Abstract
Endoscopic biliary stenting is today the most common palliative treatment for patients suffering from obstructive jaundice associated with malignant hepatobiliary tumors or benign strictures. However, recurrent jaundice, with or without cholangitis, is a major complication of a biliary endoprosthesis insertion. Thus, stent removal and replacement with a new one frequently occurs as a consequence of device blockage caused by microbial biofilm growth and biliary sludge accumulation in the lumen. Factors and mechanisms involved in plastic stent clogging arising from epidemiological, clinical and experimental data, as well as the possible strategies to prevent biliary stent failure, will be reviewed and discussed.
Keywords: Biliary stent, Cholangitis, Endoscopic insertion, Microbial biofilm, Prevention, Stent occlusion
Since the late 1970s, when this surgical procedure was first introduced,1 endoscopic and percutaneous insertion of biliary stents has provided effective relief in obstructive diseases of the biliary tract. It is presently considered the treatment of choice for unresectable malignant hepatobiliary tract obstructions and the minimally invasive treatment of strictures of the biliary duct. However, late stent occlusion, the major complication with biliary stents, occurs in a significant number of cases (10% to 30%)2 and is associated with recurrent jaundice, with or without cholangitis. Thus, stent removal and replacement with a new one is often needed incurring additional health care costs and worsening the patient’s quality of life.
In addition to microbial colonization and duodenal reflux of food constituents (e.g., fibers), several other factors have been suggested to be involved in the occlusion of these endoprostheses. These factors include stent design, physicochemical properties of the constitutive materials, surface irregularities of the devices promoting microbial biofilm formation, and biliary sludge accumulation.3–5
Biliary Obstruction and Jaundice Relief
Palliative relief of biliary obstruction for patients with malignant strictures of bile ducts or inoperable hepatobiliary cancers may be accomplished with surgical bypass by means of choledochoduodenostomy, choledochojejunostomy or hepaticojejunostomy.6 Alternatively, nonoperative biliary drainage can be achieved through either the endoscopic or the percutaneous transhepatic approach.7 In most clinical settings, as detailed in the American Society for Gastrointestinal Endoscopy guidelines,8 endoscopic retrograde cholangiopancreatography is the intervention of choice since it offers the great advantage of establishing internal drainage without the need for an external catheter. On the other hand, the percutaneous transhepatic cholangiopancreatography is generally performed in those clinical settings where endoscopic retrograde cholangiopancreatography is not possible or in the cases of its failure. However, the inserted catheter can be the origin of ascending cholangitis due to the aspiration of duodenal content and can create discomfort for the patient.
According to a randomized study by Speer and colleagues9 that compared patients with endoscopically inserted stents (n=37) versus patients with percutaneously inserted transhepatic stents (n=33), the success rate for biliary drainage (expressed as bilirubin decrease greater than 20%) was significantly higher in the endoscopic group (81% versus 61%). In addition, the overall complication rate was 19% in the endoscopic group versus 67% in the percutaneous group. On the basis of this and other randomized comparative trials6,10 of surgical versus endoscopic palliation for obstructive jaundice, both approaches appear to be effective with regard to short-term outcome. However, in patients with obstructive jaundice, endoscopic placement of biliary stents is currently the palliative strategy of choice because of its lower incidence of complications, as well as its lower procedure-associated morbidity and mortality, and reduced length of hospital stay.11 In fact, jaundice may be associated with numerous complications, including malabsorption and consequent progressive malnutrition, coagulopathy, hepatocellular dysfunction, pruritus and recurrent cholangitis. Ballinger et al12 have shown that endoscopically inserted biliary stents also improve quality of life in patients with malignant obstructive jaundice due to unresectable pancreatic cancer. Only a few additional investigations have been carried out to further compare these procedures. Of interest, the first prospective randomized clinical trial and reported in 2002 by Pinol and colleagues13 that compared percutaneous placement of self-expanding metal stents with endoscopic insertion of polyethylene endoprostheses demonstrated therapeutic success. Overall median survival was higher in the endoscopic group (71% versus 42% of patients, and 3.7 versus 2.0 months, respectively), whereas major complications were more common in the percutaneous group (61% versus 35% of patients).
Types of Biliary Stents
With regard to materials used to manufacture stents, biliary endoprostheses currently used in clinics fall into two broad categories, plastic (teflon, polyethylene or polyurethane) and metallic (stainless steel or nickel-titanium alloy) stents. First introduced in 1979,1 straight, slightly curved or pigtailed plastic stents are today the most commonly used, even if different designs did not have an effect on their patency.14
Polymeric stents are available in different lengths (5 to 18 cm) and diameters (7 to 12 Fr), with or without side holes, and are provided with anchoring flaps at their ends to reduce possible migration after positioning. Interestingly, in the frame of a study aimed to chemically analyze the clogging material of polymeric biliary endoprostheses,15 it was determined that 94% of the stents retrieved in the study because of clogging were made of ethylene-vinylacetate copolymer instead of polyethylene as declared by manufacturers. Thus, the authors concluded that this copolymer, although probably preferred to the polyethylene because of its higher flexibility, is not a suitable material for biliary endoprostheses, since it appears to facilitate clogging phenomena.15
The clogging tendency of plastic stents represents a major disadvantage leading the patient to recurrent jaundice and pruritus. If signs of cholangitis develop, the stent should be replaced in order to avoid the occurrence of life-threatening sepsis.16
The risk of occlusion of standard polyethylene stents appears to increase progressively after 3 months. In fact, a median patency of 4 to 5 months has been estimated for the 10 Fr plastic stents.17,18 Although stents larger in diameter allow higher bile flow rates and exhibit later onset of clogging,19 duodenoscopes available to date cannot accept stents with a diameter higher than 12 Fr.
