Abstract
Ig class switch recombination (CSR) and somatic hypermutation serve to diversify antibody responses and are orchestrated by the activity of activation-induced cytidine deaminase and many proteins involved in DNA repair and genome surveillance. Msh5, a gene encoded in the central MHC class III region, and its obligate heterodimerization partner Msh4 have a critical role in regulating meiotic homologous recombination and have not been implicated in CSR. Here, we show that MRL/lpr mice carrying a congenic H-2b/b MHC interval exhibit several abnormalities regarding CSR, including a profound deficiency of IgG3 in most mice and long microhomologies at Ig switch (S) joints. We found that Msh5 is expressed at low levels on the H-2b haplotype and, importantly, a similar long S joint microhomology phenotype was observed in both Msh5 and Msh4-null mice. We also present evidence that genetic variation in MSH5 is associated with IgA deficiency and common variable immune deficiency (CVID) in humans. One of the human MSH5 alleles identified contains two nonsynonymous polymorphisms, and the variant protein encoded by this allele shows impaired binding to MSH4. Similar to the mice, Ig S joints from CVID and IgA deficiency patients carrying disease-associated MSH5 alleles show increased donor/acceptor microhomology, involving pentameric DNA repeat sequences and lower mutation rates than controls. Our findings suggest that Msh4/5 heterodimers contribute to CSR and support a model whereby Msh4/5 promotes the resolution of DNA breaks with low or no terminal microhomology by a classical nonhomologous end-joining mechanism while possibly suppressing an alternative microhomology-mediated pathway.
Keywords: immunoglobulin subclass deficiency, mismatch repair, Msh4
After appropriate stimulation, B cells undergo class switch recombination (CSR), whereby the functionally rearranged V(D)J DNA segment is recombined with a downstream Ig constant region segment. The biochemistry of CSR is complex and involves the B cell-specific gene activation-induced cytidine deaminase, which initiates both CSR and somatic hypermutation (1). CSR also requires many ubiquitously expressed genes important for detecting DNA mismatches and breaks and regulating DNA repair (2). CSR occurs at specific DNA segments called switch (S) regions, which lie upstream of each constant region and contain hotspots for activation-induced cytidine deaminase-mediated cytosine deamination. The ligation of the Sμ region with the downstream S regions is carried out by protein factors that comprise the nonhomologous end joining machinery for DNA repair (1, 2).
Mismatch repair proteins play a critical role in safeguarding genetic stability. The key proteins for initiation of eukaryotic mismatch repair are homologues of bacterial MutS and MutL. In mammals, there are five MutS (Msh2, Msh3, Msh4, Msh5, and Msh6) and four MutL (Mlh1, Mlh3, Pms1, and Pms2) homologues. Each Mut homologue acts at the DNA repair or recombination site by forming heterodimers; Msh2-Msh6 (MutSα), Msh2-Msh3 (MutSβ), Msh4-Msh5 (MutSγ), Mlh1-Pms2 (MutLα), and Mlh1-Mlh3 (MutLγ) (3). MutS heterodimers are thought to recruit MutL heterodimers. Experiments using Mut homologue gene-knockout (KO) mice revealed that Msh2-, Msh6-, Mlh1-, and Pms2-deficient animals had decreased efficiency of CSR and somatic hypermutation (4). Deficiencies of MutS and MutL genes often result in differences in microhomology lengths at S joints and show three phenotypes, decreased (Msh2−/− and Mlh3−/−) (4, 5), no change (Msh6−/−) (6), or increased (Mlh1−/− and Pms2−/−) (5, 7) microhomology. The differences in S joint phenotypes between Msh2−/− mice and Mlh1−/− or Pms2−/− mice suggest the existence of other proteins that function in the same pathway of CSR as Mlh1 and Pms2.
Msh5 and Msh4 are involved in the resolution of DNA Holliday junctions, the four-stranded DNA structures that form during homologous recombination in meiosis (8). Msh4 and Msh5 KO mice are sterile due to an inability to resolve these meiotic chromosomal crossovers (9–11).
Based on these studies in mice, the Mut homologues are attractive candidate genes for human Ig deficiencies. Selective IgA deficiency (IgAD) (serum IgA <0.05 g/liter) is the most common primary immunodeficiency disorder in man, with a prevalence of ≈1/600 Caucasian individuals (12). The selective nature of the CSR defect in IgAD is not understood. Common variable immune deficiency (CVID) is a more severe disease and affects ≈1/25,000 Caucasians. Patients show a marked reduction in serum levels of both IgG (usually <3 g/liter) and IgA (<0.05 g/liter), together with reductions of IgM in about half the cases (<0.3 g/liter). CVID patients have a high incidence of infectious complications and, paradoxically, are prone to autoimmune disorders (13).
The available evidence suggests a common genetic basis for IgAD and CVID (14) and individuals with IgAD may transition into CVID. Haplotypes of the MHC show genetic association with IgAD, notably HLA (HLA) A1-B8-DR3 and B14-DR1 (15–17). Homozygosity for the A1-B8-DR3 haplotype is a particularly strong risk factor for IgAD in Caucasians, with an incidence reported as high as 13% (18). Whereas the association of IgAD and CVID with the MHC is clearly documented, the identity of the genetic effect(s) within the MHC remains controversial, with studies suggesting that class II molecules and/or genes in the centromeric class III region are involved (17, 19, 20). Other genes that contribute to CVID include rare mutations in the T cell costimulatory molecule ICOS (21) and TACI (TNFRSF13B) (22, 23).
Here, we provide evidence that Msh5 contributes to dysregulated Ig CSR in mice and identify a possible role for MSH5 in human IgAD and CVID.
