary table 3). We note that although genetic analysis demonstrates tam-1 is a pha-4 enhancer, fold enhancement in RNAi experiments is often less than 2-fold (For example, Supplementary table 3, experiments 5, 8 show enhancement at 1.3-fold). We have the greatest confidence in genes that like tam-1, reproducibly enhanced pha-4 with statistical significance, and therefore only these were scored as enhancers.
test(RNAi) on pha-4 strain
synMuv, NuRD RNAi was fed to control smg-1(ts) and smg-1(ts);pha-4(zu225) at permissive temperature (24°). Control experiments confirmed the ability of synMuv(RNAi) to produce a Muv phenotype in the appropriate synMuv background strain lin-15(n767) or lin-37(n758). Because smg-1(ts);pha-4 had an average background of 5% lethality when fed control (gfp) dsRNA (n≥100, 3 trials), statistical significance and fold difference were calculated compared to a baseline of 5% lethality. test(RNAi) displayed low background lethality (≤5%) when fed to control strain (n>50, ≥ 3 trials), except lin-53(RNAi) and ubc-9(RNAi) which had an average of 82% and 17% background lethality, respectively (n>50, ≥3 trials). lin-53(RNAi) and ubc-9(RNAi) background lethality [“control strain; test(RNAi)” value] was subtracted from “smg(ts);pha-4;test(RNAi)” lethality value before calculating statistical significance and fold-enhancement (Supplementary Table 4).
Nuclear Spot Assay
Microinjections
Transgenic lines were as follows: no target control (SM1413, SM1414), lin-26 CISg target (SM1398, SM1420), lin-26 CISg target with mutagenized FoxA sites (SM1455, SM1459). SM1413, SM1414 were created by injecting cha-1(p1182) worms with Xho1-linearized pha-4::yfp plasmid (SEM691) (1ng/μl), H2A.Zpromoter::CFP::LacI PCR product (Updike and Mango, 2006) (4ng/μl), an Sph1/Kpn1 fragment from lacO multimeric plasmid pSV2-dhfr-8.23 (4.5 ng/μl) (Straight et al., 1996), cha-1 plasmid (RM527P, a gift from J. Rand) linearized with Apa1 (2ng/μl), and sheared herring sperm DNA to make 100ng/ul total DNA. SM1398, SM1420 were created by injecting lin-26 CISg target PCR product (10ng/μl), and SM1455 and SM1459 were created by injecting lin-26 CISg mutagenized PCR product (10ng/μl) in addition to components listed for SM1413 and SM1414. PHA-4::YFP rescues pharyngeal cells in pha-4 null animals to the same extent as a pha-4 genomic clone (both rescue partially).
Analysis
Nuclear spot assays were performed as described previously (Carmi et al., 1998; Gonzalez-Serricchio and Sternberg, 2006; Kaltenbach et al., 2000; Updike and Mango, 2006). The following modifications were made: Images were acquired from live embryos using a Leica DM RXE confocal microscope with LCS software. A multitrack setting was used to acquire separate CFP and YFP images from three random slices through the pharynges of embryos at 8E – 20E stages (in 2E and 4E embryos, PHA-4::YFP photobleached too rapidly for analysis). PHA-4::YFP binding to the array appears as an intense YFP spot within a background of weaker nuclear YFP expression (where PHA-4::YFP binds to endogenous targets). Images were merged in Photoshop, and YFP nuclear spots were analyzed for co-localized CFP expression. In embryos designated positive, up to 4 co-localized nuclear spots were observed per slice. All co-localized spots were exclusively observed in the pharynx. Two transgenic lines, 20 embryos each line, were imaged for each control or target array.
RESULTS
Three experiments were performed to address the role of pha-4 during pharyngeal development. First, by challenging pharyngeal precursors with a heterologous regulator, we established when pharyngeal precursors transfate to pharyngeal fate. Second, to determine when pha-4 functions, we examined whether pharyngeal cells were generated when pha-4 expression was reduced at different times in development. Third, we examined whether pharyngeal cells could transfate to an alternative cell type when pha-4 expression was reduced at different developmental stages. These experiments defined the 4E-8E stages as a critical time for commitment to pharyngeal fate and revealed that pha-4 activity is required for pharyngeal cell fate specification at or before this time. pha-4 is also required subsequently for pharyngeal differentiation and morphogenesis.
4E-8E stages are a critical period for commitment to the pharynx fate
To determine when pharynx precursors were committed to pharyngeal fate, exogenous cell fate regulators were expressed at different stages to challenge wild-type pharyngeal cells to adopt alternate cell fates. Activation of gene expression by heat shock is very rapid, providing an accurate means to assess developmental timing (Fukushige and Krause, 2005; Horner et al., 1998; Labouesse and Mango, 1999; Zhu et al., 1998).
When induced by a heat shock (HS) promoter, the midgut GATA transcription factor end-1, and the muscle bHLH transcription factor hlh-1 transfate precursors to midgut and muscle, respectively (Fukushige and Krause, 2005; Zhu et al., 1998). To follow pharyngeal cell development specifically, transcription of HS::end-1 or HS::hlh-1 was activated briefly at the 2E, 4E, 8E, or 16E stages, and cell fates assayed after overnight incubation. Terminal embryos were co-immunostained for the pharyngeal marker PHA-4, and for one of the following: the early midgut marker ELT-2, the late midgut marker 1CB4, or the muscle marker 4C6.3 (Figure 1). Although PHA-4 is expressed in both midgut and pharyngeal cells (Horner et al., 1998; Kalb et al., 1998), pharyngeal cells are clearly distinguishable both by their stronger PHA-4 expression relative to endodermal cells, and their lack of 1CB4 staining (compare Figures 1A, D).
Figure 1. 4E-8E stages are a critical period for commitment to the pharynx fate.

Pharyngeal precursors were challenged to adopt endodermal or muscle fates by activating expression of (A–O) HS::end-1, or (P–U) HS::hlh-1, respectively. Embryos challenged at (A–C, G–I, P–R) 2E, (D–F, J–L, S–U) 4E and (M–O) 8E stages are shown. (A,D,G,J,M,P,S) Embryos stained with anti-PHA-4. Midgut cells show weak PHA-4 expression, as expected. Pharyngeal bright PHA-4 stain does not co-localize with gut or muscle markers, and is marked with dotted circles. Embryos stained with (B,E) the early endodermal marker, ELT-2, (H,K,N) the late endodermal marker, 1CB4, and (Q,T) the muscle differentiation marker, paramyosin. (C,F,I,L,O,R,U) merge. (V) Graph shows percentage of embryos heat shock treated at the 2E, 4E, 8E, and 16E stages that exhibit pharyngeal cells. Embryos either bear HS::end-1 (red) or HS::hlh-1 (blue).
The pharynx challenge revealed that most pluripotent pharyngeal precursors become committed to pharyngeal fate at the 4E–8E stages. When pharyngeal precursors were challenged with HS::end-1 at the 2E stage, all pharyngeal precursors transfated in the majority of embryos. There was ubiquitous expression of midgut markers and a lack of bright PHA-4 stain (Figure 1A–C, 1G–I). This finding is in agreement with published results (Zhu et al., 1998). By contrast, when challenged at the 4E stage, most embryos had at a small number of precursors that were refractory to the challenge and differentiated as pharynx. This conclusion is based on the emergence of bright PHA-4 staining at the expense of midgut marker expression in subsets of cells (Figure 1D–F, 1J–L). Embryos challenged at the 8E stage had a larger complement of precursors that were refractory to the challenge (Figure 1M–O), and embryos treated at the 16E stage were completely unaffected by the challenge and appeared wild-type (data not shown). Similar results were obtained when the pharyngeal precursors were challenged to acquire body wall muscle fate (Figure 1P–U, data not shown).
These results were quantified to determine when precursors commit to pharyngeal fate (Figure 1V). When challenged to become midgut at the 2E 4E, 8E, and 16E stages (n≥65 each stage), 18%, 64%, 91%, and 100% animals exhibited pharyngeal cells, respectively. When challenged to become muscle at the 2E, 4E, 8E, and 16E stages (n≥20 each stage), 18%, 55%, 80% and 100% embryos exhibited pharyngeal cells, respectively. In summary, at the 2E stage, a relatively small percentage of embryos are unresponsive to exogenous developmental regulators. This may reflect initiation of commitment to the pharynx fate, alternatively some embryos may have been exiting the 2E stage at the time of heat shock. By the 4E stage, more than half the embryos are refractory to cell fate change. One cell division later, at the 8E stage, the vast majority of embryos have pharyngeal cells, suggesting the window of plasticity is largely complete.
pha-4 contributes to early and late pharyngeal development
To determine when pha-4 was required to establish pharyngeal fate, pha-4 temperature-sensitive (ts) animals [smg-1(cc546ts);pha-4(q500)] (Kaltenbach et al., 2005) were shifted from permissive to restrictive temperature at incrementally later stages in development (2E, 4E, 8E, 16E) and allowed to mature overnight.
