Abstract
Phosphocholine (PC) is the immunodominant epitope found on the surface of Streptococcus pneumoniae (SPn). T15-idiotype Abs, whose heavy (H) chain variable region is encoded by the V1 gene, are dominant in the anti-PC response in adult mice and protect mice from lethal pneumococcal infection. The ability of anti-PC Abs using H chains other than the V1 H chain to protect against pneumococcal infection remains controversial. We generated V1−/− knockout mice to determine whether protective anti-PC Abs could be produced in the absence of the V1 gene. No anti-PC Abs were produced in V1−/− mice immunized with avirulent SPn; however, PC-BSA binding Abs were induced after immunization with PC-keyhole limpet hemocyanin but at significantly lower levels than those in wild-type mice. These Abs provided poor protection against virulent SPn; thus, <25% of V1−/− mice survived challenge with 104 bacteria as compared with 100% survival of V1+/+ mice. The anti-PC Abs in V1−/− mice were heteroclitic, binding to nitrophenyl-PC better than to PC. None of nine hybridomas produced from V1−/− mice provided passive protection. However, the V1−/− mice produced normal amounts of Ab to SPn proteins that can partially protect mice against SPn. These data indicate that the V1 gene is critical for the production of anti-PC Abs providing optimum protection against infection with SPn, and the V1−/− mice could be useful in unmasking epitopes other than the immunodominant PC epitope on SPn capable of providing cross protection.
Phosphocholine (PC) is the immunodominant epitope found on the surface of a number of microorganisms, including Streptococcus pneumoniae (SPn) (1). PC is thought to play a vital role in the pathogenesis of SPn (2). By binding to the platelet activating factor (PAF) receptor on epithelial and endothelial cells, PC facilitates transport of the bacteria into the blood and brain (2, 3). Anti-PC Abs can block the binding of both SPn and PAF to PAF receptor (ref. 2 and our unpublished data). It has been shown that anti-PC Abs are protective against lethal pneumococcal infection in mice (4). Our laboratory previously has demonstrated that a PC conjugate vaccine could be used to provide protection against SPn in both normal and X-linked immune deficient (Xid) mice (5, 6). In normal mice, the immune response to avirulent, nonencapsulated SPn is dominated by a single clone of B cells bearing the T15-idiotype (id) (7, 8), whereas, Xid mice fail to respond to PC on the bacterial C polysaccharide (5, 6, 9). The immune response of normal mice to PC conjugated to keyhole limpet hemocyanin (KLH) also is dominated by T15-id Abs, but Xid mice produce T15-id− Abs with heavy (H) chains, like T15, encoded by the V1 gene (10–12). Each V1 variant expresses a different V:D junction and pairs with a different light (L) chain to form a PC-binding Ab. The germ line T15 V1 H chain (95H-Asp) associates with κ22 L chain, the M603 variant (95H-Asn) associates with κ8 L chain, the M167/M511 variants (96H-Ala) associate with the κ24 L chain, and the D16 variant (95H-Gly) associates with the κ1c L chain (13, 14). These VH1-id+ Abs, with the exception of the D16-like variants, are generally highly protective against SPn in passive transfer assays (6, 12).
T15-id+ anti-PC Abs generally provide better protection against infection with SPn than do T15-id−/VH1-id+ anti-PC Abs (our unpublished data). The ability of anti-PC Abs using H chains other than the V1 H chain to protect against pneumococcal infection remains controversial. When T15-id+ Abs were suppressed by injection of anti-T15-id Ab, adult mice produced the same amount of anti-PC Abs as did nonsuppressed mice after SPn immunization (15). T15-id− Abs from idiotype-suppressed mice bound to PC with approximately the same affinity as did T15-id+ Abs (16). Characterization of the H chains from T15-id− Abs revealed that they were encoded by genes from a number of VH families, including 7183, J558, X-24, VQ52, and S107 (16–18), and some of these T15-id− anti-PC Abs provided the same degree of protection against SPn as did T15-id+ Abs (16, 18). Protective T15-id−/VH1-id− anti-PC Abs, which crossreact with DNA, also have been induced in anti-I-Jd-treated mice (18); however, it is not clear whether protective VH1-id− anti-PC Abs can be induced in normal mice without suppressing T15-id or eliminating suppressor T cells.
Aged mice and elderly humans both are highly susceptible to infection with SPn (19–22). In aged mice, T15-id+ Abs no longer dominate the immune response to SPn, and they produce anti-PC Abs that use H chains other than VH1 and L chains other than Vκ22 (21, 23, 24). In humans, it is still unclear whether PC-specific Abs can provide protection against infection with SPn (25, 26). A VH gene homologous to the V1 gene of the mouse has not been found in humans, although PC-specific Abs have been readily detected (27–31). It also remains controversial whether humans produce a T15-id dominant response (30, 31).
To determine whether a set of highly protective non-VH1-id anti-PC Abs could be induced by immunization with PC in mice lacking a V1 gene, we generated V1 gene-deficient mice by gene targeting. When these V1-deficient mice were immunized with a PC-conjugate vaccine, they did not produce PC-specific Abs capable of providing optimum protection against virulent SPn, and these mice were unable to respond at all to PC after immunization with the bacteria itself. These data demonstrated that the V1 gene is critical for the production of anti-PC Abs providing optimum protection against infection with SPn.
Materials and Methods
Construction of the V1 Targeting Vector and Generation of Mutant Chimeras.
