Abstract
In Arabidopsis, resistance to Turnip Crinkle Virus (TCV) depends on the resistance (R) gene, HRT, and the recessive locus rrt. Resistance also depends on salicylic acid (SA), EDS1, and PAD4. Exogenous application of SA confers resistance in RRT-containing plants by increasing HRT transcript levels in a PAD4-dependent manner. Here we report that reduction of oleic acid (18:1) can also induce HRT gene expression and confer resistance to TCV. However, the 18:1-regulated pathway is independent of SA, rrt, EDS1, and PAD4. Reducing the levels of 18:1, via a mutation in the SSI2-encoded stearoyl-acyl carrier protein-desaturase, or by exogenous application of glycerol, increased transcript levels of HRT as well as several other R genes. Second-site mutations in the ACT1-encoded glycerol-3-phosphate acyltransferase or GLY1-encoded glycerol-3-phosphate dehydrogenase restored 18:1 levels in HRT ssi2 plants and reestablished a dependence on rrt. Resistance to TCV and HRT gene expression in HRT act1 plants was inducible by SA but not by glycerol, whereas that in HRT pad4 plants was inducible by glycerol but not by SA. The low 18:1-mediated induction of R gene expression was also dependent on ACT1 but independent of EDS1, PAD4, and RAR1. Intriguingly, TCV inoculation did not activate this 18:1-regulated pathway in HRT plants, but instead resulted in the induction of several genes that encode 18:1-synthesizing isozymes. These results suggest that the 18:1-regulated pathway may be specifically targeted during pathogen infection and that altering 18:1 levels may serve as a unique strategy for promoting disease resistance.
Keywords: glycerol, salicylic acid, stearoyl-acyl carrier protein-desaturase, Turnip Crinkle Virus, ssi2
Plants respond to pathogen perception by triggering a cascade of responses. Perception involves strain-specific detection of a pathogen-encoded elicitor, through direct or indirect interaction, with the corresponding resistance (R) gene product. Such an interaction (also known as incompatible interaction) triggers one or more defense signaling pathways and is often associated with the induction of the hypersensitive response (HR) at the site of pathogen entry. HR is one of the first visible manifestations of the host-induced defense response and is thought to prevent development and movement of the pathogen by inducing deliberate death of the infected cells. R protein-mediated recognition of pathogen can also lead to the accumulation of various phytohormones including salicylic acid (SA), jasmonic acid, and/or ethylene, which in turn signal the activation of defense gene expression. Each hormone activates a specific pathway, and these act individually, synergistically, or antagonistically, depending on the pathogen involved, the ultimate effect of which confers disease resistance and prevents spread of the pathogen to uninoculated parts of the plant.
Several components of the SA-mediated pathway have been identified, mutations in which lead to enhanced susceptibility to various pathogens [supporting information (SI) Table 2] (1–3). Mutations in eds1 (4), eds5 (5), pad4 (6), and sid2 (7) lower or abolish the pathogen-induced increase in SA levels. The EDS1, EDS5, PAD4, and SID2 proteins participate in basal disease resistance to virulent pathogens as well as R protein-mediated resistance to avirulent pathogens. Defense signaling mediated via a majority of R proteins, which contain Toll–IL1-like domains at their N termini, depends on EDS1. Conversely, the NDR1 protein is required for many R proteins that contain coiled-coil domains at their N termini. Besides EDS1 and NDR1, R protein-mediated signaling is also known to require the RAR1 and SGT1 proteins, which are implicated as possible regulators of protein ubiquitylation (8, 9). However, unlike EDS1 and NDR1, RAR1 and SGT1 proteins can mediate signaling via R proteins that contain either Toll–IL1-like or coiled-coil domains at their N termini.
Unlike most R genes encoding for coiled coil-containing proteins, HRT, which confers resistance to Turnip Crinkle Virus (TCV), depends on EDS1. HRT-mediated resistance is also dependent on PAD4, EDS5, and SID2, but is independent of RAR1 and SGT1 (10). In addition to HRT, resistance to TCV also requires the recessive locus rrt (11). However, this requirement for rrt can be overcome by increasing the levels of HRT transcript via exogenous application of SA (10). Although SA appears to be downstream of the initial recognition event, it cannot confer resistance in the absence of HRT and PAD4 (10). Mobilizing HRT into susceptible mutant backgrounds carrying high SA, such as ssi2 or cpr5, can constitutively increase expression of HRT and, thereby, resistance to TCV (10). The ssi2 and cpr5 plants contain mutations in genes encoding for stearoyl-acyl carrier protein-desaturase (S-ACP-DES) (12) and a transmembrane protein of unknown function (13, 14), respectively. Although molecular mechanisms responsible for constitutive defense in cpr5 are not clear, characterization of ssi2 and its suppressors has implicated low levels of oleic acid (18:1) in altering defense signaling (15–17). The diverse biology of ssi2 and cpr5 mutants suggests that high levels of endogenous SA may be responsible for the up-regulation of HRT in HRT ssi2 and HRT cpr5 plants (10–21).
In the present study we demonstrate the presence of an alternate, SA-, rrt-, PAD4-, and EDS1-independent pathway, which confers resistance to TCV by increasing HRT transcript levels. This pathway, controlled by the levels of oleic acid, also regulates expression of several other R genes, which encode structurally different R proteins. These data present insights into the regulation of plant defense pathways.
Results
The ssi2 Mutation Up-Regulates HRT Gene Expression in an SA- and PAD4-Independent Manner.