Metallic stents are larger in diameter, self-expanding, and inclined to be covered by biliary epithelial cells soon after implantation. The major disadvantages of these stents compared to plastic ones are represented by their higher cost and difficulty in repositioning or removal once deployed, and therefore, do not allow their use in benign strictures.
The commonly used Wallstent (Schneider Stent, Minneapolis, MN) is a metal stent consisting of a stainless steel alloy tubular mesh kept unexpanded within an 8 Fr delivery system. When inserted, its diameter increases to 30 Fr and length decreases about 30%, reaching the designated sizes of 42, 68 or 90 mm. Although metallic stents are associated with a lower obstruction rate (median duration of patency = 8–10 months) than plastic ones, the growth of normal or tumor tissues throughout the voids of the metal mesh may lead to stent obstruction.20,21 In an attempt to circumvent tumor ingrowth of such stents, silicone-covered metal stents were developed.22 According to a recent prospective randomized controlled trial on the patency of 10 Fr polyethylene stents versus silicone-covered 30 Fr steel self-expanding stents, the latter are recommended in unresectable patients with malignant common bile duct strictures (median survival of 4.5 months), while plastic stents are preferred in one-third of patients who have distant metastases.23 When blocked, stents can be unclogged by dragging an extraction balloon through their obstructed lumen, by brachytherapy or by diathermic devices. Alternatively, a standard polyethylene stent or an additional metal expandable stent can be inserted through the blocked endoprosthesis. However, it is important to note that duodenal perforation and acute bleeding have been reported as a result of erosion of the duodenal wall caused by metal stents.24
According to a recent Cochrane review, endoscopic stenting is preferable to surgery in palliation of malignant biliary obstructions. The choice of plastic or metal stent depends on the life expectancy of the patient.25
Causative Factors and Dynamics of the Clogging Process in Plastic Stents
A number of papers have examined the phenomenon of obstruction of the inner lumen of biliary endoprostheses. Some explanatory hypotheses have been proposed on the basis of attributing a major role in the occlusion to one or more sludge components.
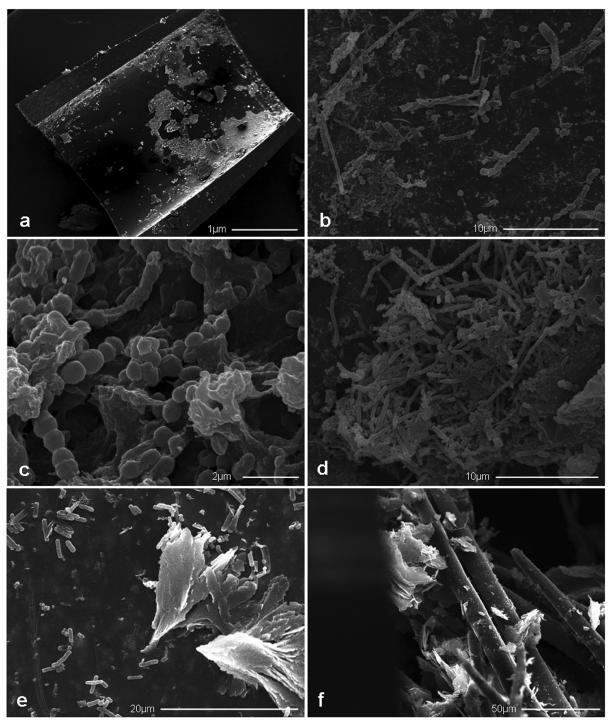
The occluding sludge removed from an explanted plastic biliary stent has the appearance of a brownish, soft and heterogeneous material. Scanning electron microscopy observations of polyethylene occluded stents (figure 1 ▶) and biochemical analyses of biliary sludge have revealed that this amorphous material is composed of bacteria and/or fungi, microbial byproducts, proteins, dietary fibers, crystals of fatty acid calcium salts, and amorphous calcium bilirubinate.26–29 The commonly used polymeric stents are made of hydrophobic materials and, after insertion, their inner surface is rapidly covered by a layer of host proteins, such as fibronectin, vitronectin, laminin, fibrin or collagen to form a conditioning film that promotes microbial adherence.30–34 Surface irregularities are also thought to enhance bacterial adherence and biofilm formation.4,28,35 Another promoting factor has been suggested to be the bile immunoglobulin-bacteria complex.32 In fact, both IgA and IgG have been demonstrated to bind bacteria and to deposit as a complex on the inner surface of polyethylene stents. Bile glycoprotein mucin, shown to line the inner surface of occluded stents, has also been proposed to affect bacterial adherence acting as a cement among water insoluble compounds, such as bilirubin and bacterial clumps.36
Figure 1.
Scanning electron micrographs of clogged polyethylene stents showing (a) fragments of a protein conditioning film layered on the inner surface of a blocked stent, as visible after removal of the sludge material, (b) different bacterial and fungal species colonizing the inner surface of the stent, (c) a mature biofilm of Enterococcus spp., (d) a Candida spp. mature biofilm exhibiting different stages of fungal growth, (e) typical flock-shaped cholesterol crystals, as well as numerous bacilli and (f) plant fibers found in the biliary sludge.
