Abstract
Vagus nerve stimulation potentiates hippocampal LTP in freely moving rats. --Previous studies have demonstrated that electrical stimulation of the vagus nerve (VNS) delivered at a moderate intensity following a learning experience enhances memory in laboratory rats and human subjects, while VNS at lower or higher intensities has little or no effect. This finding suggests that VNS may affect memory processes by modulating neural plasticity in brain structures associated with memory storage such as the hippocampus. To test this hypothesis, the present study investigated the modulatory effect of VNS on the development of long-term potentiation (LTP) in the dentate gyrus of freely-moving rats. Rats receiving 0.4 mA VNS showed enhanced potentiation of the population spike amplitude for at least 24 hr after tetanus relative to the sham-stimulation group. In contrast, no such effect was observed with 0.2 mA VNS. Stimulation at 0.8 mA had a short-term effect and tended to enhance early LTP, but to a lesser extent than did 0.4 mA. The 0.4 mA stimulation was the same intensity that was previously shown to enhance retention performance in an inhibitory avoidance task. These findings suggest that the neural mechanisms underlying the mnemonic effect of VNS may involve modulating synaptic plasticity in the hippocampus. These data also suggest that neural activity in the vagus nerve, occurring as a result of changes in peripheral state, is an important mechanism by which emotional experiences and arousal can enhance the storage of memories of those experiences.
Keywords: vagus nerve stimulation, long-term potentiation, hippocampus, arousal, rats
Introduction
Learning, memory storage, and recall are all known to be modulated by arousal. Typically, moderate levels of arousal tend to facilitate mnemonic processes while either low or very high levels of arousal result in poor retention performance [1]. The release of a variety of neurohormones such as ACTH, epinephrine, corticosterone, and opioid peptides is associated with arousal and these substances are thought to mediate arousal’s effects on memory. This is because when these neurohormones are administered either shortly before or immediately after training, they influence retention performance measured some time later [2]. As with arousal, there is an inverted U-shaped relationship between neurohormone dose and retention performance. Interestingly, many of the neurohormones that modulate memory do not freely cross the blood-brain barrier to enter the CNS and influence mnemonic processes [3]. For instance, in a pioneering study, Gold and van Buskirk [4] reported in rats that parenteral post-training injections of epinephrine, a neurohormone which enters the brain only poorly from the periphery, enhanced retention of an inhibitory avoidance task. The effects of epinephrine were dose-dependent and an inverted U-shaped function was observed such that moderate doses enhanced memory while high doses impaired retention performance. This finding has been replicated in many subsequent studies using a wide variety of drugs and behavioral measures, including appetitively as well as aversively motivated tasks in laboratory rats [5] and human subjects [6]. Two mechanisms have been proposed to address the question of how peripherally acting substances, such as epinephrine, exert their modulatory effects on memory.
One possible mechanism involves the hyperglycemic effects of epinephrine. Findings from a number of studies indicate that an epinephrine-induced increase in circulating glucose concentrations may mediate epinephrine’s effects on memory [7] and considerable evidence supports the hypothesis that glucose modulates memory by direct brain action [8–9]. On the other hand, peripherally administered L-glucose, an isomer of glucose, that does not readily enter the brain, also facilitates retention performance [10]. The reports of a mnemonic effect of L-glucose do not fit well with the view that glucose crosses the blood-brain barrier and acts on the brain directly to influence memory processes. Interestingly, Talley et al. [10] reported that the enhanced performance of rats on a four-arm alternation task seen with injections of L-glucose, but not seen with D-glucose, was attenuated by bilateral subdiaphragmatic vagotomy, suggesting that substances, such as L-glucose, may act by activating vagal afferents to influence brain processes that underlie memory storage.
Evidence from a number of studies suggests that neural messages, such as those produced by the activation of peripheral receptors by memory modulatory agents such as epinephrine, may be carried to the CNS by the afferent fibers of the vagus nerve (for a review, see [3]). In an early study, Williams & Jensen [11] reported that the dose-response curve for the peripherally-acting drug 4-OH-amphetamine was shifted to the right in vagotomized rats. In the sham-operated control animals, a posttraining injection of 2.0 mg/kg of the drug significantly enhanced retention performance on an inhibitory avoidance task and the 4.0 mg/kg dose produced impairment. This is the expected inverted U-shaped dose-response function. In contrast, in the vagotomized animals the 2.0 mg/kg dose had little effect, whereas the previously impairing 4.0 mg/kg dose produced memory enhancement [11]. In addition, reversible inactivation of the nucleus tractus solitarius (NTS), the primary relay site of vagal afferents in the brain stem, also attenuates the enhancement of memory produced by peripherally administered epinephrine [12].
Furthermore, electrical stimulation of the vagus nerve (VNS) can mimic the effects of peripheral receptor activation to modulate memory storage. Moderate-intensity VNS (0.4 mA), delivered shortly after a learning experience, enhanced retention performance of rats in an inhibitory avoidance task [13–14] and a similar level of stimulation (0.5 mA) enhanced retention in a word recognition task in human subjects [15]. In both cases, inverted U-shaped functions were seen and either higher or lower stimulation intensities were without effect.
Findings from anatomical, electrophysiological, and neurochemical studies also lend considerable support for the idea that the vagus nerve is well-suited to carry neural messages associated with changes in peripheral states to the brain [16–20]. However, the question of what processes associated with memory formation in the CNS are modulated by activation of the vagus nerve remains unanswered.