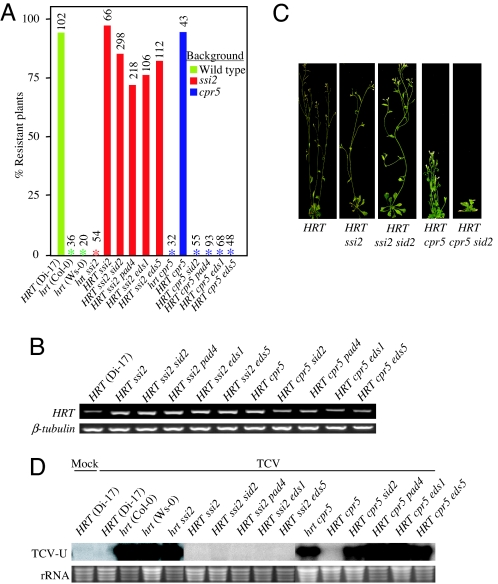
To examine the role of high endogenous SA in the up-regulation of HRT gene expression, we mobilized the sid2 mutation into HRT ssi2 and HRT cpr5 plants and obtained HRT ssi2 sid2 and HRT cpr5 sid2 plants. A mutation in sid2 was expected to abolish the elevated levels of SA in these plants (7, 17) and, thereby, down-regulate HRT gene expression, resulting in susceptibility to TCV. As expected, HRT cpr5 sid2 plants showed basal level expression of HRT and pronounced susceptibility (Fig. 1), confirming that high endogenous SA levels in HRT cpr5 plants were likely responsible for elevated HRT gene expression and enhanced resistance. In contrast to these results, HRT ssi2 sid2 plants continued to show high levels of HRT transcript (Fig. 1B). Consistent with the increased transcript levels of HRT, the HRT ssi2 sid2 plants continued to show heightened resistance to TCV (Fig. 1 A, C, and D). This suggested that induction of HRT and enhanced TCV resistance in HRT ssi2 sid2 plants were independent of SA. The ssi2-mediated induction of HRT was also independent of mutations in eds1, pad4, and eds5, indicating that, in ssi2 plants, a factor(s) other than SA was responsible for the induced expression of HRT. However, as compared with HRT ssi2 plants, the HRT ssi2 sid2, HRT ssi2 eds5, HRT ssi2 eds1, and HRT ssi2 pad4 plants showed ≈15%, ≈18%, ≈20%, and ≈25% reductions in the number of resistant plants, respectively (Fig. 1A). These findings indicate that both SA-dependent and -independent signaling pathways triggered in HRT ssi2 plants have an additive effect on resistance to TCV. In contrast to HRT ssi2 plants, mutations in pad4, eds1, and eds5 abolished induced expression of HRT as well as resistance to TCV in HRT cpr5 plants (Fig. 1).
Fig. 1.
ssi2- and cpr5-mediated resistance signaling in various double mutant backgrounds. (A) Percentage of TCV-resistant plants in various genetic backgrounds. The number of plants tested is indicated above each bar. All plants were analyzed 3 weeks after inoculation. Asterisks indicate 100% susceptibility. (B) RT-PCR analyses showing HRT transcript levels in indicated genotypes. The level of β-tubulin was used as an internal control to normalize the amount of cDNA template. (C) Typical morphological phenotypes of TCV-inoculated plants; susceptible plants showed crinkling, stunted bolt development, and drooping of bolts. The resistant plants were morphologically similar to the mock-inoculated plants. The plants were photographed 2 weeks after inoculation. (D) Systemic spread of TCV to uninoculated tissue in TCV-inoculated plants. RNA was extracted from the uninoculated tissues at 18 days after inoculation and analyzed for the presence of the viral transcripts (TCV-U). Ethidium bromide staining of rRNA was used as a loading control.
HRT Promoter Responds to SA and Low 18:1 Conditions.
Because altered defense signaling in the ssi2 plants has been attributed to the reduced levels of 18:1 (15–17, 22), we checked the responsiveness of HRT promoter to SA and low 18:1 conditions. Histochemical assays for transient β-glucuronidase (GUS) activity in cells expressing the GUS protein under control of the HRT promoter were carried out. Agrobacterium cells transformed with an HRT promoter–GUS fusion vector were infiltrated into leaves from sid2 or ssi2 sid2 plants. Leaves from sid2 plants displayed GUS activity only when pretreated with SA (Fig. 2A) or glycerol (data not shown). By comparison, GUS activity was detected constitutively in ssi2 sid2 leaves. Taken together, these observations suggest that the HRT promoter responds to both SA and low 18:1 conditions.
Fig. 2.
HRT promoter assay and HRT transcript levels, 18:1 content, TCV resistance, and viral replication in leaves containing normal or higher oleate levels. (A) Transient GUS assay. Leaves were infiltrated with untransformed cells (-ve) or Agrobacterium transformed with HRT–GUS fusion construct. The sid2 plants were treated with water (−) or SA (+) for 2 days before infiltration. Leaf discs (sid2) or whole leaves (ssi2 sid2) were processed for GUS histochemical staining as described before (45). (B) RT-PCR analyses showing basal-level expression of HRT in HRT ssi2 act1 and HRT ssi2 gly1 plants. The level of β-tubulin was used as an internal control to normalize the amount of cDNA template. The 18:1 levels are a mean of six independent replicates. (C) Systemic spread of TCV to uninoculated tissue (TCV-U) in TCV-inoculated plants. RNA was extracted from the uninoculated tissues at 18 days after inoculation and analyzed for the presence of the viral transcripts. Ethidium bromide staining of rRNA was used as a loading control. (D) Typical morphological phenotypes of mock- and TCV-inoculated HRT ssi2 act1 and HRT ssi2 gly1 plants. The susceptible plants showed crinkling, stunted bolt development, and drooping of bolts. Plants were photographed at 8 days after inoculation. (E) Effect of 18:1 infiltrations on viral replication in the inoculated leaf. Oleic acid (O, 1 mM) or water (W) was injected 24 h before or after (last two lanes) TCV inoculation, and the samples were harvested 72 h after inoculation. Ethidium bromide staining of rRNA was used as a loading control..
