Abstract
The impact of agostic interactions (i.e., 3-center–2-electron M H
H C bonds) on the structures and reactivity of organotransition metal compounds is reviewed.
C bonds) on the structures and reactivity of organotransition metal compounds is reviewed.
Introduction and Historical Perspective
Soon after the discovery of transition metal alkyl compounds, it became clear that the presence of the transition metal imparted properties to the alkyl group that were unprecedented in the normal ambient chemistry of simple organic compounds. For instance, the occurrence of the reversible α-elimination process and the ability to extract hydride from the β-carbon of a transition metal–ethyl compound are two examples of the impact of a transition metal on an alkyl group (1).e
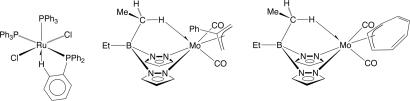
Further, as the methodology of single crystal structure determination rapidly advanced, there were reports of C H systems in which there appeared to be an unusually close approach of the hydrogen to a metal center, as illustrated by the examples shown in Fig. 1 (2–7). However, although the x-ray diffraction studies provided evidence for the close approach of the hydrogen to the metal, there was no evidence to distinguish whether this reflected an attraction of the C
H systems in which there appeared to be an unusually close approach of the hydrogen to a metal center, as illustrated by the examples shown in Fig. 1 (2–7). However, although the x-ray diffraction studies provided evidence for the close approach of the hydrogen to the metal, there was no evidence to distinguish whether this reflected an attraction of the C H bond to the metal or whether the ligand structure was holding the C
H bond to the metal or whether the ligand structure was holding the C H bond close the metal. The former explanation was, nevertheless, strongly advanced by Cotton (8).
H bond close the metal. The former explanation was, nevertheless, strongly advanced by Cotton (8).
Fig. 1.
Early examples of compounds with M H–C agostic interactions.
H–C agostic interactions.
In an attempt to resolve this matter, the titanium compound (Me2PCH2CH2PMe2)TiEtCl3 illustrated in Fig. 2 was prepared and structurally characterized by x-ray diffraction (9, 10). (Me2PCH2CH2PMe2)TiEtCl3 was chosen as a target compound for the following reasons: (i) the titanium center is d0, and because the electron count is formally 12 there are empty d-orbitals available for accepting electron density from the C H bond; (ii) the ligands are relatively small and would not sterically inhibit the close approach of a C
H bond; (ii) the ligands are relatively small and would not sterically inhibit the close approach of a C H bond; and (iii) the ethyl group would break the threefold symmetry about the Ti
H bond; and (iii) the ethyl group would break the threefold symmetry about the Ti C bond (as compared with the corresponding methyl compound) and thereby make identification of a Ti–H–C interaction easier.
C bond (as compared with the corresponding methyl compound) and thereby make identification of a Ti–H–C interaction easier.
Fig. 2.
The first structurally characterized β-agostic-metal-alkyl compound. The bond lengths and angles for the agostic ethyl group are shown.
Indeed, the crystal structure of (Me2PCH2CH2PMe2)TiEtCl3 shows a remarkably acute angle of 85.9(6)° at the β-carbon whereas a normal angle close to 109° would have been expected in the absence of any unusual interaction. This result provided the first unambiguous evidence that the short H Ti distance and acute angle must arise from an attractive force between the titanium center and C
Ti distance and acute angle must arise from an attractive force between the titanium center and C H bond. Soon after, a similar but smaller distortion of an α-hydrogen was observed by neutron diffraction studies for the corresponding methyl–titanium compound (11).
H bond. Soon after, a similar but smaller distortion of an α-hydrogen was observed by neutron diffraction studies for the corresponding methyl–titanium compound (11).
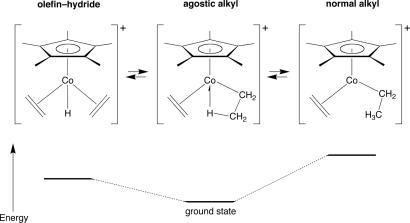
The next important development in the story of β-agostic ethyl complexes came from the studies of the dynamic equilibria in the agostic compound Cp*Co(η2–C2H4)(β-agostic–C2H5) as studied by variable temperature 1H NMR spectroscopy. The identified equilibria and energetics are summarized in Fig. 3 (12).
Fig. 3.
The dynamic equilibria present in the compound Cp*Co(η2-C2H4)(β-agostic-C2H5). The agostic complex is the ground state structure.
Very quickly it became apparent that in suitable circumstances the C H bond could act as a ligand to a transition metal center by virtue of the formation of a 3-center–2-electron covalent bond. To emphasize this point, a review on the subject was published in 1983 (13), after which the area developed rapidly, and a second review was published in 1988 (14). These reviews have been cited 1,097 and 715 times, respectively (as of November 2006).
H bond could act as a ligand to a transition metal center by virtue of the formation of a 3-center–2-electron covalent bond. To emphasize this point, a review on the subject was published in 1983 (13), after which the area developed rapidly, and a second review was published in 1988 (14). These reviews have been cited 1,097 and 715 times, respectively (as of November 2006).
The term “agostic bond” was coined because the carbon–hydrogen bond in simple alkyl groups had been long known as a stable and “inert” bond in organic chemistry. This new behavior with transition metal centers was clearly likely to have considerable relevance to organometallic chemistry and in particular to organometallic catalytic reactions. Therefore, it was thought to be worthwhile to draw attention to this phenomenon by introducing the word “agostic” to describe such interactions.
In the first review (13) we defined agostic as follows:
“We propose the term ‘agostic' which will be used to discuss the various manifestations of covalent interactions between carbon-hydrogen groups and transition metal centers in organometallic compounds. The word agostic will be used to refer specifically to situations in which a hydrogen atom is covalently bonded simultaneously to both a carbon atom and a transition metal atom.”
The original article (13) also pointed out that agostic bonds were likely to be “very much more common than hitherto suspected” and that because “carbon–hydrogen bonds are a ubiquitous feature of organometallic chemistry it is useful to define the new term agostic which serves to emphasize the phenomena and to differentiate between terminal hydridoalkyl (C–M H) systems.”
H) systems.”