Results
H-2b Congenic MRL/lpr Mice Show Defects in CSR.
We generated H-2b/b congenic MRL/lpr mice by introgressing the H-2b MHC haplotype from 129/Sv mice onto the MRL/lpr background. After nine generations of backcrossing, animals were genotyped for 136 polymorphic microsatellites, which confirmed that all markers outside the H-2 region were MRL/lpr derived. The congenic H-2b interval measured ≈13 Mb and included the entire MHC region (Fig. 1A). H-2b/b MRL/lpr mice exhibited no differences in disease compared with wild-type animals (24). Strikingly, however, 11/16 (68%) H-2b/b congenics had undetectable serum IgG3 antibodies (Fig. 1B), diminished levels of serum IgA antibodies in older mice, together with elevated serum levels of IgM and IgG2a antibodies. Serum IgG1 and IgG2b levels were similar in H-2b/b and H-2k/k MRL/lpr mice [supporting information (SI) Fig. 5]. The deficiency of IgG3 in the H-2b/b congenics was confirmed by ELISpot assays of splenic antibody secreting cells (SI Fig. 6). Importantly, the antibody phenotypes were similar in congenic H-2b/b MRL/lpr animals backcrossed nine generations, and those animals backcrossed >20 generations (data not shown), demonstrating that the genetic effect is stable, shows consistent incomplete penetrance, and is localized to the H-2 region.
Fig. 1.
Serum IgG3 deficiency, Msh5 gene expression, and CSR in H-2b/b congenic MRL/lpr mice. (A) Map of the 129/Sv congenic interval in F9 and F≥20 congenic H-2b/b MRL/lpr mice. The microsatellite markers and gene polymorphisms used to characterize the introgressed region are shown. (B) Serum IgG3 levels in the F9 H-2k/k, H-2b/k, and H-2b/b MRL/lpr mice. n = 12–16 mice in each group at 12 weeks of age. The number of mice in each group decreased with aging because of mortality. Bars indicate mean values. (C) Msh5 mRNA expression levels were measured in cDNA from splenic B cells of H-2k/k MRL/lpr mice and IgGpos and IgGneg H-2b/b MRLlpr congenic mice (n = 3 each) (D) CSR of splenic B cells was induced in vitro with LPS for class switch induction to IgG3. Representative FACS plots show the percentage of CD19+ IgG3 positive cells from IgG3pos H-2k/k and IgG3neg H-2b/b MRL/lpr mice. Numbers shown are average percentage ± SEM switched cells for three mice in each group. (E) IgG3pos H-2k/k and IgG3neg H-2b/b MRL/lpr mice were immunized with TNP-LPS or TNP-Ficoll, and IgG2b (Right) and IgG3 (Left) anti-TNP responses were measured at 2 weeks. Serum OD380 values are represented on the y axis. Data shown represent the mean ± SEM; n = 10 in each group. (F) Msh5 expression profile in BALB/c (H-2d) (n = 4), 129/Sv (H-2b) (n = 2), C57BL/6 (H-2b) (n = 3), and FVB (H-2q) (n = 3) mice, using quantitative PCR (mean ± SEM). (C and F) Data represent relative Msh5 mRNA copy numbers when compared with resting B cells from H-2k/k MRL/lpr mice (H-2k/k MRL/lpr = 100%; mean ± SEM). ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001. P values were calculated by using two-tailed Student's t tests.
Hypomorphic Allele of Msh5 on the H-2b Haplotype.
To identify the gene(s) from the H-2 region contributing to the IgG3 deficiency, we used gene expression microarrays to assay spleen RNA from 8-week-old congenic IgG3pos H-2b/b, IgG3neg H-2b/b, and H-2k/k MRL/lpr littermates. Essentially all of the significant differences in gene expression, with the exception of IgG3 mRNA, were genes encoded within the MHC congenic interval. IgG3 mRNA expression was significantly higher in H-2k/k MRL/lpr mice (average 36,385 affymetrix expression units) compared to IgG3neg H-2b/b mice (average 2,837 affymetrix expression units; P = 1 × 10−4) (SI Table 2). The H-2 Ea gene is deleted on the H-2b haplotype (25) and showed low expression in the H-2b/b congenic spleens. Expression differences were also observed for other class I and II MHC genes, which likely reflect polymorphisms between the H-2b and H-2k haplotypes. Msh5, which is located in the MHC class III region (Fig. 1A), was the only other differentially expressed gene within the MHC region and showed ≈6-fold lower expression in the H-2b congenic spleens (IgG3pos H-2b/b, 100 expression units; IgG3neg H-2b/b, 135 expression units) compared with the wild-type H-2k/k mice (668 expression units; P = 9 × 10−3 vs. IgG3pos H-2b/b and P = 8.2 × 10−3 vs. IgG3neg H-2b/b) (SI Table 2). The microarray expression results for Msh5 were confirmed by using TaqMan real-time quantitative PCR (Fig. 1C).
We next evaluated the influence of the low Msh5 levels on B cell class switching in vitro. Surprisingly, there were no differences in the efficiency of in vitro switching to IgG3 (LPS) or IgG1 (LPS + IL-4) between the IgG3neg H-2b/b congenic and control H-2k/k MRL/lpr B cells (Fig. 1D and data not shown). The ability of H-2b congenic B cells to switch in vitro is reminiscent of human IgAD, where in vitro stimulation of B cells from IgAD patients with CD40 and IL-10 induces normal levels of IgA secretion (26). Interestingly, immunization of IgG3neg H-2b/b MRL/lpr mice with T-independent stimuli TNP-LPS or TNP-Ficoll failed to elicit an IgG3 anti-TNP response, whereas IgM (data not shown) and IgG2b responses were intact (Fig. 1E).