Based on pharynx morphology using DIC microscopy (Figure 2A–D), terminal embryos were placed into one of four categories. Ranked from most to least severe, the categories were: (1) “No detectable pharynx” (Figure 2A), (2) “Pun” or pharynx unattached, where pharynges failed to attach to the mouth due to fewer pharyngeal cells or aberrant morphogenesis (Figure 2B), (3) “Misshapen” where pharynges attached but had clear morphological defects (Figure 2C), and (4) “Wild-type” where pharyngeal morphology appeared normal (Figure 2D). Control smg-1(ts) animals shifted at different embryonic stages had Wild-type pharynges (n≥15 each stage, data not shown). We confirmed pharyngeal phenotypes by immunostaining for three markers indicative of different cell types and developmental stages: α-intermediate filament, 3NB12, and 9.2.1 label marginal cells (Figure 2E–H; (Pruss et al., 1981), early pharyngeal muscle (Figure 2I–L; (Priess and Thomson, 1987), and terminal, differentiated pharyngeal muscle (Figure 2M–P; (Miller et al., 1983), respectively. As in pha-4 null embryos, “No pharynx” embryos displayed trace amounts of cell-type specific markers (Figure 2E,I,M; (Mango et al., 1994a). Antibody staining of “Pun”, “Misshapen”, or “Wild-type” embryos mirrored overall pharyngeal morphology, demonstrating that the categories accurately reflected the terminal phenotype.
Figure 2. pha-4 is required for pharyngeal specification and morphogenesis/differentiation.


Representative phenotypes of pha-4(ts) embryos shifted from permissive to restrictive temperature at different stages in development. (A,E,I,M) No pharynx phenotype, (B,F,J,N) pharynx unattached (Pun), (C,G,K,O) Misshapen pharynx, (D,H,L,P) Wild-type pharynx morphology. Phenotypes were examined by morphology and expression of pharyngeal markers. (A–D) Differential interference contrast (DIC) images, (E–H) α-intermediate filament antibody stain marks marginal cells, (I–L) 3NB12 antibody stains early (e.) pharyngeal muscle, (M–P) 9.2.1 antibody stains late (l.) pharyngeal muscle. Arrows indicate buccal cavity; arrowheads outline edges of Pun and Misshapen pharynges; bracket and parenthesis indicate anterior bulb, and posterior bulb, respectively.
pha-4(ts) embryos shifted at 2-cell, 2E, 4E, 8E, 16E stages exhibit an array of pharyngeal phenotypes. (Q) Percentage of pha-4(ts) embryos shifted from permissive to restrictive temp. with designated pharynx phenotypes (R) Percentage of pha-4(ts) embryos shifted from restrictive to permissive temp. with designated pharynx phenotypes.
Quantification of observed phenotypes (Figure 2Q) demonstrated that pha-4 was required first to specify pharyngeal identity (Mango et al., 1994a) and subsequently to drive morphogenesis/differentiation. Under the most restrictive conditions, when pha-4(ts) L4 stage animals developed to adulthood and laid eggs at restrictive temperature, 60% (20/34) of embryos had no pharynx, indicating that this strain could mimic the pha-4 null phenotype. Similarly, a large proportion of embryos shifted to restrictive temperature at the 2-cell stage had No pharynx (37%, 22/60). Embryos shifted at later stages showed progressively less severe morphological phenotypes. Of embryos shifted at the 2E (n=87), 4E (n=43), 8E (n=45), and 16E (n=25) stages, the highest represented phenotypes were Pun (2E, 76%), Misshapen (4E, 42%), and Wild-type (8E, 82%; 16E, 97%), respectively. The majority of Pun pharynges observed among 2-cell and 2E shifted animals appeared considerably smaller than wild-type pharynges (Compare Figure 2J, N, to 2L, P), consistent with the idea that fewer cells were specified to the pharynx fate. These data suggest that pha-4 activity is required by approximately the 4E stage to establish the normal complement of pharyngeal precursors.
Conversely, shifting pha-4(ts) embryos from restrictive to permissive temperature demonstrated that pha-4 must be active within an early developmental window to rescue pharynx development fully (Figure 2R, Supplementary Figure 1). Only embryos shifted to permissive temperature at the 2-cell (n=16), and 2E (n=57) stages exhibited Wild-type pharyngeal morphology (96% and 68%, respectively). Embryos shifted later showed more severe morphologies. Most embryos shifted at the 4E stage (73%, n=41) were Pun with wild-type size pharynges (Supplementary Figure 1B). A large proportion of embryos shifted at the 8E stage (37%, n=38), and most shifted at the 16E stage (79%, n=28), had No pharynx, and the majority of Pun animals observed at these stages had small pharynges (Supplementary Figure 1C). We conclude that re-activation of pha-4 expression after the 4E stage is not sufficient to rescue pharyngeal specification.
We reasoned that when cells are committed to the pharyngeal fate, they should not be able to adopt an alternative fate. We examined whether cells can adopt an alternative fate when pha-4 activity is reduced at the 2-cell to 8E stages. We focused on ectoderm since previous studies had shown that pharyngeal precursors from pha-4 null animals can develop into LIN-26+ ectoderm (Horner et al., 1998). pha-4(ts) embryos were shifted to restrictive temperature and assayed for ectopic expression of ectodermal markers LIN-26 and elt-3::gfp in the presumptive pharynx (Figure 3). Early shifted animals exhibited ectopic expression of LIN-26 (Figure 3A) and the transgene elt-3::gfp (Figure 3C) in the presumptive pharynx, whereas late shifted animals did not (Figure 3B, D). Specifically, pha-4(ts);elt-3::gfp embryos shifted at the 2-cell (n=26), 2E (n=23), 4E (n=28), and 8E (n=10) stages exhibited 62%, 27%, 13%, and 0% transfated cells, respectively (Figure 3E). Therefore, only animals shifted prior to the 8E stage exhibited ectopic expression of ectodermal markers in the pharynx. Collectively, these experiments suggest that pha-4 is required at the Specification stage to establish the pharyngeal precursors. After commitment to the pharyngeal fate, pha-4 is required for Morphogenesis/Differentiation, based on the unusual morphologies of treated embryos.
Figure 3. pharyngeal precursors are pluripotent in early development.

pha-4(ts) embryos shifted from permissive to restrictive temp. at the (A,C) 2E and (B,D) 8E stages, and stained for the ectodermal markers (A,B) LIN-26 and (C,D) elt-3::gfp (closeup of head). Ectodermal LIN-26 and elt-3::gfp is expressed along the periphery of the embryo. Expression of ectodermal markers in presumptive pharynx is indicated with dotted circles. Boundaries of pharynges are marked with solid lines. (E) Proportion of pha-4(ts) embryos shifted from permissive to restrictive temp. at the 2-cell, 2E, 4E, and 8E stages that exhibit expression of the ectodermal marker, elt-3::gfp in the pharynx.
PHA-4 binds the ectodermal gene lin-26 in pharyngeal cells
How does pha-4 regulate cell fate restriction? Previous work from our lab showed that PHA-4 functions as a transcriptional activator that promotes expression of pharyngeal genes throughout development (Gaudet and Mango, 2002). Intriguingly, there is also evidence that PHA-4 may repress expression of the ectodermal gene, lin-26. First, pha-4 null animals ectopically express LIN-26 in pharyngeal precursors (Mango et al., 1994a). Second, widespread, ectopic expression of PHA-4 promotes the pharyngeal fate at the expense of LIN-26 (Horner et al., 1998). Third, the lin-26 promoter bears multiple consensus FoxA binding sites within a region containing several blocks of sequence conserved with C. briggsae, called conserved intron sequence (CIS) a–h (Dufourcq et al., 1999; Landmann et al., 2004). GFP reporters made from promoter fragments revealed that the CISe-i region, or lin-26 3’UTR, bears sequences that prevents pharyngeal expression. Within this region, CISg (~900kb) has three consensus FoxA sites (Landmann et al., 2004). Based on these observations, we hypothesized that PHA-4 might repress lin-26 by binding to CISg.
The nuclear spot assay (Carmi et al., 1998; Gonzalez-Serricchio and Sternberg, 2006; Kaltenbach et al., 2000; Updike and Mango, 2006) was used to determine whether PHA-4 associates directly with lin-26 CISg (Figure 4). pha-4::yfp and lin-26 CISg DNAs were co-injected with lacI::cfp and lacO to generate an extrachromosomal array. LacI::CFP binding to lacO was visualized as a small CFP “spot” that marked the location of the array in cell nuclei. In vivo binding of PHA-4::YFP to target promoters was visualized as a yellow spot that co-localized with LacI::CFP.
Figure 4. Association of PHA-4::YFP with lin-26 promoter.

(A,E,I) PHA-4::YFP (green) is expressed in pharyngeal cells. Extrachromosomal target arrays are marked with (B,F,J) LACI::CFP (red) in a nuclear spot assay. (C,G,K) merged YFP and CFP spots are yellow. (D,H,L) illustrations of target arrays, and proportion of target arrays with co-localized PHA-4::YFP and LACI::CFP. (M) illustration of lin-26 promoter highlighting CISg region. (A–D) PHA-4::YFP is not enriched (arrowheads) at sites of arrays with no target. (E–H) PHA-4::YFP associates with target arrays containing the ectodermal gene lin-26 promoter CISg (arrows indicate yellow overlap), (I–L) PHA-4::YFP is not enriched (arrowheads) at sites of arrays containing lin-26 CISg with conserved FoxA binding sites mutated.