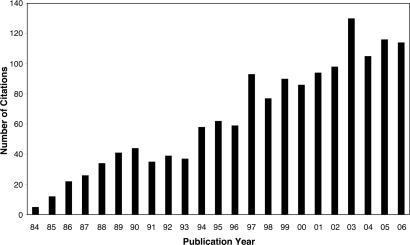
The prediction that there would be many examples of agostic bonds in organometallic chemistry has been amply justified over the last 23 years. For example, a search (in November 2006) for the word “agostic” in ISI Web of Science (Thomson, Philadelphia, PA) and SciFinder Scholar (Chemical Abstracts Service, Columbus, OH) yielded 1,031 and 1,483 items, respectively, and there were 29 papers with >100 citations. The rapid rise in the discovery of agostic systems is reflected in the histogram in Fig. 4.
Fig. 4.
The number of publications per year found from SciFinder Scholar by using the search term “agostic.”
In addition to metal alkyl compounds, there are also metal alkane compounds, so-called σ-complexes (15), where the alkane is attached to the metal by an agostic interaction (16–19), i.e., a 3-center–2-electron M H–C interaction. By comparison with agostic alkyl compounds, however, metal alkane compounds have very limited stability, and bona fide examples have not been isolated in the solid state (20, 21).f Excellent evidence for their existence, however, comes from (i) low-temperature IR spectroscopic and NMR spectroscopic studies (16);g (ii) isotopic labeling experiments, such as the observation of deuterium exchange between hydride and alkyl sites, e.g., [M](CH3)D → [M](CH2D)H; and (iii) the measurement of kinetic isotope effects.h
H–C interaction. By comparison with agostic alkyl compounds, however, metal alkane compounds have very limited stability, and bona fide examples have not been isolated in the solid state (20, 21).f Excellent evidence for their existence, however, comes from (i) low-temperature IR spectroscopic and NMR spectroscopic studies (16);g (ii) isotopic labeling experiments, such as the observation of deuterium exchange between hydride and alkyl sites, e.g., [M](CH3)D → [M](CH2D)H; and (iii) the measurement of kinetic isotope effects.h
Agostic and Anagostic Interactions
The 1983 article (13) drew attention to the fact that “the agostic C H
H M bond is similar to the familiar and long-known bridging hydrogen systems which occur in B
M bond is similar to the familiar and long-known bridging hydrogen systems which occur in B H
H B, M
B, M H
H M and B
M and B H
H M groups.” However, the latter interactions, which are very common examples of 3-center–2-electron bonds, are not encompassed by the definition of “agostic.” Part of the reason for introducing the term “agostic” was to emphasize that 3-center–2-electron interactions involving C
M groups.” However, the latter interactions, which are very common examples of 3-center–2-electron bonds, are not encompassed by the definition of “agostic.” Part of the reason for introducing the term “agostic” was to emphasize that 3-center–2-electron interactions involving C H bonds are unusual. As such, “agostic” is not synonymous with “3-center–2-electron.” In recent years, however, certain authors, who appear to be unaware of the reason for introducing the term, have taken “agostic” to be synonymous with “3-center–2-electron.” For example, M
H bonds are unusual. As such, “agostic” is not synonymous with “3-center–2-electron.” In recent years, however, certain authors, who appear to be unaware of the reason for introducing the term, have taken “agostic” to be synonymous with “3-center–2-electron.” For example, M H–B groups have been described by certain authors as agostic interactions.i However, this is an inappropriate use of the term agostic, which refers specifically to 3-center–2-electron interactions involving M
H–B groups have been described by certain authors as agostic interactions.i However, this is an inappropriate use of the term agostic, which refers specifically to 3-center–2-electron interactions involving M H–C groups, and does not refer to all 3-center–2-electron interactions. Although the original definition of agostic specifically mentioned transition metal compounds, we do, nevertheless, consider it appropriate to expand the definition to all metals.
H–C groups, and does not refer to all 3-center–2-electron interactions. Although the original definition of agostic specifically mentioned transition metal compounds, we do, nevertheless, consider it appropriate to expand the definition to all metals.
With respect to the definition of agostic compounds, it is important to emphasize that not all compounds that possess M H–C interactions should be classified as agostic. Specifically, it has recently become evident that there is a growing class of molecules that exhibit M
H–C interactions should be classified as agostic. Specifically, it has recently become evident that there is a growing class of molecules that exhibit M H–C interactions for which the bonding is not appropriately described as 3-center–2-electron; as such, molecules of this type should not be classified as agostic. This notion was first put forth by Brammer et al. (29, 30), who analyzed complexes with M
H–C interactions for which the bonding is not appropriately described as 3-center–2-electron; as such, molecules of this type should not be classified as agostic. This notion was first put forth by Brammer et al. (29, 30), who analyzed complexes with M H–N interactions and concluded that short linear M
H–N interactions and concluded that short linear M H–N arrangements are best described as a “hydrogen bond” involving a 3-center–4-electron orbital interaction and an electrostatic contribution in which the metal serves as a hydrogen bond acceptor. An exemplary illustration of such a complex that features an M···H–N hydrogen bond is provided by [Et3NH][Co(CO)4] (31). In this complex, the [Co(CO)4]− anion has an 18-electron configuration and is devoid of a vacant orbital in the valence shell; as such, the metal is incapable of participating in a 3-center–2-electron M
H–N arrangements are best described as a “hydrogen bond” involving a 3-center–4-electron orbital interaction and an electrostatic contribution in which the metal serves as a hydrogen bond acceptor. An exemplary illustration of such a complex that features an M···H–N hydrogen bond is provided by [Et3NH][Co(CO)4] (31). In this complex, the [Co(CO)4]− anion has an 18-electron configuration and is devoid of a vacant orbital in the valence shell; as such, the metal is incapable of participating in a 3-center–2-electron M H–N interaction. Because C
H–N interaction. Because C H groups are known to be weak hydrogen bond donors, Brammer et al. (29) raised the possibility that some M
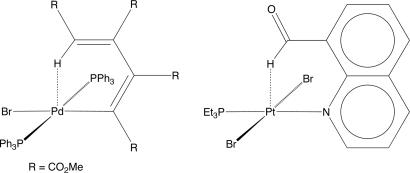
H groups are known to be weak hydrogen bond donors, Brammer et al. (29) raised the possibility that some M H–C interactions could be better described as “hydrogen bonds” rather than agostic interactions. In this regard, it is pertinent to note that, well before the realization of agostic interactions, Maitlis and coworkers (32) actually reported the structure of trans-Pd(PPh3)2{C4(CO2Me)4H}Br (Fig. 5) in 1972 and suggested that “… some interaction (hydrogen bonding?) is occurring” between the δ-butadienyl hydrogen and the palladium. The presence of the question mark in this quote, however, clearly indicates that the nature and significance of the interaction was, understandably, far from being properly recognized at that time.
