Abstract
The role continuous contact with self-peptide/MHC molecules (self ligands) in the periphery plays in the function of mature T cells remains unclear. Here, we elucidate a role for MHC class II molecules in T cell trafficking and antigen responsiveness in vivo. We find that naïve CD4 T cells deprived of MHC class II molecules demonstrate a progressive and profound defect in motility (measured by real-time two-photon imaging) and that these cells have a decreased ability to interact with limiting numbers of cognate antigen-bearing dendritic cells, but they do not demonstrate a defect in their responsiveness to direct stimulation with anti-CD3 monoclonal antibody. Using GST fusion proteins, we show that MHC class II availability promotes basal activation of Rap1 and Rac1 but does not alter the basal activity of Ras. We propose that tonic T cell receptor signaling from self-ligand stimulation is required to maintain a basal state of activation of small guanosine triphosphatases critical for normal T cell motility and that T cell motility is critical for the antigen receptivity of naïve CD4 T cells. These studies suggest a role for continuous self-ligand stimulation in the periphery for the maintenance and function of mature naïve CD4 T cells.
Keywords: GTPases, MHC class II deficiency, self ligand
T cells developing in the thymus depend on signals from self-peptide/MHC molecules (self ligands) for lineage commitment and T cell receptor (TCR) repertoire selection. Positively selected thymocytes are able to migrate out of the thymus whereas unselected ones remain (1). In the periphery, naïve T cells are in constant motion, making incessant, short-lived contact with self-ligand-bearing dendritic cells (DCs) residing in secondary lymphoid tissues. The movement of T cells within secondary lymphoid tissue facilitates scanning for limiting numbers of DCs bearing antigen allowing T cells to reach the critical threshold for optimal TCR signaling (2). Lymphocyte movement is a complex process composed of interactions and changes between the cytoskeleton, adhesion molecules, and motor proteins within the cell compartment, and is regulated by a number of small guanosine triphosphatases (GTPases) (3, 4). These small GTPases transmit extracellular signals to downstream effectors within the cell. The role self ligands play in mediating such signals is unknown.
In recent years it has been increasingly recognized that self-ligand signals play critical roles in the biology of mature T cells in the periphery, although these roles are still being defined. Mounting evidence suggests that this self-recognition is an active process that occurs continuously in noninflammatory conditions and in the absence of cognate antigen (5–7). In addition, nonagonist peptide/MHC molecules have been shown to accumulate in the immunological synapse and contribute to TCR signaling (8). Signals from self ligands are important for the overall “well-being” of mature T cells and maintain clonal heterogeneity (9) by conferring the ability to survive and proliferate during periods of lymphopenia (10, 11).
Two seemingly conflicting views have recently emerged to define the role of peripheral contact with self ligands on the function of CD4 T cells. One view is that continuous self-ligand stimulation contributes to self-tolerance, whereas the opposing view is that self-ligand stimulation enhances immunity. Loss of contact with MHC molecules for weeks results in down-modulation of CD5 expression (a negative regulator of T cell reactivity) (12, 13) and in hyperresponsiveness to TCR stimulation (12, 14). These observations are consistent with the idea that continuous self-ligand stimulation raises the threshold for TCR responsiveness, thereby avoiding the emergence of autoreactivity (15–17). More recently, Germain and colleagues (18) have suggested that self ligands regulate the recruitment of the tyrosine phosphatase SHP-1, thereby regulating TCR reactivity. The opposing view is that continuous stimulation from self ligands expressed by antigen-presenting cells (APCs) is an important tonic signal for naïve T cells, lowering the threshold for T cell activation and keeping the T cells in a state of readiness to respond to foreign antigens (19–22).
Thus, whether repeated encounter with self ligands enhances or diminishes T cell reactivity to foreign peptide/MHC ligands remains unclear. In this study we report a perspective on how self-ligand interactions promote antigen reactivity. We found that CD4 T cells deprived of contact with self-MHC class II molecules displayed an impaired motility within lymph nodes that resulted in decreased ability to interact with limiting numbers of cognate antigen-bearing DCs and blunted proliferation. We propose that tonic TCR signaling from continuous self-ligand stimulation is required to maintain T cell motility and that normal T cell motility is critical for antigen receptivity of naïve CD4 T cells.