We then surveyed several mouse strains and identified two distinct groups: high Msh5 expressers [MRL/lpr (H-2k), AKR (H-2k) (data not shown) and BALB/c (H-2d)] and low Msh5 expressers [129/Sv (H-2b), C57BL/6 (H-2b) and FVB (H-2q)] (Fig. 1F). The difference in B cell Msh5 mRNA expression levels between the two groups was ≈100-fold. The higher relative levels of Msh5 in the B cells of MRL/lpr H-2b congenics, compared with native H-2b strains, may reflect the activated state of B cells in MRL/lpr mice (27). Consistent with this idea, Msh5 was inducible in B cells from C57BL/6 mice (SI Fig. 7), although induced Msh5 expression levels were at least 10-fold lower than high expressers at baseline. Msh5 expression in B cells from high-expressing strains was not inducible (data not shown). We conclude that H-2b/b congenic MRL/lpr mice express a hypomorphic allele of Msh5 and hypothesize that the low expression of Msh5 on the MRL background contributes to the observed antibody phenotype.
Increased Microhomology at IgG3 Switch Junctions in H-2b/b MRL/lpr B Cells.
We next asked whether the IgG3 deficiency observed in the congenic MRL/lpr animals was accompanied by phenotypic differences in IgG3 S joints. Sμ-Sγ3 joints, amplified from splenic B cells of IgG3neg MRL/lpr H-2b/b mice, showed significantly longer segments of microhomology than the S joints from IgG3pos congenic (P = 0.0012) or H-2k/k MRL/lpr B cells (P = 0.0014; Fig. 2). The H-2b/b IgG3neg mice also had longer microhomology segments at Sμ-Sα joints (SI Fig. 8A). No other significant abnormalities were observed at the S junctions (SI Tables 3 and 4).
Fig. 2.
Increased microhomology at Sμ-Sγ3 junctions in IgG3neg H-2b/b congenic MRL/lpr, Msh5−/− FVB, and Msh4−/− C57BL/6 mice. S joints were amplified from three 6- to 8-week-old mice in the H-2k/k MRL/lpr, IgG3pos H-2b/b MRL/lpr, wild-type FVB, Msh5−/− FVB, and wild-type C57BL/6 groups, and four 6- to 8-week-old mice in the IgG3neg H-2b/b MRL/lpr and Msh4−/− C57BL/6 groups. Each dot represents the number of nucleotides of donor/acceptor identity at the junction for an individual S joint. P values were calculated by using two-tailed Mann–Whitney tests.
To extend these findings, we evaluated B cells in Msh5 KO FVB mice (9). Serum antibody levels and in vitro switching showed no significant differences between Msh5−/− mice and littermates (SI Fig. 9A and data not shown). Importantly, the Sμ-Sγ3 (Fig. 2) and Sμ-Sα (SI Fig. 8A) joints from splenic B cells of Msh5−/− mice showed significantly increased microhomology compared with wild-type littermates. To address the concern that other genes in tight linkage disequilibrium with Msh5 might be contributing to the observed phenotype, in both the congenics and the Msh5 KOs, we took two approaches. First, we studied KO mice for complement Factor B (Bf), another MHC class III region gene located <500 Kb centromeric of Msh5. Gene targeting for Bf was performed on the 129/Sv background, and the mice studied were backcrossed seven generations onto the C57BL/6 genetic background (28). Importantly, Sμ-Sγ3 and Sμ-Sα joints from splenic B cells of C57BL/6 wild-type and Bf−/− C57BL/6 mice showed no significant differences in the lengths of microhomologies (SI Fig. 8 B and C). Second, we evaluated mice deficient for Msh4, the heterodimeric partner of Msh5. Msh4 is located on mouse chromosome 3, a location distinct from Msh5. Similar to Msh5−/− mice, there were no significant differences in serum antibody levels (SI Fig. 9B) or in vitro class switching (SI Fig. 10). However, splenic Sμ-Sγ3 and Sμ-Sα joints from Msh4−/− mice showed significantly longer microhomologies compared with wild-type littermates (Fig. 2 and SI Fig. 8A). Taken together, these data strongly support a role for Msh5 and Msh4 in the regulation of microhomology at Ig S joints in mice.
Genetic Variants of MSH5 Are Associated with Human CVID and IgAD.
The selective antibody isotype deficiency observed in the H-2b/b congenic MRL/lpr mice prompted us to investigate MSH5 as a candidate gene for human IgAD and CVID. MSH5 mRNA is expressed in human tonsillar B lymphocytes and is present at levels that are higher in CD77+ germinal center B cells than in naïve or memory B cells (29) (SI Fig. 11). Using quantitative PCR, we confirmed the constitutive expression of MSH5 mRNA in both purified peripheral blood human B cells and Epstein–Barr virus transformed B cells (SI Table 5).
To identify genetic variation in MSH5, we sequenced the 25 coding and noncoding exons of MSH5 together with the promoter region in 96 IgAD and CVID cases and identified five nonsynonymous polymorphisms and a number of SNPs in noncoding regions of the gene (SI Fig. 12 and data not shown). To determine whether the identified variation in MSH5 contributed genetic susceptibility to IgAD or CVID, we genotyped relevant SNPs in 207 Swedish IgAD cases, 83 Swedish CVID cases, and 198 Swedish control cases and compared allele frequencies (Table 1).
Table 1.