The nuclear spot assay shows that PHA-4::YFP associates with lin-26 Cisg. In control animals with no target promoter in the extrachromosomal array, a background of 15% of embryos exhibited co-localized spots (2 strains, n=20 embryos each; Figure 4A–D). Background binding may be non-specific or from cryptic PHA-4 sites in co-injected DNA. We observed PHA-4::YFP binding to lin-26 CISg in approximately 50% of pharynges in two independent lines (n=20 embryos each; Figure 4E–H). Importantly, when the three FoxA binding sites in CISg were mutated, the frequency of co-localized spots was reduced to background levels (16%, 2 strains, n=20 embryos; Figure 4I–L). These results demonstrate that PHA-4 associates with a repressed, ectodermal target within the pharynx.
tam-1 cooperates with pha-4 to specify the pharyngeal cell fate
How does pha-4 function as a repressor? To begin to address this question, we surveyed potential PHA-4 interacting proteins for genes that enhance pha-4 lethality. The C. elegans Interactome previously identified twelve proteins that interacted with PHA-4 in a yeast two-hybrid study (Li et al., 2004). We surveyed genes encoding each of the twelve candidate PHA-4 interacting proteins to determine if any could enhance a partial loss of pha-4 function observed in smg-1(0);pha-4 animals (Gaudet and Mango, 2002; Kaltenbach et al., 2005). Our positive control F38A6.1 (pha-4), produced embryonic and larval lethality in WT and smg(0);pha-4 strains as expected (Supplementary table 1; (Mango et al., 1994a). Of the candidate PHA-4 interactors, only tam-1 had synthetic effects with pha-4. tam-1(RNAi) of smg-1(0);pha-4 animals lead to 30% arrested progeny at the first larval (L1) stage (n=20, Supplementary table 1). Arrested L1s exhibited one of the following pharyngeal defects: Pun, Misshapen pharynges, or pharynges stuffed with bacteria, a sign of a malfunctioning pharynx. By contrast, fewer than 5% of control smg-1(0);tam-1(RNAi) or smg-1(0);pha-4 animals arrested at the L1 stage (data not shown). These findings reveal that thattam-1 interacts with pha-4 genetically.
We verified the genetic interaction between pha-4 and tam-1 in two ways. For these experiments we used tam-1(cc567), which carries a nonsense mutation in tam-1 and behaves as a null at restrictive temperature (Hsieh et al., 1999). First, we found that smg-1(0);pha-4;tam-1(cc567) triple mutants exhibited a 12-fold increase in synthetic embryonic/L1 lethality at restrictive temperature compared to smg-1(0);pha-4 alone (61±12% vs. 5±5% lethality), and a 20-fold enhancement compared to tam-1(cc567) alone (61±12% vs. 3±2% lethality; >100 embryos per trial, 3 trials, p-value ≤0.0001)(Figure 5A). Thus, the synergy that we saw did not depend on RNAi. Second, weak pha-4(RNAi) was performed on tam-1(cc567) animals at restrictive temperature by diluting bacteria producing pha-4 double-stranded (ds) RNA with bacteria producing GFP dsRNA. In 8/8 trials, tam-1(cc567) significantly enhanced weak pha-4(RNAi) by an average of 2-fold (p-value=0.0138, Supplementary table 2). Thus, enhancement did not depend on the smg-1 mutant allele. We note that enhancement values were sometimes compressed due to lethality induced by weak pha-4(RNAi) in control animals. For example, in one trial, weak pha-4(RNAi) produced 48% lethality in control and 100% lethality in tam-1(cc567) strains, thus producing the maximum enhancement possible at 2.1-fold (Supplementary table 3).
Figure 5. tam-1 enhances pha-4.

(A) Proportion of lethal L1 larvae observed in tam-1(cc567), smg-1(0);pha-4, and smg-1(0);pha-4;tam-1(cc567) mutant strains (B,C) Embryos subjected to weak pha-4(RNAi) and stained for the ectodermal marker, LIN-26. Ectopic LIN-26 in presumptive pharynx marked with arrows. (B) smg-1(0);pha-4, (C) smg-1(0);pha-4;tam-1(cc567). (D) N2 and (E) tam-1(cc567) 2-fold embryos at restrictive temperature (25°) stained with anti-PHA-4.
Does tam-1 impact the Specification stage of pharyngeal development? We examined the extent of cell fate transformations in either smg-1(0);pha-4 or smg-1(0);pha-4;tam-1(cc567) embryos administered weak pha-4(RNAi). 33% of smg-1(0);pha-4;tam-1(cc567) embryos exhibited ectopic LIN-26 expression in the presumptive pharynx (n=20), compared to 0% in smg-1(0);pha-4 animals (n=20) (Figures 5B, C). Control tam-1(cc567); gfp(RNAi) animals did not display pharyngeal defects or cell fate transformations (n=20, data not shown). To determine whether tam-1 functions in pharyngeal specification by regulating PHA-4 expression, wild-type (N2) and tam-1(cc567) were raised at restrictive temperature and immunostained with anti-PHA-4. PHA-4 expression is unchanged in tam-1 animals compared to wild-type (Figure 5D, E), demonstrating that tam-1 is not required for PHA-4 expression. These data support the notion that tam-1 functions with pha-4 to regulate specification of pharyngeal cells.
NuRD components interact genetically with pha-4
In genetic tests, tam-1 behaves as a synMuv B gene (Hsieh et al., 1999), suggesting that other synMuv genes might also interact genetically with pha-4. TAM-1 contains a tripartite motif (TRIM, or RBCC domain), consisting of RING finger, B-box, and coiled-coil domains. Many TRIM domain proteins mediate transcriptional repression (Frank and Roth, 1998; Meroni and Diez-Roux, 2005; Peng et al., 2002; Wu et al., 2001). Therefore, we focused our analysis on NuRD components to test whether other mediators of transcriptional repression enhance pha-4.
We performed two RNAi surveys to demonstrate that inactivation of some NuRD components enhance pha-4 (Table 1, Supplementary Tables 2, 3). First, weak pha-4(RNAi) was performed on NuRD mutant strains, and progeny examined for enhanced lethality. In this assay, three NuRD mutants, MTA/egl-27(n170), MTA/egr-1(ku285) and Mi-2/chd-3(eh4), each showed significant enhancement with pha-4 (p-value<0.05), whereas Mi-2/chd-4(ar113) and RbAp48/lin-53(n833) did not. In addition to tam-1 and NuRD, four synMuv B genes (lin-9, lin-13, lin-54, ubc-9) and no synMuv A genes interacted genetically with pha-4 (Supplementary table 2). We have the greatest confidence in genes that like tam-1, reproducibly enhanced pha-4 with statistical significance, and therefore only these were scored as enhancers. As lin-9 and lin-13 are reported to augment RNAi (Lehner et al., 2006; Wang et al., 2005), we cannot rule out RNAi effects for these two genes.
Table 1.
NuRD components enhance pha-4
| RNAi | Worm strain | Brief description | Avg. fold enhancement | maximum p-value | total trials |
|---|---|---|---|---|---|
| NuRD | |||||
| pha-4 | egl-27(n170) | MTA-1 | 3.4 | .0372 | 3 |
| egl-27 | smg(ts); pha-4 | 19.0 | .0001 | 3 | |
| pha-4 | egr-1(ku285) | MTA-1 | 5.5 | .0003 | 3 |
| egr-1 | smg(ts); pha-4 | 4.6 | .0051 | 4 | |
| pha-4 | chd-3(eh4) | Mi-2 | 2.5 | .0093 | 5 |
| pha-4 | lin-53(n833) | RbAp48 | 1.3 | .4497 | 3 |
| lin-53 | smg(ts); pha-4 | 1.2 | .0001 | 4 | |
| pha-4 | chd-4(ar113) | Mi-2 | 0.3 | .3360 | 3 |
| mep-1 | smg(ts); pha-4 | CHD-4 interactor | 1.7 | 1.000 | 3 |
| synMuv B (control) | |||||
| pha-4 | lin-52(n771) | novel | 0.9 | .9261 | 3 |
embryonic/L1 lethality was compared between smg(ts);pha-4 and smg(ts) strains fed NuRD(RNAi), or between NuRD genetic mutants and wild-type worms fed diluted pha-4(RNAi).
In the converse experiment, RNAi against NuRD components egl-27, egr-1, lin-53 and mep-1 combined with loss of function pha-4 worms, smg(ts);pha-4 at permissive temperature, revealed significant enhancement by egl-27 and egr-1 (Table 1). Enhancement by lin-53 (1.2 average fold enhancement, p-value .0001) may be compressed due to high lethality induced by lin-53(RNAi) in control animals. For example in one trial, lin-53(RNAi) produced 79% lethality in control and 99.5% lethality in smg(ts);pha-4 strains, thus producing the maximum enhancement possible at 1.3-fold (Supplementary Table 4). The observation that vulva development is particularly sensitive to the partial loss-of-function mutation lin-53(n833), while embryonic roles are unaffected, may explain the apparent discrepancy between results obtained with lin-53(RNAi) and lin-53(n833) (Lu and Horvitz, 1998). Another possibility is that since lin-53(RNAi) causes a high percentage of lethality on its own, it could be non-specifically hypersensitive to the reduction of a second gene. These findings suggest that some NuRD components interact synergistically with pha-4 mutations to control viability.