Results
OT-II TCR transgenic CD4 T cells (OT-II cells) were highly purified from OT-II B6.PL RAG−/− mice by using negative selection with magnetic microbeads to eliminate I-Ab-, CD11b-, and CD16-positive cells before adoptive transfer. Self-ligand deprivation was achieved by transferring OT-II cells into MHC class II-deficient recipients. To control for potential homeostatic proliferation due to the CD4 lymphopenia found in MHC class II-deficient mice, we transferred OT-II cells into CD4-deficient (MHC class II-sufficient) mice. As an additional control, OT-II cells were transferred into wild-type C57BL/6 (B6) mice. In some experiments, the OT-II cells were labeled before transfer with a fluorescent dye, 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE), to track cell division and/or visualize the cells by immunofluorescence microscopy. Antigen could not be administered alone in this model because the endogenous DCs in the MHC class II-deficient mice are unable to provide the essential peptide/MHC class II complex to the OT-II cells. We therefore used a system of immunizing with peptide/MHC class II-bearing DCs (23) by purifying DCs from Act-mOVA transgenic mice (24). These mice ubiquitously express a membrane-bound (nonsecreted) form of chicken ovalbumin (mOVA) under the β-actin promoter and OT-II cells proliferate when cultured with irradiated splenocytes or purified DCs obtained from these Act-mOVA mice (E.I., unpublished data). Exogenously administered Act-mOVA DCs were used to determine whether the OT-II cells were responsive to stimulation [supporting information (SI) Fig. 5].
Prolonged MHC Class II Deprivation Leads to a Progressive Defect in the Ability of Naïve Antigen-Specific CD4 T Cells to Become Activated and Proliferate in Response to Cognate Antigen-Bearing DCs.
As in other studies using TCR transgenic or polyclonal T cells (12, 13), we noted progressively lower levels of CD5 expression on OT-II cells with longer periods of residence in MHC class II-deficient recipients (SI Fig. 6). In vivo responsiveness of MHC class II-deprived CD4 T cells was tested by analyzing cell division and expression of CD69 in response to highly purified Act-mOVA or wild-type DCs. Because CD5 is a negative regulator of TCR-mediated signaling (25–28), we anticipated that this change would result in hyperresponsiveness to antigenic stimulation. In contrast, we found that the ability of OT-II cells to proliferate in response to Act-mOVA-expressing DCs injected on the same day as the OT-II cells was lower in MHC class II-deficient compared with MHC class II-sufficient recipients. This defect became more pronounced when the OT-II cells were deprived of MHC class II contact for 24 h, and was most profound with additional days of deprivation (Fig. 1A). A similar progressive defect was noted in the expression of CD69 (Fig. 1B). Importantly, after 1–2 days of deprivation, despite the defect in proliferation, 70% of the OT-II cells expressed CD69 and, thus, had contacted MHC class II-expressing DCs. CD69 expression dropped to 28% with 7 days of deprivation, but very little cell division was noted at that time. These results were not confounded by homeostatic proliferation, because there was no CFSE dilution of OT-II cells in recipients in the absence of DC injection within 8 days (data not shown). To test whether the defect in antigen responsiveness results from loss of specific or general interactions with MHC class II, we transferred CFSE-labeled purified OT-II cells into single peptide (YAe) mice. These mice carry a transgene encoding MHC class II I-Ab molecules occupied by a single antigenic peptide (Eα52–68) covalently linked to the β chain (29). Proliferation in response to antigen-bearing DCs was significantly reduced in these recipients (SI Fig. 7), indicating that peptides are, at least in part, responsible for the observed functional defect.
Fig. 1.
Prolonged MHC class II deprivation leads to progressive defects in T cell activation and proliferation in response to antigen-bearing DCs. OT-II cells were transferred into MHC class II-deficient, CD4-deficient, or wild-type B6 mice on indicated days before i.v. injection of 1 × 106 Act-mOVA DCs on day 0. In the circumstance where DCs were transferred on the same day as T cells, the T cells were injected before the DCs. (A) Spleens were harvested 3 days after DC injection. Similar results were found with peripheral lymph nodes (data not shown). (Left) Histograms show CFSE content of OT-II cells (CD4+ Thy1.1+) transferred into MHC class II-deficient (unshaded) and CD4-deficient (shaded) recipients. The mean number of cell divisions (M) as experienced by an average OT-II cell recovered on day 3 was calculated according to the following formula: M = Σ0→n(Tn × n)/Σ0→nTn, where Tn represents the number of OT-II cells within each cell division bin n; n = number of cell divisions. The graph shows data from spleens of CD4-deficient (filled squares) and MHC class II-deficient (open circles) recipients. Each data point shows the mean (±SD) of four animals. These data are representative of five independent experiments. (B) Spleens were harvested 18 h after injection of Act-mOVA DCs. Histograms show CD69 expression by OT-II (CD4+ Thy1.1+) cells transferred into MHC class II−/−, CD4−/−, and wild-type B6 recipients. Each histogram is representative of two animals per group and three independent experiments.
The progressive phenomenon discussed above suggests that the proliferation defect is acquired. However, it is possible that the defect in proliferation could be explained by lack of indirect antigen presentation or impaired T cell survival in MHC-deficient hosts. To test whether “cross-presentation” played a significant role in the proliferation of CFSE-labeled OT-II cells in CD4-deficient or wild-type mice, we eliminated the ability of the injected DCs to directly present to CFSE-labeled transferred OT-II cells. We found that MHC class II-deficient Act-mOVA DCs induced little proliferation of OT-II cells in MHC class II-sufficient hosts (SI Fig. 8). Thus, the direct pathway of antigen presentation is dominant within the time course of these experiments. Other studies (23) using injected DCs as APCs have also found the direct pathway of antigen presentation to be dominant.