Association of MSH5 alleles with CVID and IgAD in Sweden and the U.S.
| Cohort |
MSH5 alleles |
|
|---|---|---|
| L85F/P786S (exons 3, 24) | rs3131378 (intron 12) | |
| Allele frequency (n)* | ||
| Sweden | ||
| Controls (N = 396)† | 1.8% (7) | 11.9% (47) |
| IgAD (N = 414) | 3.6% (15)P = 0.104‡ | 31.4% (128)P = 2.1 × 10-1 |
| CVID (N = 166) | 2.4% (4) | 15.7% (26)P = 0.22 |
| U.S. | ||
| Controls (N = 976) | 3.2% (31) | 9.7% (95) |
| IgAD (N = 6) | 50% (3) | 0 (0) |
| CVID (N = 204) | 5.4% (11)P = 0.12 | 13.2% (27)P = 0.135 |
| Pooled odds ratio (confidence interval, 95%)§ | ||
| Combined | ||
| All IgAD | 2.85 (1.24–6.51) | 3.28 (2.28–4.72) |
| (N = 420 cases, 1,372 controls) | P = 5.8 × 10−3 | P = 7.9 × 10−11 |
| All CVID | 1.63 (0.88–3.02) | 1.40 (1.00–1.98) |
| (N = 370 cases, 1,372 controls) | P = 0.058 | P = 0.026 |
| All IgAD and CVID | 2.04 (1.29–3.30) | 2.15 (1.69–2.73) |
| (N = 790 cases, 1,372 controls) | P = 1.8 × 10−3 | P = 2.6 × 10−10 |
*N = total number of chromosomes genotyped in each group.
†n = number of positive alleles.
‡P values determined by using χ2 tests.
§Pooled odds ratios and P values determined by using Mantel-Haenszel tests.
We identified two rare nonsynonymous SNPs: a G/T SNP, Q292H, in exon 11, which was present in one Swedish CVID case and absent in all controls tested; and a G/T SNP, C580G, in exon 19, which was present in 2 of 212 IgAD patients and not found in either controls or CVID cases (SI Table 6). We also identified SNPs in exon 3 (L85F, rs28381349) and exon 24 (P786S, rs28399984). Interestingly, the L85F SNP was always found together with the P786S SNP (D′ = 1), indicating they are located on the same chromosomal segment. By oligotyping HLA-B and DR alleles in the Swedish cases, we determined that the L85F/P786S allele is present on the ancestral HLA B14-DR1 haplotype. Fourteen of 16 L85F/P786S cases (88%) were DR1 and/or B14 positive (SI Fig. 12). Importantly, B14-DR1 is one of the MHC haplotypes that has shown strong genetic association with IgAD and CVID (16). The MSH5 L85F/P786S allele was present in the Swedish controls at a frequency of 1.8% and was enriched ≈2-fold in IgAD cases (3.6%) and to a lesser extent in CVID (2.4%). These differences did not reach statistical significance (Table 1). Another nonsynonymous SNP, P29S, in exon 2 (rs2075789) was a frequent polymorphism in the control population (12.1%) and was not enriched in patients (SI Table 6).
SNP rs3131378, located in intron 12 of MSH5, is present on the extended A1-B8-DR3 MHC haplotype. Of 124 haplotypes carrying SNP rs3131378, 111 were positive for DR3 (90%), and 107/127 were positive for B8 (84%). rs3131378 was strongly associated with IgAD (31.4% allele frequency compared with 11.9% in controls, P = 2.1 × 10−11) (Table 1). CVID patients showed only a modestly increased allele frequency of rs3131378 (15.7%).
We next typed these SNPs in an independent cohort of 102 United States Caucasian CVID cases and 488 U.S. controls. Three U.S. IgAD cases were also typed. Although not reaching statistical significance, the L85F/P786S double missense allele of MSH5 was more frequent in the CVID cohort (5.4%) than in controls (3.2%). All three U.S. IgAD cases typed were heterozygous for the L85F/P786S allele. The MSH5 allele that was present on the extended DR3 haplotype was also modestly increased in U.S. CVID cases (13.2% vs. 9.7% in controls) (Table 1).
In a combined analysis of the Swedish and U.S. cohorts, the L85F/P786S allele showed significant association with IgAD (P = 5.8 × 10−3), borderline significance in CVID (P = 0.058), and evidence for association in the combined IgAD+CVID analysis (P = 1.8 × 10−3). rs3131378 showed strong association in the combined IgAD analysis (P = 7.9 × 10−11) and significant evidence in the pooled CVID analysis (P = 0.026) (Table 1).
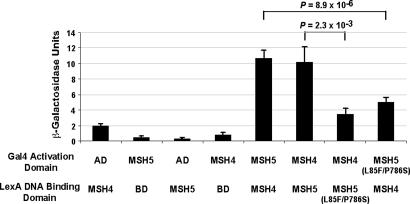
We next performed yeast two-hybrid assays to measure the interaction of the L85F/P786S variant MSH5 protein with MSH4, because both L85F and P786S are located within identified MSH4-interacting domains of MSH5 (SI Fig. 12) (30). Using sensitive liquid β-galactosidase assays, we found that the L85F/P786S MSH5 protein variant showed a diminished ability to bind to MSH4 as compared with the wild-type MSH5 (Fig. 3).
Fig. 3.
MSH5 L85F/P786S variant has reduced binding affinity to MSH4. Yeast two-hybrid assays were performed to assess the ability of the wild-type MSH5 and MSH5 L85F/P786S variant proteins, fused with either a LexA DNA binding domain (BD) or a Gal4 activation domain (AD) to interact with wild-type MSH4. The strength of interaction was measured by using a liquid β-galactosidase assay. Data represent mean ± SE of nine replicates from three independent experiments for the AD-MSH5 L85F/P786S interaction with BD-MSH4, and 12 replicates for all other conditions in four independent experiments. Western blots of whole yeast lysates confirmed equivalent expression of the wild-type and MSH5 L85F/P786S proteins (SI Fig. 17).