Restoration of 18:1 Levels in HRT ssi2 Plants Compromises Resistance to TCV.
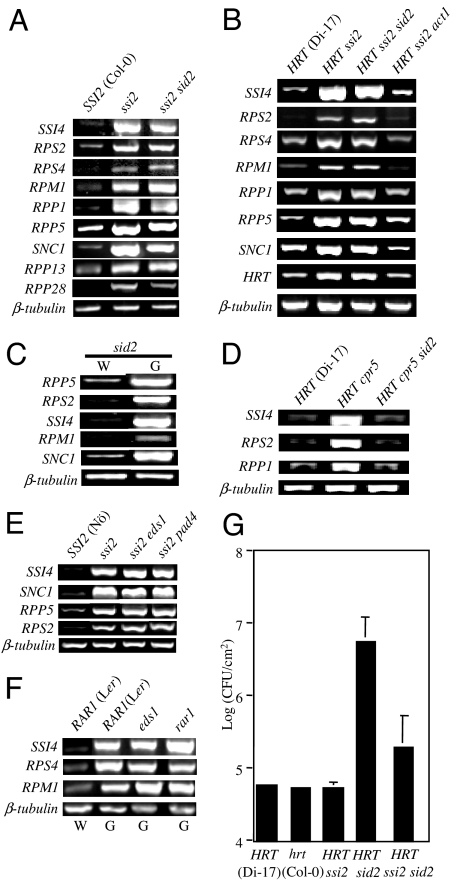
We next asked whether restoration of 18:1 levels would down-regulate HRT gene expression to WT-like levels. We crossed Di-17 plants (resistant, HRT/HRT rrt/rrt) with ssi2 act1 and ssi2 gly1 plants (both susceptible, hrt/hrt RRT/RRT); mutations in act1 and gly1 restore WT phenotypes in ssi2 plants by increasing the 18:1 content (15, 16) (SI Fig. 6A). Interestingly, HRT ssi2 act1 and HRT ssi2 gly1 plants showed basal level expression of HRT (Fig. 2B). Furthermore, resistance to TCV segregated with the recessive rrt allele; only 25% of HRT ssi2 act1 or HRT ssi2 gly1 plants showed resistance to TCV (Table 1 and Fig. 2 C and D). Fatty acid (FA) profiling revealed that, unlike HRT ssi2 and HRT ssi2 sid2 plants (17), the HRT ssi2 act1 and HRT ssi2 gly1 plants showed higher than or WT-like levels of 18:1, respectively (Fig. 2B). These data indicate that restoration of 18:1 levels in HRT ssi2 plants was sufficient to restore the basal level expression of HRT as well as susceptibility to TCV. Control crosses between Di-17 and act1 or gly1 segregated normally for HR and resistance (Table 1), suggesting that these phenotypes were not influenced by mutations in act1 or gly1.
Table 1.
Epistatic analyses of F2 populations generated by crossing Di-17 with various WT or mutant lines
| Cross | Total no. of plants analyzed | Genotype | No. of plants obtained | HR | Resistant | Susceptible | χ2 | P value* |
|---|---|---|---|---|---|---|---|---|
| Di-17 × Col-0 | 136 | HRT/− | 93 | + | 18 | 75 | 1.58 | 0.21 |
| Di-17 × Nössen | 132 | HRT/− | 94 | + | 22 | 72 | 0.13 | 0.72 |
| Di-17 × ssi2 act1 | 378 | HRT/− SSI2/− ACT1/− | 157 | + | 36 | 121 | 0.36 | 0.55 |
| HRT/− ssi2 | 66 | ND | 62 | 4 | 167.2 | 0.00† | ||
| HRT/− act1 | 54 | + | 11 | 43 | 0.62 | 0.43 | ||
| HRT/− ssi2 act1 | 37 | + | 9 | 28 | 0.03 | 0.86 | ||
| Di-17 × ssi2 gly1-3 | 301 | HRT/− SSI2/− GLY1/− | 108 | + | 26 | 82 | 0.05 | 0.82 |
| HRT/− ssi2 | 53 | ND | 51 | 2 | 143.4 | 0.00† | ||
| HRT/− gly1 | 44 | + | 12 | 32 | 0.12 | 0.73 | ||
| HRT/− ssi2 gly1 | 31 | + | 6 | 25 | 0.53 | 0.47 | ||
| Di-17 × act1 | 123 | HRT/− ACT1/− | 84 | + | 19 | 65 | 0.25 | 0.62 |
| HRT/− act1 | 12 | + | 3 | 9 | 0.00 | 1.00 | ||
| Di-17 × gly1-3 | 126 | HRT/− GLY1/− | 71 | + | 16 | 55 | 0.23 | 0.63 |
| HRT/− gly1 | 22 | + | 8 | 14 | 1.51 | 0.22 |
The genotype at HRT and various mutant loci was determined by cleaved amplified polymorphic sequence analysis. ND, not determined; these plants show spontaneous HR.
*One degree of freedom.
†Statistically significant.