The hippocampus is thought to be one of several brain structures important in explicit memory storage processes [21]. Long-term potentiation (LTP) in the hippocampus is viewed as a useful model of the synaptic plasticity that may underlie memory formation [22–23]. LTP has two phases: a protein synthesis-independent early LTP lasting up to 4 or 6 hours and a protein synthesis-dependent late LTP that may last for days (e.g., [24]). If indeed those changes in synaptic function that mediate LTP are actually involved in mnemonic processes, then the same sort of influences that modulate memory in the behaving animal, such as afferent activity in the vagus nerve, should also modulate the development of LTP. Therefore, the aim of the present study was to determine whether VNS, at an intensity previously shown to facilitate memory, also has the capacity to facilitate the development of early and late LTP in the hippocampus.
Methods
Animals
Fifty-four male Long-Evans hooded rats weighing 350–450 g served as subjects. They were housed individually and maintained on a 12/12 h light/dark cycle (lights on at 6:00 a.m.) with food and water freely available. The protocol for the study was reviewed and approved by the Institutional Animal Care and Use Committee (Southern Illinois University Carbondale).
Vagus Nerve Stimulating Electrodes
VNS electrodes were constructed from two 7-mm sections of pure silver wire. Each was soldered to a 30-ga, Kynar-insulated, silver-plated stranded wire 9.5 cm in length. Polyvinyl chloride (PVC) tubes (O.D. = 0.76 mm, 5-mm long) were then placed on the silver wires and a 2-mm-long strip of PVC was removed from one side of each tube thereby exposing a segment of silver wire to contact the vagus nerve. The two silver wires were then inserted into a 3.5-mm section of PVC tubes (O.D. = 1.78 mm), which provided a framework to keep the electrodes stable and separated (1.5 mm). Epoxylite was used to insulate the end of each electrode pole and also the seams joining the wire to the PVC tubing. Each stimulating pole was then formed into a helix which, when implanted, encircled the nerve (for a diagram of the VNS electrode, see Fig. 1, Page 1487 in [25]).
Figure 1.
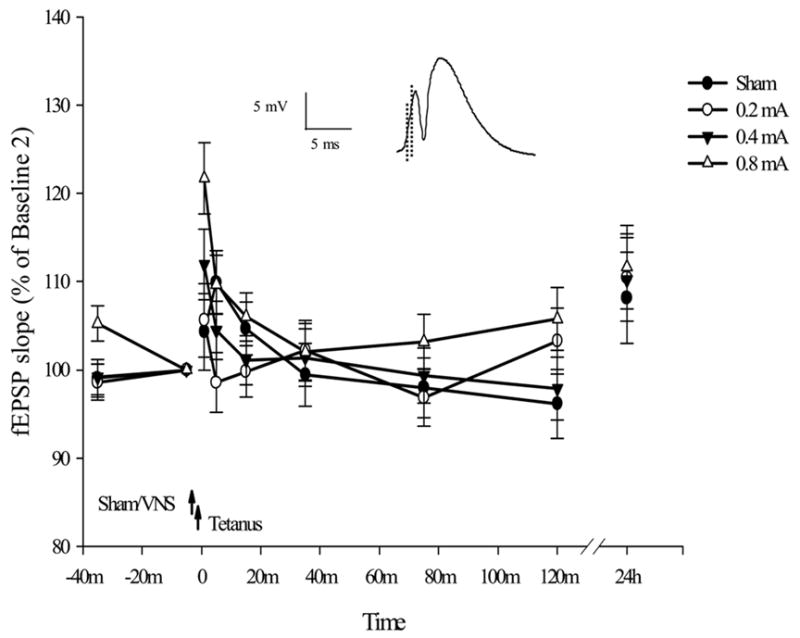
Mean percent changes (relative to Baseline 2 values) in field EPSP (fEPSP) slope in freely-moving and awake rats receiving 0.2 (n = 12), 0.4 (n = 13), or .8 (n = 13) mA VNS compared to rats receiving sham VNS stimulation (n = 11) prior to tetanization. Slope of the fEPSP (see inset) was calculated as the maximum slope over a 0.5-ms segment of the averaged evoked responses (between the two dashed lines in the left portion of the waveform). The small magnitude of the observed fEPSP slope changes after tetanization indicates that no LTP of the fEPSP was induced under the present experimental conditions. The leftmost values displayed are those at Baseline 1, followed by Baseline 2 which served as the reference values. The left and right arrows illustrate the time points for VNS delivery and LTP induction, respectively. Means ± SEM are shown.
Vagus Nerve Stimulating Electrode Implantation
Rats were anesthetized with sodium pentobarbital (i.p., 60.0 mg/kg). To access the left vagus at the cervical level, an incision approximately 2.0 cm in length was made on the left ventral side of the neck just lateral to the midline. The sternohyoid and sternomastoid muscles were separated longitudinally using small forceps and then retracted laterally until the carotid artery could be seen. The vagus nerve was then carefully separated from the surrounding connective tissue until a length of nerve sufficient for electrode placement was exposed. The nerve was then gently placed into the bend of the helix of each electrode pole. Once the nerve was within each helix, the poles were gently tightened to ensure good electrical contact with the nerve. The electrode assembly was then sutured to the sternohyoid muscle. The stranded wires from the electrode were then passed subcutaneously to the dorsal side of the neck exiting through a small incision just posterior to the skull. The incision on the ventral side of the neck was then sutured.