An S107 cDNA probe was used to screen a 129SvJ mouse genomic library (Stratagene), and 15 positive clones were screened again with a V1-specific oligo probe, 5′-TACTTGCAGCAAT CCACTCCAGTC-3′. Two overlapping V1 clones were confirmed, restriction-mapped, sequenced, and subcloned into pBluescript II KS(+) (Stratagene). To produce the targeting vector, a 3.5-kb EcoRI-BamHI fragment containing 5′ V1 genomic DNA and a 5-kb BamHI fragment from 3′ V1 genomic DNA were inserted into the NotI-XhoI and EcoRI-BamHI sites of the pPNT vector (32), respectively. The assembled targeting vector was linearized with NotI and electroporated into J1 embryonic stem (ES) cells. The transfected cells first were cultured in nonselective medium overnight and then subjected to selection with G418 at 350 μg/ml and ganciclovir at 2 μM. Correctly targeted ES cells (Fig. 1B) identified by Southern blots with 5′ and 3′ probes were injected into C57BL/6 blastocysts. Chimeric male mice were mated to C57BL/6 females to test for germ line transmission.
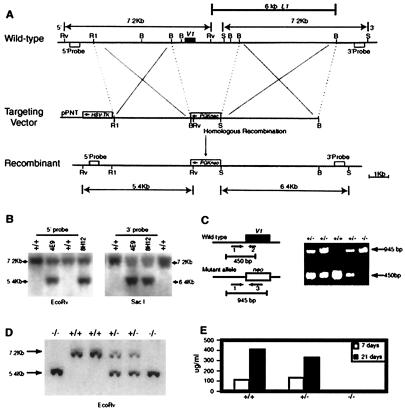
Figure 1.
Generation of V1 knockout mice. (A) Maps of V1 wild-type genome and gene-targeting strategy. The partial restriction map of the wild-type 129Sv/J genomic DNA containing the V1 gene (Top), gene-targeting vector (Middle), and targeted allele (Bottom) are shown. The positions of the relevant restriction sites for the enzymes BamHI (B), EcoRV (Rv), XbaI (X), EcoRI (R1), and SacI (S) are marked. The 5′ probe detects EcoRV-digested genomic fragments of 7.2 kb (wild-type allele) and 5.4 kb (targeted allele), and the 3′ probe detects SacI-digested genomic fragments of 7.2 kb (wild-type allele) and 6.4 kb (targeted allele). (B) Southern blot analysis of ES cell genomic DNA. The hybridization shows clones 4E9 and 8H12 were correct targeted ES cells. (C) PCR genotype strategy. Amplified fragments of expected size were obtained by using primers 1, 2, and 3, i.e., 450 bp for the wild-type allele and 945 bp for the mutant allele. (D) Southern blot analysis of genomic DNA from the offspring of a heterozygous mating. Genomic DNA was extracted from mice tails, digested with EcoRV, and hybridized with 5′ probe. (E) ELISA analysis of anti-PC Abs using the VH1 H chain. V1 KO mice were immunized with EPC-KLH. Serum was taken at 7 days after the first or second PC-KLH immunization. VH1-id+ PC-BSA binding Abs were detected with a rat anti-mouse VH1-id Ab (33). +/+, wild type; +/−, heterozygous; −/−, homozygous.
Mice Genotyping.
The mutant mice were genotyped by either PCR or Southern blotting. PCR typing was performed with primers derived from genomic V1 sequences (primer 1, 5′-TGAAGTTTTCTGAGCACAATTGTTACGA-3′, and primer 2, 5′-GTGTTGGG GA GGGTGTACTCATGTAGGA-3′) and from the Neo gene (primer 3, 5′-CGACCACCAAG C GAAACAT-3′). Primers 1 and 2 amplify an 450-bp DNA fragment from the wild-type allele, and primers 1 and 3 amplify an 945-bp DNA fragment from the mutant allele (Fig. 2C). The 5′ probe is a 131-bp fragment obtained by PCR on the EcoRV/EcoRI subclone with the primers 5′-TTCTGTG GATGCAAGCAC-3′ and 5′-TGGATACAAGACACAGGT-3′. The 3′ probe is a 414-bp fragment obtained by PCR on the BamHI/NotI subclone with the primers 5′-CATCACAC ATAACACCTA-3′ and 5′-ATCAGGATCAGCCACTCT-3′.
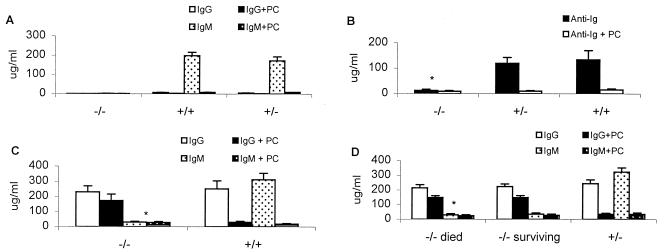
Figure 2.
ELISA analysis of PC-BSA binding Abs in serum from V1 KO mice after immunization with SPn R36a or EPC-KLH. (A) Sera taken 7 days after the second SPn R36a immunization. (B) Sera taken 7 days after the first EPC-KLH immunization. (C) Sera taken 7 days after the second immunization with EPC-KLH and analyzed for IgG and IgM anti-PC Abs in V1 KO mice. (D) Sera taken 7 days after the third immunization with EPC-KLH and analyzed for the production of anti-PC Abs in V1 KO mice that either died or survived after challenge with WU-2 bacteria (n = 10; *, P < 0.01, relative to V1+/+ or V1 +/− mice).
Immunogens and Immunization Protocol.