To test whether CD4 T cells deprived of MHC class II are viable, we determined the numbers of OT-II cells after adoptive transfer into MHC class II-sufficient or deficient hosts by flow cytometry (SI Fig. 9A) or confocal immunofluorescence microscopy (SI Fig. 9B). MHC class II-deprived T cells versus nondeprived T cells did not die at a faster rate. We next documented that OT-II cells stimulated with anti-CD3 mAb showed little or no difference in proliferative capacity between the MHC class II-sufficient and -deficient environments (SI Fig. 9C). In addition, T cell proliferation was dose-dependent in both recipient groups. These results demonstrate that MHC class II deprivation neither impaired T cell survival nor prevented TCR signaling. Thus, we considered the alternative possibility that the T cells are defective in encountering antigen-bearing DCs.
MHC Class II Deprivation Leads to a Progressive Defect in the Ability of Naïve CD4 T Cells to Physically Interact with Antigen-Bearing DCs.
Although OT-II cells transferred into MHC class II-deficient recipients were present within the T cell regions of secondary lymphoid tissue, were viable, and were capable of signaling through TCR with anti-CD3 mAb, they were not activated by antigen-bearing DCs. We used confocal immunofluorescence microscopy to colocalize the two cell populations within the T cell regions of secondary lymphoid tissue to determine whether OT-II cells deprived of MHC class II molecules physically interact with antigen-bearing DCs (Fig. 2). The numbers of Act-mOVA DCs in the MHC-sufficient and MHC-deficient sections were similar (data not shown). In the condition where OT-II cells were transferred into MHC class II-deficient recipients (Fig. 2G, open circles), there was an increase in the percentage of antigen-bearing DCs not contacting (not adjacent to/overlapping with) OT-II cells within the periarterial lymphoid sheaths of the spleen with 4 days of MHC class II deprivation (73%) as compared with 1 day of deprivation (53%, P < 0.001) or 0 days of deprivation (46%, P < 0.01). In contrast DCs not contacting OT-II cells in the MHC class II-sufficient environment (Fig. 2G, filled squares) were a minority and were unchanged over time (31%, 23%, and 35%, with 0, 1, and 4 days after transfer, respectively). The percent of OT-II cells contacting antigen-bearing DCs was greater in an MHC class II-sufficient versus -deficient environment with 1 or 4 days (P < 0.001, for each) but not with 0 days of MHC class II deprivation. Thus, when OT-II cells are exposed to an MHC class II-deprived environment, the lack of in vivo contact of the cells with DCs correlates with decreased CD69 expression (Fig. 1B), the latter being an indicator of TCR stimulation. In addition, after 4 days of MHC class II deprivation, <1% of antigen-bearing DCs were found to have large (≥4) surrounding OT-II cell clusters compared with 34% in an MHC class II-sufficient environment (P < 0.05). Although the functional relevance of large DC/T cell clusters has not been clearly demonstrated, this has been shown to correlate with CD4 T cell effector function in similar systems (i.e., delayed-type hypersensitivity response) (23, 30, 31). These visualization studies strongly suggest that self-ligand-deprived T cells have an impaired ability to contact and/or adhere to antigen-bearing DCs.
Fig. 2.
OT-II T cells fail to form clusters with antigen-bearing DCs after transfer into MHC class II-deficient recipients. CFSE-labeled OT-II cells were adoptively transferred into MHC class II-deficient or CD4−/− mice on the day of (day 0) (A, B, D, and E), 1 day before (day −1), or 4 days before (day −4) (C and F) injection of seminaphthorhodafluor (SNARF)-labeled Act-mOVA DCs. (A–F) Photomicrographs show CFSE-labeled OT-II cells (green) and SNARF-labeled mOVA DCs (red) in spleens 24 h after DC injection into CD4−/− (A–C) mice and MHC class II−/− (D–F). High-power view of an individual cluster (B and E) is representative of multiple clusters formed within the periarterial lymphoid sheaths of the spleen. (Scale bars: A, C, D, and F, 50 μm; B and E, 25 μm.) (G) The percent of DC (mean ± SD) with no OT-II cells making contact in CD4−/− (filled squares) or MHC class II−/− (open circles) recipients is indicated. This graph shows combined data from three individual experiments. ∗, P < 0.001.
MHC Class II Deprivation Leads to Reduced CD4 T Cell Motility in Secondary Lymphoid Tissue.