H–C interactions could be better described as “hydrogen bonds” rather than agostic interactions. In this regard, it is pertinent to note that, well before the realization of agostic interactions, Maitlis and coworkers (32) actually reported the structure of trans-Pd(PPh3)2{C4(CO2Me)4H}Br (Fig. 5) in 1972 and suggested that “… some interaction (hydrogen bonding?) is occurring” between the δ-butadienyl hydrogen and the palladium. The presence of the question mark in this quote, however, clearly indicates that the nature and significance of the interaction was, understandably, far from being properly recognized at that time.
Fig. 5.
Early examples of compounds that have M H–C interactions that are not characterized as agostic.
H–C interactions that are not characterized as agostic.
In addition to trans-Pd(PPh3)2{C4(CO2Me)4H}Br, Brammer and coworkers noted that a variety of other compounds could also possibly be described as possessing M H–C hydrogen bonds (for example, see Fig. 5).j In many of these cases, although it had been recognized that the M
H–C hydrogen bonds (for example, see Fig. 5).j In many of these cases, although it had been recognized that the M H–C interactions were not the same as that in traditional agostic compounds, the distinction suggested by Brammer had not been emphasized. Indeed, Venanzi and coworkers (37) actually introduced the term “pregostic” to describe such complexes, with the interpretation being that the interaction is preagostic, i.e., on the way to becoming agostic.k However, because agostic refers to a 3-center–2-electron M
H–C interactions were not the same as that in traditional agostic compounds, the distinction suggested by Brammer had not been emphasized. Indeed, Venanzi and coworkers (37) actually introduced the term “pregostic” to describe such complexes, with the interpretation being that the interaction is preagostic, i.e., on the way to becoming agostic.k However, because agostic refers to a 3-center–2-electron M H–C interaction, it is evident that neither “pregostic” nor “preagostic” are appropriate descriptions for these complexes. A more appropriate descriptor for M
H–C interaction, it is evident that neither “pregostic” nor “preagostic” are appropriate descriptions for these complexes. A more appropriate descriptor for M H–C interactions that do not involve 3-center–2-electron interactions is, therefore, “anagostic” (38), a term that was introduced by Lippard and coworkers and which may be used to refer generally to any M
H–C interactions that do not involve 3-center–2-electron interactions is, therefore, “anagostic” (38), a term that was introduced by Lippard and coworkers and which may be used to refer generally to any M H–C interaction that is not agostic (39).l
H–C interaction that is not agostic (39).l
Agostic and anagostic M H–C interactions are characterized by significantly different structural and spectroscopic properties (Fig. 6). Thus, agostic M
H–C interactions are characterized by significantly different structural and spectroscopic properties (Fig. 6). Thus, agostic M H–C interactions are characterized by relatively short M
H–C interactions are characterized by relatively short M H distances (≈1.8–2.3 Å) and small M
H distances (≈1.8–2.3 Å) and small M H
H C bond angles (≈90–140°), whereas anagostic interactions are characterized by relatively long M···H distances (≈2.3–2.9 Å) and large M
C bond angles (≈90–140°), whereas anagostic interactions are characterized by relatively long M···H distances (≈2.3–2.9 Å) and large M H
H C bond angles (≈110–170°) (40, 41). With respect to 1H NMR spectroscopy, a signature of an agostic interaction is an unusually low 1JCH value, which can range from 50 to 100 Hz (13, 14). Furthermore, the chemical shifts of agostic hydrogen atoms are typically observed upfield of the uncoordinated group, whereas anagostic hydrogen atoms are typically observed downfield; the latter observation is in accord with the hydrogen-bonded description of the interaction.
C bond angles (≈110–170°) (40, 41). With respect to 1H NMR spectroscopy, a signature of an agostic interaction is an unusually low 1JCH value, which can range from 50 to 100 Hz (13, 14). Furthermore, the chemical shifts of agostic hydrogen atoms are typically observed upfield of the uncoordinated group, whereas anagostic hydrogen atoms are typically observed downfield; the latter observation is in accord with the hydrogen-bonded description of the interaction.
Fig. 6.
Structural and spectroscopic differences between agostic and anagostic interactions.
Complexes that contain anagostic M H
H C interactions are typically associated with d8 transition metals centers that are square planar prior to the interaction, as illustrated by a variety of rhodium(I) phosphinate complexes synthesized by Bergman and coworkers (43).m Crabtree, Eisenstein, and coworkers (44) examined the structural characteristics of complexes that featured M
C interactions are typically associated with d8 transition metals centers that are square planar prior to the interaction, as illustrated by a variety of rhodium(I) phosphinate complexes synthesized by Bergman and coworkers (43).m Crabtree, Eisenstein, and coworkers (44) examined the structural characteristics of complexes that featured M H–N and M
H–N and M H–C interactions in otherwise square planar d8 complexes and concluded that although the former are appropriately described as hydrogen bonds because the M···H
H–C interactions in otherwise square planar d8 complexes and concluded that although the former are appropriately described as hydrogen bonds because the M···H N bonds are close to the linear, the situation is ambiguous for the M
N bonds are close to the linear, the situation is ambiguous for the M H–C interactions. For example, although the M
H–C interactions. For example, although the M H–C interactions are not linear, they do not adopt the “side-on” arrangement observed in unambiguously agostic compounds.
H–C interactions are not linear, they do not adopt the “side-on” arrangement observed in unambiguously agostic compounds.
Prompted by the conclusion that the M H–C interaction is not unambiguously described as a hydrogen bond, the nature of the anagostic M···H–C interaction in a variety of square planar d8 compounds was recently addressed theoretically by Bergman, Ellman, Oldfield, and coworkers (40). The principal conclusions of this study are that the dz2 orbital is not involved to a significant degree and that the interactions vary from purely electrostatic to electrostatic with partial covalence, with the latter corresponding to compounds with the shorter M···H distances and stronger interaction. Thus, although the “hydrogen bonding” description of anagostic M
H–C interaction is not unambiguously described as a hydrogen bond, the nature of the anagostic M···H–C interaction in a variety of square planar d8 compounds was recently addressed theoretically by Bergman, Ellman, Oldfield, and coworkers (40). The principal conclusions of this study are that the dz2 orbital is not involved to a significant degree and that the interactions vary from purely electrostatic to electrostatic with partial covalence, with the latter corresponding to compounds with the shorter M···H distances and stronger interaction. Thus, although the “hydrogen bonding” description of anagostic M H–C interactions would be an extreme view of the bonding, it does nevertheless capture the essence of the difference between agostic and anagostic compounds. The important point is, therefore, that M
H–C interactions would be an extreme view of the bonding, it does nevertheless capture the essence of the difference between agostic and anagostic compounds. The important point is, therefore, that M H–C interactions come in more than one variety, and the nature of the interaction depends critically on the metal center.