Electrophysiological Surgery and Electrode Implantation
Following VNS electrode implantation, the animal was placed in a stereotaxic device. Head position was adjusted to place bregma and lambda in the same horizontal plane. A midline incision was made along the top of the head, which joined the incision made to access the VNS electrode wires. The scalp was reflected and the calvaria freed of the periosteum. A burr hole was drilled on each side approximately 3 mm anterior to bregma and 2.5 mm lateral to the midline. Stainless steel jewelers’ screws affixed in these holes served as reference and ground electrodes. Two additional jewelers’ screws were affixed to the skull approximately 3 mm posterior and 3 mm lateral to lambda to anchor the head post. Two 2-mm holes were then trephined above the stimulation and recording sites in the left hemisphere. A monopolar recording electrode was then electrophysiologically guided into the granule cell layer of the dentate gyrus (AP: −3.6 mm; ML: 2.0 mm from bregma; DV: −3.2~−3.5 mm from the dura) and a bipolar stimulation electrode was implanted into the medial perforant path (AP: −7.8 mm; ML: 4.3 mm from bregma; DV: −2.3~−2.8 mm from the dura). Both electrodes were made of Teflon-coated stainless steel wire 125 μm in diameter. Biphasic test stimulations (0.1 Hz, 0.15 msec per half wave, 150 ~ 400 μA) were delivered through an electrically isolated pulse stimulator (Model 2000, A-M Systems, Carlsborg, WA) and the resultant evoked potentials were amplified (Microelectrode AC Amplifier, Model 1800, A-M Systems; band pass 1–10 kHz) and monitored with an oscilloscope. Both electrodes were moved slowly to the target positions to maximize the amplitude of the population spike. Additionally, placement of the stimulating electrode into the medial but not lateral perforant path was verified by distinct profile changes observed as both electrodes were lowered [26]. Gelfoam was used to cover the dura surface around both electrodes. Both electrodes were then fixed in position with dental acrylic cement applied to the skull. After the cement hardened, male gold-plated pins of all wires (2 for the vagus nerve stimulating electrode, 2 for the bipolar stimulating electrode, 1 for the dentate recording electrode, 1 for ground, and 1 for the reference electrode) were inserted into a miniature 9-pin connector (Ginder Scientific, Ontario, Canada), which was then secured to the skull with dental acrylic and with all wires fully covered. The incision area was then cleaned and antibiotic ointment applied. The incision was then sutured. The animals were monitored until normal locomotion was observed and then returned to the housing room.
Vagus nerve stimulation and electrophysiological recording procedures
Six days after surgery each rat was placed into an electrically shielded Plexiglas recording chamber (25 × 30 × 45 cm). Each rat was connected by a flexible cable to a 10-channel swivel that allowed the animal to move freely throughout the chamber. Animals were habituated to the recording chamber and setting for 20 min. An I/O curve was obtained by varying stimulation intensity. A stimulation intensity that evoked 40% of the maximal population spike was then established for each animal and used for all subsequent recordings. Each animal was then unplugged and returned to its home cage.
Baseline data collection, VNS delivery, LTP induction, and the subsequent recordings (up to 2 hour post tetanization) took place on the next day. Experiments always started between 10:00 and 10:30 am to control for diurnal rhythms. Each animal was again placed into the recording box and left undisturbed for 40 min before recordings began. During this habituation period, test stimulations were given to ensure that the stimulation intensity determined from the I/O curve on the previous day still evoked a response that was 40% of the maximal population spike amplitude. Slight adjustments of the stimulation intensity were sometimes necessary. Evoked responses were recorded at two baselines separated by 30 min. During each recording session, perforant path stimulation (10 trials, 0.1 Hz, 0.15 msec per half wave) was controlled by and recordings acquired with NACGather software (Theta Burst Corp., Irvine, CA). Measurements of the population spike amplitude and fEPSP slope were then extracted with NACShow software (Theta Burst Corp.) from an average of 10 evoked responses recorded during each session. After a stable baseline was established (spike amplitude did not vary more than 15% from the previous baseline recording), each animal was randomly assigned to a group receiving either 0.2, 0.4, or 0.8 mA biphasic VNS (3 trains of 20.0 Hz, 0.5 msec pulses for 10.0 sec; intertrain interval 1.0 min) or sham stimulation. VNS was administered and all recordings were collected while the animal was in an alert state. Immediately after the VNS was delivered, unsaturated LTP was induced with weak tetanic stimulation (3 bursts of 15 pulses, 200.0 Hz, 0.2 msec duration; interburst interval 10.0 sec) delivered to the perforant path, at the same intensity used for single pulse stimulations [27]. Evoked responses were then recorded immediately after tetanization and again 5, 15, 35, 75, and 120 min later. Data were stored on a laboratory computer. Following completion of recordings at 120 min after tetanization, each rat was unplugged and returned to the housing room.
Twenty-four hour after tetanization, each rat was returned to the recording chamber, habituated for 30 min, and one last recording was obtained. After the 24-hour data were collected, the impedance of VNS electrode was measured and the rat euthanized.
Electrode Impedance Testing
Cyberonic Model 250 NCP programming software was used to program a Cyberonic Model 302 pulse generator used to assess the impedance of the VNS electrodes. Six rats (2 in sham, 2 in 0.2 mA, and 2 in 0.4 mA VNS group) were a priori excluded from final data analysis because the impedance of their stimulating electrodes exceeded 5–8 k ohm.