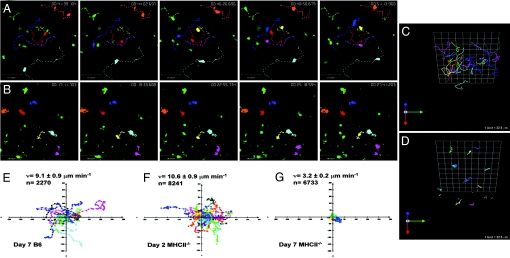
We next wanted to determine why, despite the presence of antigen-bearing DCs within the paracortex of the lymph node, the CD4 T cells fail to cluster with the DCs and proliferate. We hypothesized that there was a defect in motility of the CD4 T cells. A defect in motility could result in impaired DC contact and reduced TCR signaling and proliferation. We performed video microscopy on explanted lymph nodes to determine OT-II cell velocity and path of movement after transfer into MHC class II-deficient and wild-type animals. We observed a profound defect in the motility of OT-II cells deprived of MHC class II molecules in lymph nodes in the absence of antigen (SI Movie 1) compared with OT-II cells transferred into an MHC class II-sufficient environment (SI Movie 2). In fact, the longer the cells resided in the MHC class II-deficient animals, the more profound the defect, in both their velocity and their tracking path (Fig. 3). As an additional measure of the sessile nature of the cells, we analyzed the numbers of OT-II cells in the blood and the spleen 7 days after transfer. Compared with MHC class II-sufficient recipients, MHC class II-deficient recipients had fewer OT-II cells in the blood despite similar numbers in the spleens (SI Fig. 10). These data suggest that motility, before antigen encounter, is an essential component of CD4 T cell activation and that it is influenced by contact with MHC class II molecules.
Fig. 3.
MHC class II deprivation impairs CD4 T cell motility. (A and B) Time-lapse images of CFSE-labeled OT-II T cells in explanted lymph nodes of wild-type (A) and MHC class II-deficient (B) mice. The center of mass of individual cells (colored dots) was tracked in 3D and plotted on z projections of the imaging volume (200 × 225 × 75 μm). Seven representative T cell tracks (colored lines) are shown for both and a time stamp (hr:min:sec:msec) is placed in the upper right corner of each frame. (C and D) Three-dimensional plots of day 7 OT-II T cell trajectories of B6 (C) and MHC class II-deficient (D) mice. (E–G) Two-dimensional projections of OT-II T cell tracks (colored lines) are plotted normalized to their starting positions OT-II T cell trajectories in wild-type hosts (E) and MHC class II-deficient hosts on day 2 (F) and day 7 (G). Representative tracks are shown ranging from 20 to 45 min in length. The axes are in micrometers. Mean velocity (v) for each group is shown with SE and the total number of velocity measurements in the imaging record (n). These data are representative of three independent experiments.
Reduced CD4 T Cell Motility Is Associated with Reduced Levels of Active Rap1 and Rac1 but Not Ras.
The mechanism(s) of reduced motility after MHC class II deprivation are unclear. Cell adhesion and cytoskeletal reorganization are critical for lymphocyte attachment and migration. The signaling pathways responsible for regulating cell adhesion and cytoskeletal reorganization involve small GTPases (32) and are likely candidates to be altered in our system. Rap1 is a small G protein in the Ras superfamily and in its active form has been shown to be an important regulator of integrin function and transmigration (3). Rac1, a member of the Rho GTPase family, is involved in actin cytoskeletal reorganization and cell protrusion (4). We therefore hypothesized that basal levels of active Rap1 and Rac1 may be altered in the absence of self-ligand stimulation. In addition, we hypothesized that no alteration would be found in the basal activation of Ras, a GTPase shown to be important for TCR signaling pathways essential for cell proliferation but not motility (33, 34). To obtain sufficient numbers (2–10 million cells) of self-ligand-deprived OT-II CD4 T cells for biochemical analysis, OT-II TCR transgenic mice were treated with anti-MHC class II antibody (Y3P). Treatment with this antibody has been shown by others to deprive CD4 T cells of contact with self ligands (19). OT-II cells were purified (>90%) 7 days after Y3P or control antibody injection and assayed for the presence of both active and total Rap1 and Rac1. We found a 50% reduction in the basal levels of active Rap1 (Fig. 4A) and a 70% reduction in the basal levels of active Rac1 (Fig. 4B) in self-ligand-deprived CD4 T cells but no change in the basal activity of active Ras (Fig. 4C). These data suggest that tonic TCR signaling from continuous self-ligand stimulation is required to maintain a basal state of activation of GTPases critical for T cell motility.
Fig. 4.
MHC class II availability promotes basal Rap1 and Rac1 activity. OT-II CD4 T cells were purified from transgenic mice treated with Y3P or mouse IgG (control) for 7 days. Samples containing 2–7 × 106 cells were lysed and basal active Rap1 (A), Rac1 (B), or Ras (C) was precipitated with a GST-RalGDS-RBD fusion protein, GST-Pak1 fusion protein, or GST Raf-RBD fusion protein respectively, as previously described (49). The resulting precipitates were analyzed by Western blotting with an anti-Rap1, anti-Rac1, or anti-Ras antibody to assess the content of active Rap1, Rac1, or Ras in samples. Aliquots of total lysates were analyzed in the same way to assess the total Rap1, Rac1, or Ras content. Bands were quantified and the total number of pixels in each image from two independent experiments was used to calculate the average active to total ratio. Error bars represent standard deviations.