H–C interactions come in more than one variety, and the nature of the interaction depends critically on the metal center.
This distinction between agostic and anagostic interactions does not, however, appear to be widely recognized, and some compounds have been mischaracterized. For example, a copper(II) complex was recently reported to possess a Cu–H–C agostic interaction (45), but a subsequent analysis indicates that is better described as a weak multicentered hydrogen bond (46), i.e., an anagostic interaction.
Consideration of M···H–C and M···C distances suggest that the magnitude of interaction is typically greater for agostic compounds. For example, metrical details pertaining to M H–C interactions in calixarene compounds are summarized in Table 1, from which it is evident that the interaction is more significant for the d2 agostic molybdenum compound [CalixBut(OH)2(O)2]Mo(PMe3)3H2 than the d8 anagostic rhodium and platinum derivatives, as illustrated by the Mo–H distance in [CalixBut(OH)2(O)2]Mo(PMe3)3H2 being ≈0.25 Å shorter than the corresponding value in [CalixBut(OH)2(O)2]Pt(dppp), the complex with the next shortest value. The 1H NMR spectroscopic properties of the agostic and anagostic calixarene compounds are also quite distinct, with the methylene hydrogen involved in the agostic interaction of [CalixBut(OH)2(O)2]Mo(PMe3)3H2 being shifted significantly to high field (−4.6 ppm), whereas that for [CalixBut(OH)2(O)2]Pt(dppp) is observed at 6.5 ppm.
H–C interactions in calixarene compounds are summarized in Table 1, from which it is evident that the interaction is more significant for the d2 agostic molybdenum compound [CalixBut(OH)2(O)2]Mo(PMe3)3H2 than the d8 anagostic rhodium and platinum derivatives, as illustrated by the Mo–H distance in [CalixBut(OH)2(O)2]Mo(PMe3)3H2 being ≈0.25 Å shorter than the corresponding value in [CalixBut(OH)2(O)2]Pt(dppp), the complex with the next shortest value. The 1H NMR spectroscopic properties of the agostic and anagostic calixarene compounds are also quite distinct, with the methylene hydrogen involved in the agostic interaction of [CalixBut(OH)2(O)2]Mo(PMe3)3H2 being shifted significantly to high field (−4.6 ppm), whereas that for [CalixBut(OH)2(O)2]Pt(dppp) is observed at 6.5 ppm.
Table 1.
Comparison of M···H and M···C bond lengths involving methylene groups in various agostic and anagostic calixarene complexes (data taken from ref. 49).
| Compound | d(M···H), Å | d(M···C), Å | rcov(M), Å |
|---|---|---|---|
| [CalixBut(OH)2(O)2]Mo(PMe3)3H2 | 2.01 | 2.73 | 1.31 |
| [CalixBut(OH)2(O)2]Pt(dppp) | 2.25 | 3.04 | 1.24 |
| [CalixBut(OH)2(O)2]{Rh(cod)}2 | 2.44 | 3.18 | 1.21 |
| [CalixBut(OCH2PPH2)4]PtCl2(AuPPh3)2 | 2.61 | 3.49 | 1.24 |
| [CalixBut(OH)2(OCH2PPH2)2]PtCl2 | 2.67 | 3.55 | 1.24 |
Factors Influencing the Formation of Agostic Compounds
The agostic M H–C interaction involves donation of the electron density associated with the C
H–C interaction involves donation of the electron density associated with the C H bond to a metal center that has a ≤16-electron configuration.n For non-d0 metal centers, the interaction may also be supplemented by backbonding into the C
H bond to a metal center that has a ≤16-electron configuration.n For non-d0 metal centers, the interaction may also be supplemented by backbonding into the C H σ* orbital.o The ability to isolate an agostic compound, therefore, depends critically on the magnitude of these interactions. Thus, if the σ-donation is too weak, an agostic interaction would not form, whereas if the backbonding interaction is too strong, cleavage of the C
H σ* orbital.o The ability to isolate an agostic compound, therefore, depends critically on the magnitude of these interactions. Thus, if the σ-donation is too weak, an agostic interaction would not form, whereas if the backbonding interaction is too strong, cleavage of the C H bond would ensue, thereby resulting in the formation of an alkyl–hydride derivative. A simple illustration of the latter point is provided by the molybdenum and tungsten calixarene compounds, [CalixBut(OH)2(O)2]Mo(PMe3)3H2 and [Calix-HBut(OH)2(O)2]W(PMe3)3H3, which exist as agostic and alkyl hydride derivatives, respectively, in the solid state (49).
H bond would ensue, thereby resulting in the formation of an alkyl–hydride derivative. A simple illustration of the latter point is provided by the molybdenum and tungsten calixarene compounds, [CalixBut(OH)2(O)2]Mo(PMe3)3H2 and [Calix-HBut(OH)2(O)2]W(PMe3)3H3, which exist as agostic and alkyl hydride derivatives, respectively, in the solid state (49).
Role of Agostic Interactions in Reaction Intermediates and Transition States
In the 1988 review of complexes exhibiting agostic bonds (14), we speculated that “… it seems likely that many of the proposed 16-electron intermediates are in fact 18-electron agostic compounds and the [true] 16-electron species would then become transition states.” We further suggested that “… if there are these agostic 18-electron intermediates, they may facilitate understanding of selectivities and other stereochemical factors arising in catalytic reactions.” These predictions have been borne out in a large number of subsequently studied systems. Proof that agostic interactions stabilize unsaturated reaction intermediates and play a role in determining transition states structures has come largely from either direct observation of intermediates by low-temperature spectroscopic techniques or through H/D isotope effects. As an illustration of the significance of agostic interactions in intermediates and transition states, we highlight here the crucial role that such interactions play in insertion of olefins into both early and late transition metal hydrogen and carbon bonds as well as how such interactions are proposed to control the stereochemistry of polymers produced via coordination/insertion chain propagation.