6-(O-phosphocholine) hydroxyhexanoate (EPC) was synthesized by the Science Applications International Corporation Chemical Synthesis and Analysis Laboratory (National Cancer Institute-Frederick Cancer Research and Development Center, Frederick, MD). Immunizations were performed i.p. with 200 μg of EPC-KLH in complete Freund's adjuvant for primary responses and with 100 μg of EPC-KLH in incomplete Freund's adjuvant (IFA) for the second and third immunizations. Heat-inactivated and lyophilized R36a (a type 2, unencapsulated, and avirulent strain of SPn was a gift from Jan Cerny, University of Maryland, Baltimore) was administered i.p. as a reconstituted vaccine in saline at 50 μg per mouse. Animals received antigen on day 0 and were boosted on days 14 and 28. Serum was obtained at day 7 for primary responses, day 21 for secondary responses, and day 35 for tertiary responses.
Abs and ELISA Procedure.
The rat anti-V1 hybridoma T68.3, which recognizes all Abs carrying the V1 H chain, was obtained from M. Scharff (Albert Einstein Medical College, Bronx, NY) (33). The biotinylated goat anti-mouse IgG and IgM and streptavidin alkaline phosphatase were obtained from American Type Culture Collection, Fisher Biotech, and Calbiochem, respectively. The levels of anti-PC Ab and anti-R36a Ab were measured by an ELISA assay as described previously (6).
Solid-Phase Enzyme-Linked Immunospot (ELISPOT) Assay.
Serial dilutions of spleen cells in RPMI medium 1640 were put into duplicate wells of flat-bottom 96-well plates that had been coated with 100 μl of PC-BSA (5 μg/ml) and blocked with BSA. Plates were incubated at 37°C for 4–5 h in a 5% CO2 incubator, washed five times with PBS containing 3% BSA and 0.05% Tween-20, and developed by the addition of biotin-labeled Abs, followed by avidin-alkaline phosphatase, each for 1 h at room temperature. Final color development was achieved by using 5-bromo-4-chloro-3-indolyl phosphate (Sigma) in 2-AMP buffer according to the manufacturer's recommendations.
Hybridoma Production and Ab Purification.
Hybridomas were generated from spleen cells of homozygous V1 knockout mice immunized twice with EPC-KLH as described (34). Hybridomas secreting EPC-BSA-binding Abs were selected by ELISA as described above and subcloned. Hybridoma cells were injected into pristane-primed C.CBA/N mice to produce ascites fluid. Abs were purified on protein A or protein G Sepharose columns and eluted with glycine-HCl, pH 2.8.
Bacteria and Protection Assays.
WU-2, a virulent type 3 strain of SPn, was obtained from David Briles (University of Alabama, Birmingham). Bacteria were maintained in glycerol stocks at −70°C until needed, and were passaged frequently through mice to maintain virulence. Glycerol stocks were plated on trypticase soy agar supplemented with 5% sheep blood (Life Technologies, Grand Island, NY) and incubated for 14 h at 37°C. Bacteria then were transferred to fresh Todd Hewitt broth supplemented with 0.5% yeast extract and incubated 4–6 h at 37°C, centrifuged at 4,000 × g for 10 min at 4°C, and the pellet was resuspended in PBS. An OD420 reading was taken to determine the concentration of bacteria. Bacteria were diluted in PBS and mice were injected with 0.2 ml of solution containing 101-104 bacteria for the challenges. All dilutions of bacteria were plated to confirm the number of colony-forming units (cfu) per ml given to each animal. Blood samples from moribund animals were plated on blood agar plates to confirm the presence of α-hemolytic bacteria. Animals were immunized two or three times as described above, randomly assigned to challenge groups (10 mice per group), and challenged i.p. with serial 10-fold dilutions of WU-2 bacteria (101 to 104) at 7 days after their second or third immunizations. For passive protection assay, purified Abs (50, 100, and 150 μg per mouse) were injected i.p. into naïve C.CBA/N (Xid) recipients along with the serial 10-fold dilutions of WU-2 bacteria. Anti-PC T15-id Abs HPC G11 (IgG3) and PC2 (IgM) were used as positive controls. Infected mice were monitored for 10 days postinjection.
Results
Generation of V1-Deficient Mice.
To generate a V1 deletion mutation, a replacement-type targeting vector was prepared in which the 3.4-kb BamHI fragment containing the V1 gene was replaced by a PGK-Neo gene cassette whose 3′ end was flanked by a PGK-TK gene cassette, thus allowing positive selection by G418 and negative selection by ganciclovir (Fig. 1A). The linearized vector was electroporated into a 129/Sv J1 ES cell line, and clones were selected in the presence of G4l8 and ganciclovir. Genomic DNA from surviving clones was digested with EcoRV or SacI and hybridized with a 5′ or 3′ probe (Fig. 1B). Correct targeting in ES cell clones was indicated by the generation of a 5.4-kb EcoRV fragment instead of the wild-type 7.2-kb EcoRV fragment, and by the generation of a 6.4-kb SacI fragment instead of the wild-type 7.2-kb SacI fragment (Fig. lB). Only two V1 mutant ES cell clones were obtained by screening 3,600 double-selected ES colonies. This low frequency is probably attributable to erroneous recombination of the 6-kb genomic sequence downstream of the V1 gene that is homologous to the Line 1 repeat fragment (35) (Fig. lA). The 4E9 cell line was injected into C57BL/6J blastocysts to obtain male chimeric mice that transmitted the targeted allele through the germ line. The offspring of chimeras mated with C57BL/6 mice were screened for the presence of the targeted allele by a PCR amplification strategy (Fig. 1C) or by Southern blotting analysis (Fig. 1D). V1+/− mice then were mated to obtain experimental animals and V1−/− breeders.