Given the background frequency of the MSH5 L85F/P786S allele in the population, it was important to determine whether controls carrying this allele were IgA deficient. We measured IgA levels in the plasma of 11 controls heterozygous for the L85F/P786S allele. All showed normal IgA levels (ranging from 1.0 to 4.4 g/liter), suggesting there is incomplete penetrance for the Ig deficiency phenotype associated with the L85F/P786S allele, consistent with the hypothesis that IgAD is a complex multigenic disease.
Increased Microhomology and Lower Mutation Rate at Sμ-Sα1 Joints of CVID Patients Carrying Disease Associated MSH5 Alleles.
We next investigated whether patients with IgAD and CVID carrying the identified MSH5 alleles showed differences in Ig S joint phenotypes. Sμ-Sα1 joints were amplified from peripheral blood DNA in three groups of controls: healthy donors lacking MSH5 or TACI polymorphisms (Control 1), healthy donors homozygous for rs3131378 on the B8-DR3 extended haplotype (Control 2, DR3++), and healthy donors heterozygous for the MSH5 L85F/P786S allele (Control 3, L85F/P786S). These sequences were compared with those amplified from three patient groups: CVID patients carrying TACI mutations and lacking MSH5 nonsynonymous or DR3 alleles (Patient 1, TACI*), DR3++ patients (Patient 2, DR3++), and patients carrying nonsynonymous alleles of MSH5 (Patient 3, MSH5*). The CVID group carrying TACI mutations tested the hypothesis that S joint phenotypes would differ between patients carrying TACI and MSH5 alleles.
Strikingly, CVID patients carrying MSH5 nonsynonymous polymorphisms displayed significantly longer stretches of Sμ-Sα1 microhomology than the various control groups (median 9 bp vs. 2 bp in controls; P = 1.9 × 10−7) (Fig. 4A). DR3++ CVID patients demonstrated a similar long microhomology phenotype (median 8 bp, P = 3 × 10−4). Importantly, S junction microhomology in CVID patients carrying alleles of the TACI gene and lacking any of the disease associated MSH5 alleles showed levels of microhomology similar to controls (Fig. 4A and SI Table 7). Thus, the long microhomology phenotype was specific to CVID cases carrying disease-associated MSH5 alleles.
Fig. 4.
Extended microhomology at B cell Ig switch joints of CVID patients carrying associated alleles of MSH5. (A) Distribution of microhomology length in Sμ-Sα1 junctions from CVID patients and controls. Each dot represents the length of microhomology of an independent S joint. The unlabeled group of controls lack any MSH5 nonsynonymous or DR3 alleles. MSH5 (L85F/P786S), heterozygote for the L85F/P786S allele. DR3++, homozygous for the rs3131378 SNP on the extended B8-DR3 MHC haplotype. TACI*, carrying one or more TACI missense mutations. Red, 0–1 bp microhomology; blue, 2–7 bp microhomology; orange, ≥ 8 bp microhomology. P values were calculated by using Mann–Whitney tests. (B) The percentage of S joints where there was “in-frame” alignment of pentamer repeat units between germline Sμ and Sα1 is represented. n = number of joints sequenced and examined. Statistical significance was determined by using two-tailed Fisher's exact tests.
We also found differences in S joint mutation rates between the groups. In controls lacking disease-associated MSH5 alleles, the mutation rate across the entire S joints averaged five mutations per 1,000 bp (SI Table 8). In contrast, there were far fewer mutations in joints from patients carrying MSH5 nonsynonymous polymorphisms (1.3 mutations per 1,000 bp; P = 2 × 10−12) or DR3++ (0.3 mutations per 1,000 bp; P = 1.3 × 10−7). Interestingly, mutation rates were also significantly lower in the DR3++ controls (3.2 mutations per 1,000 bp, P = 0.01) compared with other control groups. Across all of the groups studied, ≈90% of the S region mutations were targeted to dG:dC base pairs, but no differences were observed for the rate of transitions at dG:dC base pairs (SI Fig. 13 and SI Table 8). We also analyzed Sμ-Sα joints from IgAD patients carrying the associated MSH5 alleles, and the results mirrored those observed in CVID (SI Table 9).
To investigate whether mutations in MSH5 could also be contributing to alterations in the CSR process to other Ig isotypes, we characterized Sμ-Sγ3 junctions from a group of CVID patients carrying MSH5 disease-associated alleles. No significant differences in the length of microhomology were observed among the different groups (SI Table 10). Of interest, and similar to the findings in Sμ-Sα1 joints, the mutation rate across Sμ-Sγ3 joints was lower for both MSH5 L85F/P786S and DR3+/+ patients compared with controls (SI Table 11).
Finally, we examined the targeting of breakpoints to either pentamers or activation-induced cytidine deaminase hotspots and the pattern of alignment of pentamer repeats at the S junctions. In controls, ≈50% of the Sμ and Sα1 breakpoints occurred within pentamer repeats. In contrast, breakpoints from MSH5 L85F/P786S patients were significantly targeted to pentamer motifs at Sμ (82%, P = 5.4 × 10−3) (SI Table 7) but not at Sα1 (SI Table 11). Controls carrying the MSH5 L85F/P786S allele showed a similar preferential targeting of Sμ breakpoints to pentamers (75%, P = 0.013) as observed in L85F/P786S patients. More significantly, we found that the vast majority of Sμ-Sα1 junctions from MSH5 L85F/P786S (95%) and DR3++ (100%) patients showed an “in-phase” alignment of pentamer motifs, whereas in control junctions, only ≈50% of pentamer motifs were aligned (Fig. 4B and SI Fig. 14).