Electron microscopy observations of occluded stents have shown that clogging material, the so-called biliary sludge, is mainly formed by the accumulation of multispecies microbial colonies growing in sessile mode, microbial byproducts, and crystals of calcium bilirubinate and calcium palmitate. Within this complex, large plant fibers arising from duodenal reflux have been observed as constituents of intraluminal networks contributing to stent clogging.37
In healthy individuals, bile flow and duodenal reflux to both the bile and pancreatic duct are under the control of the sphincter of Oddi, which prevents the ascension of microorganisms, and therefore, maintains the sterility of bile and pancreatic juice. When a biliary stent is inserted across the sphincter of Oddi, the loss of the antimicrobial barrier, represented by the sphincter itself, and the low pressure in the common bile duct allow reflux of intestinal content to promote ascending microbial colonization.38
Microorganisms isolated from occluded biliary stents include both aerobic and anaerobic bacterial species, as well as fungi (table 1 ▶).28,36,39–41 The Gram-positive Enterococcus species and Gram-negatives Escherichia coli and Klebsiella species are the most common aerobic bacteria isolated from biliary sludge, while Clostridium species are the prevalent anaerobes. However, the ratio between aerobic and anaerobic species, as well as all isolated species, varies in different studies, presumably depending on either the portion (proximal, median or distal) of the stent analyzed or the delay in performing microbiological analysis after stent removal. Therefore, in stents explanted from humans, anaerobes have rarely been reported,29,39 while a variety of both aerobic and anaerobic bacteria were isolated in a study performed on a pig model of polyethylene stent clogging42 where surgically extracted stents allowed a contamination-free microbial isolation. On the other hand, the finding of an early attachment of anaerobic bacteria belonging to different species, including Clostridium perfringens, Clostridium bifermentans and Bacteroides fragilis, reported in a study of unblocked biliary stents removed after a relatively short implantation period (mean of 33 days),41 seems to indicate a predominant role of anaerobic bacteria at least in initiating the process leading to the stent clogging.
Table 1.
Microbial species isolated from blocked or unblocked plastic biliary stents and their incidence according to data reported in the literature.
| Microbial species | Speer et al28(%)* | Dowidar et al39(%)† | Di Rosa et al40(%)‡ | Leung et al41(%)δ | Zhang et al36(%) || |
| Aerobic bacteria | |||||
| Gram positive | |||||
| Bacillus spp | - | - | - | 9 | - |
| Enterococcus spp | 3 | 12 | 44 | 23 | 19 |
| Pediococcus spp | - | - | 2 | - | - |
| Streptococcus spp | 7 | 5 | 2 | - | 10 |
| Staphylococcus spp | 9 | 2 | 15 | 6 | 10 |
| Gram negative | |||||
| Aeromonas hydrophila | 1 | - | - | - | - |
| Citrobacter spp | 10 | - | 2 | - | 10 |
| Escherichia coli | 32 | 20 | 10 | - | 14 |
| Enterobacter cloacae | 6 | 8 | 2 | - | 14 |
| Haphnia alvey | 3 | 3 | - | - | - |
| Klebsiella spp | 14 | 22 | 7 | - | 10 |
| Morganella morganii | 1 | 2 | - | - | - |
| Proteus spp | 6 | 3 | - | - | - |
| Pseudomonas spp | 1 | 8 | 2 | - | 5 |
| Serratia spp | - | 10 | |||
| Yersinia enterocolitica | 1 | - | - | - | - |
| Anaerobic bacteria | |||||
| Bacteroides spp | - | 2 | - | 6 | - |
| Clostridium bifermentans | - | - | - | 11 | - |
| Clostridium perfringens | 1 | 5 | - | 37 | - |
| Fusobacterium spp | - | 2 | - | - | - |
| Veillonella spp | - | - | 2 | - | - |
| Fungi | |||||
| Aspergillus spp | - | 6 | |||
| Candida spp | 1 | 7 | 10 | 3 | - |
* Microbial species were isolated from the sludge removed from the whole blocked biliary stents.
† Microbial species were isolated from the sludge removed from the central part of blocked biliary stents.
‡ Microbial species were isolated from the sludge removed from a 2.5 mm segment of both duodenal and biliary ends of blocked and unblocked biliary stents.
δ Microbial species were isolated from the sludge removed from a 5 mm segment of the proximal end of unblocked biliary stents.
|| Microbial species were isolated from the sludge removed from whole unblocked biliary stents.
A synergistic effect on bacterial adherence and biofilm formation between Gram-positive and Gram-negative bacterial species has also been reported.43 Numerous studies have demonstrated the multispecies nature of microbial biofilm which progressively increases in thickness and contributes significantly to occlusion of the stent lumen.28,29,40,43–45 To address the issue of how a biofilm could reach such a thickness to significantly narrow the lumen of the stent, one must remember that a biofilm, by definition, is a microbial community embedded within an exopolysaccharide matrix and which also engulfs a number of “foreign bodies” of different sizes. Further, the three-dimensional structure of biofilm recognizes the presence of voids and channels needed for the diffusion of nutrients and signal molecules required for intercellular communication. In biliary stent clogging, “foreign bodies” engulfed in the growing biofilm are not only microbial byproducts and proteins but also large-sized dietary fibers, crystals of fatty acid calcium salts and deposits of amorphous calcium bilirubinate.