To determine whether exogenous application of 18:1 in HRT ssi2 plants had the same effect as the act1 or gly1 mutations, we infiltrated Di-17, Col-0 (susceptible, hrt/hrt RRT/RRT), and HRT ssi2 sid2 plants with 18:1 (Fig. 2E). Infiltration of 18:1 did not appear to alter TCV replication in the inoculated leaves of WT plants; similar levels of TCV transcript were detected in water- and 18:1-treated Di-17 or Col-0 leaves. As expected, the susceptible Col-0 plants supported increased replication of the virus; TCV transcript levels in the inoculated leaves were severalfold higher than in the resistant Di-17 plants. In contrast, the levels of TCV transcript in the inoculated leaves of HRT ssi2 sid2 plants were 2- to 3-fold lower than those in Di-17, suggesting that these plants repressed viral replication as compared with Di-17. Strikingly, infiltration of 18:1 into HRT ssi2 sid2 leaves increased the amount of TCV transcript by ≈6- to 8-fold. However, TCV replication remained unaffected if 18:1 was infiltrated 24 h after TCV inoculation (Fig. 2E, last lane), indicating that 18:1 levels during the initial stages of pathogen perception were crucial for resistance signaling against TCV. Interestingly, although 18:1 injections increased TCV transcript at the localized site of inoculation, it did not allow systemic spread of the virus in HRT ssi2 sid2 plants (data not shown). This could be because constitutive expression of HRT is likely to initiate downstream events leading to resistance, and a temporary surge in 18:1 levels at the localized site of infiltration may not be sufficient to promote systemic spread of the virus. Indeed, exogenous application only normalized 18:1 levels locally, at the site of infiltration, whereas the remaining untreated leaves continued to show low levels of 18:1 (SI Fig. 6B and SI Table 3). The above assumption is further supported by our results related to light-mediated resistance signaling to TCV (18), which showed that dependence on light can be overcome by initiating downstream signaling before dark treatment. Thus, exogenous application of SA before dark treatment of Di-17 plants, or the presence of the ssi2 or cpr5 mutations in HRT plants, prevents dark-triggered susceptibility (18).
Exogenous Application of Glycerol Confers Resistance to TCV in an SA-Independent but ACT1-Dependent Manner.
Additional evidence supporting a role for 18:1 in regulating HRT gene expression was obtained by glycerol treatment of several genotypes. Previously, we have shown that exogenous application of glycerol lowered 18:1 content in WT, sid2, and pad4 backgrounds but not in act1 plants (16, 17). This is because the act1 plants are blocked in the step leading to the acylation of glycerol-3-phosphate with 18:1 (SI Fig. 6A). In addition, we earlier demonstrated that glycerol application induced high levels of SA and that this induction depended on the presence of a functional SID2 protein (17). As predicted, exogenous application of glycerol lowered 18:1 content in WT, HRT sid2, and HRT pad4 plants, but not in HRT act1 plants (Fig. 3A). Consequently, WT and HRT pad4 plants were up-regulated in PR-1 gene expression as opposed to HRT act1 plants (Fig. 3A). HRT sid2 plants failed to induce PR-1 gene expression in response to exogenous glycerol because of their inability to increase SA levels. Most importantly, glycerol-mediated reduction in 18:1 levels induced the levels of HRT transcript in WT, HRT sid2, and HRT pad4, but not in HRT act1, plants (Fig. 3B).
Fig. 3.
PR-1 and R gene expression levels, 18:1 levels, HR formation, and TCV resistance in plants treated with water (W), SA, or glycerol (G). (A) PR-1 gene expression and 18:1 content in treated plants. The glycerol-treated HRT ssi2 plants were harvested 24 h after treatment. Ethidium bromide staining of rRNA was used as a loading control. The 18:1 levels are a mean of six independent replicates. (B) RT-PCR analyses showing expression of HRT and SSI4 genes in treated plants. The level of β-tubulin was used as an internal control to normalize the amount of cDNA template. (C) Visible HR formation in treated plants at 3 days after inoculation. (D) Percentage of TCV-resistant plants obtained after exogenous application of water, SA, or glycerol. Resistance was analyzed 3 weeks after inoculation. The number of plants tested is indicated above each bar. Asterisks indicate 100% susceptibility. (E) Systemic spread of TCV to uninoculated tissue in TCV-inoculated plants. RNA was extracted from the uninoculated tissues at 18 days after inoculation and analyzed for the presence of the viral transcripts (TCV-U). Ethidium bromide staining of rRNA was used as a loading control. (F) Typical morphological phenotypes of TCV-inoculated plants. The susceptible plants showed crinkling, stunted bolt development, and drooping of bolts. Plants were photographed at 18 days after inoculation.
Because the up-regulation of HRT abolishes visible HR to TCV, we next monitored the effect of glycerol pretreatment on TCV-induced HR development in various genotypes. With the exception of HRT act1 plants, all other genotypes, including HRT (Di-17), HRT sid2, and HRT pad4 plants, did not develop visible HR if they were pretreated with glycerol (Fig. 3C; data not shown for Di-17). Thus, although the SA-mediated repression of HR to TCV depends on PAD4 (10), 18:1-mediated repression of HR to TCV is independent of PAD4. The glycerol bioassay further confirmed that HRT expression and HR to TCV can be induced in an SA-independent manner by lowering the levels of 18:1 and that this effect was specific, because act1 plants failed to show these responses upon glycerol application.
Consistent with the increase in HRT transcript levels, exogenous application of glycerol significantly enhanced resistance to TCV in HRT pad4 and HRT sid2 plants but not in HRT act1 plants (Fig. 3 B and D–F). By contrast, SA pretreatment resulted in a substantial increase of TCV resistance in HRT act1 plants but only a marginal increase in HRT pad4 plants (Fig. 3D). SA or glycerol were unable to confer resistance in hrt-containing plants. These results suggest that SA- and glycerol-triggered pathways are mutually exclusive. This was further confirmed by generating HRT cpr5 act1 and HRT cpr5 ssi2 act1 plants; unlike HRT ssi2 act1 plants, resistance in HRT cpr5 act1 and HRT cpr5 ssi2 act1 plants was comparable to that seen in HRT cpr5 plants (data not shown). Taken together, these data indicate that there are at least two mechanisms leading to the induction of HRT, one dependent on SA and PAD4 and the other responsive to reduced levels of 18:1. Furthermore, these data suggest that glycerol treatment is more effective at enhancing resistance; in comparison to SA, pretreatment with glycerol produced ≈30% more resistant HRT sid2 plants (Fig. 3D).
Low 18:1 Conditions Up-Regulate Expression of Structurally Divergent R Genes.