Discussion
We found that ≈70% of H-2b/b congenic MRL/lpr mice carrying a hypomorphic Msh5 allele were deficient in serum IgG3, and nucleotide sequence analysis of Ig S junctions revealed increased donor/acceptor microhomology. Similar long microhomologies at S junctions were observed in KO mice for Msh5 and Msh4. Furthermore, we identified several alleles of MSH5 in humans that show genetic association with CVID and IgAD, including one (L85F/P786S) where the encoded mutant protein showed reduced binding affinity to its heterodimerization partner MSH4. Similar to the phenotype observed in the mice, Sμ-Sα1 joints from patients with associated MSH5 alleles showed increased microhomology, together with a reduced mutation rate, an increased in-phase alignment of pentamer repeats at the junctions, and targeting of Sμ breaks to pentamers (SI Fig. 15). Given these findings, what might be the mechanism by which Msh5 participates in the complex biochemistry of CSR?
Msh5 has a well-characterized role in resolving Holliday junctions that form between homologous DNA strands during meiosis (8–10). Heterodimers of Msh5 and Msh4 are postulated to form a “sliding clamp” on DNA and serve as scaffolding for the recombination machinery including the DNA repair proteins Mlh1 and Pms2 (8). We envision a similar function for Msh4/5 during the early stages of intra-chromosomal “synapsis” of Sμ to Sγ3 or Sα, thereby facilitating the recruitment of proteins required for nonhomologous end joining.
An important observation relevant to the current data are the higher level of sequence homology between Sμ and Sγ3 regions than between Sμ and any of the other S regions in mice (1). Similarly, in humans the Sμ region shows high levels of overall homology with the Sα region (≈70%) and much less homology (≈20%) with the various IgG S regions (31). We speculate that, given its potential contribution to IgAD, MSH5 in humans may have a specific role in facilitating CSR between Sμ and Sα. There is more IgA produced in the body than any other Ig isotype and this function of MSH5 may have evolved to ensure high-level IgA production for mucosal defenses.
Another important question is the relationship between Msh5 and the long S joint microhomologies. S junctions with extended microhomology are rarely found in the B cells of healthy individuals and may reflect the activity of an alternative microhomology mediated end-joining (MMEJ) pathway, which uses homology searching and exonuclease activity to ligate homologous DNA strands with 3′ or 5′ overhangs. Increased S joint microhomology is also found in humans with ATM and DNA Ligase IV missense mutations (32, 33) and in Pms2−/− (7) and Mlh1−/− (5) mice, suggesting that these proteins may form a complementation group for CSR. If this group of genes is important for recruitment of the nonhomologous end joining machinery, reduced function of these proteins may result in a net loss in efficiency of CSR, as observed in IgAD and CVID, and an increased dependence on microhomology-directed mechanisms for alignment and ligation of S joints. The low mutation rate noted in long microhomology S joints may reflect exonuclease activity that is required for MMEJ, which, we postulate, could “erase” the footprints of activation-induced cytidine deaminase activity at the initial double-strand break, such that the ligated ends of the resulting S regions are well upstream or downstream from the initial site of double-strand break and in an area of reduced mutation frequency. We are also intrigued by the possibility that Msh5 may have anti-recombinational activity and secondarily function to suppress MMEJ of S joints (SI Fig. 16). Many oncogenic chromosome translocations in the Ig S region contain short stretches of microhomology between the donor and acceptor DNA strands (34, 35). Thus, active suppression of the MMEJ pathway may reduce the number of chromosomal translocations resulting from CSR. Interestingly, the frequency of malignant B cell lymphomas is increased in patients with CVID (13).
Antibody deficiencies were observed in congenic H-2b/b MRL/lpr mice, but not in Msh4−/− or Msh5−/− mice, whereas long S joint microhomologies were found in each strain. MRL/lpr mice have strong spontaneous self-antigen driven B cell responses in vivo, with antibody levels up to 10-fold higher than non-autoimmune animals (27). We speculate that the genetically based activated B cell phenotype in MRL/lpr mice accentuates the defects in CSR caused by the hypomorphic allele of Msh5 in the congenics. It seems likely that the IgG3 deficiency in MRL/lpr H-2b/b mice reflects specific interactions between the hypomorphic H-2b Msh5 allele and other genes in the MRL/lpr background.
As for the human studies, the nonsynonymous alleles of MSH5 identified in the current study are rare (Q292H and C580G) or uncommon (L85S/P786S), and thus our power to definitively conclude that these alleles contribute to immune deficiency based on genetic data is limited. We believe it is important to interpret the human genetic data under the hypothesis that IgAD and CVID are complex genetic diseases. As shown here, Ig deficiencies were not observed in controls heterozygous for MSH5 nonsynonymous alleles. However, there were subtle, yet significant, changes in S joint phenotypes in controls carrying the various MSH5 alleles: decreased switch joint mutation rates in DR3++ controls and increased targeting of Sμ breakpoints to pentamers in MSH5 L85F/P786S controls (SI Fig. 15 and SI Table 8). Furthermore, B8-DR3 is a common MHC haplotype in the Caucasian population (≈10% allele frequency), yet the vast majority of DR3++ individuals are not immune deficient. We have not yet identified functional effects of the MSH5 allele carried on the A1-B8-DR3 extended haplotype. Although there are seven unique SNPs within the MSH5 gene found only on this extended haplotype, there are no nonsynonymous polymorphisms, and further work will be required to determine whether this allele is associated with altered splicing, expression, folding or inducibility of MSH5 during CSR. Because of the high level of linkage disequilibrium on the B8-DR3 haplotype, it is currently not possible to rule out the potential role of additional genes on the haplotype contributing to CSR.