Polymerization of nonpolar olefins such as ethylene and propylene is carried out commercially on a massive scale employing group 4 d0 metal complexes, particularly Ti(IV) derived catalysts (50–53). Chain growth clearly occurs by a coordination/insertion mechanism, and the precise details of the transition state for insertion have received intense scrutiny from a number of research groups. The simplest view of the insertion process was proposed in the early 1960s by Cossee and Arlman (54, 55) and involves olefin coordination followed by migration of the growing alkyl chain to the bound olefin (Scheme 1). In our 1983 review (56), we suggested as an alternative, a “modified Green–Rooney” mechanism in which the insertion is facilitated by an α-agostic interaction, which is present in both the ground and transition states (Scheme 2). There is now considerable experimental and theoretical evidence to support an insertion mechanism in which an α-agostic interaction occurs in the transition state of many such insertion reactions involving early transition metal–alkyl complexes (57).
Scheme 1.
Scheme 2.
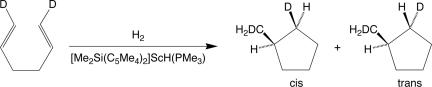
The most frequently used method for detecting α-agostic interactions in the transition states for olefin insertions has been isotopic perturbation of stereochemistry as first proposed by Grubbs and coworkers (58). The use of this probe is best illustrated by the work of Bercaw and coworkers (59, 60) who showed that [Me2Si(C5Me4)2]Sc(PMe3)H catalyzes the hydrocyclization 1,6-dideuterio-1,5-hexadiene to produce a 1.23:1.00 ratio of the trans:cis products (Scheme 3). This result supports a transition state for cyclization involving an α-agostic interaction with the favored transition state being the one in which the lighter isotope, H, occupies the bridging position as shown in Fig. 7.
Scheme 3.
Fig. 7.
Isomeric transition states for cyclization involving an α-agostic interaction ([Sc] = {[Me2Si(C5Me4)2]Sc).
Although numerous studies have established a secondary H/D isotope effect supporting an α-agostic interaction, the magnitude of these effects vary and in some cases, including the original Grubbs study (58) employing Cp2Ti(Cl)R/EtAlCl2, no effect is observed. In an insightful analysis, Grubbs and Coates (57) proposed a general mechanism shown in Scheme 4 to account for the variable isotope effects. Specifically, no isotope effect is observed if coordination of olefin is rate-determining (k2 > k−1), whereas an isotope effect is observed if olefin insertion (k2) is rate-determining.
Scheme 4.
Agostic interactions are proposed to play an important role in controlling polyolefin stereochemistry which is critical in determining the physical properties and utility of these materials. Numerous homogeneous C2 symmetric metallocene catalysts have been shown to produce highly isotactic polypropylene through an “enantiomorphic site control” mechanism in which the same enantioface of propylene binds and inserts each time (50, 57). The transition state proposed for insertion in a typical C2-symmetric ansa-metallocene is shown in Fig. 8. The α-agostic interaction orients the remaining α-hydrogen and α-polymeryl (P) groups in a vertical plane with the large P group occupying the least crowded position. Propene insertion then occurs through a four-centered transition state in which the methyl and polymeryl groups prefer to lie trans to one another. The agostic interaction clearly plays a key role in controlling the enantiofacially selective insertion because insertion into a Ti–CH3 bond in an analogous species is not facially selective (61, 62).
Fig. 8.
α-Agostic transition state for propene insertion.
In addition to α-agostic interactions, both β- and γ-agostic interactions are of significance in early metal olefin polymerization systems. β-Agostic species have been identified as the ground-state structures in several d0 metal alkyl complexes and are thought in many cases to be the catalyst resting state in olefin polymerizations (63–65). Species exhibiting γ-agostic interactions are formed upon insertion of olefins into an α-agostic species (Scheme 5) and have also been proposed as possible catalyst resting states (66–72). These γ interactions are thought to inhibit inversion at metal (“chain-swinging”) and thus allow for highly syndiospecific polymerization of propylene, where strict alternation (accomplished through monomer insertion) between sites preferring the si and re faces of propylene is required for high syndiospecificity (57). Bercaw and coworkers (73) recently reported experimental evidence for γ-agostic assistance in β-methyl elimination from a zirconium neopentyl complex, the microscopic reverse of α-agostic assistance in olefin insertion.
Scheme 5.
Agostic interactions figure heavily in migratory insertion and olefin polymerization reactions involving late transition complexes, but in these cases β-agostic species are the rule. cis-Olefin hydrides can exist as either classical terminal hydrides or β-agostic structures. Such species play a key role in olefin isomerization and olefin dimerization reactions. In general, agostic structures are favored for first and (less so) second row metals, especially cationic species or species possessing ligands that enhance the Lewis acidity of the metal center. Classical hydride structures appear to generally be favored for third row complexes or electron-rich systems.
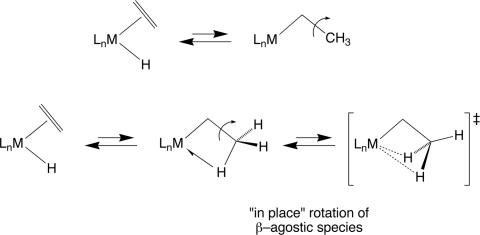
There are many cases studied in which the metal hydride undergoes exchange with the olefinic hydrogens through migratory insertion reactions. Such reactions are particularly important in metal-catalyzed olefin isomerizations. The classical view of this process is that insertion occurs to form an unsaturated species followed by C C bond rotation and return of a different β-hydrogen to metal as shown in Scheme 6 Upper. There is growing evidence (74–79) that a true 16e intermediate (or 14e intermediate in the case of square planar d8 systems) is never formed in these reactions: exchange occurs by formation of a β-agostic intermediate followed by “in-place” rotation of the Cα–Cβ bond as shown in Scheme 6 Lower. The unsaturated metal center never relinquishes contact with electron density in β–CH bonds.
C bond rotation and return of a different β-hydrogen to metal as shown in Scheme 6 Upper. There is growing evidence (74–79) that a true 16e intermediate (or 14e intermediate in the case of square planar d8 systems) is never formed in these reactions: exchange occurs by formation of a β-agostic intermediate followed by “in-place” rotation of the Cα–Cβ bond as shown in Scheme 6 Lower. The unsaturated metal center never relinquishes contact with electron density in β–CH bonds.