Discussion
Studies have shown that self-ligand deprivation over days to weeks leads to T cell hyperresponsiveness that may be due to down-regulation of CD5 expression (12). This finding is consistent with the idea that self-ligand stimulation tunes down TCR responsiveness to avoid autoreactivity (15–17). As expected, we saw down-regulation of CD5 expression. However, we could not show that this caused significant hyperresponsiveness of T cells toward TCR stimulation. In our study, the proliferative response toward stimulation with anti-CD3 mAb was comparable in the MHC class II-sufficient and -deficient environments. Perhaps longer periods of deprivation are required for an enhanced responsiveness.
In contrast, short-term (15–30 min) self-ligand deprivation has been shown to lead to acute loss of TCR ζ phosphorylation and TCR polarization, which correlates with decreased IL-2 production and proliferation to cognate antigen in vitro (19). Indeed, our studies support the notion that continuous stimulation from self ligands expressed by APCs is likely to be of considerable significance for the functional responsiveness of T cells toward cognate antigens (Fig. 1A and SI Fig. 7). However, with more prolonged deprivation (i.e., days rather than hours) we found a defect in the expression of CD69 (Fig. 1B), a sensitive measure of TCR signaling, that was not evident in the short-term studies (19). Although more prolonged deprivation induces more profound defects, our preliminary data (not shown) demonstrated that these defects were reversible. That is, OT-II cells were re-isolated from MHC class II-deficient or -sufficient recipients after being “parked” in those recipients 7 days and retransferred into wild-type recipients and allowed to park in those recipients for 4–7 days. After an in vivo challenge of antigen, the previously MHC class II-deprived OT-II cells proliferated as well as the control OT-II cells that were not previously MHC class II-deprived. These data suggest that the T cells were unable to effectively contact antigen-bearing DCs in vivo.
It is well appreciated that under steady-state conditions naïve T cells present in secondary lymphoid tissues are not “resting,” but are in constant motion (30, 35). Germain and colleagues (36) have recently shown that T cells crawl across a fibroblast reticular cell network within the paracortex of the lymph node frequently forming short-lived contacts with self-ligand expressing DCs which are tightly adherent to the fibroblast reticular cells. During this period the T cells cover a significant distance but are restricted within the T cell zone. This exploratory behavior may allow T cells to scan many DCs for presence of cognate antigen. When they contact cognate antigen-bearing DCs, over time, the T cells slow down, swarm around the DCs, and begin to form longer contacts with antigen-bearing DCs that eventually evolve into long-lived dynamic clusters that last for hours (30). In our study, with increased MHC class II deprivation, there is a defect in the motility of the T cells, before encountering antigen on DCs. The velocity and the distance traveled by the CD4 T cells within the paracortex are reduced (Fig. 3). Our interpretation is that impaired T cell trafficking within lymphoid tissue decreases the probability of contacting a limited number of antigen-bearing DCs and precludes TCR signaling (Fig. 1B), T cell clustering on DCs (Fig. 2), and T cell proliferation (Fig. 1A). These data suggest that prolonged deprivation results in a T cell motility defect ex vivo that is not evident in the in vitro assays because the T cells are able to contact APCs passively. However, the defective motility which we report for CD4 T cells deprived of MHC class II molecules may result from loss of either a specific interaction (involving TCR recognition) and/or a general interaction (for example, CD4 binding). Our experiments using single-peptide YAe mice suggest that the defect is at least in part related to TCR signaling. Regardless of the individual roles of CD4 and TCR in dictating the response, we know of no other reports which demonstrate that denying access of CD4 T cells to endogenous class II MHC molecules impacts their motility and impairs their ability to encounter antigen-laden APCs.
Lymphocyte movement is a complex process composed of interactions and changes between the cytoskeleton, adhesion molecules, and motor proteins within the cell compartments. Motility depends on the ability of cells to assume a “motile” form, including reorganization of actin cytoskeleton and possibly alterations of adhesion molecules. Rap1 is a potent activator of integrins and has been shown to induce cell polarization, cell surface receptor redistribution, and migration (3, 32, 37, 38). Rac1 is important for actin reorganization and cell protrusion (39–43). Ras has been shown to be important for TCR signaling pathways essential for cell proliferation, e.g., ERK and JNK (33, 34). We show that reduced basal activity of both Rap1 and Rac1 is associated with in vivo self-ligand deprivation, but we found no decrease in basal activity of Ras (Fig. 4). These data suggest that continuous self-ligand stimulation in the periphery provides multiple tonic TCR signals essential for cell motility and function.
Materials and Methods
Mice.