Another critical parameter influencing biofilm growth is bile viscosity which differs on the basis of a patient’s health status. According to Poiseuille’s law, if bile viscosity increases, the maintenance of the same bile flow requires an increase in the inner stent diameter, and it has been calculated that an increase of 0.2 mm in inner stent diameters corresponds to a 300% increase in bile flow.46 Thus, narrowing of the stent lumen, as a consequence of the increase in biofilm thickness, causes the slowing of bile flow promoting both spontaneous and bacteria-driven bile salt precipitation. Considering that the mean diameter of slime-producing, biofilm-forming bacteria is around 1 μm, a reduction of 0.2 mm in a 10 Fr polyethylene stent (inner diameter 2.4 mm) would correspond to a biofilm constituted by 200 tight bacterial layers. However, as previously discussed, the effective thickness of each bacterial layer is certainly much higher and strongly depends on the continuous engulfment of large-sized “foreign bodies.” In conclusion, biofilm growth in biliary stents represents a multi-parameter system that is difficult to describe by a simple mathematical model that only considers the increase of biofilm thickness as a function of size of bacterial cells and their duplication rate. At any rate, biofilm formation occurs when a number of individual microbial cells attach to the polymeric surfaces of the stent.47 The ability of bacteria to perform this initial attachment event is controlled by both environmental factors, such as pH, temperature, nutrient levels and genetic factors that include the presence of genes such as those which encode motility structures and adhesins.47,48 In fact, the presence of the protein conditioning film is thought to mediate microbial attachment via specific microbial adhesins able to recognize the different host proteins (table 2 ▶) layered on the polymeric stent surface. After the initial phase of attachment, microorganisms begin to grow as a monolayer to form microcolonies. During their sessile growth, bacteria undergo a series of phenotypic changes through the expression of various enzymes which lead to the production of an exopolysaccharide matrix, known as slime,49,50 that gives rise to the complex architecture of the mature biofilm.47,51 Slime enables the microorganisms to better adhere to the stent surface and protects the embedded bacteria from the action of antimicrobial agents. As the biofilm continues to grow, bacteria may spread into uncolonized areas by detaching from the biofilm and re-entering their planktonic mode. If this occurs, these planktonic bacteria can repeat the cycle on new surfaces. Sessile microbial growth almost always implies a marked decrease in susceptibility to antimicrobial agents compared with planktonic growth.52,53
Table 2.
Bacterial enzymes and other sludge components involved in the occlusive process of plastic biliary stents.
| Component | Role | |
| Bacterial enzymes | ||
| β-glucuronidase from C. perfringens | Deconjugate bilirubin and other bile constituents | |
| Lecithinase and phospholipase C from Clostridium spp | Break down lipids and lecithin and cause precipitation of calcium salts of fatty acids44 | |
| Other components | ||
|
Bile acid (9 ± 4%) Bilirubin (12 ± 7%) Cholesterol (5 ± 3%) Lecithin (6 ± 3%) Plant fibers (20 ± 10%) Proteins (25 ± 12%) |
} Groen et al27 | Plant fibers decrease bile flow and form a biomaterial entrapping network37 13–16 KDa proteins tightly bound to the inner stent walls form a conditioning film27 |
| Bile glycoprotein mucin (12.4%)36 | Affect bacterial adhesion? Promote stent-sludge accumulation?36 | |
| Bile immonoglobulins32 | Facilitate bacterial adhesion, clumping and biofilm formation?32 | |
| Fibronectin, vitronectin, laminin, fibrin, collagen30–34 | Form a conditioning film on the inner stent surface enhancing microbial adhesion | |
In a recent paper from our group, Enterococcus faecalis and Enterococcus faecium strains isolated from biliary stents have been investigated for plasmid content, sex-pheromone response, presence of genes encoding for aggregation substance and adhesive properties. Virulence genes encoding for aggregation substance have been detected by polymerase chain reaction and the ability of clinical isolates to adhere to in vitro cultured cells and to produce biofilm, as well as their susceptibility to various antibiotics, has been assessed. This study indicates that the production of slime exhibited by most enterococcal isolates plays an important role in the colonization and subsequent occlusion of biliary stents, suggesting that aggregation substance is implicated in the occlusion process and that enterococci carrying aggregation substance genes have a selective advantage in endoprosthesis colonization.54–56
The accumulation of biliary sludge is thought to be a multifactorial process in which, other than microbial growth, slime production and biofilm formation, a significant role is played by some bacterial enzymes (table 2 ▶). Chemical analyses have shown that, in addition to the nonbacterial constituents listed in table 2 ▶, calcium bilirubinate and calcium palmitate are commonly present in biliary sludge.15,27 In fact, β-glucuronidase, produced by E. coli and Clostridium species,57–59 deconjugates bilirubin which combines with calcium ions, precipitating as calcium bilirubinate, thus increasing the amount of sludge.60 The free glucuronic acids can be easily utilized by bacteria as an energy source and can form glycocalyx, which contributes to the increase of sludge mass. In addition to the previously discussed enzymatic activity, phospholipase C, which is able to hydrolyze biliary lecithin causing the precipitation of calcium palmitate, has been evidenced in Clostridium species.41
Cholesterol crystals are also a common finding in biliary sludge. In fact, cholesterol is solubilized in phospholipid vesicles, as well as together with phospholipids and bile salts in mixed micelles.61 Since the formation of mixed micelles requires more phospholipids than cholesterol, a larger amount of phospholipids is removed during the conversion of vesicles into mixed micelles. As a result, the residual vesicles become progressively richer in cholesterol which tend to aggregate, leading to the precipitation of cholesterol crystals.
Preventing Stent Occlusion
It is well known that larger diameter plastic biliary stents have a prolonged patency, but also that the threshold value of 12 Fr cannot be exceeded because of the maximum inner diameter of currently available duodenoscopes.62 The stent design, particularly the presence or absence of side holes, has also been considered as a preventive strategy. However, comparative studies on traditional polyethylene stents versus the newly designed Tannenbaum Teflon stents (Cook UK, Letchworth, Hertfordshire) without side holes found no significant advantage with regard to stent patency and/or patient survival.63,64 On the other hand, more recent data obtained from a retrospective, nonrandomized comparative study on 130 patients receiving plastic stents with and without side holes, suggest the use of plastic stents without side holes would be more effective for primary biliary stenting of middle and lower biliary strictures.65
A recent randomized prospective trial66 compared a DoubleLayer stent (Olympus, Tokyo, Japan) without side holes with a standard polyethylene stent with side holes in a total of 120 patients with jaundice due to malignant strictures. The study showed that the DoubleLayer stent had a longer patency period and a reduced risk of occlusion.