Data Analysis
LTP was assessed by measuring the percentage difference between the fEPSP slope and population spike amplitude taken from an average of 10 evoked potentials recorded at each time point after tetanization relative to those recorded at the second baseline. The fEPSP slope was calculated as the maximum slope over a 0.5-ms period for the segment of the averaged evoked responses that fell between the onset and the apex of the initial positive component of the waveform (see Fig. 1). The population spike amplitude was calculated as the difference between the apex of the negativity and the intersection point of an upward vertical line drawn from the apex of the negativity and the line from the apex of the initial positivity and that of the second positivity (Fig. 2). A 4 (treatment groups) × 7 (times after tetanization) repeated measures analysis of variance (ANOVA) was used to test for a significant main effect of group. All ANOVA probability values were calculated based on the Greenhouse-Geisser [28] correction for sphericity of repeated measures. Non-corrected degrees of freedom are reported. Follow-up analyses were performed to determine if LTP was significantly increased in any VNS group relative to the sham group and whether there was an inverted-U shaped relationship between VNS intensity and observed LTP. Bonferroni post hoc tests were used for multiple group comparisons. All tests were two-tailed, and the level of significance was set at p < 0.05.
Figure 2.
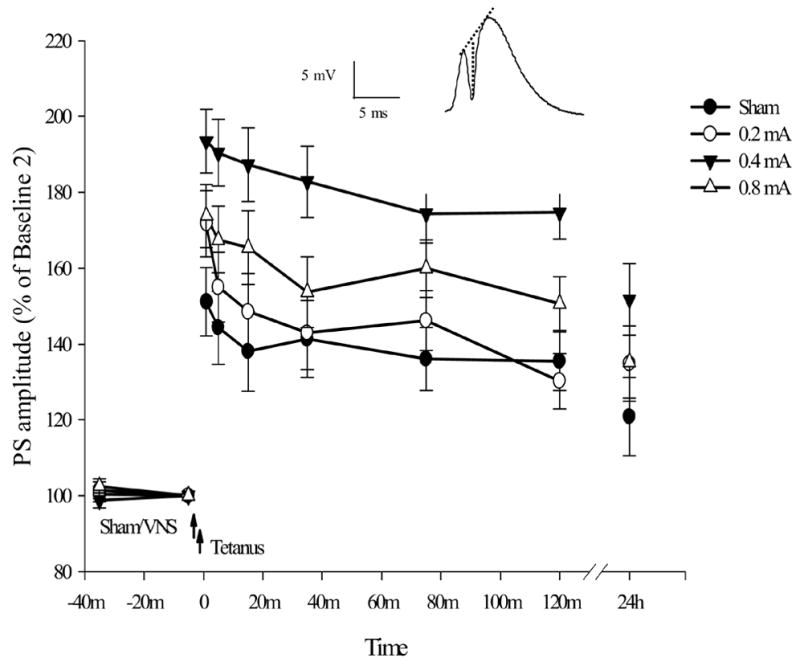
Mean percent changes (relative to Baseline 2 values) in population spike amplitude in freely-moving and awake rats receiving 0.2 (n = 12), 0.4 (n = 13), or .8 (n = 13) mA VNS compared to rats receiving sham VNS stimulation (n = 11) prior to tetanization. The height of the vertical dashed line in the inset indicates amplitude of the population spike. Rats receiving 0.4 mA VNS showed significantly enhanced LTP relative to rats in the sham group at all time points (see Results for all statistical values and results). The leftmost values displayed are those at Baseline 1, followed by Baseline 2 which served as the reference values. The left and right arrows illustrate the time points for VNS delivery and LTP induction, respectively. Means ± SEM are shown.
Results
One-way ANOVAs indicated that there were no significant differences in baseline stability as measured by mean percentages of Baseline 2 fEPSP slopes (F(3, 45) = 2.187, p > .1) or population spike amplitudes (F(3, 45) = 0.678, p > .5) relative to Baseline 1 values among the four groups. In addition, there were no significant group differences in perforant path stimulation intensities (F(3, 45) = 0.917, p > .4). These findings indicate that all four groups were similar in terms of baseline stability of evoked potentials as well as the perforant path stimulation intensities used to elicit evoked responses and induce LTP in the dentate gyrus.
VNS and LTP of fEPSP Slope
The results indicated no significant overall group effect (F(3, 45) = 1.337, p > .2) or group by time interaction (F(18, 270) = 1.398, p > .1) in the mean percentage values of fEPSP slope after tetanization relative to baseline values (Fig. 1). Pooled across the four groups, the averages of percentage values relative to Baseline 2 fEPSP slope measurements were small in magnitude (111.3 ± 2.2%, 105.6 ± 1.8%, 102.9 ± 1.4%, 101.3 ± 1.6%, 99.4 ± 1.6%, 100.9 ± 1.9%, and 110.2 ± 2.4 %, for immediately after, and 5, 15, 35, 75, 120 min, 24 h after tetanization, respectively). These data suggest that the weak tetanizing stimulation used in this study induced little or no LTP of fEPSP in the granule cell layer of the dentate gyrus, and VNS at any intensity did not facilitate the process. The repeated measures ANOVA revealed a significant time effect (F(6, 270) = 9.526, p < .001). However, given the small changes of fEPSP slope after tetanization we did not conduct further analysis on the time effect in this report.