Inasmuch as the V1 H chain is used to produce PC-specific Abs, loss of the V1 gene should result in loss of the VH1-id component of the anti-PC response. To confirm the complete inactivation of V1 gene in our knockout (KO) mice, we immunized them with EPC-KLH and detected anti-PC-secreting B cells in the spleen by ELISPOT and anti-PC Abs in the serum by ELISA. VH1-id+ Abs were assessed by using the rat anti-mouse VH1 Ab T68.3, which recognizes all Abs carrying a V1 H chain (33). Homozygous knockout mice produced neither VH1-id+ anti-PC Ab-secreting B cells (Table 1) nor VH1-id+ anti-PC Abs (Fig. 1E) after the first or second immunization. These data demonstrate that the V1 gene had been deleted.
Table 1.
Reduced numbers of anti-PC Ab-secreting B cells
| KO mice | Anti-PC-secreting B cells per 106 spleen cells
|
|||
|---|---|---|---|---|
| V1 | κ + λ | PC inhibited* | NPPC inhibited* | |
| +/+ | 440 ± 65 | 490 ± 63 | 91 ± 3 | 94 ± 2 |
| +/− | 370 ± 58 | 380 ± 82 | 90 ± 2 | 91 ± 3 |
| −/− | 0 | 48 ± 8 | 38 ± 3 | 81 ± 3 |
Mice were sacrificed 5 days after EPC-KLH immunization, and the number of anti-PC Ab-secreting B cells were measured in the presence or absence of PC or NPPC by ELISPOT.
*The percentage of Ab-secreting cells inhibited by PC and NPPC (n = 10).
Anti-PC Abs Are Not Produced in V1−/− Mice After Immunization with SPn.
The thymus-independent immune response of normal mice to the unencapsulated R36a strain of SPn is dominated by protective IgM anti-PC Abs bearing the T15-id (7, 36, 37). As shown in Table 2 and Fig. 2A, V1 KO mice cannot produce anti-PC Abs after immunization with SPn R36a. However, the wild-type mice produced >150 μg/ml anti-PC IgM Abs. ELISPOT analysis also showed that no anti-PC-secreting B cells were detected in the spleen of V1−/− mice after the first or second immunization with R36a (data not shown). Thus, V1−/− mice do not response to the PC epitope on the cell wall of SPn.
Table 2.
Anti-PC Ab response after immunization with the different kinds of PC
| V1 KO mice | Immunogens | ELISA Ag
|
||||
|---|---|---|---|---|---|---|
| R36a
|
EPC-BSA
|
|||||
| Ab, OD | PC inhibited | Ab, μg/ml | PC inhibited† | NPPC inhibited† | ||
| −/− | R36a in saline | 1.49 ± 0.13 | 0 | <2* | 0 | 0 |
| PC-KLH | 0.62 ± 0.05* | 15.4 ± 7.3* | 267 ± 43* | 29.3 ± 6.6* | 86.4 ± 3.5 | |
| +/+ | R36a in saline | 1.38 ± 0.12 | 15.5 ± 3.9 | 199 ± 18 | 92.2 ± 4.5 | 92.1 ± 2.5 |
| PC-KLH | 1.17 ± 0.1 | 73.2 ± 4.5 | 567 ± 53 | 91.2 ± 2.8 | 93.3 ± 3.9 | |
KO mice were immunized twice i.p. with EPC-KLH or SPn R36a. The sera taken 7 days after the second immunization were assayed with or without PC or NPPC inhibition by ELISA on microtiter plates coated either with EPC-BSA or with SPn R36. The serum was diluted 1∶500 for detection of anti-R36a Abs.
*P < 0.001 relative to V1 KO wild-type mice; n = 10.
†The percentage of Ab binding inhibited by PC and NPPC.
Reduced Production of Anti-PC Abs and Changed PC Binding Affinity in V1 KO Mice After EPC-KLH Immunization.
To address whether V1 KO mice can produce PC-specific Abs using H chains other than VH1 after immunization with a thymus-dependent form of PC, mice were immunized with EPC-conjugated KLH and their spleens were analyzed for anti-PC secreting cells by ELISPOT. Five days after immunization, V1−/− KO mice produced 10-fold fewer B cells secreting PC-BSA binding Abs than did V1+/− or V1+/+ mice (Table 1). The number of Ab secreting B cells in V1−/− mice increased 5-fold after secondary immunization, but was still significantly lower than that in V1+/− or V1+/+ mice (data not shown). Furthermore, anti-PC Abs were not detected in the serum of V1−/− mice until 7 days postimmunization, a time when wild-type mice already produce about 100μg/ml of serum anti-PC Abs (Fig. 2B). Serum anti-PC Abs in V1−/− mice also increased dramatically after secondary immunization (Fig. 2C), but the level still was significantly lower (P < 0.01) than that in V1+/− or V1+/+ mice, even after three immunizations (Fig. 2D). Anti-PC-BSA Abs induced in V1 KO mice also were analyzed by inhibition ELISA and ELISPOT in the presence of PC or nitrophenylphosphocholine (NPPC) to determine their binding specificity. As shown in Table 1, only 38% of anti-PC-BSA Ab-secreting B cells in V1−/− mice were PC-inhibitable, whereas 81% were NPPC-inhibitable. ELISA data showed that <30% of serum anti-PC-BSA-binding Ab in V1−/− mice was PC-inhibitable, whereas 86% was NPPC-inhibitable (Table 2). By comparison, 90% of anti-PC Abs (Table 2) and anti-PC-secreting B cells (Table 1) in wild-type mice were PC-inhibitable. These data indicated that the anti-PC Abs produced in V1−/− mice are mostly heteroclitic Abs, having higher specificity for phenyl-PC than for PC.