Regarding duodenobiliary reflux as a relevant factor in stent clogging, stents with a one-directional valve mechanism allowing only anterograde bile flow were recently developed. However, these so-called anti-reflux stents still need large randomized trials to clearly establish their clinical efficacy.67
Other approaches demonstrating in vitro reduction of bacterial adhesion concerned new biomaterials for biliary stents,68 hydrophilic polymer-coated polyurethane stents69 and silver-coated stents,70 and also the impregnation of polymeric biliary stents with antimicrobial agents.71,72 However, results obtained so far have not reached enough clinical relevance to consider these innovative stents as definitive substitutes for the conventional ones.
In the hope that planktonic bacteria are killed before their attachment on the stent surface, prophylactic treatment with antibiotics has also been proposed to prevent clogging. In the treatment of suppurative cholangitis, quinolones have been reported to be effective antimicrobial agents exhibiting a wide antimicrobial activity against most Gram-negative bacilli and some Gram-positive cocci.73 In vitro and in vivo studies have reported that ciprofloxacin has good tissue penetration and, reaching a high concentration in the bile, is able to reduce bacterial adhesions.74–76 However, despite the benefits expected from in vitro studies, clinical trials based on ciprofloxacin prophylaxis have failed to demonstrate significant prolonging of stent patency, whereas quality of life improved and, after stenting, cholangitis was reduced.77,78 Other antibiotics, including ampicillin-sulbactam,79 ursodeoxycholic acid with cyclical antibiotics80 or plus norfloxacin,81 have also been tested for their in vitro and/or in vivo ability to prevent stent clogging. The Cochrane review of this area82 investigated antibiotics and choleretic agent efficacy in preventing stent clogging and prolonging patient survival after endoscopic stenting for malignant biliary obstruction. The meta-analysis of five randomized trials, including 258 patients treated with polyethylene stents, did not show a significant effect of treatment on the duration of stent patency or mortality.
In conclusion, further studies are required to assess the antibiotic susceptibility of biofilm-forming microorganisms normally found in biliary sludge based on the knowledge of their 100-fold to 1000-fold increase in antimicrobial resistance.83 Such resistance, presumably, is related to the emergence of persister cells.53
References
- 1.Soehendra N, Reynders-Frederix V. Palliative bile duct drainage - a new endoscopic method of introducing a transpapillary drain. Endoscopy 1980;12:8–11. [DOI] [PubMed] [Google Scholar]
- 2.Huibregtse K, Tytgat GN. Palliative treatment of obstructive jaundice by transpapillary introduction of large bore bile duct endoprosthesis. Gut 1982;23:371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dowidar N, Kolmos HJ, Matzen P. Experimental clogging of biliary endoprostheses. Role of bacteria, endoprosthesis material, and design. Scand J Gastroenterol 1992;27:77–80. [DOI] [PubMed] [Google Scholar]
- 4.McAllister EW, Carey LC, Brady PG, Heller R, Kovacs SG. The role of polymeric surface smoothness of biliary stents in bacterial adherence, biofilm deposition, and stent occlusion. Gastrointest Endosc 1993;39:422–425. [DOI] [PubMed] [Google Scholar]
- 5.Weickert U, Venzke T, Konig J, Janssen J, Remberger K, Greiner L. Why do bilioduodenal plastic stents become occluded? A clinical and pathological investigation on 100 consecutive patients. Endoscopy 2001;33:786–790. [DOI] [PubMed] [Google Scholar]
- 6.Smith AC, Dowsett JF, Russell RC, Hatfield AR, Cotton PB. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bileduct obstruction. Lancet 1994;344:1655–1660. [DOI] [PubMed] [Google Scholar]
- 7.McLean GK, Burke DR. Role of endoprostheses in the management of malignant biliary obstruction. Radiology 1989;170:961–967. [DOI] [PubMed] [Google Scholar]
- 8.Adler DG, Baron TH, Davila RE, Egan J, Hirota WK, Leighton JA, Qureshi W, Rajan E, Zuckerman MJ, Fanelli R, Wheeler-Harbaugh J, Faigel DO; Standards of Practice Committee of American Society for Gastrointestinal Endoscopy. ASGE guideline: the role of ERCP in diseases of the biliary tract and the pancreas. Gastrointest Endosc 2005;62:1–8. [DOI] [PubMed] [Google Scholar]
- 9.Speer AG, Cotton PB, Russell RC, Mason RR, Hatfield AR, Leung JW, MacRae KD, Houghton J, Lennon CA. Randomised trial of endoscopic versus percutaneous stent insertion in malignant obstructive jaundice. Lancet 1987;2:57–62. [DOI] [PubMed] [Google Scholar]
- 10.Andersen JR, Sorensen SM, Kruse A, Rokkjaer M, Matzen P. Randomised trial of endoscopic endoprosthesis versus operative bypass in malignant obstructive jaundice. Gut 1989;30:1132–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanapa P, Williamson RC. Surgical palliation for pancreatic cancer: developments during the past two decades. Br J Surg 1992;79:8–20. [DOI] [PubMed] [Google Scholar]