Scheme 6.
Cationic nickel(II) and palladium(II) complexes of type {[κ2–ArN=C(R)C(R)=NAr]MR}+ are highly active late metal catalysts for polymerization of a broad spectrum of olefinic monomers (80–84). The polymer microstructures obtained by using these systems are unique and quite different from those obtained from polymerizations by using early metal catalysts. For example, polymerization of ethylene results in branched polymers whose extent of branching varies depending on metal (Ni less branched, Pd more branched), ligand substituents, temperature, and ethylene pressure. Polymers ranging from lightly branched semicrystalline to completely amorphous materials can be produced (80, 81, 83).
The catalyst resting states are generally the alkyl ethylene species, but after migratory insertion, β-agostic alkyl complexes have been shown to be intermediates through independent synthesis and spectroscopic characterization at low temperatures (Scheme 7) (80, 85–89). These β-agostic species undergo rapid “chain walking” by means of a series of formally β-elimination/readdition reactions as shown in Scheme 7; however, density functional theory (DFT) studies suggest that a true olefin hydride intermediate actually never forms in these isomerizations (90). The relative stabilities of these agostic species and, more importantly, the rates of isomerization of these intermediates relative to their rate of trapping by ethylene, in large measure control the extent and nature of the branches in the polyolefins formed.
Scheme 7.
Although C H bonds are weak ligands, the examples of intramolecular coordination of C
H bonds are weak ligands, the examples of intramolecular coordination of C H bonds to transition metal centers cited here demonstrate that these agostic interactions can nevertheless play a critical role in determining structures of reaction intermediates and transition states and thereby control reaction products. The structures and chemistry of complexes exhibiting intramolecular M
H bonds to transition metal centers cited here demonstrate that these agostic interactions can nevertheless play a critical role in determining structures of reaction intermediates and transition states and thereby control reaction products. The structures and chemistry of complexes exhibiting intramolecular M H–C interactions discussed here are clearly related to the structures and chemistry of intermolecular alkane complexes discussed in articles in this special feature.
H–C interactions discussed here are clearly related to the structures and chemistry of intermolecular alkane complexes discussed in articles in this special feature.
Concluding Remarks
At the time of the 1983 review (13), intramolecular interactions of ligand C H bonds with transition metal centers was a phenomenon not widely recognized and was assumed to be a rare occurrence due to the poor coordinating ability of the C
H bonds with transition metal centers was a phenomenon not widely recognized and was assumed to be a rare occurrence due to the poor coordinating ability of the C H bond. The function of the 1983 review, in addition to summarizing data available at the time concerning such interactions, was to suggest that agostic interactions may be significantly stronger and much more prominent in organometallic chemistry than recognized or anticipated. Indeed, as exemplified in Fig. 4, such interactions are now widely recognized to occur in complexes involving metals across the periodic table and the term “agostic” is in common usage. Literally hundreds of examples presently show that agostic interactions influence ground-state structures and can play a critical role in dictating the structures and stabilities of reaction intermediates and transition states. Consequently, we predict that agostic interactions will continue to be an important component of organometallic chemistry in the future.
H bond. The function of the 1983 review, in addition to summarizing data available at the time concerning such interactions, was to suggest that agostic interactions may be significantly stronger and much more prominent in organometallic chemistry than recognized or anticipated. Indeed, as exemplified in Fig. 4, such interactions are now widely recognized to occur in complexes involving metals across the periodic table and the term “agostic” is in common usage. Literally hundreds of examples presently show that agostic interactions influence ground-state structures and can play a critical role in dictating the structures and stabilities of reaction intermediates and transition states. Consequently, we predict that agostic interactions will continue to be an important component of organometallic chemistry in the future.
Acknowledgments
This work was supported by Department of Energy, Office of Basic Energy Sciences Grant DE-FG02-93ER14339 and National Science Foundation Grant CHE-0615704.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
For a review of α- and β-hydrogen elimination processes, see ref. 1.
Although there are two reports of complexes in which alkanes are in the vicinity of the metal center (refs. 20 and 21), there is no spectroscopic evidence that the alkane remains coordinated to the metal in solution. Furthermore, for one of these complexes (ref. 21), both the metal and alkane are disordered so that the precise details of the interaction are uncertain.
For recent examples of σ-complexes that have been characterized by NMR spectroscopy, see refs. 22–24.
For a recent example, see ref. 28.
Venanzi and coworkers (37) also offered another interpretation for “pregostic”: agostic of the weak type described by Pregosin.
M H–C interactions that are not agostic have also been referred to as pseudo-agostic (see ref. 39).
H–C interactions that are not agostic have also been referred to as pseudo-agostic (see ref. 39).
For a recent analysis of M H–C interactions in d8 Ni, Pd, and Pt compounds, see ref. 43.
H–C interactions in d8 Ni, Pd, and Pt compounds, see ref. 43.
For a recent review of theoretical aspects of agostic interactions, see ref. 47.
This view of the bonding in agostic compounds is necessarily simplistic. For a more detailed discussion, see ref. 48.