In summary, these data provide evidence that the contribution of Msh5 to Ig CSR is complex and likely regulatory. Antibody deficiency was only observed in the congenic MRL/lpr mice, and inbred strains carrying hypomorphic alleles of Msh5 (e.g., C57BL/6, 129/Sv) did not show Ig deficiencies, possibly due to balancing selection of the MHC region (36). In humans, the various mutant MSH5 alleles identified may not be sufficient by themselves to cause clinically significant antibody deficiencies and may be compensated by other genes that confer disease resistance. Thus, the disease state in IgAD and CVID is likely a result of complex interactions with other susceptibility genes and possibly environmental factors, similar to other multigenic complex diseases in humans.
Methods
Mice.
129/Sv (H-2b), C57BL/6 (H-2b), BALB/c (H-2d), FVB (H-2q) and MRL/lpr (H-2k) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). H-2b/b MRL/lpr mice were generated as described in ref. 24. Msh5-deficient mice were described in ref. 9 and were bred onto the FVB (H-2q) background for >15 generations. Bf-gene KO mice were provided by J. Thurman (University of Colorado, Boulder, CO) (28), and Msh4-gene KO mice, backcrossed to C57BL/6, were provided by W. Edelmann (Albert Einstein College of Medicine, Bronx, NY) (11).
Human DNA Samples.
DNA from 207 IgAD and 83 CVID Swedish cases and 198 controls were collected at the Karolinska Institute (Stockholm, Sweden). Three IgAD and 102 CVID U.S. cases were collected at the Mt. Sinai Hospital (New York, NY) and at the NCI/NIH (Bethesda, MD). 488 healthy U.S. controls were selected from the New York Health Project (37). All U.S. patients and controls are of self-reported European-Caucasian ancestry. Informed consent was obtained from all subjects, and the studies were approved by human subjects research institutional review boards.
MSH5 Sequencing and Genotyping.
The 25 exons and 1 Kb of the promoter of MSH5 were sequenced in 63 CVID cases from the U.S. or Sweden, and 33 IgAD cases from Sweden at the Broad Institute. Automated sequence analysis software (SNP_COMPARE) and manual examination was used to screen the sequencing files. Twenty-seven “high-quality” SNPs were identified, of which, 17 were already described in dbSNP (v124), and 10 SNPs were new. Additional sequencing was performed to resolve discrepancies or to fill-in missing data. Genotyping assays for the identified SNPs were performed by using both Taqman and Sequenom platforms. Primer and probe sequences are shown in SI Table 12. Additional details are provided in SI Materials and Methods.
Mouse and human switch junction sequence alignments are also provided in SI Appendices 1 and 2.
Supplementary Material
Acknowledgments
We thank the many patients and physicians for their contributions. These studies were supported by Fundação para a Ciência e Tecnologia, Portugal Fellowship SFRH/BD/16281/2004 (to R.C.F.), National Institutes of Health Grants U19 AI067152 and AR043274, and the Swedish Research Council.
Abbreviations
- CSR
class switch recombination
- CVID
common variable immune deficiency
- IgAD
IgA deficiency
- KO
knockout.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700815104/DC1.
References
- 1.Min IM, Selsing E. Adv Immunol. 2005;87:297–328. doi: 10.1016/S0065-2776(05)87008-7. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y. Nat Rev Immunol. 2006;6:261–270. doi: 10.1038/nri1804. [DOI] [PubMed] [Google Scholar]
- 3.Svetlanov A, Cohen PE. Exp Cell Res. 2004;296:71–79. doi: 10.1016/j.yexcr.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Wu X, Tsai CY, Patam MB, Zan H, Chen JP, Lipkin SM, Casali P. J Immunol. 2006;176:5426–5437. doi: 10.4049/jimmunol.176.9.5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrader CE, Vardo J, Stavnezer J. J Exp Med. 2002;195:367–373. doi: 10.1084/jem.20011877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z, Scherer SJ, Ronai D, Iglesias-Ussel MD, Peled JU, Bardwell PD, Zhuang M, Lee K, Martin A, Edelmann W, Scharff MD. J Exp Med. 2004;200:47–59. doi: 10.1084/jem.20040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrenstein MR, Rada C, Jones AM, Milstein C, Neuberger MS. Proc Natl Acad Sci USA. 2001;98:14553–14558. doi: 10.1073/pnas.241525998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snowden T, Acharya S, Butz C, Berardini M, Fishel R. Mol Cell. 2004;15:437–451. doi: 10.1016/j.molcel.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 9.de Vries SS, Baart EB, Dekker M, Siezen A., de Rooij DG, de Boer P, te Riele H. Genes Dev. 1999;13:523–31. doi: 10.1101/gad.13.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edelmann W, Cohen PE, Kneitz B, Winand N, Lia M, Heyer J, Kolodner R, Pollard JW, Kucherlapati R. Nat Genet. 1999;21:123–127. doi: 10.1038/5075. [DOI] [PubMed] [Google Scholar]