- 12.Ballinger AB, McHugh M, Catnach SM, Alstead EM, Clark ML. Symptom relief and quality of life after stenting for malignant bile duct obstruction. Gut 1994;35:467–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinol V, Castells A, Bordas JM, Real MI, Llach J, Montana X, Feu F, Navarro S. Percutaneous self-expanding metal stents versus endoscopic polyethylene endoprostheses for treating malignant biliary obstruction: randomized clinical trial. Radiology 2002;225:27–34. [DOI] [PubMed] [Google Scholar]
- 14.Catalano MF, Geenen JE, Lehman GA, Siegel JH, Jacob L, McKinley MJ, Raijman I, Meier P, Jacobson I, Kozarek R, Al-Kawas FH, Lo SK, Dua KS, Baille J, Ginsberg GG, Parsons W, Meyerson SM, Cohen S, Nelson DB, McHattie JD, Carr-Locke DL. “Tannenbaum” Teflon stents versus traditional polyethylene stents for treatment of malignant biliary stricture. Gastrointest Endosc 2002;55:354–358. [DOI] [PubMed] [Google Scholar]
- 15.Costa Costa L, Bracco P, Vada S, Trossarelli L, Jacobson K. A chemical analysis of the clogging process of polymeric biliary endoprostheses. Biomaterials 2001;22:3113–3119. [DOI] [PubMed] [Google Scholar]
- 16.Motte S, Deviere J, Dumonceau JM, Serruys E, Thys JP, Cremer M. Risk factors for septicemia following endoscopic biliary stenting. Gastroenterology 1991;101:1374–1381. [DOI] [PubMed] [Google Scholar]
- 17.Shepherd HA, Royle G, Ross AP, Diba A, Arthur M, Colin-Jones D. Endoscopic biliary endoprosthesis in the palliation of malignant obstruction of the distal common bile duct: a randomized trial. Br J Surg 1988;75:1166–1168. [DOI] [PubMed] [Google Scholar]
- 18.Schmassmann A, von Gunten E, Knuchel J, Scheurer U, Fehr HF, Halter F. Wallstents versus plastic stents in malignant biliary obstruction: effects of stent patency of the first and second stent on patient compliance and survival. Am J Gastroenterol 1996;91:654–659. [PubMed] [Google Scholar]
- 19.Speer AG, Cotton PB, MacRae KD. Endoscopic management of malignant biliary obstruction: stents of 10 French gauge are preferable to stents of 8 French gauge. Gastrointest Endosc 1988;34:412–417. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien S, Hatfield AR, Craig PI, Williams SP. A three year follow up of self expanding metal stents in the endoscopic palliation of longterm survivors with malignant biliary obstruction. Gut 1995;36:618–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Berkel AM, Bergman JJ, Waxman I, Andres P, Huibregtse K. Wallstents for metastatic biliary obstruction. Endoscopy 1996;28:418–421. [DOI] [PubMed] [Google Scholar]
- 22.Tsang TK, Pollack J, Chodash HB. Silicone-covered metal stents: an in vitro evaluation for biofilm formation and patency. Dig Dis Sci 1999;44:1780–1785. [DOI] [PubMed] [Google Scholar]
- 23.Soderlund C, Linder S. Covered metal versus plastic stents for malignant common bile duct stenosis: a prospective, randomized, controlled trial. Gastrointest Endosc 2006;63:986–995. [DOI] [PubMed] [Google Scholar]
- 24.Marano BJ Jr, Bonanno CA. Metallic biliary endoprosthesis causing duodenal perforation and acute upper gastrointestinal bleeding. Gastrointest Endosc 1994;40:257–258. [DOI] [PubMed] [Google Scholar]
- 25.Moss AC, Morris E, MacMathuna P. Palliative biliary stents for obstructing pancreatic carcinoma. Cochrane Database Syst Rev 2006;2:CD004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basoli A, Fiocca F, Di Rosa R, Baldassarri L, Donelli G. Biliary stent occlusion: a microbiological and scanning electron microscopy (SEM) investigation. In: Zanella E, ed. Advances in abdominal surgery. Dordrecht, The Netherlands: Kluwer Academic Publishers;1999. 69–80.
- 27.Groen AK, Out T, Huibregtse K, Delzenne B, Hoek FJ, Tytgat GN. Characterization of the content of occluded biliary endoprostheses. Endoscopy 1987;19:57–59. [DOI] [PubMed] [Google Scholar]
- 28.Speer AG, Cotton PB, Rode J, Seddon AM, Neal CR, Holton J, Costerton JW. Biliary stent blockage with bacterial biofilm. A light and electron microscopy study. Ann Intern Med 1988;108:546–553. [DOI] [PubMed] [Google Scholar]
- 29.Leung JW, Ling TK, Kung JL, Vallance-Owen J. The role of bacteria in the blockage of biliary stents. Gastrointest Endosc 1988;34:19–22. [DOI] [PubMed] [Google Scholar]
- 30.Moesch C, Sautereau D, Cessot F, Berry P, Mounier M, Gainant A, Pillegand B. Physicochemical and bacteriological analysis of the contents of occluded biliary endoprostheses. Hepatology 1991;14:1142–1146. [PubMed] [Google Scholar]
- 31.An YH, Friedman RJ. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J Biomed Mater Res 1998;43:338–348. [DOI] [PubMed] [Google Scholar]
- 32.Chan FK, Suen M, Li JY, Sung JJ. Bile immunoglobulins and blockage of biliary endoprosthesis: an immunohistochemical study. Biomed Pharmacother 1998;52:403–407. [DOI] [PubMed] [Google Scholar]
- 33.Yu JL, Andersson R, Ljungh A. Protein adsorption and bacterial adhesion to biliary stent materials. J Surg Res 1996;62:69–73. [DOI] [PubMed] [Google Scholar]
- 34.Yu JL, Andersson R, Wang LQ, Bengmark S, Ljungh A. Fibronectin on the surface of biliary drain materials—a role in bacterial adherence. J Surg Res 1995;59:596–600. [DOI] [PubMed] [Google Scholar]