References
- 1.Parkin G, Bunel E, Burger BJ, Trimmer MS, van Asselt A, Bercaw JE. J Mol Catal. 1987;41:21–39. [Google Scholar]
- 2.La Placa S, Ibers JA. Inorg Chem. 1965;4:778–783. [Google Scholar]
- 3.Trofimenko S. J Am Chem Soc. 1968;90:4754–4755. [Google Scholar]
- 4.Trofimenko S. Inorg Chem. 1970;9:2493–2499. [Google Scholar]
- 5.Cotton FA, Jeremic M, Shaver A. Inorg Chim Acta. 1972;6:543–551. [Google Scholar]
- 6.Cotton FA, LaCour T, Stanislowski AG. J Am Chem Soc. 1974;96:5074–5082. [Google Scholar]
- 7.Cotton FA, Day VW. J Chem Soc Chem Commun. 1974:415–416. [Google Scholar]
- 8.Cotton FA. Inorg Chem. 2001;41:643–658. doi: 10.1021/ic010972n. [DOI] [PubMed] [Google Scholar]
- 9.Dawoodi Z, Green MLH, Mtetwa VSB, Prout K, Schultz AJ, Williams JM, Koetzle TF. J Chem Soc Dalton Trans. 1986:1629–1637. [Google Scholar]
- 10.Dawoodi Z, Green MLH, Mtetwa VSB, Prout K. J Chem Soc Chem Commun. 1982:802–803. [Google Scholar]
- 11.Dawoodi Z, Green MLH, Mtetwa VSB, Prout K. J Chem Soc Chem Commun. 1982:1410–1411. [Google Scholar]
- 12.Brookhart M, Green MLH, Pardy RBA. J Chem Soc Chem Commun. 1983:691–693. [Google Scholar]
- 13.Brookhart M, Green MLH. J Organomet Chem. 1983;250:395–408. [Google Scholar]
- 14.Brookhart M, Green MLH, Wong LL. Prog Inorg Chem. 1988;36:1–124. [Google Scholar]
- 15.Buchanan JM, Stryker JM, Bergman RG. J Am Chem Soc. 1986;108:1537–1550. [Google Scholar]
- 16.Hall C, Perutz RN. Chem Rev. 1996;96:3125–3146. doi: 10.1021/cr9502615. [DOI] [PubMed] [Google Scholar]
- 17.Crabtree RH. Chem Rev. 1995;95:987–1007. [Google Scholar]
- 18.Crabtree RH. Angew Chem Int Ed Engl. 1993;32:789–805. [Google Scholar]
- 19.Kubas GJ. Metal Dihydrogen and s-Bond Complexes: Structure, Theory, and Reactivity. New York: Kluwer Academic/Plenum; 2001. [Google Scholar]
- 20.Castro-Rodriguez I, Nakai H, Gantzel P, Zakharov LN, Rheingold AL, Meyer K. J Am Chem Soc. 2003;125:15734–15735. doi: 10.1021/ja0379316. [DOI] [PubMed] [Google Scholar]
- 21.Evans DR, Drovetskaya T, Bau R, Reed CA, Boyd PD. J Am Chem Soc. 1997;119:3633–3634. [Google Scholar]
- 22.Geftakis S, Ball GE. J Am Chem Soc. 1998;120:9953–9954. [Google Scholar]
- 23.Lawes DJ, Geftakis S, Ball GE. J Am Chem Soc. 2005;127:4134–4135. doi: 10.1021/ja044208m. [DOI] [PubMed] [Google Scholar]
- 24.Lawes DJ, Darwish TA, Clark T, Harper JB, Ball GE. Angew Chem Int Ed Engl. 2006;45:4486–4490. doi: 10.1002/anie.200600313. [DOI] [PubMed] [Google Scholar]
- 25.Jones WD. Acc Chem Res. 2003;36:140–146. doi: 10.1021/ar020148i. [DOI] [PubMed] [Google Scholar]
- 26.Bullock RM, Bender BR. In: Encyclopedia of Catalysis. Horváth IT, editor. Vol 4. New York: Wiley; 2002. pp. 281–348. [Google Scholar]
- 27.Janak KE. In: Comprehensive Organometallic Chemistry III. Crabtree RH, Mingos DMP, editors. Vol 1. Oxford: Elsevier; 2006. Chap 1.20. [Google Scholar]
- 28.Çetin A, Ziegler CJ. Dalton Trans. 2006:1006–1008. doi: 10.1039/b516558j. [DOI] [PubMed] [Google Scholar]
- 29.Brammer L, Charnock JM, Goggin PL, Goodfellow RJ, Koetzle TF, Orpen AG. J Chem Soc Chem Commun. 1987:443–445. [Google Scholar]
- 30.Brammer L, Charnock JM, Goggin PL, Goodfellow RJ, Orpen AG, Koetzle IF. J Chem Soc Dalton Trans. 1991:1789–1798. [Google Scholar]
- 31.Brammer L, McCann MC, Bullock RM, McMullan RK, Sherwood P. Organometallics. 1992;11:2339–2341. [Google Scholar]
- 32.Roe DM, Bailey PM, Moseley K, Maitlis PM. J Chem Soc Chem Commun. 1972:1273–1274. [Google Scholar]
- 33.Albinati A, Anklin CG, Pregosin PS. Inorg Chim Acta. 1984;90:L37–L38. [Google Scholar]
- 34.Albinati A, Arz C, Pregosin PS. Inorg Chem. 1987;26:508–513. [Google Scholar]
- 35.Albinati A, Anklin CG, Ganazzoli F, Rüegger H, Pregosin PS. Inorg Chem. 1987;26:503–508. [Google Scholar]
- 36.Albinati A, Pregosin PS, Wombacher F. Inorg Chem. 1990;29:1812–1817. [Google Scholar]
- 37.Bortolin M, Bucher UE, Rüegger H, Venanzi LM, Albinati A, Lianza F, Trofimenko S. Organometallics. 1992;11:2514–2521. [Google Scholar]
- 38.Sundquist WI, Bancroft DP, Lippard SJ. J Am Chem Soc. 1990;112:1590–1596. [Google Scholar]
- 39.Braga D, Grepioni F, Tedesco E, Biradha K, Desiraju GR. Organometallics. 1997;16:1846–1856. [Google Scholar]
- 40.Zhang Y, Lewis JC, Bergman RG, Ellman JA, Oldfield E. Organometallics. 2006;25:3515–3519. [Google Scholar]
- 41.Braga D, Grepioni F, Biradha K, Desiraju GR. J Chem Soc Dalton Trans. 1996:3925–3930. [Google Scholar]
- 42.Lewis JC, Wu J, Bergman RG, Ellman JA. Organometallics. 2005;24:5737–5746. [Google Scholar]
- 43.Mukhopadhyay A, Pal S. Eur J Inorg Chem. 2006:4879–4887. [Google Scholar]
- 44.Yao W, Eisenstein O, Crabtree RH. Inorg Chim Acta. 1997;254:105–111. [Google Scholar]
- 45.Castro M, Cruz J, López-Sandoval H, Barba-Behrens N. J Chem Soc Chem Commun. 2005:3779–3880. doi: 10.1039/b505554g. [DOI] [PubMed] [Google Scholar]