The enhancement of lethality between pha-4 and NuRD reflected a failure to specify cells to the pharyngeal fate. We used the adherens junction marker MH27 to show that 9%, 13%, and 25% of tam-1, Mi-2/chd-3(eh4), and MTA/egl-27(n170) animals, respectively, did not develop pharynges when combined with weak pha-4(RNAi) (Figure 6; n>30 embryos per strain (Francis and Waterston, 1991). Weak pha-4(RNAi) alone with WT animals did not produce any pharynx-less embryos (n=59)(Figure 6A). Comparing egl-27 and chd-3 to negative controls (WT and the synMuv B gene lin-52(n771) which does not enhance pha-4 (Table 1), there was a 7–13 fold enhancement of the No pharynx phenotype (Figure 6C–E), and a 2–3 fold enhancement of the Pun phenotype (Figure 6E). Importantly, enhancement of pharyngeal phenotypes was specific, since non-pharyngeal tissues appeared normal (for example, compare Figure 6A, C, right of dotted line). These data show that MTA/chd-3, Mi-2/egl-27, and Mi-2/egr-1 affect pharyngeal cell fate specification.
Figure 6. NuRD components enhance pha-4.
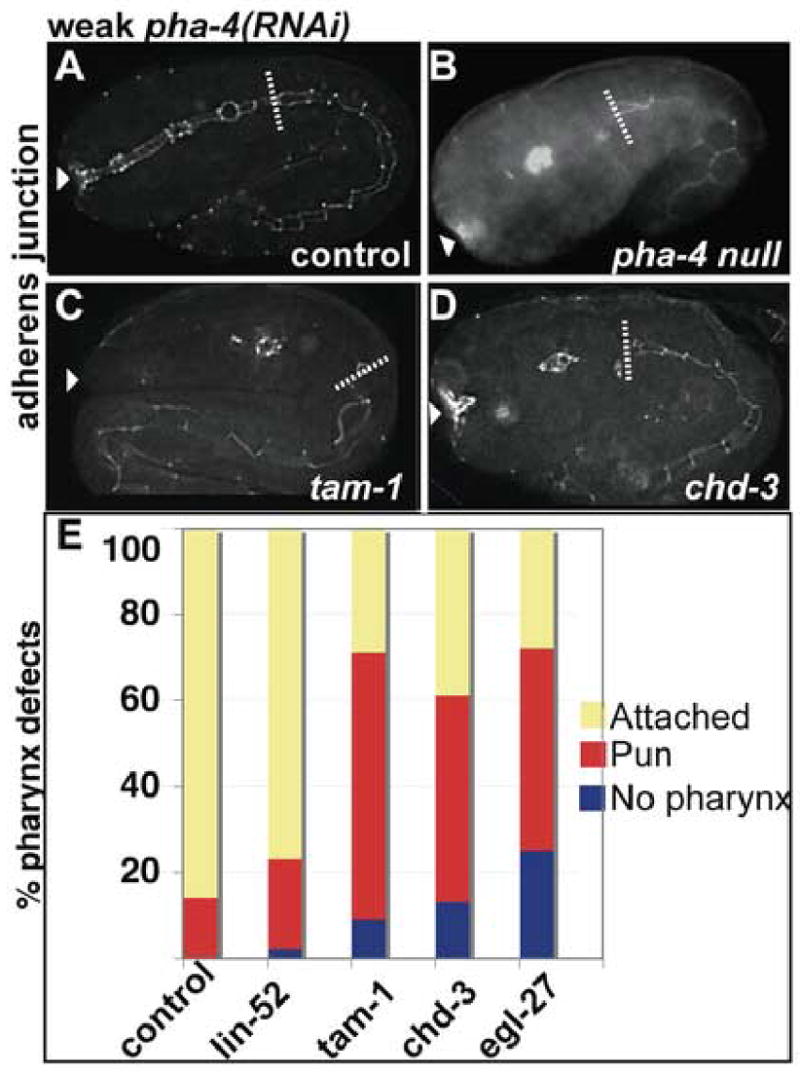

Embryos administered weak pha-4(RNAi) and assayed for pharyngeal morphology by staining for adherens junction marker MH27. (A) control (WT), (B) pha-4(q490) null, (C) tam-1(cc567), (D) chd-3(eh4). Arrowhead indicates buccal cavity, dotted line indicates posterior end of pharynx. Intact midgut MH27 stain lies posterior to the phaynx. (E) Percentage of embryos with pharyngeal phenotypes observed in control [WT, lin-52(n771)] or tam-1 and NuRD mutant strains [tam-1(cc567), chd-3(eh4), egl-27(n170)] fed weak pha-4(RNAi).
Taken together we find that Mi-2/egl-27, and Mi-2/egr-1, enhance pha-4 by influencing cell fate specification whereas MTA/chd-4 and mep-1 do not. Results with chd-3 could not be confirmed due to the failure of controls; feeding and injecting chd-3(RNAi) did not produce the Muv phenotype in lin-15A(n767) animals (data not shown). As expected, in none of these experiments did we observe a Muv phenotype when pha-4 loss-of-function was combined with loss of synMuv components (data not shown).
DISCUSSION
A central question in developmental biology concerns how a cell transitions from the pluripotent state to become a single, differentiated cell type. Our findings reveal that the 4E–8E stages are a critical period when early precursors of the C. elegans pharynx lose their developmental plasticity and become committed to the pharyngeal fate. This transition depends on the selector gene pha-4, which cooperates with the TRIM protein TAM-1 and components of the NuRD complex. tam-1 and pha-4 are required to repress ectodermal genes within nascent pharyngeal precursors. We suggest that PHA-4 functions as both a transcriptional activator and a repressor to promote pharyngeal fate and inhibit alternative fates.
Two stages of restriction for pharyngeal precursors
Three experiments indicate that pha-4 functions during the 2E (when expression initiates) to 8E stages to promote the transition from a pluripotent to a committed developmental state. First, when challenged with an exogenous selector gene, the ability of most wildtype pharyngeal precursors to transfate to endoderm or body wall muscle is lost between the 4E–8E stages. Second, pharyngeal precursors transfate to ectoderm when pha-4 is inactivated prior to the 8E stage. Third, pharyngeal development in pha-4(ts) mutants can be rescued only when pha-4 activity is restored before the 8E stage. We note that transition from the pluripotent to the committed state reflects commitment to pharyngeal identity, but not to a specific cell type within the pharynx. Until the terminal cell divisions, pharyngeal precursors exist that will generate distinct pharyngeal cell types, such as the gland cell g1AL (MSaaapaapaa) and neurosecretory-motor cells NSML (ABaraapapaav) and NSMR (ABaraapppaav) (Sulston et al., 1983). Thus, cells whose descendents will become pharyngeal cells undergo at least two stages of developmental restriction: first to a pharyngeal identity at the 4E–8E stages (Specification stage) and subsequently to a pharyngeal cell type (Morphogenesis/Differentiation stage).
The timing we observe agrees well with the cell lineage, in which blastomeres of the 4E–8E stages produce either pharyngeal cells or non-pharyngeal cells, but not both (Sulston et al., 1983). We note that although we observed the shift in pha-4(ts) animals at approximately the 4E stage, the transition was not precise. This effect could reflect a lag in knock-down of pha-4 RNA and protein, a concern with any temperature-shift experiment, or it could reflect a broad temporal requirement for pha-4. Either way, our data suggest that pha-4 is required at the Specification stage to establish pharyngeal precursors. Activation of gene expression by heat shock is rapid, therefore the midgut and muscle challenge experiments accurately reflect the timing of commitment to the pharyngeal fate.
Other studies have also implicated the ~4E–8E stages as the transitional stage for developmental competence (Labouesse and Mango, 1999). For example, ectopic expression of pha-4 throughout the embryo can convert a proportion of embryonic cells to a pharyngeal fate, provided pha-4 is expressed prior to the 4E stage (Horner et al., 1998). Similarly, widespread end-1 or hlh-1 expression can induce embryonic blastomeres to become intestine or body wall muscles, respectively, until the 4E–10E stages (Fukushige and Krause, 2005; Zhu et al., 1998). We note that a previous study implicated that pharyngeal precursors first became refractory to HS::hlh-1 at the 10E stage (Fukushige and Krause, 2005). One possible reason for the observed difference compared to our work is that the marker for pharynx fate in the previous study is expressed in fewer cells (3NB12, in a subset of pharyngeal muscles) compared to the marker used in this work (PHA-4), which is a global marker of pharyngeal cells. Together these observations suggest that early embryonic blastomeres are receptive to different selector genes and can follow alternative developmental pathways during the Specification stage, whereas older blastomeres lose pluripotency while still retaining plasticity with regard to pharyngeal cell type. However, because these studies relied on exogenous regulators, the mechanisms guiding this transition during normal development were mysterious.