Immunization with EPC-KLH Poorly Protects V1 KO Mice from SPn.
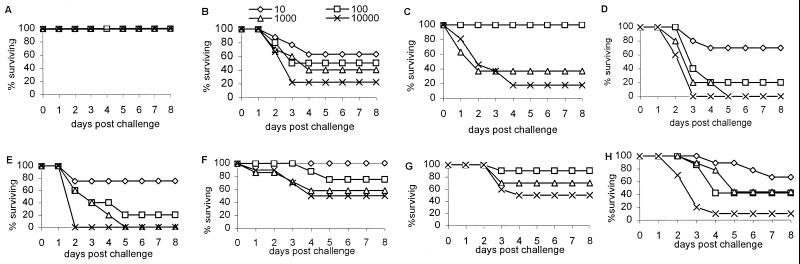
Previous studies from our laboratory have demonstrated that EPC-KLH is capable of protecting not only normal mice but also Xid mice from SPn (5, 6). To examine the protective ability of anti-PC Abs in V1−/− mice, the mice were immunized with EPC-KLH and challenged with WU-2, a virulent type 3 strain of SPn. After two immunizations, V1+/− or V1+/+ mice were totally protected from challenge with up to l04 cfu per mouse (Fig. 3A). In contrast, 63%, 55%, 40%, and 22% of the V1−/− mice survived challenge with 10, 102, 103, and 104 bacteria, respectively (Fig. 3B). After three immunizations with EPC-KLH, V1−/− mice were totally protected against 10 to 100 bacteria, but no increase in survival was seen in mice challenged with 103 and 104 bacteria (Fig. 3C). EPC-KLH-immunized V1−/− mice produced greater than 200 μg/ml serum Abs, which bound to PC-BSA (Fig. 2 C and D), and some of this Ab bound nonencapsulated SPn; however, <20% of binding to R36a was PC-inhibitable, whereas 70% of the R36a binding Ab in wild-type mice was PC-inhibitable (Table 2). No significant difference (P > 0.05) in the PC-BSA binding Ab levels occurred between V1−/− animals that died and those that survived after bacterial challenge (Fig. 2D). These data demonstrate that the anti-PC-BSA binding Abs from V1−/− mice are much less protective than VH1-id+Abs.
Figure 3.
Protection of mice against infection with SPn. (A) V1+/+ mice challenged after the second EPC-KLH immunization. (B) V1−/− mice challenged after the second EPC-KLH immunization. (C) V1−/− mice challenged after the third EPC-KLH immunization. (D) Naïve V1+/+ mice. (E) Naïve V1−/− mice. (F–H) V1+/+ (F), V1+/− (G), and V1−/− (H) mice challenged after immunizing twice with SPn R36a in saline.
Immunization with Avirulent Spn R36a Partially Protects V1−/− Mice Against Virulent SPn.
Recent research has suggested that some pneumococcal cell wall proteins crossprotect against multiple serotypes of SPn (38, 39). To determine whether the loss of the V1 gene affected the animals' ability to mount an immune response to other bacterial antigens, V1 KO mice were immunized twice with unencapsulated avirulent SPn R36a and then challenged with virulent bacteria WU-2. After immunization with R36a, there was no significant difference (P > 0.05) in the production of total Ab binding to R36a between V1−/− and V1+/+ mice (Table 2). This finding suggests that V1−/− mice have a normal immune response to other antigens from SPn. Although V1−/− mice cannot produce anti-PC Abs in response to R36a immunization (Fig. 2A and Table 2), 42% survived a bacterial challenge after immunization with R36a as compared with 25% in the unimmunized control (compare panels E and H in Fig. 3; P < 0.05), and the LD50 increased from 50 to 500 cfu in V1−/− mice. However, the wild-type and heterozygous mice immunized with R36a produced >150μg/ml IgM anti-PC Ab (Fig. 2A), and their survival increased to 75% (compare panels F, G, and H in Fig. 3).
Hybridoma Abs from V1−/− Mice Were Incapable of Passive Protection.
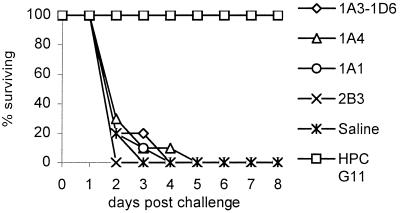
Previous studies (6, 12, 37, 40) demonstrated that T15-id+ anti-PC Abs were generally better at passive protection against SPn than were either VH1-id+/T15-id− Abs or V1-id− anti-PC Abs. It was therefore of interest to see whether the PC -specific Abs generated in PC-KLH-immune V1−/− mice were capable of transferring immunity to naïve recipients. Affinity-purified hybridoma Abs (50, 100, and 150 μg) were tested for their ability to protect naïve Xid mice from challenge with 10 to 104 cfu of WU-2. The Xid mice have an X-linked point mutation in the pleckstrin homology domain of their Bruton's tyrosine kinase gene that makes them unable to respond to carbohydrate antigens such as C polysaccharide. Thus, these mice are highly susceptible to infection with encapsulated bacteria like SPn (40). Fig. 4 shows that none of the hybridoma Abs from V1−/− mice were capable of protecting Xid mice challenged with even 10 cfu of bacteria, whereas 100 μg of the T15+ IgG anti-PC Ab HPC G11 protected all mice against challenge with up to 104 cfu.