- 35.van Berkel AM, van Marle J, van Veen H, Groen AK, Huibregtse K. A scanning electron microscopic study of biliary stent materials. Gastrointest Endosc 2000;51:19–22. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Tsang TK, Jack CA. Bile glycoprotein mucin in sludge occluding biliary stent. J Lab Clin Med 2003;142:58–65. [DOI] [PubMed] [Google Scholar]
- 37.van Berkel AM, van Marle J, Groen AK, Bruno MJ. Mechanisms of biliary stent clogging: confocal laser scanning and scanning electron microscopy. Endoscopy 2005;37:729–734. [DOI] [PubMed] [Google Scholar]
- 38.Sung JY, Leung JW, Shaffer EA, Lam K, Olson ME, Costerton JW. Ascending infection of the biliary tract after surgical sphincterotomy and biliary stenting. J Gastroenterol Hepatol 1992;7:240–245. [DOI] [PubMed] [Google Scholar]
- 39.Dowidar N, Kolmos HJ, Lyon H, Matzen P. Clogging of biliary endoprostheses. A morphologic and bacteriologic study. Scand J Gastroenterol 1991;26:1137–1144. [DOI] [PubMed] [Google Scholar]
- 40.Di Rosa R, Basoli A, Donelli G, Penni A, Salvatori FM, Fiocca F, Baldassarri L. A microbiological and morphological study of blocked biliary stents. Microbial Ecology in Health and Diseases 1999;11:84–88. [Google Scholar]
- 41.Leung JW, Liu Y, Chan RC, Tang Y, Mina Y, Cheng AF, Silva J Jr. Early attachment of anaerobic bacteria may play an important role in biliary stent blockage. Gastrointest Endosc 2000;52:725–729. [DOI] [PubMed] [Google Scholar]
- 42.Maillot N, Aucher P, Robert S, Richer JP, Bon D, Moesch C, Grollier G, Irani J, Carretier M, Beauchant M. Polyethylene stent blockage: a porcine model. Gastrointest Endosc 2000;51:12–18. [DOI] [PubMed] [Google Scholar]
- 43.Leung JW, Liu YL, Desta T, Libby E, Inciardi JF, Lam K. Is there a synergistic effect between mixed bacterial infection in biofilm formation on biliary stents? Gastrointest Endosc 1998;48:250–257. [DOI] [PubMed] [Google Scholar]
- 44.Sung JY, Leung JW, Shaffer EA, Lam K, Costerton JW. Bacterial biofilm, brown pigment stone and blockage of biliary stents. J Gastroenterol Hepatol 1993;8:28–34. [DOI] [PubMed] [Google Scholar]
- 45.Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, Marrie TJ. Bacterial biofilms in nature and disease. Annu Rev Microbiol 1987;41:435–464. [DOI] [PubMed] [Google Scholar]
- 46.Rey JF, Maupetit P, Greff M. Experimental study of biliary endoprosthesis efficiency. Endoscopy 1985;17:145–148. [DOI] [PubMed] [Google Scholar]
- 47.Costerton JW. Overview of microbial biofilms. J Ind Microbiol 1995;15:137–140. [DOI] [PubMed] [Google Scholar]
- 48.O’Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu Rev Microbiol 2000;54:49–79. [DOI] [PubMed] [Google Scholar]
- 49.Baldassarri L, Donnelli G, Gelosia A, Voglino MC, Simpson AW, Christensen GD. Purification and characterization of the staphylococcal slime-associated antigen and its occurrence among Staphylococcus epidermis clinical isolates. Infect Immun 1996;64:3410–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baldassarri L, Donelli G, Gelosia A, Simpson AW, Christensen GD. Expression of slime interferes with in vitro detection of host protein receptors of Staphylococcus epidermidis. Infect Immun 1997;65:1522–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Danese PN, Pratt LA, Kolter R. Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J Bacteriol 2000;182:3593–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donelli G, Guaglianone E. Emerging role of Enterococcus spp in catheter-related infections: biofilm formation and novel mechanisms of antibiotic resistance. J Vasc Access 2004;5:3–9. [DOI] [PubMed] [Google Scholar]
- 53.Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother 2001;45:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waar K, van der Mei HC, Harmsen HJ, Degener JE, Busscher HJ. Adhesion to bile drain materials and physicochemical surface properties of Enterococcus faecalis strains grown in the presence of bile. Appl Environ Microbiol 2002;68:3855–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waar K, van der Mei HC, Harmsen HJ, Degener JE, Busscher HJ. Enterococcus faecalis surface proteins determine its adhesion mechanism to bile drain materials. Microbiology 2002;148:1863–1870. [DOI] [PubMed] [Google Scholar]
- 56.Donelli G, Paoletti C, Baldassarri L, Guaglianone E, Di Rosa R, Magi G, Spinaci C, Facinelli B. Sex pheromone response, clumping, and slime production in enterococcal strains isolated from occluded biliary stents. J Clin Microbiol 2004;42:3419–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buehler HJ, Katzman PA, Doisy EA. Studies on beta-glucuronidase from E. coli. Proc Soc Exp Biol Med 1951;76:672–676. [DOI] [PubMed] [Google Scholar]
- 58.Sakaguchi Y, Murata K, Kimura M. Clostridium perfringens and other anaerobes isolated from bile. J Clin Pathol 1983;36:345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leung JW, Liu YL, Leung PS, Chan RC, Inciardi JF, Cheng AF. Expression of bacterial beta-glucuronidase in human bile: an in vitro study. Gastrointest Endosc 2001;54:346–350. [DOI] [PubMed] [Google Scholar]
- 60.Maki T. Pathogenesis of calcium bilirubinate gallstone: role of E. coli, beta-glucuronidase and coagulation by inorganic ions, polyelectrolytes and agitation. Ann Surg 1966;164:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holzbach RT. Nucleation of cholesterol crystals in native bile. Hepatology 1990;12:155S–159S. [PubMed] [Google Scholar]