OT-II TCR transgenic mice (44) were bred to the Thy1.1 RAG−/− background. The OT-II TCR is specific for chicken ovalbumin peptide 323–339 (pOVA)/I-Ab complex. I-Aβ-deficient (MHC class II-deficient) and CD4-deficient mice were obtained from Taconic (Germantown, NY). I-A/I-E-deficient (MHC class II-deficient) mice were obtained from The Jackson Laboratory (Bar Harbor, ME) (45). Act-mOVA mice (24) were bred to I-Aβ-deficient mice to produce Act-mOVA MHC class II-deficient mice. Wild-type B6 mice were purchased from the National Cancer Institute (Frederick, MD). YAe SP mice were a gift from P. Marrack (29). Mice used were generally 6–12 weeks of age and maintained in microisolator cages with filtered air in the specific pathogen-free facility according to the National Institutes of Health guidelines. All experiments were approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
Adoptive Transfer of Cells.
Unless otherwise specified, donor OT-II cells from the secondary lymphoid tissues (axillary, brachial, cervical, inguinal, and mesenteric lymph nodes, and spleen) were labeled with CFSE (Molecular Probes, Eugene, OR) by using the technique previously described (46), and were injected into the tail vein at 1–5 × 106 cells per specified recipient mouse. The OT-II cells were always purified by negative selection before the adoptive transfer by using magnetic streptavidin microbeads (Miltenyi Biotec, Auburn, CA) and biotinylated anti-I-Aβ, anti-CD11c, anti-CD11b, and anti-CD16/32 antibodies (BD Biosciences and eBioscience, San Diego, CA). Single-cell suspensions of DCs were obtained after mechanical disruption of spleens from Act-mOVA or wild-type B6 mice by incubating tissue with collagenase D (Roche Diagnostics, Indianapolis, IN) for 20 min at 37°C under 5% CO2/95% O2 and then briefly with 10 mM EDTA as previously described (47). DCs were enriched from the single-cell suspensions by using anti-CD11c microbeads according to the manufacturer's protocol (Miltenyi Biotec). Enriched DCs were injected into the tail vein at indicated times, 0.5–1.5 × 106 cells per mouse. In some instances the DCs were labeled with seminaphthorhodafluor (SNARF) (Molecular Probes) or CellTracker Orange CMTMR dye (Molecular Probes) before injection. Some of the mice received i.v. injections of the anti-CD3 mAb 2C11 at indicated doses.
Flow Cytometry.
Lymph nodes and spleens were harvested at the indicated time points and a single-cell suspension was created by using mechanical disruption. Cells were incubated on ice with fluorochrome-labeled mAbs: anti-TCR Vα2, anti-CD4, and anti-Thy1.1. Events were collected by using a Becton Dickinson (Mountain View, CA) FACScan or FACSCalibur flow cytometer and analyzed by using FlowJo software (Tree Star, San Carlos, CA).
Immunofluorescence Microscopy.
Lymph nodes and spleens were snap frozen in precooled isopentane, sectioned (25 μm), and fixed in 2% formaldehyde. Slides were mounted with Vectashield (Vector Laboratories, Burlingame, CA). Separate red and green images were acquired by using a BioRad MRC-1000 confocal microscope equipped with a krypton/argon laser and COMOS version 7.0a software (Bio-Rad Life Sciences, Hercules, CA). Image processing was done using Confocal Assistant (BIPL, Minneapolis, MN) and Photoshop 7.0 (Adobe, San Jose, CA) software (23).
Two-Photon Microscopy.
Mice were euthanized by CO2 asphyxiation and cervical lymph nodes were removed for microscopy. Lymph nodes were secured in the flow chamber with a thin film of VetBond (3M, St. Paul, MN) and maintained at 37°C by perfusing the chamber with warm RPMI medium 1640 bubbled with a mixture of 95% O2/5% CO2 (carbogen gas) (48). Time-lapse imaging was performed with a custom-built multiphoton microscope at the Washington University School of Medicine. CFSE-labeled T cells were excited by a Chameleon Ti:sapphire laser (Coherent, Santa Clara, CA) tuned to 780 nm, fluorescence at 490–550 nm was collected by photomultiplier tube, and the signal was acquired by a RAVEN (BitFlow, Woburn, MA) analog video acquisition board. ImageWarp (A&B Software/BitFlow) was used to control the various hardware devices during acquisition and to process and archive the image data. For each time-point, 15 video frames (200 μm × 250 μm at 0.5 μm per pixel) were averaged to increase signal contrast and z-stacks were acquired by taking 31 sequential z-steps at 2.5-μm spacing with an automated z-stepper motor (Prior, Rockland, MA). Image rendering and analysis were performed with either Volocity (Improvision, Lexington, MA) or PicViewer (John Dempster, University of Strathclyde, Glasgow, Scotland) programs.
Immunoprecipitation and Immunoblotting.