Figure 4.
Passive transfer protection. C.CBA/N mice were injected i.p. with 100 μg of hybridomas Abs generated from EPC-KLH-immunized V1 KO mice, and they were challenged with 102 cfu per mouse of SPn strain WU-2 at the same time. HPC G11, a T15-id anti-PC Ab, served as the positive control (n = 10).
Discussion
In this study, we describe the generation and characterization of V1-deficient mice. The data presented here indicate that the V1 gene is critical for production of anti-PC Abs capable of providing optimum protection from infection with SPn. Deletion of the V1 gene eliminated the production of the prototypic germ line-encoded T15-id anti-PC Abs that previously have been shown to be highly protective against infection with SPn (6, 37). When homozygous V1 KO mice were immunized with nonencapsulated, avirulent SPn, they were unable to produce any PC-specific Abs, even though their Ab titer against whole bacteria was indistinguishable from that of wild-type mice. Less than a 2-fold increase in survival over that seen in the unimmunized controls occurred after immunization of V1 KO mice with avirulent bacteria, whereas 75% of the immunized wild-type and heterozygous littermates survived challenge with encapsulated, virulent pneumococci. These wild-type mice produce >150 μg of IgM anti-PC Ab after bacterial immunization. We assume that these IgM anti PC-specific Abs are responsible for the significant (P < 0.01) increase in protection seen at each challenge dose in the V1+/− and V1+/+ mice as compared with the V1 KO mice (Fig. 3 F--H). Thus, the deletion of the V1 gene leaves a potentially fatal hole in the protective Ab repertoire.
Xid mice are similar to V1 KO mice in that they also are unable to respond to PC after immunization with nonencapsulated, avirulent SPn. The basis of this defect seems to be twofold. First, the xid mutation causes a loss in response to TI-2 antigens, and, second, PC-specific B cells appear to undergo clonal deletion in Xid mice (41). However, previous studies from our laboratory (5, 6) have shown that immunization with an EPC-KLH vaccine can provide 100% protection in Xid mice against challenge with 104 virulent SPn. Some of EPC-KLH-induced T15-id−, PC-specific Abs in Xid mice were able to provide protection in passive transfer assays, but these protective Abs still expressed the VH1-id (6, 12). When the V1 KO mice were immunized twice with EPC-KLH, <25% of the mice were protected against the highest dose of bacteria, and only 63% of the mice could be protected against as few as 10 bacteria, whereas 100% of wild-type mice were protected against 104 bacteria. Even after three immunizations, 100% of V1 KO mice could not be protected against >100 bacteria. No PC-specific Abs or Ab-secreting cells expressing the VH1-id were detectable in the serum or spleens of EPC-KLH-immunized V1 KO mice. V1 KO mice did, however, produce Abs that bound to PC-BSA. PC-BSA-specific Ab-secreting cells were 10-fold lower in the V1 KO than in the heterozygous littermates after primary immunization and 2-fold lower after secondary immunization. This finding suggests that very few PC-specific B cells are present in naïve V1 KO mice as compared with wild-type mice, which have >103 T15-id B cells in their spleens (10). The B cells in V1 KO mice that respond to EPC-KLH are rapidly expanded after secondary immunization such that the level of circulating anti-PC-BSA Ab is >200 μg/ml; however, these Abs provide poor protection against virulent bacteria as compared with the Abs present in wild-type mice.
Most of the Abs induced in V1 KO mice after EPC-KLH immunization were unusual in that they were heteroclitic; thus, binding to PC-BSA was inhibited better by NPPC than by PC. The IgM PC-BSA binding Ab levels in V1 KO mice were also 10-fold lower than those found in control wild-type mice. These data suggest that V1 KO mice produce atypical Abs in response to EPC-KLH immunization. In normal mice, very few heteroclitic Abs are produced in response to EPC-KLH, and the response remains dominated by the germ line T15-id (5). The response in V1 KO mice seems to be similar to that seen in Xid mice or to the secondary response of normal mice after immunization with diazophenylphosphocholine-KLH (42), except that these heteroclitic Abs seem to be of very low affinity. Because EPC-KLH contains no phenyl ring, the B cells cannot undergo affinity maturation for this epitope. The heteroclitic phenylphosphocholine-specific Abs produced in V1 KO and Xid mice also are similar in that they bind poorly to SPn (our unpublished data) and do not provide protection in passive transfer assays (12). However, the naïve, unimmunized V1 KO mouse, unlike the Xid mouse, is not more susceptible to SPn than are normal mice. Fewer than 10 bacteria will kill Xid mice, whereas the LD50 in both wild-type and V1 KO mice was 5-fold higher. The V1 KO, unlike the Xid mouse, should respond to TI-2 carbohydrate antigens and is therefore only selectively immunodeficient to the PC-epitope on SPn. There may be other non-PC, carbohydrate-specific, crossreactive Abs in unimmunized V1 KO mice that are missing in Xid mice that account for the increase in LD50 seen in V1 KO mice. The naturally occurring anti-PC Abs in normal mice are not adequate to prevent death in these mice after infection with SPn; however, they appear to be responsible for the delay in time-to-death seen in wild-type mice as compared with the V1 KO mice (Fig. 3 D and E).