- 11.Kneitz B, Cohen PE, Avdievich E, Zhu L, Kane MF, Hou H, Jr, Kolodner RD, Kucherlapati R, Pollard JW, Edelmann W. Genes Dev. 2000;14:1085–1097. [PMC free article] [PubMed] [Google Scholar]
- 12.Burrows PD, Cooper MD. Adv Immunol. 1997;65:245–276. [PubMed] [Google Scholar]
- 13.Cunningham-Rundles C, Bodian C. Clin Immunol. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 14.Vorechovsky I, Zetterquist H, Paganelli R, Koskinen S, Webster AD, Bjorkander J, Smith CI, Hammarstrom L. Clin Immunol Immunopathol. 1995;77:185–192. doi: 10.1006/clin.1995.1142. [DOI] [PubMed] [Google Scholar]
- 15.Hammarstrom L, Smith CI. Tissue Antigens. 1983;21:75–79. doi: 10.1111/j.1399-0039.1983.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 16.Olerup O, Smith CI, Hammarstrom L. Nature. 1990;347:289–290. doi: 10.1038/347289a0. [DOI] [PubMed] [Google Scholar]
- 17.Schaffer FM, Palermos J, Zhu ZB, Barger BO, Cooper MD, Volanakis JE. Proc Natl Acad Sci USA. 1989;86:8015–8019. doi: 10.1073/pnas.86.20.8015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alper CA, Marcus-Bagley D, Awdeh Z, Kruskall MS, Eisenbarth GS, Brink SJ, Katz AJ, Stein R, Bing DH, Yunis EJ, Schur PH. Tissue Antigens. 2000;56:207–216. doi: 10.1034/j.1399-0039.2000.560302.x. [DOI] [PubMed] [Google Scholar]
- 19.Vorechovsky I, Webster AD, Plebani A, Hammarstrom L. Am J Hum Genet. 1999;64:1096–1109. doi: 10.1086/302326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vorechovsky I, Cullen M, Carrington M, Hammarstrom L, Webster AD. J Immunol. 2000;164:4408–4416. doi: 10.4049/jimmunol.164.8.4408. [DOI] [PubMed] [Google Scholar]
- 21.Grimbacher B, Hutloff A, Schlesier M, Glocker E, Warnatz K, Drager R, Eibel H, Fischer B, Schaffer AA, Mages HW, et al. Nat Immunol. 2003;4:261–268. doi: 10.1038/ni902. [DOI] [PubMed] [Google Scholar]
- 22.Salzer U, Chapel HM, Webster AD, Pan-Hammarstrom Q, Schmitt-Graeff A, Schlesier M, Peter HH, Rockstroh JK, Schneider P, Schaffer AA, et al. Nat Genet. 2005;37:820–828. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 23.Castigli E, Wilson SA, Garibyan L, Rachid R, Bonilla F, Schneider L, Geha RS. Nat Genet. 2005;37:829–834. doi: 10.1038/ng1601. [DOI] [PubMed] [Google Scholar]
- 24.Sekine H, Graham KL, Zhao S, Elliott MK, Ruiz P, Utz PJ, Gilkeson GS. J Immunol. 2006;15:7423–7434. doi: 10.4049/jimmunol.177.10.7423. [DOI] [PubMed] [Google Scholar]
- 25.Mathis DJ, Benoist C, Williams VE, 2nd, Kanter M, McDevitt HO. Proc Natl Acad Sci USA. 1983;80:273–277. doi: 10.1073/pnas.80.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briere F, Bridon JM, Chevet D, Souillet G, Bienvenu F, Guret C, Martinez-Valdez H, Banchereau J. J Clin Invest. 1994;94:97–104. doi: 10.1172/JCI117354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Theofilopoulos AN, Dixon FJ. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 28.Taube C, Thurman JM, Takeda K, Joetham A, Miyahara N, Carroll MC, Dakhama A, Giclas PC, Holers VM, Gelfand EW. Proc Natl Acad Sci USA. 2006;103:8084–8089. doi: 10.1073/pnas.0602357103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein U, Tu Y, Stolovitzky GA, Keller JL, Haddad J, Jr, Miljkovic V, Cattoretti G, Califano A, Dalla-Favera R. Proc Natl Acad Sci USA. 2003;100:2639–2644. doi: 10.1073/pnas.0437996100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yi W, Wu X, Lee TH, Doggett NA, Her C. Biochem Biophys Res Commun. 2005;332:524–532. doi: 10.1016/j.bbrc.2005.04.154. [DOI] [PubMed] [Google Scholar]
- 31.Dunnick W, Hertz GZ, Scappino L, Gritzmacher C. Nucleic Acids Res. 1993;21:365–372. doi: 10.1093/nar/21.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan Q, Petit-Frere C, Lahdesmaki A, Gregorek H, Chrzanowska KH, Hammarstrom L. Eur J Immunol. 2002;32:1300–1308. doi: 10.1002/1521-4141(200205)32:5<1300::AID-IMMU1300>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 33.Pan-Hammarstrom Q, Jones AM, Lahdesmaki A, Zhou W, Gatti RA, Hammarstrom L, Gennery AR, Ehrenstein MR. J Exp Med. 2005;201:189–194. doi: 10.1084/jem.20040772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramiro AR, Jankovic M, Eisenreich T, Difilippantonio S, Chen-Kiang S, Muramatsu M, Honjo T, Nussenzweig A, Nussenzweig MC. Cell. 2004;118:431–438. doi: 10.1016/j.cell.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Weinstock DM, Elliott B, Jasin M. Blood. 2006;107:777–780. doi: 10.1182/blood-2005-06-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hughes AL, Yeager M. Annu Rev Genet. 1998;32:415–435. doi: 10.1146/annurev.genet.32.1.415. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell MK, Gregersen PK, Johnson S, Parsons R, Vlahov D. J Urban Health. 2004;81:301–310. doi: 10.1093/jurban/jth116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.