To examine whether the SA-independent and 18:1-modulated induction was specific for HRT, we analyzed the expression of several other R genes in the ssi2, ssi2 sid2, HRT ssi2, and HRT ssi2 sid2 backgrounds (Figs. 3B and 4A and B). A majority of the R genes analyzed were up-regulated in their expression levels in ssi2 sid2 and HRT ssi2 sid2 plants, indicating that low 18:1 levels induced R gene expression in an SA-independent manner. This was further confirmed upon exogenous application of glycerol on sid2 plants, which induced the expression of several R genes (Fig. 4C). As predicted, R gene expression was restored to basal levels in the presence of the act1 mutation, which normalizes 18:1 levels in ssi2 plants (Fig. 4B). In contrast to ssi2 plants, induction of R genes in the cpr5 background was SA-dependent (Fig. 4D).
Fig. 4.
Oleic acid-modulated expression of R genes and resistance to bacterial pathogen. (A) RT-PCR analysis of various R genes in WT (SSI2), ssi2, and ssi2 sid2 backgrounds. (B) RT-PCR analysis of various R genes in WT (HRT), HRT ssi2, HRT ssi2 sid2, and HRT ssi2 act1 backgrounds. (C) RT-PCR analysis of various R genes in water (W)- or glycerol (G)-treated sid2 plants. (D) RT-PCR analysis of various R genes in WT (HRT), HRT cpr5, and HRT cpr5 sid2 backgrounds. (E) RT-PCR analysis of various R genes in WT (SSI2), ssi2, ssi2 eds1, and ssi2 pad4 backgrounds. (F) RT-PCR analysis of various R genes in water (W)- or glycerol (G)-treated WT (RAR1), eds1, or rar1 plants. The levels of β-tubulin were used as internal control to normalize the amount of cDNA template in experiments shown in A–F. (G) Growth of P. syringae on WT (HRT or hrt), HRT ssi2, HRT sid2, and HRT ssi2 sid2 leaves. The Nössen ecotype was also tested and showed resistance similar to that seen in Di-17 and Col-0 plants (data not shown). Four leaf discs were harvested from infected leaves 3 days after inoculation and ground in 10 mM MgCl2, and the bacterial numbers were titered. The bacterial numbers ± SD (n = 4) are presented as log of cfu per cm2. The experiment was independently performed twice with similar results.
R protein-mediated signaling requires EDS1 and/or PAD4 functions, and mutations in these proteins have been shown to down-regulate R gene expression (20, 21, 23–26). Therefore, we next analyzed low 18:1-mediated induction of R genes in the presence of eds1 or pad4 mutations. Interestingly, both ssi2 eds1 and ssi2 pad4 plants showed elevated transcripts of all R genes tested (Fig. 4E). Glycerol treatment of eds1 plants also elevated R gene transcript levels comparable to those induced in WT plants (Fig. 4F). Glycerol treatment of rar1, which encodes another essential regulator of R gene-mediated signaling, also resulted in an increased accumulation of R gene transcripts. Taken together, these results suggest that 18:1-regulated expression of R genes does not depend on EDS1, PAD4, or RAR1.
To investigate whether increased accumulation of R gene transcripts in the HRT ssi2 sid2 background also conferred SA-independent disease resistance to nonviral pathogens, we assayed RPS2-mediated resistance to the avirulent bacterial pathogen Pseudomonas syringae containing AvrRPT2 (Fig. 4G). The HRT sid2 plants showed severe susceptibility, consistent with the SA-dependent nature of RPS2-mediated resistance (27). By comparison, HRT ssi2 and the WT ecotypes (Di-17, Col-0, or Nössen) showed resistance (Fig. 4G; data not shown for Nössen). Interestingly, although HRT ssi2 sid2 plants were more susceptible than HRT ssi2 or WT plants, these plants showed an ≈70-fold decrease in bacterial titer as compared with HRT sid2 plants. These results indicate that SA-independent enhanced resistance, mediated by reduced 18:1 levels, was not specific to a certain group of pathogen and was conferred because of increased expression of the corresponding R gene. Because the SA-depleted ssi2 nahG plants have previously been shown to display enhanced resistance to virulent pathogens (28) and insects (29), the SA-independent induction of multiple R genes is also likely to contribute to generalized defense in these plants.
TCV Inoculation Induces Expression of Various S-ACP-DES Isoforms.
Because a reduction in oleic acid levels induces defense signaling, we next asked whether resistance response to TCV triggers the 18:1-mediated pathway. FA profiling of mock- or TCV-inoculated plants did not show significant differences between the 18:1 levels, suggesting that the 18:1-derived pathway may not be activated during a resistance response to TCV (Fig. 5A). The Arabidopsis genome contains seven S-ACP-DES enzymes, and four of these are expressed in leaves and are capable of synthesizing 18:1 (22). To determine whether TCV inoculation of Di-17 plants altered the expression profile of any of these seven S-ACP-DES isoforms, we analyzed their transcript levels in mock- and TCV-inoculated plants. Inoculation of TCV increased the transcript levels of SSI2, S-ACP-DES3, and S-ACP-DES5 genes (Fig. 5B). Because 18:1 levels in plants are under posttranscriptional and posttranslational controls (22), induction of various desaturases is unlikely to increase 18:1 above WT levels, although it is likely to prevent a decline in the 18:1 content. Taken together, these data suggest that induction of several S-ACP-DES isoforms in TCV-inoculated Di-17 plants is likely to ensure normal 18:1 levels. Because SA-dependent and 18:1-regulated pathways have additive effects, it is possible that pathogens have developed mechanisms to shut off one or more defense pathway(s) to increase their chances of survival in the host. The ability of the bacterial effector proteins to suppress host immune responses further supports this notion (30).
Fig. 5.