Nine hybridomas that bound to PC-BSA were produced from spleens of EPC-KLH-immunized V1 KO mice. Inasmuch as none of these were capable of providing protection against SPn in a passive protection assay, the nature of the Abs providing the low level of protection seen in immune V1 KO mice still is not known. Additional hybridomas are being produced and characterized. However, it is clear from the data presented in this paper that Abs carrying V1-encoded H chains provide the optimum binding specificity for PC and give the best crossprotection against SPn. The loss or lack of the V1 gene leaves the animal highly susceptible to infection by microorganisms bearing PC and makes it difficult to provide protection by using PC as the target epitope. It is still unclear why T15-id+ Abs are more protective than are other V1 anti-PC Abs; however, their property of self-binding may increase the overall avidity of these Abs for PC (43).
The current commercially available pneumococcal vaccine is composed of the purified capsular polysaccharides from 23 of the more than 90 different strains of SPn. In normal adults, this vaccine is 80% effective at preventing infection with SPn (44), but it is much less effective in infants or in the elderly (45, 46). Although anti-PC Ab titers increase significantly after immunization with the capsular polysaccharide vaccine, it is unclear whether human anti-PC Abs can provide protection against SPn and whether humans can produce a T15-id Ab because no VH gene homologous to the mouse VH1 gene has been found in humans (27–31). Inasmuch as infants can't respond to the capsular polysaccharides or PC, there is a critical need for a more comprehensive vaccine. The V1 KO mouse could be useful in unmasking epitopes other than the immunodominant PC epitope on SPn capable of providing crossprotection. Because B cells expressing the T15-id totally dominate the immune response to SPn in normal mice, they may mask or out-compete B cells responding to less immunogenic epitopes or B cells present at a low precursor frequency. The inability of V1 KO mice to respond to the immunodominant PC-epitope on SPn may permit the development of more widely protective vaccine based on recognition of other SPn determinants.
Acknowledgments
We thank Z. Liu for blastocyst injection, J. Nagel for DNA sequencing, A. Carter for bleeding mice, J. Cerny for lyophilized R36a, M. Scharff for anti-V1 hybridoma T68.3 and PC2 hybridoma, D. Briles for WU-2 bacteria, and P. J. Gearhart for anti-PC T15-id HPC G11 Ab.
Abbreviations
- ELISPOT
solid-phase enzyme-linked immunospot
- EPC
6-(O-phosphocholine) hydroxyhexanoate
- ES
embryonic stem
- id
idiotype
- KLH
keyhole limpet hemocyanin
- KO
knockout
- PC
phosphocholine
- SPn
Streptococcus pneumoniae
- Xid
X-linked immune deficient
- H chain
heavy chain
- L chain
light chain
- cfu
colony-forming units
- NPPC
nitrophenylphosphocholine
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.110039497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.110039497
References
- 1.Potter M. Ann NY Acad Sci. 1971;190:306–321. doi: 10.1111/j.1749-6632.1971.tb13543.x. [DOI] [PubMed] [Google Scholar]
- 2.Cundell D R, Gerard N P, Gerard C, Idanpaan-Heikkila I, Tuomanen E I. Nature (London) 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 3.Tuomanen E I, Austrian R, Masure H R. N Engl J Med. 1995;332:1280–1284. doi: 10.1056/NEJM199505113321907. [DOI] [PubMed] [Google Scholar]
- 4.Briles D E, Forman C, Crain M. Infect Immun. 1992;60:1957–1962. doi: 10.1128/iai.60.5.1957-1962.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenny J J, Guelde G, Fischer R T, Longo D L. Int Immunol. 1994;6:561–568. doi: 10.1093/intimm/6.4.561. [DOI] [PubMed] [Google Scholar]
- 6.Fischer R T, Longo D L, Kenny J J. J Immunol. 1995;154:3373–3382. [PubMed] [Google Scholar]
- 7.Cosenza H, Kohler H. Science. 1972;176:1027–1029. doi: 10.1126/science.176.4038.1027. [DOI] [PubMed] [Google Scholar]
- 8.Claflin J L, Cubberley M. J Immunol. 1980;125:551–558. [PubMed] [Google Scholar]
- 9.Quintans J. Eur J Immunol. 1977;7:749–751. doi: 10.1002/eji.1830071020. [DOI] [PubMed] [Google Scholar]
- 10.Gearhart P J, Sigal N H, Klinman N R. J Exp Med. 1977;145:876–891. doi: 10.1084/jem.145.4.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crews S J, Griffin J, Huang H, Calame K, Hood L. Cell. 1981;25:59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- 12.Guo W-X, Burger A M, Fischer R T, Sieckmann D G, Longo D L, Kenny J J. Int Immunol. 1997;9:665–677. doi: 10.1093/intimm/9.5.665. [DOI] [PubMed] [Google Scholar]