OT-II mice were injected i.p. with anti-MHC class II antibody (clone Y3P) or control mouse IgG antibody (0.5 mg) every other day for 7 days before harvest. Lymph nodes were harvested and CD4 T cells were isolated by negative selection (CD4 isolation kit, Miltenyii Biotec). Samples containing 2–7 × 106 cells were lysed and basal active Rap1 was precipitated with a GST-RalGDS-RBD fusion protein (49), basal active Rac1 was precipitated with a GST-Pak1 fusion protein (50), and basal active Ras was precipitated with a GST-Raf-RBD fusion protein (51). The resulting precipitates were analyzed by Western blotting with an anti-Rap1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), an anti-Rac antibody (Upstate Biotechnology, Lake Placid, NY), and an anti-Ras antibody (Transduction Laboratories, Lexington, KY) to assess the content of the active GTPase in the samples. Aliquots of total lysates were analyzed in the same way to assess the total Rap1, Rac1, and Ras contents. Images were captured and quantified with Odyssey Infrared Imager (LI-COR Biosciences, Lincoln, NE).
Supplementary Material
Acknowledgments
We thank Drs. Stephen Jameson and Matthew Mescher for helpful discussions and criticisms. This work was supported by the American Society of Nephrology–American Society of Transplantation John Merrill Transplant Research Scholar Award (to E.I.), Vikings Grant (to E.I.), and National Institutes of Health Grants AI069836 (to E.I.), AI031126 (to Y.S.), AI038474 (to Y.S.), and DK061961 (to A.K.).
Abbreviations
- APC
antigen-presenting cell
- B6
C57BL/6
- CFSE
5(6)-carboxyfluorescein diacetate N-succinimidyl ester
- DC
dendritic cell
- GTPase
guanosine triphosphatase
- mOVA
membrane-bound form of chicken ovalbumin
- TCR
T cell receptor.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0608299104/DC1.
References
- 1.Ladi E, Yin X, Chtanova T, Robey EA. Nat Immunol. 2006;7:338–343. doi: 10.1038/ni1323. [DOI] [PubMed] [Google Scholar]
- 2.Viola A, Lanzavecchia A. Science. 1996;273:104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 3.Bos JL. Curr Opin Cell Biol. 2005;17:123–128. doi: 10.1016/j.ceb.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Yamazaki D, Kurisu S, Takenawa T. Cancer Sci. 2005;96:379–386. doi: 10.1111/j.1349-7006.2005.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoll S, Delon J, Brotz TM, Germain RN. Science. 2002;296:1873–1876. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- 6.Delon J, Stoll S, Germain RN. Immunol Rev. 2002;189:51–63. doi: 10.1034/j.1600-065x.2002.18906.x. [DOI] [PubMed] [Google Scholar]
- 7.Reichert P, Reinhardt RL, Ingulli E, Jenkins MK. J Immunol. 2001;166:4278–4281. doi: 10.4049/jimmunol.166.7.4278. [DOI] [PubMed] [Google Scholar]
- 8.Wulfing C, Sumen C, Sjaastad MD, Wu LC, Dustin ML, Davis MM. Nat Immunol. 2002;3:42–47. doi: 10.1038/ni741. [DOI] [PubMed] [Google Scholar]
- 9.Jameson SC. Nat Rev Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 10.Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 11.Takeda S, Rodewald HR, Arakawa H, Bluethmann H, Shimizu T. Immunity. 1996;5:217–228. doi: 10.1016/s1074-7613(00)80317-9. [DOI] [PubMed] [Google Scholar]
- 12.Smith K, Seddon B, Purbhoo MA, Zamoyska R, Fisher AG, Merkenschlager M. J Exp Med. 2001;194:1253–1261. doi: 10.1084/jem.194.9.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polic B, Kunkel D, Scheffold A, Rajewsky K. Proc Natl Acad Sci USA. 2001;98:8744–8749. doi: 10.1073/pnas.141218898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhandoola A, Tai X, Eckhaus M, Auchincloss H, Mason K, Rubin SA, Carbone KM, Grossman Z, Rosenberg AS, Singer A. Immunity. 2002;17:425–436. doi: 10.1016/s1074-7613(02)00417-x. [DOI] [PubMed] [Google Scholar]
- 15.Grossman Z, Paul WE. Proc Natl Acad Sci USA. 1992;89:10365–10369. doi: 10.1073/pnas.89.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grossman Z, Paul WE. Curr Opin Immunol. 2001;13:687–698. doi: 10.1016/s0952-7915(01)00280-1. [DOI] [PubMed] [Google Scholar]
- 17.Grossman Z, Paul WE. Semin Immunol. 2000;12:197–203. doi: 10.1006/smim.2000.0232. discussion 257–344. [DOI] [PubMed] [Google Scholar]