Oleic acid and S-ACP-DES transcript levels after mock and TCV inoculation. (A) The inoculated leaves from Di-17 plants were sampled at 0–72 h after inoculation and processed for FA levels. The values are a mean of six independent replicates. The error bars represent SD. (B) S-ACP-DES transcript levels in mock- and TCV-inoculated plants 72 h after inoculation. Data from two independently extracted RNA samples are shown here. The level of β-tubulin was used as an internal control to normalize the amount of cDNA template. Transcript levels of S-ACP-DES1, S-ACP-DES2, S-ACP-DES4, and S-ACP-DES6 either were not detected or showed no difference between mock- and TCV-inoculated plants.
Discussion
Oleic acid, a major monounsaturated FA in plants and animals, is known to influence mammalian immunity by participating in a variety of cellular processes. These include activation of signaling cascades (31), regulation of protein activities (32), and transport of proteins across cellular compartments (33). The human α-lactalbumin, in its 18:1-conjugated form (HAMLET), induces apoptotic cell death specifically in tumor cells (34). The cancer protective properties of olive oil were also recently attributed to 18:1 (35).
In mammalian cells 18:1 biosynthesis is catalyzed by stearoyl-CoA desaturase (SCD), and altered expression of SCD is associated with abnormal physiology, as in diabetes, obesity, and neurological disorders (36). The soluble plant S-ACP-DES is functionally similar to SCD and catalyzes de novo synthesis of 18:1 in plastids. Our earlier work has shown that a mutation in the SSI2-encoded S-ACP-DES affects many cellular responses in the plant, and we have demonstrated that the altered defense-related phenotypes in ssi2 plants are specifically associated with their low 18:1 levels (12, 15–17, 22). Previous research by Nandi et al. (37, 38) has implicated a reduction in hexadecatrienoic acid (16:3) to be responsible for restoration of ssi2 phenotypes. However, several results contradict a role for 16:3. First, we have characterized several suppressor mutants in ssi2 background, which restore all of the ssi2-trigerred phenotypes but contain normal levels of 16:3 (ref. 39 and unpublished data). Second, ssi2 plants themselves show a slight reduction in 16:3 levels as compared with the WT plants (15–17). Third, age-dependent reappearance of ssi2 phenotypes in ssi2 gly1 plants does not alter 16:3 levels (ref. 16 and unpublished data). Fourth, mutations in fad5, fad6, fad7, fad8, and dgd1 lower 16:3 levels but do not restore ssi2 phenotypes (15, 17). Fifth, glycerol application converts WT plants into ssi2 mimics but is not associated with any alteration of 16:3 levels (ref. 16 and unpublished data). Sixth, overexpression of SSI2 (15) or SSI2 isoform (22) complements ssi2 phenotypes by increasing 18:1 levels but does not lower 16:3 content. Thus, it is possible that a reduction in 16:3 levels in various ssi2 suppressors is merely coincidental because most mutations that influence 18:1 levels are likely to affect the prokaryotic pathway of glycerolipid biosynthesis.
Oleic acid is already known to both activate and inhibit protein activities in plants. Examples include the activation of phospholipase D activity in Arabidopsis (40) and inhibition of glucose-6-phosphate transporter activity in Brassica embryos (41). Thus, induction of R gene transcripts by low 18:1 conditions could be a result of activation/inactivation of molecules directly or indirectly involved in their regulation. Indeed, 18:1 is known to up-regulate a transcriptional repressor of the oncogene HER2 (42) and induces genes required for neuronal differentiation by activating a transcription factor (43). An alternate possibility is that the altered ratio of saturated to unsaturated FAs is responsible for induction of R genes. In either case, the SSI2-catalyzed synthesis of 18:1 appears to influence defense responses by regulating R gene expression. Furthermore, this regulation is independent of R gene-signaling components EDS1, PAD4, and RAR1. It is intriguing that the 18:1-regulated pathway does not distinguish between R genes based on the structure of the encoded protein and influences transcription of R genes encoding either coiled-coil domain-carrying or Toll–IL1-like-domain-carrying proteins. We propose that this constitutive up-regulation of a large variety of R genes in ssi2 plants is responsible for their altered defense-related phenotypes.
In conclusion, we report here an oleic acid-dependent signaling pathway that confers resistance in an SA-independent manner. Further molecular and biochemical characterization of 18:1-dependent regulation of R genes should provide exciting insights into the role of 18:1 in plant defense.
Methods
Plant Growth Conditions, Genetic Analysis, and Pathogen Inoculations.
Plants were grown in MTPS 144 Conviron (Winnipeg, MB, Canada) walk-in-chambers at 22°C, 65% relative humidity, and 14-h photoperiod. Crosses were performed by pollinating flowers of Di-17 plants with pollen from Col-0, Nössen, ssi2, cpr5, ssi2 act1, ssi2 gly1, act1, and gly1. The double-mutant combinations in ssi2 and cpr5 backgrounds were created by crossing HRT ssi2 or HRT cpr5 plants with eds1, sid2, eds5, and pad4. The HRT ssi2 act1 cpr5 plants were derived from a cross between HRT ssi2 act1 and HRT cpr5. The HRT cpr5 act1 plants were derived from a cross between HRT cpr5 and act1. The ssi2 eds1, ssi2 sid2, and ssi2 pad4 plants have been described earlier (17). All genotypes were initially screened in the F2 generation and confirmed further in F3 and F4 generations. The genotypes at various loci were determined by conducting cleaved amplified polymorphic sequence analysis (CAPS) or derived CAPS (d-CAPS), as described before (10–12, 15–17). Inoculations with TCV and P. syringae were conducted as described before (10, 17).
Construction of HRT Promoter–GUS Fusions and GUS Assays.