- 13.Kenny J J, Moratz C M, Guelde G, O'Connell C D, Dell C, Penner S J, Weber J S, Berry J, Claflin J L, Longo D L. J Exp Med. 1992;176:1637–1643. doi: 10.1084/jem.176.6.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C, Roberts V A, Stevens S, Brown M, Stenzel-Poore M P, Rittenberg M B. EMBO J. 1995;14:2784–2794. doi: 10.1002/j.1460-2075.1995.tb07278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Accolla R S, Gearhart P J, Sigal N H, Cancro M P, Klinman N R. Eur J Immunol. 1977;7:876–881. doi: 10.1002/eji.1830071211. [DOI] [PubMed] [Google Scholar]
- 16.Nicoletti C. Exp Mol Pathol. 1995;62:99–108. doi: 10.1006/exmp.1995.1011. [DOI] [PubMed] [Google Scholar]
- 17.Nicoletti C, Borghsi-Nicoletti C, Yang X, Schulze D H, Cerny J. J Immunol. 1991;147:2750–2755. [PubMed] [Google Scholar]
- 18.Limpanasithikul W, Ray S, Diamond B. J Immunol. 1995;155:967–973. [PubMed] [Google Scholar]
- 19.Nicoletti C, Yang X, Cerny J. J Immunol. 1993;150:543–549. [PubMed] [Google Scholar]
- 20.Song H, Price P W, Cerny J. Immunol Rev. 1997;160:55–62. doi: 10.1111/j.1600-065x.1997.tb01027.x. [DOI] [PubMed] [Google Scholar]
- 21.Yang X, Stedra J, Cerny J. J Immunol. 1994;152:2214–2221. [PubMed] [Google Scholar]
- 22.Musher D M, Chapman A J, Goree A, Jonsson S, Briles D, Baughn R E. J Infect Dis. 1986;154:245–256. doi: 10.1093/infdis/154.2.245. [DOI] [PubMed] [Google Scholar]
- 23.Nicoletti C, Borghesi-Nicoletti C, Yang X H, Schulze D H, Cerny J. J Immunol. 1991;147:2750–2755. [PubMed] [Google Scholar]
- 24.Nicoletti C, Cerny J. Cell Immunol. 1992;144:332–346. doi: 10.1016/0008-8749(92)90249-o. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen S V, Sorensen U B, Henrichsen J. Microb Pathog. 1993;14:299–305. doi: 10.1006/mpat.1993.1029. [DOI] [PubMed] [Google Scholar]
- 26.Ekdahl K, Braconier J H, Svanborg C. Clin Infect Dis. 1997;25:654–660. doi: 10.1086/513763. [DOI] [PubMed] [Google Scholar]
- 27.Gray B M, Dillon H C J, Briles D E. J Clin Microbiol. 1983;18:1102–1107. doi: 10.1128/jcm.18.5.1102-1107.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown M, Schiffman G, Rittenberg M B. J Immunol. 1984;132:1323–1328. [PubMed] [Google Scholar]
- 29.Freijd A, Jonsson A, Rynnel-Dagoo B. APMIS. 1988;96:901–905. doi: 10.1111/j.1699-0463.1988.tb00959.x. [DOI] [PubMed] [Google Scholar]
- 30.Nishinarita S, Sawada S, Horie T. Med Microbiol Immunol. 1990;179:205–214. doi: 10.1007/BF00195251. [DOI] [PubMed] [Google Scholar]
- 31.Halpern R, Kaveri S V, Kohler H. J Clin Invest. 1991;88:476–482. doi: 10.1172/JCI115328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tybulewicz V L J, Crawford C E, Jackson P K, Bronson R T, Mulligan R C. Cell. 1992;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 33.Desaymard C, Giusti A M, Scharff M D. Mol Immunol. 1984;21:961–967. doi: 10.1016/0161-5890(84)90154-8. [DOI] [PubMed] [Google Scholar]
- 34.Sieckmann D S, Stall A M, Subbarao B. Hybridoma. 1991;10:121–135. doi: 10.1089/hyb.1991.10.121. [DOI] [PubMed] [Google Scholar]
- 35.Lewin B. In: Genes VI. Lewin B, editor. New York: Oxford Univ. Press; 1997. pp. 597–619. [Google Scholar]
- 36.Lee W, Cosenza H, Kohler H. Nature (London) 1974;247:55–57. doi: 10.1038/247055a0. [DOI] [PubMed] [Google Scholar]
- 37.Yother J, Forman C, Gray B M, Briles D E. Infect Immun. 1982;36:184–188. doi: 10.1128/iai.36.1.184-188.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee C J, Wang T R, Tai S S. Crit Rev Microbiol. 1997;23:121–142. doi: 10.3109/10408419709115133. [DOI] [PubMed] [Google Scholar]
- 39.Goldblatt D. J Med Microbiol. 1998;47:563–567. doi: 10.1099/00222615-47-7-563. [DOI] [PubMed] [Google Scholar]
- 40.Briles D E, Horowitz J, McDaniel L S, Benjamin W H J, Claflin J L, Booker C, Scott G, Forman C. Curr Top Microbiol Immunol. 1986;124:103–120. doi: 10.1007/978-3-642-70986-9_7. [DOI] [PubMed] [Google Scholar]
- 41.Kenny J J, Stall A M, Sieckmann D G, Lamers M C, Finkleman F, Finch L, Longo D L. J Immunol. 1991;146:2568–2577. [PubMed] [Google Scholar]
- 42.Chang S P, Brown M, Rittenberg M B. J Immunol. 1982;128:702–706. [PubMed] [Google Scholar]
- 43.Kang C Y, Brunck T K, Kieber-Emmons T, Blalock J E, Kohler H. Science. 1988;240:1034–1036. doi: 10.1126/science.3368787. [DOI] [PubMed] [Google Scholar]
- 44.Shapiro E D, Berg A T, Austrian R, Schroeder D, Parcellis V, Margolis A, Adair R K, Clemens J D. N Engl J Med. 1991;325:1453–1460. doi: 10.1056/NEJM199111213252101. [DOI] [PubMed] [Google Scholar]
- 45.Cowan M, Ammann A J, Wara D, Howie V, Schultz L, Doyle N, Kaplan M. Pediatrics. 1978;62:721–727. [PubMed] [Google Scholar]
- 46.Forrester H, Jahnigen D, LaForce F. Am J Med. 1987;83:425–430. doi: 10.1016/0002-9343(87)90751-0. [DOI] [PubMed] [Google Scholar]