- 62.Kadakia SC, Starnes E. Comparison of 10 French gauge stent with 11.5 French gauge stent in patients with biliary tract diseases. Gastrointest Endosc 1992;38:454–459. [DOI] [PubMed] [Google Scholar]
- 63.England RE, Martin DF, Morris J, Sheridan MB, Frost R, Freeman A, Lawrie B, Deakin M, Fraser I, Smith K. A prospective randomised multicentre trial comparing 10 Fr Teflon Tannenbaum stents with 10 Fr polyethylene Cotton-Leung stents in patients with malignant common duct strictures. Gut 2000;46:395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Terruzzi V, Comin U, De Grazia F, Toti GL, Zambelli A, Beretta S, Minoli G. Prospective randomized trial comparing Tannenbaum Teflon and standard polyethylene stents in distal malignant biliary stenosis. Gastrointest Endosc 2000;51:23–27. [DOI] [PubMed] [Google Scholar]
- 65.Nakamura S, Ohara H, Yamada T, Nakazawa T, Sano H, Ando H, Kajino S, Hashimoto T, Ando T, Nomura T, Joh T, Okayama Y, Uchida A, Iida M, Itoh M. Efficacy of plastic tube stents without side holes for middle and lower biliary strictures. J Clin Gastroenterol 2002;34:77–80. [DOI] [PubMed] [Google Scholar]
- 66.Tringali A, Mutignani M, Perri V, Zuccala G, Cipolletta L, Bianco MA, Rotondano G, Philipper M, Schumacher B, Neuhaus H, Schmit A, Deviere J, Costamagna G. A prospective, randomized multicenter trial comparing DoubleLayer and polyethylene stents for malignant distal common bile duct strictures. Endoscopy 2003;35:992–997. [DOI] [PubMed] [Google Scholar]
- 67.Reddy DN, Banerjee R, Choung OW. Antireflux biliary stents: are they the solution to stent occlusions? Curr Gastroenterol Rep 2006;8:156–160. [DOI] [PubMed] [Google Scholar]
- 68.Cetta F, Rappuoli R, Montalto G, Baldi C, Gori M, Cetta D, Zuckermann M, Magnani A, Barbucci R. New biliary endoprosthesis less liable to block in biliary infections: description and in vitro studies. Eur J Surg 1999;165:782–785. [DOI] [PubMed] [Google Scholar]
- 69.Jansen B, Goodman LP, Ruiten D. Bacterial adherence to hydrophilic polymer-coated polyurethane stents. Gastrointest Endosc 1993;39:670–673. [DOI] [PubMed] [Google Scholar]
- 70.Leung JW, Lau GT, Sung JJ, Costerton JW. Decreased bacterial adherence to silver-coated stent material: an in vitro study. Gastrointest Endosc 1992;38:338–340. [DOI] [PubMed] [Google Scholar]
- 71.Rees EN, Tebbs SE, Elliott TS. Role of antimicrobial-impregnated polymer and Teflon in the prevention of biliary stent blockage. J Hosp Infect 1998;39:323–329. [DOI] [PubMed] [Google Scholar]
- 72.Sung JY, Shaffer EA, Lam K, Rususka I, Costerton JW. Hydrophobic bile salt inhibits bacterial adhesion on biliary stent material. Dig Dis Sci 1994;39:999–1006. [DOI] [PubMed] [Google Scholar]
- 73.Sung JJ, Lyon DJ, Suen R, Chung SC, Co AL, Cheng AF, Leung JW, Li AK. Intravenous ciprofloxacin as treatment for patients with acute suppurative cholangitis: a randomized, controlled clinical trial. J Antimicrob Chemother 1995;35:855–864. [DOI] [PubMed] [Google Scholar]
- 74.Leung JW, Liu YL, Desta TD, Libby ED, Inciardi JF, Lam K. In vitro evaluation of antibiotic prophylaxis in the prevention of biliary stent blockage. Gastrointest Endosc 2000;51:296–303. [DOI] [PubMed] [Google Scholar]
- 75.Libby ED, Leung JW. Prevention of biliary stent clogging: a clinical review. Am J Gastroenterol 1996;91:1301–1308. [PubMed] [Google Scholar]
- 76.Leung Leung JW, Libby ED, Morck DW, McKay SG, Liu Y, Lam K, Olson ME. Is prophylactic ciprofloxacin effective in delaying biliary stent blockage? Gastrointest Endosc 2000;52:175–182. [DOI] [PubMed] [Google Scholar]
- 77.Luman W, Ghosh S, Palmer KR. A combination of ciprofloxacin and Rowachol does not prevent biliary stent occlusion. Gastrointest Endosc 1999;49:316–321. [DOI] [PubMed] [Google Scholar]
- 78.Chan G, Barkun J, Barkun AN, Valois E, Cohen A, Friedman G, Parent J, Love J, Enns R, Baffis V, Jabbari M, Szego P, Stein L, Abraham N. The role of ciprofloxacin in prolonging polyethylene biliary stent patency: a multicenter, double-blinded effectiveness study. J Gastrointest Surg 2005;9:481–488. [DOI] [PubMed] [Google Scholar]
- 79.Tsang TK, Pollack J, Chodash HB. Inhibition of biliary endoprostheses occlusion by ampicillin-sulbactam in an in vitro model. J Lab Clin Med 1997;130:643–648. [DOI] [PubMed] [Google Scholar]
- 80.Ghosh S, Palmer KR. Prevention of biliary stent occlusion using cyclical antibiotics and ursodeoxycholic acid. Gut 1994;35:1757–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barrioz T, Ingrand P, Besson I, de Ledinghen V, Silvain C, Beauchant M. Randomised trial of prevention of biliary stent occlusion by ursodeoxycholic acid plus norfloxacin. Lancet 1994;344:581–582. [DOI] [PubMed] [Google Scholar]
- 82.Galandi D, Schwarzer G, Bassler D, Allgaier HP. Ursodeoxycholic acid and/or antibiotics for prevention of biliary stent occlusion. Cochrane Database Syst Rev 2002;3:CD003043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smith AW. Biofilms and antibiotic therapy: is there a role for combating bacterial resistance by the use of novel drug delivery systems? Adv Drug Deliv Rev 2005;57:1539–1550. [DOI] [PubMed] [Google Scholar]