The HRT promoter was PCR-amplified from a clone obtained from the Di-17 genomic library (44) by using HindIII- and BamHI-linkered primers TGAGAAGCTTGTTGTTGCTGTCTCCTCTC and AAGAGGATCCAACAACTAGTCGTCGAG. The PCR fragment was cloned upstream of the GUS ORF in pBI121. The HRT–GUS fusion construct was transformed into Agrobacterium strain MP90, and a culture grown overnight was used for agroinfiltrations as described before (45).
FA Analysis and Oleic Acid Infiltration.
FA analysis was carried out by using gas chromatography as described previously (16). Oleic acid (Sigma–Aldrich, St. Louis, MO) was dissolved in water and infiltrated into leaves by using a needle-less syringe. To determine the 18:1 concentration required to elevate the leaf 18:1 content in ssi2 plants to WT levels, we injected 50 μm to 10 mM into HRT ssi2 sid2 leaves followed by FA profiling (SI Table 3). A concentration of 500 μm to 1 mM was considered optimal, with similar results being obtained upon infiltration of either of these concentrations.
SA and Glycerol Treatments.
SA and glycerol treatments were carried out by spraying 500 μM and 50 mM solutions, respectively, prepared in sterile water. Control plants were treated with water. Plants were sampled or inoculated 2 days after SA treatment and 3 days after glycerol treatment, unless otherwise mentioned.
RNA Extraction, RT-PCR, and Northern Blot Analyses.
RNA extraction, RT-PCR, and Northern blot analyses were conducted as described before (10, 22). For RT-PCR, the number of amplification cycles was reduced to 22–25 to evaluate and quantify any differences among transcript levels before they reached saturation. Primers used for PCR amplifications are described in SI Table 4.
Supplementary Material
Acknowledgments
We thank Amy Crume for help with managing the plant growth facility, John Johnson for help with gas chromatography, and Thomas Muse and Ludmila Lapchyk for help with genotyping. We thank Daniel Klessig (The Boyce Thompson Institute for Plant Research, Ithaca, NY) for providing HRT-containing clones from the Di-17 genomic library. We thank June Nasrallah and David Smith for critical comments on the manuscript. This work was supported by National Science Foundation Grant MCB-0421914 (to A.K. and P.K.), U.S. Department of Agriculture National Research Initiative Grant 2004-03287 (to P.K.), and Kentucky Science and Engineering Foundation Grants 419-RDE-004, 04RDE-006, and 820-RDE-007 (to A.K. and P.K.). This study is publication 07-12-044 of the Kentucky Agricultural Experiment Station.
Abbreviations
- FA
fatty acid
- TCV
Turnip Crinkle Virus
- HR
hypersensitive response
- SA
salicylic acid
- GUS
β-glucuronidase
- S-ACP-DES
stearoyl-acyl carrier protein-desaturase.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0609259104/DC1.
References
- 1.Durrant W, Dong X. Annu Rev Phytopathol. 2004;42:185–209. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- 2.Kachroo A, Kachroo P. In: Genetic Engineering: Principles and Methods. Setlow J, editor. Vol 28. Heidelberg: Springer; 2007. pp. 55–83. [Google Scholar]
- 3.Kachroo P, Chandra-Shekara AC, Klessig DF. Adv Virus Res. 2006;66:161–191. doi: 10.1016/S0065-3527(06)66004-1. [DOI] [PubMed] [Google Scholar]
- 4.Falk A, Feys BJ, Frost LN, Jones JD, Daniels MJ, Parker JE. Proc Natl Acad Sci USA. 1999;96:3292–3297. doi: 10.1073/pnas.96.6.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nawrath C, Heck S, Parinthawong N, Metraux JP. Plant Cell. 2002;14:275–286. doi: 10.1105/tpc.010376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jirage D, Tootle TL, Reuber TL, Frost LN, Feys BJ, Parker JE, Ausubel FM, Glazebrook J. Proc Natl Acad Sci USA. 1999;96:13583–13588. doi: 10.1073/pnas.96.23.13583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wildermuth MC, Dewdney J, Wu G, Ausubel FM. Nature. 2001;414:562–565. doi: 10.1038/35107108. [DOI] [PubMed] [Google Scholar]
- 8.Dodds PN, Schwechheimer C. Plant Cell. 2002;14:S5–S8. doi: 10.1105/tpc.141330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muskett P, Parker J. Microbes Infect. 2003;5:969–976. doi: 10.1016/s1286-4579(03)00183-7. [DOI] [PubMed] [Google Scholar]
- 10.Chandra-Shekara AC, Navarre D, Kachroo A, Kang H-G, Klessig D, Kachroo P. Plant J. 2004;5:647–659. doi: 10.1111/j.1365-313X.2004.02241.x. [DOI] [PubMed] [Google Scholar]
- 11.Kachroo P, Yoshioka K, Shah J, Dooner HK, Klessig DF. Plant Cell. 2000;12:677–690. doi: 10.1105/tpc.12.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kachroo P, Shanklin J, Shah J, Whittle EJ, Klessig DF. Proc Natl Acad Sci USA. 2001;98:9448–9453. doi: 10.1073/pnas.151258398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshida S, Ito M, Nishida I, Watanabe A. Plant J. 2002;29:427–437. doi: 10.1046/j.0960-7412.2001.01228.x. [DOI] [PubMed] [Google Scholar]
- 14.Kirik V, Bouyer D, Schobinger U, Bechtold N, Herzog M, Bonneville J-M, Hulskamp M. Curr Biol. 2001;11:1891–1895. doi: 10.1016/s0960-9822(01)00590-5. [DOI] [PubMed] [Google Scholar]