- 18.Stefanova I, Hemmer B, Vergelli M, Martin R, Biddison WE, Germain RN. Nat Immunol. 2003;4:248–254. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- 19.Stefanova I, Dorfman JR, Germain RN. Nature. 2002;420:429–434. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 20.Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM. Nature. 2005;434:238–243. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- 21.Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Nature. 2002;419:845–849. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 22.Stefanova I, Dorfman JR, Tsukamoto M, Germain RN. Immunol Rev. 2003;191:97–106. doi: 10.1034/j.1600-065x.2003.00006.x. [DOI] [PubMed] [Google Scholar]
- 23.Ingulli E, Mondino A, Khoruts A, Jenkins MK. J Exp Med. 1997;185:2133–2141. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehst BD, Ingulli E, Jenkins MK. Am J Transplant. 2003;3:1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 25.Tarakhovsky A, Kanner SB, Hombach J, Ledbetter JA, Muller W, Killeen N, Rajewsky K. Science. 1995;269:535–537. doi: 10.1126/science.7542801. [DOI] [PubMed] [Google Scholar]
- 26.Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. J Exp Med. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azzam HS, DeJarnette JB, Huang K, Emmons R, Park CS, Sommers CL, El-Khoury D, Shores EW, Love PE. J Immunol. 2001;166:5464–5472. doi: 10.4049/jimmunol.166.9.5464. [DOI] [PubMed] [Google Scholar]
- 28.Wong P, Barton GM, Forbush KA, Rudensky AY. J Exp Med. 2001;193:1179–1187. doi: 10.1084/jem.193.10.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ignatowicz L, Kappler J, Marrack P. Cell. 1996;84:521–529. doi: 10.1016/s0092-8674(00)81028-4. [DOI] [PubMed] [Google Scholar]
- 30.Miller MJ, Safrina O, Parker I, Cahalan MD. J Exp Med. 2004;200:847–856. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 32.Kinashi T, Katagiri K. Immunology. 2005;116:164–171. doi: 10.1111/j.1365-2567.2005.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jo H, Sipos K, Go YM, Law R, Rong J, McDonald JM. J Biol Chem. 1997;272:1395–1401. doi: 10.1074/jbc.272.2.1395. [DOI] [PubMed] [Google Scholar]
- 34.Wan Y, Kurosaki T, Huang XY. Nature. 1996;380:541–544. doi: 10.1038/380541a0. [DOI] [PubMed] [Google Scholar]
- 35.Mempel TR, Henrickson SE, Von Andrian UH. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 36.Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medeiros RB, Dickey DM, Chung H, Quale AC, Nagarajan LR, Billadeau DD, Shimizu Y. Immunity. 2005;23:213–226. doi: 10.1016/j.immuni.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Shimonaka M, Katagiri K, Nakayama T, Fujita N, Tsuruo T, Yoshie O, Kinashi T. J Cell Biol. 2003;161:417–427. doi: 10.1083/jcb.200301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedman RS, Jacobelli J, Krummel MF. Semin Immunol. 2005;17:387–399. doi: 10.1016/j.smim.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Faure S, Salazar-Fontana LI, Semichon M, Tybulewicz VL, Bismuth G, Trautmann A, Germain RN, Delon J. Nat Immunol. 2004;5:272–279. doi: 10.1038/ni1039. [DOI] [PubMed] [Google Scholar]
- 41.Hamelers IH, Olivo C, Mertens AE, Pegtel DM, van der Kammen RA, Sonnenberg A, Collard JG. J Cell Biol. 2005;171:871–881. doi: 10.1083/jcb.200509172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katoh H, Hiramoto K, Negishi M. J Cell Sci. 2006;119:56–65. doi: 10.1242/jcs.02720. [DOI] [PubMed] [Google Scholar]
- 43.Tzima E. Circ Res. 2006;98:176–185. doi: 10.1161/01.RES.0000200162.94463.d7. [DOI] [PubMed] [Google Scholar]
- 44.Barnden MJ, Allison J, Heath WR, Carbone FR. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 45.Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, Mathis D, Fugger L. Proc Natl Acad Sci USA. 1999;96:10338–10343. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lyons AB, Parish CR. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 47.Ingulli E, Ulman DR, Lucido MM, Jenkins MK. J Immunol. 2002;169:2247–2252. doi: 10.4049/jimmunol.169.5.2247. [DOI] [PubMed] [Google Scholar]
- 48.Miller MJ, Wei SH, Parker I, Cahalan MD. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 49.van Triest M, de Rooij J, Bos JL. Methods Enzymol. 2001;333:343–348. doi: 10.1016/s0076-6879(01)33068-9. [DOI] [PubMed] [Google Scholar]
- 50.del Pozo MA, Price L, Alderson NB, Ren XD, Schwartz MA. EMBO J. 2000;19:2008–2014. doi: 10.1093/emboj/19.9.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J, Anastasiadis PZ, Liu Y, Thompson EA, Fields AP. J Biol Chem. 2004;279:22118–22123. doi: 10.1074/jbc.M400774200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.