Consistent with the 2E–8E stages representing a global transition point in cell fate potential, genes that specify cell fates are typically expressed at or before the 8E stage, including cell fate regulators for pharynx (pha-4, see below), chemosensory neurons (unc-130), mesendoderm (med-1/2), endoderm (end-1/3), and ectoderm (elt-1) (Baugh et al., 2003; Horner et al., 1998; Maduro et al., 2001; Sarafi-Reinach and Sengupta, 2000; Spieth et al., 1991; Zhu et al., 1997). Genes that control terminal differentiation generally initiate expression after the 4E stage, for example differentiation genes of the ectoderm (elt-3), pharyngeal muscle (ceh-22, myo-2), and endoderm (ges-1) (Egan et al., 1995; Gilleard et al., 1999; Granato et al., 1994; Jantsch-Plunger and Fire, 1994; Okkema and Fire, 1994). The notable exception is the muscle gene, hlh-1, which initiates expression at early 4E but affects muscle differentiation more than fate in loss of function studies (Chen et al., 1992). This observation may reflect that hlh-1, like it’s vertebrate ortholog MyoD, has the additional capacity to behave like a specification gene, and transform other cell types to muscle when mis-expressed (Fukushige and Krause, 2005; Rudnicki et al., 1993). These data support the notion that the C. elegans embryo is organized temporally to specify cell fates and promote differentiation during different phases of development.
Specification by PHA-4 involves both activator and repressor functions
Our findings suggest that PHA-4 functions both positively and negatively to restrict the developmental choices of embryonic cells during specification of pharyngeal fate. Previous work revealed that PHA-4, and its orthologs in mammals and Drosophila, activate foregut gene transcription directly (Gaudet and Mango, 2002; Liu et al., 2002; Mach et al., 1996). Targets of PHA-4 or FoxA activation are likely to contribute to cell fate choices. For example, pha-4 activates the T-box factor tbx-2, which is required to generate pharyngeal muscles from the embryonic blastomere, ABa (Smith and Mango, 2006). In vertebrates, FoxA2 directly activates the signaling molecule sonic hedgehog, which is important to specify the floor plate (Epstein et al., 1999). Thus, transcriptional activation by FoxA proteins is critical for the cell fate specification activities.
The data presented here suggest PHA-4 also functions as a transcriptional repressor to block an alternative ectodermal fate. Loss of pha-4 activity leads to expression of the ectodermal genes, lin-26 (Horner et al., 1998) and elt-3 (this work) within cells that would normally acquire a pharyngeal fate. Conversely, ectopic expression of pha-4 throughout the embryo silences expression of the ectodermal regulator lin-26 (Horner et al., 1998). The lin-26 CISg regulatory element is peppered with consensus, conserved PHA-4 sites (Landmann et al., 2004). Here, we show that PHA-4 associates with the CISg regulatory element within pharyngeal cells, suggesting direct repression by PHA-4. We note that mutation of the CISg pha-4 consensus sites in a lin-26::gfp transgene bearing the CISg-i elements is not sufficient to eliminate repression of transgene expression in pharyngeal cells (data not shown). PHA-4 may also bind additional consensus sites that are present in this region (Landmann et al., 2004).
The notion that FoxA proteins may be repressors has been suggested by Davidson and colleagues. In sea urchin embryos, foxa prevents foxb and brachyury (bra) from being expressed in the anterior archenteron. In embryos where foxa expression is blocked with morpholinos, expression of a bra cis-regulatory construct spreads to the anterior archenteron (Davidson et al., 2002). However, it has been unclear whether foxa represses bra directly or whether foxa activates a repressor of bra. Our work supports the idea that FoxA factors can function as transcriptional repressors.
There is evidence that like pha-4, other selector genes function as both activators and repressors. pax-6, a master regulatory gene for the eye, activates numerous genes that are essential for eye development, and also directly represses β 1-crystallin and δ1-crystallin (Duncan et al., 1998; Muta et al., 2002; Ogino and Yasuda, 2000). Also, MyoD, a selector gene for skeletal muscle, both activates and represses muscle-specific genes (Tapscott, 2005). Prior to muscle differentiation, MyoD recruits HDAC to repress myogenin expression. During differentiation, HDAC is replaced with the acetyltransferase P/CAF, and MyoD activates myogenin transcription (Mal and Harter, 2003). Most strikingly, there are examples where these transcription factors function as activators and repressors at the same time, in the same cells. The NFKB-related protein Dorsal, activates short gastrulation (sog) and represses zerknullt (zen) in the same set of cells to pattern the presumptive Drosophila neuroectoderm (Stathopoulos and Levine, 2002). Similarly, the selector gene GATA-1 controls hematopoietic development using both activator and repressor functions (Crispino, 2005). Our data suggest that like Dorsal and GATA-1, PHA-4 simultaneously functions as an activator and repressor in the same cells. Analysis of pha-4(ts) animals suggests pha-4 represses ectodermal gene expression around the 2-4E stage. PHA-4 also functions as an activator at this time, for example, to activate tbx-2 within a subset of pharyngeal cells (Smith and Mango, 2006). This bi-modal strategy may ensure that cells acquire the appropriate cell fate and that they do not express intermediate fates.
pha-4 interacts with tam-1 to repress the ectodermal fate
The means by which FoxA transcription factors function to regulate gene transcription is not well understood in any organism. In vitro studies from Zaret and colleagues have suggested that FoxA proteins bind compacted chromatin and modify the chromatin environment to facilitate additional transcription factor – DNA interactions (Cirillo et al., 2002; Shim et al., 1998). However, it is currently unclear whether FoxA performs these tasks alone or in combination with co-factors.
Our data show that pha-4 works in combination with tam-1 to inhibit the ectodermal cell fate. Of twelve candidate interactors, tam-1 was the only gene to dramatically enhance the lethality of weak pha-4 configurations and lead to pronounced increases in ectopic ectodermal cells (this work). Moreover, TAM-1 physically interacts with PHA-4 in a yeast two hybrid screen (Li et al., 2004). Unfortunately, TAM-1 antibodies are no longer available, and translational TAM-1::GFP reporter constructs fail consistently (J. Hsieh, A. Fire, personal communication, our unpublished observations), which precluded an analysis of possible PHA-4 - TAM-1 biochemical interactions. Although we could not test for physical interactions, the genetic interactions we observed are consistent with PHA-4 interacting with TAM-1 in some capacity to repress ectodermal genes such as lin-26.
How might TAM-1 and PHA-4 function? tam-1 was originally identified in a screen for genes that, when mutated, lead to silencing of extrachromosomal arrays bearing repetitive elements (Hsieh et al., 1999). This phenotype could reflect an activator function for TAM-1. Alternatively, TAM-1 could be involved in repression, and loss of tam-1 activity could lead to relocalization of repressors from the endogenous location to ectopic sites such as repetitive extrachromosomal arrays. Similar models have been proposed for the mes-4 methyltransferase (Garvin et al., 1998). This model implicates tam-1 in targeting of repressors to the appropriate location, rather than providing actual repression activity per se.
As a TRIM protein, TAM-1 may mediate transcriptional repression. Many TRIM/RING finger proteins downregulate gene transcription, possibly as ubiquitin ligases (Meroni and Diez-Roux, 2005). The TRIM protein TIF1α and the RING finger protein PML repress transcription by binding to the Kruppel associated box protein (KRAB1) and to HDAC1, respectively (Peng et al., 2002; Wu et al., 2001). In C. elegans, ncl-1 encodes a TRIM-like protein that is required to repress ribosomal RNA synthesis (Frank and Roth, 1998). An appealing model to be tested in future studies is that PHA-4 recruits TAM-1 to ectodermal promoters in pharyngeal cells. An alternative possibility is that tam-1 functions in parallel with pha-4 to repress ectodermal genes.
Surprisingly, tam-1 mutants are viable with normal pharynges, whereas pha-4 mutants arrest as embryos or larvae that lack a pharynx. Two alternative explanations could account for the subtlety of tam-1 mutant phenotypes. First, there are many TRIM proteins, and perhaps these are redundant for pharynx development. Second, perhaps the activator function of PHA-4 and the activities of the targets activated by PHA-4 are sufficient for pharynx development under normal circumstances. Only when PHA-4 is compromised is the dependency for tam-1 activity revealed.
We found that two NuRD components tested for a role in pharyngeal specification, Mi-2/chd-3 and MTA/egl-27, phenocopied tam-1 and enhanced phenotypes associated with this early role for pha-4. In addition, NuRD repressor complex components MTA/egl-27, and MTA/egr-1 dramatically enhanced lethality of weak pha-4 configurations (predicted NuRD components RbAp48/rba-1 and the HDAC hda-1 are embryonic lethal and thus were not analyzed in our assays). Therefore, we postulate that TAM-1 may recruit the NuRD complex to regulate pha-4 targets. Intriguingly, other proteins bearing a TRIM motif or just the RING finger domain mediate transcriptional repression via binding to the NuRD subunit Mi-2. The transcriptional repressor activity of KAP1 in vitro depends on Mi-2α/CHD3 binding to its TRIM domain (Schultz et al., 2001). The RING Finger Protein (RFP) also mediates transcriptional repression by directly associating with Mi-2α (Shimono et al., 2003). These observations suggest that TRIM/RING finger proteins may exert their effects on transcriptional repression via association with the NuRD complex.