- 15.Kachroo A, Lapchyk L, Fukushigae H, Hildebrand D, Klessig D, Kachroo P. Plant Cell. 2003;12:2952–2965. doi: 10.1105/tpc.017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kachroo A, Venugopal SC, Lapchyk L, Falcone D, Hildebrand D, Kachroo P. Proc Natl Acad Sci USA. 2004;101:5152–5157. doi: 10.1073/pnas.0401315101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kachroo P, Venugopal SC, Navarre DA, Lapchyk L, Kachroo A. Plant Physiol. 2005;139:1717–1735. doi: 10.1104/pp.105.071662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandra-Shekara AC, Gupte M, Navarre D, Raina S, Raina R, Klessig D, Kachroo P. Plant J. 2006;45:320–334. doi: 10.1111/j.1365-313X.2005.02618.x. [DOI] [PubMed] [Google Scholar]
- 19.Clarke JD, Volko SM, Ledford H, Ausubel FM, Dong X. Plant Cell. 2000;12:2175–2190. doi: 10.1105/tpc.12.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke JD, Aarts N, Feys BJ, Dong X, Parker JE. Plant J. 2001;26:409–420. doi: 10.1046/j.1365-313x.2001.2641041.x. [DOI] [PubMed] [Google Scholar]
- 21.Jirage D, Zhou N, Cooper B, Clarke JD, Dong X, Glazebrook J. Plant J. 2001;26:395–407. doi: 10.1046/j.1365-313x.2001.2641040.x. [DOI] [PubMed] [Google Scholar]
- 22.Kachroo A, Shanklin J, Whittle E, Lapchyk L, Hildebrand D, Kachroo P. Plant Mol Biol. 2007;63:257–271. doi: 10.1007/s11103-006-9086-y. [DOI] [PubMed] [Google Scholar]
- 23.Aarts N, Metz M, Holub E, Staskawicz BJ, Daniels MJ, Parker JE. Proc Natl Acad Sci USA. 1998;95:10306–10311. doi: 10.1073/pnas.95.17.10306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirano Y, Kachroo P, Shah J, Klessig DF. Plant Cell. 2002;14:3149–3162. doi: 10.1105/tpc.005348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stokes TL, Kunkel BN, Richards EJ. Genes Dev. 2002;16:171–182. doi: 10.1101/gad.952102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang S, Hua J. Plant Cell. 2004;16:1060–1071. doi: 10.1105/tpc.020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E, Ryals J. Science. 1994;266:1247–1250. doi: 10.1126/science.266.5188.1247. [DOI] [PubMed] [Google Scholar]
- 28.Shah J, Kachroo P, Nandi A, Klessig DF. Plant J. 2001;25:563–574. doi: 10.1046/j.1365-313x.2001.00992.x. [DOI] [PubMed] [Google Scholar]
- 29.Pegadaraju V, Knepper C, Reese J, Shah J. Plant Physiol. 2005;139:1927–1934. doi: 10.1104/pp.105.070433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nomura K, DebRoy S, Lee YH, Pumplin N, Jones J, He SY. Science. 2006;313:220–223. doi: 10.1126/science.1129523. [DOI] [PubMed] [Google Scholar]
- 31.Wrede CE, Dickson LM, Linghor MK, Briand I, Rhodes CJ. Biochem J. 2002;277:49676–49684. doi: 10.1074/jbc.M208756200. [DOI] [PubMed] [Google Scholar]
- 32.Klumpp S, Selke D, Hermesmeir J. FEBS Lett. 1998;437:229–232. doi: 10.1016/s0014-5793(98)01237-x. [DOI] [PubMed] [Google Scholar]
- 33.Boscal L, Diaz-Guerra MJ, Mojena M. Biochem Biophys Res Commun. 1989;160:1243–1249. doi: 10.1016/s0006-291x(89)80137-8. [DOI] [PubMed] [Google Scholar]
- 34.Svanborg C, Agerstam H, Aronson A, Bjerkvig R, Duringer C, Fischer W, Gustafsson L, Hallgren O, Leijonhuvud I, Linse S, et al. Adv Cancer Res. 2003;88:1–29. doi: 10.1016/s0065-230x(03)88302-1. [DOI] [PubMed] [Google Scholar]
- 35.Menendez JA, Vellon L, Colomer R, Lupu R. Ann Oncol. 2005;16:359–371. doi: 10.1093/annonc/mdi090. [DOI] [PubMed] [Google Scholar]
- 36.Dobrzyn A, Ntambi JM. Prostaglandins Leukotrienes Essent Fatty Acids. 2005;73:35–41. doi: 10.1016/j.plefa.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Nandi A, Krothapalli K, Buseman CM, Li M, Welti R, Enyedi A, Shah J. Plant Cell. 2003;15:2383–2398. doi: 10.1105/tpc.015529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nandi A, Welti R, Shah J. Plant Cell. 2004;16:465–477. doi: 10.1105/tpc.016907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kachroo P, Kachroo A, Lapchyk L, Hildebrand D, Klessig D. Mol Plant–Microbe Interact. 2003;11:1022–1029. doi: 10.1094/MPMI.2003.16.11.1022. [DOI] [PubMed] [Google Scholar]
- 40.Wang C, Wang X. Plant Physiol. 2001;127:1102–1112. [PMC free article] [PubMed] [Google Scholar]
- 41.Fox SR, Hill LM, Rawsthorne S, Hills MJ. Biochem J. 2000;352:525–532. [PMC free article] [PubMed] [Google Scholar]
- 42.Menendez JA, Lupu R. Curr Opin Clin Nutr Metab Care. 2006;9:346–357. doi: 10.1097/01.mco.0000232893.21050.15. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez-Rodriguez RA, Tabernero A, Velasco A, Lavado EM, Medina JM. J Neurochem. 2004;88:1041–1051. doi: 10.1046/j.1471-4159.2003.02262.x. [DOI] [PubMed] [Google Scholar]
- 44.Cooley MB, Pathirana S, Wu HJ, Kachroo P, Klessig DF. Plant Cell. 2000;12:663–676. doi: 10.1105/tpc.12.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McIntosh KB, Hulm JL, Young LW, Bontham-Smith PC. Plant Mol Biol Rep. 2004;22:53–61. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.