- 46.Thakur TS, Desiraju GR. Chem Commun. 2006:553–554. doi: 10.1039/b514427b. [DOI] [PubMed] [Google Scholar]
- 47.Clot E, Eisenstein O. Struct Bonding. 2004;113:1–36. [Google Scholar]
- 48.Scherer W, McGrady GS. Angew Chem Int Ed Engl. 2004;43:1782–1806. doi: 10.1002/anie.200200548. [DOI] [PubMed] [Google Scholar]
- 49.Buccella D, Parkin G. J Am Chem Soc. 2006;128:16358–16364. doi: 10.1021/ja066457b. [DOI] [PubMed] [Google Scholar]
- 50.Coates GW. Chem Rev. 2000;100:1223–1252. doi: 10.1021/cr990286u. [DOI] [PubMed] [Google Scholar]
- 51.Pino P, Mülhaupt R. Angew Chem Int Ed Engl. 1980;19:857–875. [Google Scholar]
- 52.Brintzinger HH, Fischer D, Mulhaupt R, Rieger B, Waymouth RM. Angew Chem Int Ed Engl. 1995;34:1143–1170. [Google Scholar]
- 53.Hlatky GG. Coord Chem Rev. 1999;181:243–296. [Google Scholar]
- 54.Arlman EJ, Cossee P. J Catal. 1964;3:99–104. [Google Scholar]
- 55.Cossee P. Tetrahedron Lett. 1960:17–21. [Google Scholar]
- 56.Brookhart M, Green MLH. J Organomet Chem. 1983;250:395–408. [Google Scholar]
- 57.Grubbs RH, Coates GW. Acc Chem Res. 1996;29:85–93. [Google Scholar]
- 58.Clawson L, Soto J, Buchwald SL, Steigerwald ML, Grubbs RH. J Am Chem Soc. 1985;107:3377–3378. [Google Scholar]
- 59.Piers WE, Bercaw JE. J Am Chem Soc. 1990;112:9406–9407. [Google Scholar]
- 60.Burger BJ, Cotter WD, Coughlan EB, Chacon ST, Hajela S, Herzog TA, Köhn R, Mitchell J, Piers WE, Shapiro PJ, Bercaw JE. In: Ziegler Catalysts. Fink G, Mülhaupt R, Brintzinger HH, editors. Berlin: Springer; 1995. pp. 317–331. [Google Scholar]
- 61.Longo P, Grassi A, Pellecchia C, Zambelli A. Macromolecules. 1987;20:1015–1018. [Google Scholar]
- 62.Zambelli A, Pellecchia C, Oliva L. Macromol Chem Macromol Symp. 1991;48/49:297–316. [Google Scholar]
- 63.Burger BJ, Thompson ME, Cotter WD, Bercaw JE. J Am Chem Soc. 1990;112:1566–1577. [Google Scholar]
- 64.Guo Z, Swenson DC, Jordan RF. Organometallics. 1994;13:1424–1432. [Google Scholar]
- 65.Jordan RF, Bradley PK, Baenziger NC, LaPointe RE. J Am Chem Soc. 1990;112:1289–1291. [Google Scholar]
- 66.Woo TK, Fan L, Ziegler T. Organometallics. 1994;13:2252–2261. [Google Scholar]
- 67.Woo TK, Fan L, Ziegler T. Organometallics. 1994;13:432–433. [Google Scholar]
- 68.Fan L, Harrison D, Woo TK, Ziegler T. Organometallics. 1995;14:2018–2026. [Google Scholar]
- 69.Kawamura-Kuribayashi H, Koga N, Morokuma K. J Am Chem Soc. 1992;114:8687–8694. [Google Scholar]
- 70.Woo TK, Margl PM, Lohrenz JDW, Blöchl PE, Ziegler T. J Am Chem Soc. 1996;118:13021–13030. [Google Scholar]
- 71.Lohrenz JCW, Woo TK, Ziegler T. J Am Chem Soc. 1995;117:12793–12800. [Google Scholar]
- 72.den Haan KH, de Boer JL, Teuben JH, Spek AL, Kojic-Prodic B, Hays GR, Huis R. Organometallics. 1986;5:1726–1733. [Google Scholar]
- 73.Chirik PJ, Dalleska NF, Henling LM, Bercaw JE. Organometallics. 2005;24:2789–2794. [Google Scholar]
- 74.Green MLH, Wong L-L. J Chem Soc Chem Commun. 1988:677–679. [Google Scholar]
- 75.Derome AE, Green MLH, Wong L-L. New J Chem. 1989;10:747–753. [Google Scholar]
- 76.Bercaw JE, Burger BJ, Green MLH, Santarsiero BD, Sella A, Trimmer M, Wong L-L. J Chem Soc Chem Commun. 1989:734–736. [Google Scholar]
- 77.McNally JP, Cooper NJ. Organometallics. 1988;7:1704–1715. [Google Scholar]
- 78.Casey CP, Yi CS. Organometallics. 1991;10:33–35. [Google Scholar]
- 79.Tempel DJ, Brookhart M. Organometallics. 1998;17:2290–2296. [Google Scholar]
- 80.Johnson LK, Killian CM, Brookhart M. J Am Chem Soc. 1995;117:6414–6415. [Google Scholar]
- 81.Ittel SD, Johnson LK, Brookhart M. Chem Rev. 2000;100:1169–1203. doi: 10.1021/cr9804644. [DOI] [PubMed] [Google Scholar]
- 82.McCord EF, McLain SJ, Nelson LTJ, Arthur SD, Coughlin EB, Ittel SD, Johnson LK, Tempel D, Killian CM, Brookhart M. Macromolecules. 2001;34:362–371. [Google Scholar]
- 83.Gates DP, Svejda SA, Onate E, Killian CM, Johnson LK, White PS, Brookhart M. Macromolecules. 2000;33:2320–2334. [Google Scholar]
- 84.Leatherman MD, Brookhart M. Macromolecules. 2001;34:2748–2750. [Google Scholar]
- 85.Svejda SA, Johnson LK, Brookhart M. J Am Chem Soc. 1999;121:10634–10635. [Google Scholar]
- 86.Shultz LH, Brookhart M. Organometallics. 2001;20:3975–3982. [Google Scholar]
- 87.Tempel DJ, Johnson LK, Huff RL, White PS, Brookhart M. J Am Chem Soc. 2000;122:6686–6700. [Google Scholar]
- 88.Shultz LH, Tempel DJ, Brookhart M. J Am Chem Soc. 2001;123:11539–11555. doi: 10.1021/ja011055j. [DOI] [PubMed] [Google Scholar]
- 89.Leatherman MD, Svejda SA, Johnson LK, Brookhart M. J Am Chem Soc. 2003;125:3068–3081. doi: 10.1021/ja021071w. [DOI] [PubMed] [Google Scholar]
- 90.Deng L, Woo TK, Cavallo L, Margl PM, Ziegler T. J Am Chem Soc. 1997;119:6177–6186. [Google Scholar]