VNS and LTP of Population Spike Amplitude
The repeated measures ANOVA of population spike amplitudes revealed that there was a significant effect of group (F(3, 45) = 5.353, p = .003). A significant time effect (F(6, 270) = 22.781, p < .001) and a lack of significant interaction between group and time (F(18, 270) = 1.081, p > .3) indicated an overall tendency for potentiated population spike amplitudes to decay over time across the four groups (Fig. 2). For sham, 0.2 mA, 0.4 mA, and 0.8 mA VNS group, mean population spike amplitudes across all time points following tetanization were 138.18 ± 8.07%, 147.08 ± 7.73%, 179.25 ± 7.43%, and 158.05 ± 7.43 % of Baseline 2, respectively. Post hoc Bonferroni multiple comparisons indicated that rats receiving 0.4 mA VNS showed enhanced LTP of population spike amplitudes relative to rats in sham-stimulation group across time (p = .003). VNS at 0.2 mA did not enhance LTP of population spike amplitudes as compared with sham-stimulated animals (p > .05). VNS at 0.8 mA showed a weak tendency to enhance LTP but animals in this group showed no significant difference from sham-stimulated rats (p > .05). VNS at 0.4 mA was associated with greater LTP than VNS at 0.2 mA across all time points post-tetanus (p = .026) while the difference in mean population spike amplitudes between 0.4 mA and 0.8 mA did not reach significance (p > .05). Taken together, these findings indicate that VNS at 0.4 mA enhanced the induction of both early and late LTP in the dentate gyrus. In contrast, VNS at 0.2 mA had no such effect. The overall results also showed that VNS at 0.8 mA did not significantly facilitate the development of either early or late LTP. Thus, the population spike data support the hypothesis that VNS at 0.4 mA, the intensity that most effectively enhances memory as measured in behavioral tasks, also enhances the induction of early LTP of population spike amplitude in the dentate gyrus while VNS at 0.2 or 0.8 mA has little or no effect. It should also be noted that VNS at 0.8 mA did show some limited tendency to affect dentate LTP as well. The data collected at 24 h post tetanus also support the idea that VNS at 0.4 mA, but not at 0.2 or 0.8 mA, enhances the development of late LTP of population spike amplitude.
Discussion
The present study found that VNS at 0.4 mA facilitated the induction of both early and late LTP of the population spike in the dentate gyrus of freely moving rats while VNS at lower and higher intensities was largely ineffective. These results closely parallel behavioral observations that VNS at 0.4 or 0.5 mA modulates memory consolidation in an intensity-dependent manner [13–15]. Preliminary data collected in our laboratory also suggest that VNS alone does not affect evoked potentials recorded from the dentate gyrus in the absence of LTP induced by high-frequency stimulation bursts [29]. Given that hippocampal LTP is widely recognized to be a model of learning and memory (e.g., [22–23]), the present findings support the idea that VNS may modulate memory formation by facilitating synaptic plasticity in the hippocampus.
Electrophysiological and neurochemical data suggest that the modulatory effect of VNS on hippocampal LTP may involve CNS noradrenergic systems. Moderate-intensity VNS increases the discharge rate of locus coeruleus neurons [18], which are the primary source of NE in the brain. Further, recent research conducted in our laboratory demonstrated that VNS at 0.4 or 0.5 mA potentiates NE release in the hippocampus [30–31]. NE or β-adrenoceptor activation facilitates the induction of LTP in all major regions of the hippocampal formation, including the dentate gyrus (e.g., [32–33]). Beta-adrenoceptors activate voltage-sensitive calcium channels including NMDA-receptor channels [34]. By inactivating ion channels mediating the calcium-sensitive voltage-independent potassium current through the stimulation of adenylate cyclase, NE decreases the hyperpolarization that occurs after an action potential thereby facilitating the generation of subsequent action potentials [35]. Norepinephrine typically has excitatory effects on the discharge rate of pyramidal and granule cells. However, NE at higher concentrations appears to have inhibitory effects on the amplitude of the evoked population spike of CA1 cells in the hippocampus [36–37], possibly through the mediation of α1 receptors [37]. A recent study also reported that low doses of NE prolong dentate LTP while a higher dose was not effective [38]. It is also noteworthy that NE has been reported to increase the spontaneous firing rate of interneurons, which can in turn lead to an inhibition of granule cells in the hippocampus [39].
The biphasic effects of NE on the excitability of the granule and pyramidal neurons in the hippocampus may perhaps explain the present findings that VNS modulated dentate LTP in an inverted-U relationship, considering preliminary data that VNS at higher intensities tends to have a greater effect on the release of NE in rat hippocampus [31]. Specifically, it was found that VNS at 0.25 mA has no effect on NE output while VNS at 0.5 mA results in a moderate increase in the extracellular NE concentration relative to baseline values while VNS at 1.0 mA potentiates NE release to an even greater extent [31].
Thus, the present finding that VNS at a low intensity, i.e. 0.2 mA, did not affect the induction of dentate LTP could be explained by the lack of sufficient NE release in this condition. The observed enhancing effect on LTP of VNS at a moderate intensity, i.e. 0.4 mA, may reflect primarily the β-adrenoceptor-mediated excitatory effects of moderate concentrations of NE on the firing activity of granule cells in the dentate gyrus. In contrast, VNS at a higher intensity, i.e. 0.8 mA, may produce both β-adrenoceptor-mediated excitatory effects and α-adrenoceptor-mediated inhibitory effects on the excitability of the granule cells and the net result may be an attenuated enhancing effect on LTP such as that seen in the present study.