We detected interactions between pha-4 and members of the synMuv pathway. One phenotype associated with synMuv B genes is augmentation of RNAi. However, the effects we detected did not track with the strength of RNAi. For example, tam-1 does not enhance RNAi (Lehner et al., 2006; Wang et al., 2005) yet it had a pronounced effect on pha-4. Conversely, lin-35 and dpl-1 had dramatic effects on RNAi (Lehner et al., 2006; Wang et al., 2005) but no synergy with pha-4. Thus, the effects we observed did not reflect indirect effects of RNAi for many of the genes. For two genes we cannot rule out RNAi effects: mutations in lin-9/Tudor and lin-13/C2H2 are reported to augment RNAi (Lehner et al., 2006; Wang et al., 2005) and also enhanced pha-4(RNAi). This interaction may reflect an indirect influence of synMuv genes on RNAi efficiency. Finally, ubc-9, an E2 ubiquitin ligase that mediates covalent attachment of small ubiquitin-related modifier (SUMO) (Boulton et al., 2002) also enhances pha-4 in our assays. There are two possibilities to explain enhancement by the sumoylation pathway. First, the TRIM motif found in TAM-1 is characteristic of E3 ubiquitin ligases, suggesting that TAM-1 and UBC-9 may be part of the same pathway. Alternatively, SUMO interacts with the pha-4 target tbx-2, and a strong SUMO loss of function has a pharynx phenotype (Roy Chowdhuri et al., 2006). It remains to be determined whether pha-4 utilizes sumoylation to regulate target genes.
Recent reports have shown that inactivation of many synMuv genes, including some NuRD components, leads to ectopic lag-2 expression (Poulin et al., 2005). The lag-2 locus encodes a ligand for Notch signaling, suggesting the phenotypes associated with synMuv inactivation reflect excess Notch signaling. In Drosophila, Notch signaling promotes ectodermal epithelial fates, which could theoretically explain the excess ectoderm in pha-4;tam-1 double mutants. However, this explanation cannot account for the interactions we detect between pha-4 and synMuv genes. For example, both mep-1 and lin-35 lead to ectopic lag-2 expression while egr-1 does not (Poulin et al., 2005). Yet egr-1, but not mep-1 or lin-35, enhances pha-4 loss-of-function mutations. We also note that Notch signaling in the embryo induces pharyngeal development and has not been shown to promote ectodermal cell fates (Schnabel and Priess, 1997). Thus, in C. elegans, there is no reason a priori to suspect excess Notch signaling would lead to loss of the pharyngeal fate. Rather, an intriguing model is that NuRD and tam-1 co-operate with pha-4 to repress ectodermal genes and thereby promote specification to pharyngeal fate.
Supplementary Material
Representative examples of pharyngeal phenotypes observed in pha-4(ts) embryos shifted from restrictive to permissive temperature at (A) 2E (B) 4E (C) 8E (D) 16E stages and allowed to develop to terminal stages. 2E-shifted embryo has Wild-type pharynx, 4E-shifted Pun embryo has long pharynx with anterior and posterior bulbs, 8E-shifted Pun embryo has small pharynx with no distinct morphological characteristics, 16E-shifted embryo has no pharynx. Arrows indicate buccal cavity; arrowheads outline edges of Pun and Misshapen pharynges; bracket and parenthesis indicate anterior bulb, and posterior bulb, respectively.
Acknowledgments
We thank Jim McGhee, Andy Fire for gifts of strains, Andy Fire and Pete Okkema for communicating unpublished information, and Colin Thacker and Bryan Wardell for technical help. J.K. was funded by NIH NRSA F32GM65728. P.S. was funded by NIH T32CA93247. S.E.M. was funded by NIH R01 GM056264 and received institutional support from the Huntsman Cancer Institute and Department of Oncological Sciences. Oligo synthesis and DNA sequencing was supported by CCSG 2P30CA42014. Some of the strains were provided by the Caenorhabditis Genetics Center, which is supported by the NIH National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agresti A. Categorical Data Analysis. Wiley-Interscience; 2002. [Google Scholar]
- Ahringer J. NuRD and SIN3 histone deacetylase complexes in development. Trends Genet. 2000;16:351–6. doi: 10.1016/s0168-9525(00)02066-7. [DOI] [PubMed] [Google Scholar]
- Azzaria M, Goszczynski B, Chung MA, Kalb JM, McGhee JD. A fork head/HNF-3 homolog expressed in the pharynx and intestine of the Caenorhabditis elegans embryo. Dev Biol. 1996;178:289–303. doi: 10.1006/dbio.1996.0219. [DOI] [PubMed] [Google Scholar]
- Baugh LR, Hill AA, Slonim DK, Brown EL, Hunter CP. Composition and dynamics of the Caenorhabditis elegans early embryonic transcriptome. Development. 2003;130:889–900. doi: 10.1242/dev.00302. [DOI] [PubMed] [Google Scholar]
- Boulton SJ, Gartner A, Reboul J, Vaglio P, Dyson N, Hill DE, Vidal M. Combined functional genomic maps of the C. elegans DNA damage response. Science. 2002;295:127–31. doi: 10.1126/science.1065986. [DOI] [PubMed] [Google Scholar]
- Bowen NJ, Fujita N, Kajita M, Wade PA. Mi-2/NuRD: multiple complexes for many purposes. Biochim Biophys Acta. 2004;1677:52–7. doi: 10.1016/j.bbaexp.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmi I, Kopczynski JB, Meyer BJ. The nuclear hormone receptor SEX-1 is an X-chromosome signal that determines nematode sex. Nature. 1998;396:168–73. doi: 10.1038/24164. [DOI] [PubMed] [Google Scholar]
- Chen L, Krause M, Draper B, Weintraub H, Fire A. Body-wall muscle formation in Caenorhabditis elegans embryos that lack the MyoD homolog hlh-1. Science. 1992;256:240–3. doi: 10.1126/science.1314423. [DOI] [PubMed] [Google Scholar]
- Cirillo LA, Lin FR, Cuesta I, Friedman D, Jarnik M, Zaret KS. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol Cell. 2002;9:279–89. doi: 10.1016/s1097-2765(02)00459-8. [DOI] [PubMed] [Google Scholar]
- Crispino JD. GATA1 in normal and malignant hematopoiesis. Semin Cell Dev Biol. 2005;16:137–47. doi: 10.1016/j.semcdb.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Cui M, Chen J, Myers TR, Hwang BJ, Sternberg PW, Greenwald I, Han M. SynMuv genes redundantly inhibit lin-3/EGF expression to prevent inappropriate vulval induction in C. elegans. Dev Cell. 2006;10:667–72. doi: 10.1016/j.devcel.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Davidson EH, Rast JP, Oliveri P, Ransick A, Calestani C, Yuh CH, Minokawa T, Amore G, Hinman V, Arenas-Mena C, Otim O, Brown CT, Livi CB, Lee PY, Revilla R, Rust AG, Pan Z, Schilstra MJ, Clarke PJ, Arnone MI, Rowen L, Cameron RA, McClay DR, Hood L, Bolouri H. A genomic regulatory network for development. Science. 2002;295:1669–78. doi: 10.1126/science.1069883. [DOI] [PubMed] [Google Scholar]
- Dufourcq P, Chanal P, Vicaire S, Camut E, Quintin S, den Boer BG, Bosher JM, Labouesse M. lir-2, lir-1 and lin-26 encode a new class of zinc-finger proteins and are organized in two overlapping operons both in Caenorhabditis elegans and in Caenorhabditis briggsae. Genetics. 1999;152:221–35. doi: 10.1093/genetics/152.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MK, Haynes JI, 2nd, Cvekl A, Piatigorsky J. Dual roles for Pax-6: a transcriptional repressor of lens fiber cell-specific beta-crystallin genes. Mol Cell Biol. 1998;18:5579–86. doi: 10.1128/mcb.18.9.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan CR, Chung MA, Allen FL, Heschl MF, Van Buskirk CL, McGhee JD. A gut-to-pharynx/tail switch in embryonic expression of the Caenorhabditis elegans ges-1 gene centers on two GATA sequences. Dev Biol. 1995;170:397–419. doi: 10.1006/dbio.1995.1225. [DOI] [PubMed] [Google Scholar]
- Epstein DJ, McMahon AP, Joyner AL. Regionalization of Sonic hedgehog transcription along the anteroposterior axis of the mouse central nervous system is regulated by Hnf3-dependent and -independent mechanisms. Development. 1999;126:281–92. doi: 10.1242/dev.126.2.281. [DOI] [PubMed] [Google Scholar]
- Fay DS, Han M. The synthetic multivulval genes of C. elegans: functional redundancy, Ras-antagonism, and cell fate determination. Genesis. 2000;26:279–84. doi: 10.1002/(sici)1526-968x(200004)26:4<279::aid-gene100>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Ferreira R, Ohneda K, Yamamoto M, Philipsen S. GATA1 function, a paradigm for transcription factors in hematopoiesis. Mol Cell Biol. 2005;25:1215–27. doi: 10.1128/MCB.25.4.1215-1227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–11. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Francis R, Waterston RH. Muscle cell attachment in Caenorhabditis elegans. J Cell Biol. 1991;114:465–79. doi: 10.1083/jcb.114.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DJ, Roth MB. ncl-1 is required for the regulation of cell size and ribosomal RNA synthesis in Caenorhabditis elegans. J Cell Biol. 1998;140:1321–9. doi: 10.1083/jcb.140.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature. 2000;408:325–30. doi: 10.1038/35042517. [DOI] [PubMed] [Google Scholar]
- Fukushige T, Krause M. The myogenic potency of HLH-1 reveals widespread developmental plasticity in early C. elegans embryos. Development. 2005;132:1795–805. doi: 10.1242/dev.01774. [DOI] [PubMed] [Google Scholar]
- Garvin C, Holdeman R, Strome S. The phenotype of mes-2, mes-3, mes-4 and mes-6, maternal-effect genes required for survival of the germline in Caenorhabditis elegans, is sensitive to chromosome dosage. Genetics. 1998;148:167–85. doi: 10.1093/genetics/148.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet J, Mango SE. Regulation of organogenesis by the Caenorhabditis elegans FoxA protein PHA-4. Science. 2002;295:821–5. doi: 10.1126/science.1065175. [DOI] [PubMed] [Google Scholar]
- Gilleard JS, McGhee JD. Activation of hypodermal differentiation in the Caenorhabditis elegans embryo by GATA transcription factors ELT-1 and ELT-3. Mol Cell Biol. 2001;21:2533–44. doi: 10.1128/MCB.21.7.2533-2544.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleard JS, Shafi Y, Barry JD, McGhee JD. ELT-3: A Caenorhabditis elegans GATA factor expressed in the embryonic epidermis during morphogenesis. Dev Biol. 1999;208:265–80. doi: 10.1006/dbio.1999.9202. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Serricchio AS, Sternberg PW. Visualization of C. elegans transgenic arrays by green fluorescent protein (GFP) BMC Genet. 2006;7:36. doi: 10.1186/1471-2156-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato M, Schnabel H, Schnabel R. Genesis of an organ: molecular analysis of the pha-1 gene. Development. 1994;120:3005–17. doi: 10.1242/dev.120.10.3005. [DOI] [PubMed] [Google Scholar]
- Hobert O. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques. 2002;32:728–30. doi: 10.2144/02324bm01. [DOI] [PubMed] [Google Scholar]
- Horner MA, Quintin S, Domeier ME, Kimble J, Labouesse M, Mango SE. pha-4, an HNF-3 homolog, specifies pharyngeal organ identity in Caenorhabditis elegans. Genes Dev. 1998;12:1947–52. doi: 10.1101/gad.12.13.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J, Liu J, Kostas SA, Chang C, Sternberg PW, Fire A. The RING finger/B-box factor TAM-1 and a retinoblastoma-like protein LIN-35 modulate context-dependent gene silencing in Caenorhabditis elegans. Genes Dev. 1999;13:2958–70. doi: 10.1101/gad.13.22.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantsch-Plunger V, Fire A. Combinatorial structure of a body muscle-specific transcriptional enhancer in Caenorhabditis elegans. J Biol Chem. 1994;269:27021–8. [PubMed] [Google Scholar]
- Kalb JM, Lau KK, Goszczynski B, Fukushige T, Moons D, Okkema PG, McGhee JD. pha-4 is Ce-fkh-1, a fork head/HNF-3alpha,beta,gamma homolog that functions in organogenesis of the C. elegans pharynx. Development. 1998;125:2171–80. doi: 10.1242/dev.125.12.2171. [DOI] [PubMed] [Google Scholar]
- Kaltenbach L, Horner MA, Rothman JH, Mango SE. The TBP-like factor CeTLF is required to activate RNA polymerase II transcription during C. elegans embryogenesis. Mol Cell. 2000;6:705–13. doi: 10.1016/s1097-2765(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Kaltenbach LS, Updike DL, Mango SE. Contribution of the amino and carboxyl termini for PHA-4/FoxA function in Caenorhabditis elegans. Dev Dyn. 2005 doi: 10.1002/dvdy.20550. [DOI] [PubMed] [Google Scholar]
- Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, Welchman DP, Zipperlen P, Ahringer J. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–7. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- Kamath RS, Martinez-Campos M, Zipperlen P, Fraser AG, Ahringer J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2001;2:RESEARCH0002. doi: 10.1186/gb-2000-2-1-research0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labouesse M, Hartwieg E, Horvitz HR. The Caenorhabditis elegans LIN-26 protein is required to specify and/or maintain all non-neuronal ectodermal cell fates. Development. 1996;122:2579–88. doi: 10.1242/dev.122.9.2579. [DOI] [PubMed] [Google Scholar]
- Labouesse M, Mango SE. Patterning the C. elegans embryo: moving beyond the cell lineage. Trends Genet. 1999;15:307–13. doi: 10.1016/s0168-9525(99)01750-3. [DOI] [PubMed] [Google Scholar]
- Landmann F, Quintin S, Labouesse M. Multiple regulatory elements with spatially and temporally distinct activities control the expression of the epithelial differentiation gene lin-26 in C. elegans. Dev Biol. 2004;265:478–90. doi: 10.1016/j.ydbio.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Lehner B, Calixto A, Crombie C, Tischler J, Fortunato A, Chalfie M, Fraser AG. Loss of LIN-35, the Caenorhabditis elegans ortholog of the tumor suppressor p105Rb, results in enhanced RNA interference. Genome Biol. 2006;7:R4. doi: 10.1186/gb-2006-7-1-r4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Armstrong CM, Bertin N, Ge H, Milstein S, Boxem M, Vidalain PO, Han JD, Chesneau A, Hao T, Goldberg DS, Li N, Martinez M, Rual JF, Lamesch P, Xu L, Tewari M, Wong SL, Zhang LV, Berriz GF, Jacotot L, Vaglio P, Reboul J, Hirozane-Kishikawa T, Li Q, Gabel HW, Elewa A, Baumgartner B, Rose DJ, Yu H, Bosak S, Sequerra R, Fraser A, Mango SE, Saxton WM, Strome S, Van Den Heuvel S, Piano F, Vandenhaute J, Sardet C, Gerstein M, Doucette-Stamm L, Gunsalus KC, Harper JW, Cusick ME, Roth FP, Hill DE, Vidal M. A map of the interactome network of the metazoan C. elegans. Science. 2004;303:540–3. doi: 10.1126/science.1091403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Shen W, Brubaker PL, Kaestner KH, Drucker DJ. Foxa3 (HNF-3gamma) binds to and activates the rat proglucagon gene promoter but is not essential for proglucagon gene expression. Biochem J. 2002;366:633–41. doi: 10.1042/BJ20020095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Horvitz HR. lin-35 and lin-53, two genes that antagonize a C. elegans Ras pathway, encode proteins similar to Rb and its binding protein RbAp48. Cell. 1998;95:981–91. doi: 10.1016/s0092-8674(00)81722-5. [DOI] [PubMed] [Google Scholar]
- Mach V, Ohno K, Kokubo H, Suzuki Y. The Drosophila fork head factor directly controls larval salivary gland-specific expression of the glue protein gene Sgs3. Nucleic Acids Res. 1996;24:2387–94. doi: 10.1093/nar/24.12.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maduro MF, Meneghini MD, Bowerman B, Broitman-Maduro G, Rothman JH. Restriction of mesendoderm to a single blastomere by the combined action of SKN-1 and a GSK-3beta homolog is mediated by MED-1 and -2 in C. elegans. Mol Cell. 2001;7:475–85. doi: 10.1016/s1097-2765(01)00195-2. [DOI] [PubMed] [Google Scholar]
- Mal A, Harter ML. MyoD is functionally linked to the silencing of a muscle-specific regulatory gene prior to skeletal myogenesis. Proc Natl Acad Sci U S A. 2003;100:1735–9. doi: 10.1073/pnas.0437843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mango SE, Lambie EJ, Kimble J. The pha-4 gene is required to generate the pharyngeal primordium of Caenorhabditis elegans. Development. 1994a;120:3019–31. doi: 10.1242/dev.120.10.3019. [DOI] [PubMed] [Google Scholar]
- Mango SE, Thorpe CJ, Martin PR, Chamberlain SH, Bowerman B. Two maternal genes, apx-1 and pie-1, are required to distinguish the fates of equivalent blastomeres in the early Caenorhabditis elegans embryo. Development. 1994b;120:2305–15. doi: 10.1242/dev.120.8.2305. [DOI] [PubMed] [Google Scholar]
- Mann RS, Carroll SB. Molecular mechanisms of selector gene function and evolution. Curr Opin Genet Dev. 2002;12:592–600. doi: 10.1016/s0959-437x(02)00344-1. [DOI] [PubMed] [Google Scholar]
- Meroni G, Diez-Roux G. TRIM/RBCC, a novel class of ‘single protein RING finger’ E3 ubiquitin ligases. Bioessays. 2005;27:1147–57. doi: 10.1002/bies.20304. [DOI] [PubMed] [Google Scholar]
- Miller DM, 3rd, Ortiz I, Berliner GC, Epstein HF. Differential localization of two myosins within nematode thick filaments. Cell. 1983;34:477–90. doi: 10.1016/0092-8674(83)90381-1. [DOI] [PubMed] [Google Scholar]
- Muta M, Kamachi Y, Yoshimoto A, Higashi Y, Kondoh H. Distinct roles of SOX2, Pax6 and Maf transcription factors in the regulation of lens-specific delta1-crystallin enhancer. Genes Cells. 2002;7:791–805. doi: 10.1046/j.1365-2443.2002.00560.x. [DOI] [PubMed] [Google Scholar]