The present finding that VNS at a moderate intensity (i.e., 0.4 mA) facilitates the development of late LTP is consistent with behavioral data showing the enhancing effect of VNS on retention in the inhibitory avoidance task as measured 24 hours after training [13–14]. Besides its role in facilitating the development of early LTP, VNS may also prolong or reinforce the development of early LTP into a lasting potentiation in the dentate gyrus. A number of studies have shown that early LTP can be transformed into late LTP in the dentate gyrus in vivo by temporal-related second heterosynaptic input provided by electrical stimulation of basolateral amygdala (BLA) [40] and by exposure to appetitive stimuli [41], or arousing novel environments [27]. In addition, direct stimulation of BLA within a time window of 30 min either before or after tetanization of the perforant path was found to be effective in the augmentation of early LTP [40]. Given that VNS potentiates NE release in the amygdala [19] and that the enhancement of dentate LTP involves β-adrenergic activation [40–41], it remains an attractive hypothesis that VNS delivered within a certain time window after weak tetanic stimulation of the perforant path may also enhance early LTP into protein synthesis-dependent late LTP. Moreover, such experimental conditions would closely resemble the behavioral studies in which VNS was given immediately after training [13–14].
Our finding that VNS had no effect in the induction of fEPSP LTP in the dentate gyrus is consistent with previous reports that the effects of increases in NE are primarily on the potentiation of the population spike, but with little or no significant effect on the fEPSP slope as measured in the dentate gyrus (e.g., [42]) or CA1 [36]. This observation may suggest that VNS affects the excitability of granule cells rather than the efficacy of synaptic transmission in the perforant pathway. However, this interpretation should be taken cautiously for two reasons. First, the parameters used in the present study were optimized to obtain a population spike and this would influence the dipole of the fEPSP in the hilus [27]. An alternative approach for evaluating the possibility that VNS potentiates fEPSP would be to place the recording electrode in midmolecular layer of the dentate gyrus where the medial perforant path terminals synapse on the dendrites of granule cells (e.g., [26, 42]). Second, the tetanization paradigm used in the present experiment, 0.1-Hz tetanic bursts, was relatively ineffective at inducing fEPSP LTP in the dentate gyrus when compared to theta frequency or 30-Hz stimulation bursts [43] and hence an effect of VNS on fEPSP could not have been seen. Future studies need to address both concerns to better examine the effect of VNS on the potentiation of synaptic transmission indexed by changes in the fEPSP in the hippocampus.
One possible explanation for the observed modulatory effects of VNS on dentate LTP is that VNS may cause changes in peripherally released stress-related hormones, which in turn affect hippocampal functioning through mechanisms other than the mediation of the vagal afferent fibers. For example, both corticosterone [44] and epinephrine [45] modulate hippocampal LTP in an inverted-U shaped dose-dependent function. The former can enter the brain and act on corticosteroid receptors [46] while the latter may have central effects on hippocampal function through its hyperglycemic actions [7]. However, rats in the present study showed only minor behavioral disturbances (e.g., behavioral freezing and an occasional slowing of respirations) during the period when VNS was delivered and they appeared to be fully recovered in one or two minutes. These behavioral observations suggest that VNS did not significantly alter levels of peripheral stress hormones, though a definite answer to this question would require close monitoring of possible hormonal changes before, during, and after the delivery of VNS. It should also be noted that hormonal-mediated release of corticosterone takes a relatively slow time course (peaks at 20 to 30 minutes after stress, see [47]) and thus can not plausibly explain the rapid onset of the effects of VNS on LTP that we observed. Moreover, by using lidocaine to block vagal action potentials below the point where VNS was given, Clark et al. [14] demonstrated that the memory-modulatory effect of VNS is mediated by vagal afferents rather than by any changes in the periphery produced by activation of vagal efferents. Taken together, these findings strongly support the idea that VNS modulates hippocampal memory-related synaptic plasticity primarily through neural messages conveyed by vagal afferents to the brain.
In conclusion, the present data provide the first evidence for the role of stimulation of the vagal-NTS pathway in the modulation of hippocampal memory-related changes in cellular functioning. Moreover, considering the evidence from behavioral and neurochemical studies that the vagus nerve conveys neural messages to the brain from the periphery caused by the release of neurohormones associated with arousal [3, 19, 48], these electrophysiological findings suggest that the modulatory effect of vagal activation on hippocampal plasticity is an important mechanism by which peripheral arousal during emotional experiences, or arousal produced from other sources (e.g., [49]), influences memory formation. Future studies combining behavioral testing with memory tasks and electrophysiological recordings in the same animals will be crucial to further establish the close relationship between VNS-induced memory enhancement and hippocampal synaptic plasticity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit formation. J Comp Neurol Psychol. 1908;18:459–482. [Google Scholar]
- 2.McGaugh JL. Memory: A century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 3.Jensen RA. Neural pathways mediating the modulation of learning and memory by arousal. In: Gold PE, Greenough WT, editors. Memory consolidation: essays in honor of James L. McGaugh. Washington, DC: American Psychological Association; 2001. pp. 129–140. [Google Scholar]
- 4.Gold PE, van Buskirk RB. Facilitation of time-dependent memory processes with posttrial epinephrine injections. Behav Biol. 1975;13:145–153. doi: 10.1016/s0091-6773(75)91784-8. [DOI] [PubMed] [Google Scholar]
- 5.McGaugh JL, Gold PE. Hormonal modulation of memory. In: Brush RB, Levine S, editors. Psychoendocrinology. New York: Academic Press; 1989. pp. 305–339. [Google Scholar]
- 6.Cahill L, Alkire MT. Epinephrine enhances human memory consolidation: interaction with arousal at encoding. Neurobiol Learn Mem. 2003;79:194–198. doi: 10.1016/s1074-7427(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 7.Gold PE. Modulation of emotional and non-emotional memories: same pharmacological systems, different neuroanatomical systems. In: Weinberger NW, McGaugh JL, Lynch G, editors. Brain and memory: modulation and mediation of neuroplasticity. New York: Oxford University Press; 1995. pp. 47–74. [Google Scholar]
- 8.Lee MK, Graham SN, Gold PE. Memory enhancement with posttraining intraventricular glucose injections in rats. Behav Neurosci. 1988;102:591–595. doi: 10.1037//0735-7044.102.4.591. [DOI] [PubMed] [Google Scholar]
- 9.McNay EC, Gold PE. Age-related differences in hippocampal extracellular fluid glucose concentration during behavioral testing and following systemic glucose administration. J Gerontol Biol Sci. 2001;56A:B66–B71. doi: 10.1093/gerona/56.2.b66. [DOI] [PubMed] [Google Scholar]
- 10.Talley CP, Clayborn H, Jewel E, McCarty R, Gold PE. Vagotomy attenuates effects of L-glucose but not of D-glucose on spontaneous alteration performance. Physiol Behav. 2002;77:243–249. doi: 10.1016/s0031-9384(02)00850-8. [DOI] [PubMed] [Google Scholar]
- 11.Williams CL, Jensen RA. Vagal Afferents: A possible mechanism for the modulation of memory by peripherally acting agents. In: Frederickson RCA, McGaugh JL, Felten DL, editors. Peripheral signaling of the brain: Role in neural- immune interactions and learning and memory. Lewiston, NY: Hogrefe & Huber; 1991. pp. 467–471.pp. 467–471. [Google Scholar]
- 12.Williams CL, McGaugh JL. Reversible lesions of the nucleus of the solitary tract attenuate the memory-modulating effects of posttraining epinephrine. Behav Neurosci. 1993;107:955–962. [PubMed] [Google Scholar]
- 13.Clark KB, Krahl SE, Smith DC, Jensen RA. Post-training unilateral vagal stimulation enhances retention performance in the rat. Neurobiol Learn Mem. 1995;63:213–216. doi: 10.1006/nlme.1995.1024. [DOI] [PubMed] [Google Scholar]
- 14.Clark KB, Smith DC, Hassert DL, Browning RA, Naritoku DK, Jensen RA. Post-training electrical stimulation of vagal afferents with concomitant vagal efferent inactivation enhances memory storage process in the rat. Neurobiol Learn Mem. 1998;70:364–373. doi: 10.1006/nlme.1998.3863. [DOI] [PubMed] [Google Scholar]
- 15.Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nature Neurosci. 1999;2:94–98. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- 16.Berthoud H, Neuhuber WL. Functional anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 17.Foley JO, DuBois FS. Quantitative studies of the vagus nerve in the cat: I. The ratio of sensory to motor fibers. J Comp Neurol. 1937;67:49–67. [Google Scholar]
- 18.Groves DA, Bowman EM, Brown VJ. Recordings from the rat locus coeruleus during acute vagal nerve stimulation in the anaesthetized rat. Neurosci Lett. 2005;379:174–179. doi: 10.1016/j.neulet.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 19.Hassert DL, Miyashita T, Williams CL. The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behav Neurosci. 2004;118:1–10. doi: 10.1037/0735-7044.118.1.79. [DOI] [PubMed] [Google Scholar]
- 20.Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153:1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- 21.Cohen NJ, Eichenbaum H, editors. Memory, amnesia, and the hippocampal system. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- 22.Bliss TVP, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 23.Martin SJ, Grimwood PD, Morris RGM. Synaptic plasticity and memory: An evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 24.Krug M, Lossner B, Ott T. Anisomycin blocks the late phase of long-term potentiation in the dentate gyrus of freely moving rats. Brain Res Bull. 1984;13:39– 42. doi: 10.1016/0361-9230(84)90005-4. [DOI] [PubMed] [Google Scholar]
- 25.Smith DC, Modglin AA, Roosevelt RW, Neese SL, Jensen RA, Browning RA, Clough RW. Electrical stimulation of the vagus nerve enhances cognitive and motor recovery following moderate fluid percussion injury in the rat. J Neurotrauma. 2005;22:1485–1502. doi: 10.1089/neu.2005.22.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNaughton BL. Evidence for two physiologically distinct perforant pathways to the fascia dentate. Brain Res. 1980;199:1–19. doi: 10.1016/0006-8993(80)90226-7. [DOI] [PubMed] [Google Scholar]