- Ogino H, Yasuda K. Sequential activation of transcription factors in lens induction. Dev Growth Differ. 2000;42:437–48. doi: 10.1046/j.1440-169x.2000.00532.x. [DOI] [PubMed] [Google Scholar]
- Okkema PG, Fire A. The Caenorhabditis elegans NK-2 class homeoprotein CEH-22 is involved in combinatorial activation of gene expression in pharyngeal muscle. Development. 1994;120:2175–86. doi: 10.1242/dev.120.8.2175. [DOI] [PubMed] [Google Scholar]
- Peng H, Feldman I, Rauscher FJ., 3rd Hetero-oligomerization among the TIF family of RBCC/TRIM domain-containing nuclear cofactors: a potential mechanism for regulating the switch between coactivation and corepression. J Mol Biol. 2002;320:629–44. doi: 10.1016/S0022-2836(02)00477-1. [DOI] [PubMed] [Google Scholar]
- Portereiko MF, Mango SE. Early morphogenesis of the Caenorhabditis elegans pharynx. Dev Biol. 2001;233:482–94. doi: 10.1006/dbio.2001.0235. [DOI] [PubMed] [Google Scholar]
- Poulin G, Dong Y, Fraser AG, Hopper NA, Ahringer J. Chromatin regulation and sumoylation in the inhibition of Ras-induced vulval development in Caenorhabditis elegans. Embo J. 2005;24:2613–23. doi: 10.1038/sj.emboj.7600726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priess JR, Thomson JN. Cellular interactions in early C. elegans embryos. Cell. 1987;48:241–50. doi: 10.1016/0092-8674(87)90427-2. [DOI] [PubMed] [Google Scholar]
- Pruss RM, Mirsky R, Raff MC, Thorpe R, Dowding AJ, Anderton BH. All classes of intermediate filaments share a common antigenic determinant defined by a monoclonal antibody. Cell. 1981;27:419–28. doi: 10.1016/0092-8674(81)90383-4. [DOI] [PubMed] [Google Scholar]
- Reddien PW, Bermange AL, Murfitt KJ, Jennings JR, Sanchez Alvarado A. Identification of genes needed for regeneration, stem cell function, and tissue homeostasis by systematic gene perturbation in planaria. Dev Cell. 2005;8:635–49. doi: 10.1016/j.devcel.2005.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy Chowdhuri S, Crum T, Woollard A, Aslam S, Okkema PG. The T-box factor TBX-2 and the SUMO conjugating enzyme UBC-9 are required for ABa-derived pharyngeal muscle in C. elegans. Dev Biol. 2006;295:664–77. doi: 10.1016/j.ydbio.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Rual JF, Ceron J, Koreth J, Hao T, Nicot AS, Hirozane-Kishikawa T, Vandenhaute J, Orkin SH, Hill DE, van den Heuvel S, Vidal M. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 2004;14:2162–8. doi: 10.1101/gr.2505604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki MA, Schnegelsberg PN, Stead RH, Braun T, Arnold HH, Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–9. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- Sarafi-Reinach TR, Sengupta P. The forkhead domain gene unc-130 generates chemosensory neuron diversity in C. elegans. Genes Dev. 2000;14:2472–85. doi: 10.1101/gad.832300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel R, Priess JR. C. elegans II. Cold Spring Harbor Laboratory press; 1997. Specification of cell fates in the early embryo; pp. 361–383. [PubMed] [Google Scholar]
- Schultz DC, Friedman JR, Rauscher FJ., 3rd Targeting histone deacetylase complexes via KRAB-zinc finger proteins: the PHD and bromodomains of KAP-1 form a cooperative unit that recruits a novel isoform of the Mi-2alpha subunit of NuRD. Genes Dev. 2001;15:428–43. doi: 10.1101/gad.869501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim EY, Woodcock C, Zaret KS. Nucleosome positioning by the winged helix transcription factor HNF3. Genes Dev. 1998;12:5–10. doi: 10.1101/gad.12.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimono Y, Murakami H, Kawai K, Wade PA, Shimokata K, Takahashi M. Mi-2 beta associates with BRG1 and RET finger protein at the distinct regions with transcriptional activating and repressing abilities. J Biol Chem. 2003;278:51638–45. doi: 10.1074/jbc.M309198200. [DOI] [PubMed] [Google Scholar]
- Simmer F, Moorman C, van der Linden AM, Kuijk E, van den Berghe PV, Kamath RS, Fraser AG, Ahringer J, Plasterk RH. Genome-wide RNAi of C. elegans using the hypersensitive rrf-3 strain reveals novel gene functions. PLoS Biol. 2003;1:E12. doi: 10.1371/journal.pbio.0000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PA, Mango SE. Role of T-box gene tbx-2 for anterior foregut muscle development in C. elegans. Dev Biol. 2006 doi: 10.1016/j.ydbio.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solari F, Ahringer J. NURD-complex genes antagonise Ras-induced vulval development in Caenorhabditis elegans. Curr Biol. 2000;10:223–6. doi: 10.1016/s0960-9822(00)00343-2. [DOI] [PubMed] [Google Scholar]
- Spieth J, Shim YH, Lea K, Conrad R, Blumenthal T. elt-1, an embryonically expressed Caenorhabditis elegans gene homologous to the GATA transcription factor family. Mol Cell Biol. 1991;11:4651–9. doi: 10.1128/mcb.11.9.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos A, Levine M. Dorsal gradient networks in the Drosophila embryo. Dev Biol. 2002;246:57–67. doi: 10.1006/dbio.2002.0652. [DOI] [PubMed] [Google Scholar]
- Straight AF, Belmont AS, Robinett CC, Murray AW. GFP tagging of budding yeast chromosomes reveals that protein-protein interactions can mediate sister chromatid cohesion. Curr Biol. 1996;6:1599–608. doi: 10.1016/s0960-9822(02)70783-5. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Schierrenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Tapscott SJ. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development. 2005;132:2685–95. doi: 10.1242/dev.01874. [DOI] [PubMed] [Google Scholar]
- Tiedemann H, Asashima M, Grunz H, Knochel W. Pluripotent cells (stem cells) and their determination and differentiation in early vertebrate embryogenesis. Dev Growth Differ. 2001;43:469–502. doi: 10.1046/j.1440-169x.2001.00599.x. [DOI] [PubMed] [Google Scholar]
- Unhavaithaya Y, Shin TH, Miliaras N, Lee J, Oyama T, Mello CC. MEP-1 and a homolog of the NURD complex component Mi-2 act together to maintain germline-soma distinctions in C. elegans. Cell. 2002;111:991–1002. doi: 10.1016/s0092-8674(02)01202-3. [DOI] [PubMed] [Google Scholar]
- Updike DL, Mango SE. Temporal Regulation of Foregut Development by HTZ-1/H2A.Z and PHA-4/FoxA. PLoS Genet. 2006;2 doi: 10.1371/journal.pgen.0020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zelewsky T, Palladino F, Brunschwig K, Tobler H, Hajnal A, Muller F. The C. elegans Mi-2 chromatin-remodelling proteins function in vulval cell fate determination. Development. 2000;127:5277–84. doi: 10.1242/dev.127.24.5277. [DOI] [PubMed] [Google Scholar]
- Wang D, Kennedy S, Conte D, Jr, Kim JK, Gabel HW, Kamath RS, Mello CC, Ruvkun G. Somatic misexpression of germline P granules and enhanced RNA interference in retinoblastoma pathway mutants. Nature. 2005;436:593–7. doi: 10.1038/nature04010. [DOI] [PubMed] [Google Scholar]
- Wu WS, Vallian S, Seto E, Yang WM, Edmondson D, Roth S, Chang KS. The growth suppressor PML represses transcription by functionally and physically interacting with histone deacetylases. Mol Cell Biol. 2001;21:2259–68. doi: 10.1128/MCB.21.7.2259-2268.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret K. Developmental competence of the gut endoderm: genetic potentiation by GATA and HNF3/fork head proteins. Dev Biol. 1999;209:1–10. doi: 10.1006/dbio.1999.9228. [DOI] [PubMed] [Google Scholar]
- Zhu J, Fukushige T, McGhee JD, Rothman JH. Reprogramming of early embryonic blastomeres into endodermal progenitors by a Caenorhabditis elegans GATA factor. Genes Dev. 1998;12:3809–14. doi: 10.1101/gad.12.24.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Hill RJ, Heid PJ, Fukuyama M, Sugimoto A, Priess JR, Rothman JH. end-1 encodes an apparent GATA factor that specifies the endoderm precursor in Caenorhabditis elegans embryos. Genes Dev. 1997;11:2883–96. doi: 10.1101/gad.11.21.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative examples of pharyngeal phenotypes observed in pha-4(ts) embryos shifted from restrictive to permissive temperature at (A) 2E (B) 4E (C) 8E (D) 16E stages and allowed to develop to terminal stages. 2E-shifted embryo has Wild-type pharynx, 4E-shifted Pun embryo has long pharynx with anterior and posterior bulbs, 8E-shifted Pun embryo has small pharynx with no distinct morphological characteristics, 16E-shifted embryo has no pharynx. Arrows indicate buccal cavity; arrowheads outline edges of Pun and Misshapen pharynges; bracket and parenthesis indicate anterior bulb, and posterior bulb, respectively.