- 27.Korz V, Frey JU. Stress-related modulation of hippocampal long-term potentiation in rats: Involvement of adrenal steroid receptors. J Neurosci. 2003;23:7281–7287. doi: 10.1523/JNEUROSCI.23-19-07281.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- 29.Watkins CR. The effects of electrical stimulation of the vagus nerve on evoked responses measured in the dentate gyrus of the hippocampus of the rat. Thesis for Master’s Degree, Southern Illinois University Carbondale; 2004. [Google Scholar]
- 30.Miyashita T, Hassert DL, Williams CL. Stimulation of a neural pathway that conveys peripheral signals to the brain potentiates the release of norepinephrine from the hippocampus. Soc Neurosci Abstr. 2002;379:11. [Google Scholar]
- 31.Roosevelt RW, Smith DC, Clough RW, Jensen RA, Browning RA. Increased extracellular concentrations of norepinephrine in rat cortex and hippocampus following vagus nerve stimulation. Brain Research. doi: 10.1016/j.brainres.2006.08.04. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanton PK, Sarvey JM. Norepinephrine regulates long-term potentiation of both the population spike and dendritic EPSP in hippocampal dentate gyrus. Brain Res Bull. 1987;18:115–119. doi: 10.1016/0361-9230(87)90039-6. [DOI] [PubMed] [Google Scholar]
- 33.Mongeau R, Blier P, de Montigny C. The serotonergic and noradrenergic systems of the hippocampus: Their interactions and the effects of antidepressant treatments. Brain Res Rev. 1997;23:145–195. doi: 10.1016/s0165-0173(96)00017-3. [DOI] [PubMed] [Google Scholar]
- 34.Gray R, Johnston D. Noradrenaline and β-adrenoceptor agonists increase activity of voltage-dependent calcium channels in hippocampal neurons. Nature. 1987;327:620–622. doi: 10.1038/327620a0. [DOI] [PubMed] [Google Scholar]
- 35.Madison DV, Nicoll RA. Noradrenaline blocks accommodation of pyramidal cell discharge in the hippocampus. Nature. 1982;299:636–638. doi: 10.1038/299636a0. [DOI] [PubMed] [Google Scholar]
- 36.Mueller A, Hoffer B, Dunwiddie T. Noradrenergic responses in rat hippocampus: Evidence for mediation by α and β receptors in the in vitro slice. Brain Res. 1981;214:113–126. doi: 10.1016/0006-8993(81)90442-x. [DOI] [PubMed] [Google Scholar]
- 37.Mynlieff M, Dunwiddie T. Noradrenergic depression of synaptic responses in hippocampus of rat: evidence for mediation by alpha 1 receptors. Neuropharmacology. 1988;27:391–398. doi: 10.1016/0028-3908(88)90148-7. [DOI] [PubMed] [Google Scholar]
- 38.Almaguer-Melian W, Rojas-Reyes Y, Alvare A, Rosillo JC, Frey JU, Bergado JA. Long-term potentiation in the dentate gyrus in freely moving rats is reinforced by intraventricular application of norepinephrine, but not oxotremorine. Neurobiol Learn Mem. 2005;83:72–78. doi: 10.1016/j.nlm.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Bijak M, Misgeld U. Adrenergic modulation of hilar neuron activity and granule cell inhibition in the guinea-pig hippocampal slices. Neuroscience. 1995;67:541– 550. doi: 10.1016/0306-4522(95)00086-x. [DOI] [PubMed] [Google Scholar]
- 40.Frey S, Bergado-Rosado J, Seidenbecher T, Pape H, Frey JU. Reinforcement of early long-term potentiation (early LTP) in dentate gyrus by stimulation of the basolateral amygdala: Heterosynaptic induction mechanisms of late-LTP. J Neurosci. 2001;21:3697–3703. doi: 10.1523/JNEUROSCI.21-10-03697.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seidenbecher T, Reymann KG, Balschun D. A post-tetanic time window for the reinforcement long-term potentiation by appetitive and aversive stimuli. Proc Natl Acad Sci USA. 1997;94:1494–1499. doi: 10.1073/pnas.94.4.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munro CAM, Walling SG, Evans JH, Harley CW. β-adrenergic blockade in the dentate gyrus in vivo prevents high frequency-induced long-term potentiation of EPSP slope, but not long-term potentiation of population spike amplitude. Hippocampus. 2001;11:322–328. doi: 10.1002/hipo.1046. [DOI] [PubMed] [Google Scholar]
- 43.Fortin DA, Bronzino JD. The effect of interburst intervals on measures of hippocampal LTP in the freely moving adult male rat. Exp Neurol. 2001;170:371– 374. doi: 10.1006/exnr.2001.7713. [DOI] [PubMed] [Google Scholar]
- 44.Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- 45.Gold PE, Delanoy RL, Merrin J. Modulation of long-term potentiation by peripherally administered amphetamine and epinephrine. Brain Res. 1984;305:103– 107. doi: 10.1016/0006-8993(84)91124-7. [DOI] [PubMed] [Google Scholar]
- 46.Pavlides C, Watanabe Y, Margarinos AM, McEwen BS. Opposing roles of type I and type II adrenal steroid receptors in hippocampal long-term potentiation. Neuroscience. 1995;68:387–394. doi: 10.1016/0306-4522(95)00151-8. [DOI] [PubMed] [Google Scholar]
- 47.De Boer SF, Koopmans SJ, Slangen JL, Van der Gugten J. Plasma catecholamine, corticosterone and glucose responses to repeated stress in rats: effect of interstressor interval length. Physiol Behav. 1990;47:1117–1124. doi: 10.1016/0031-9384(90)90361-7. [DOI] [PubMed] [Google Scholar]
- 48.Miyashita T, Williams CL. Peripheral arousal-related hormones modulates norepinephrine release in the hippocampus via influences on brainstem nuclei. Behav Brain Res. 2004;153:87–95. doi: 10.1016/j.bbr.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 49.Nielson KA, Jensen RA. Beta-adrenergic receptor antagonist antihypertensive medications impair arousal-induced modulation of working memory in elderly humans. Behav Neural Biol. 1994;62:190–200. doi: 10.1016/s0163-1047(05)80017-2. [DOI] [PubMed] [Google Scholar]


