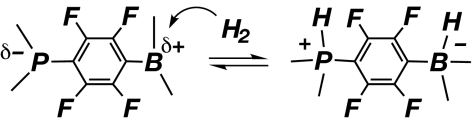Abstract
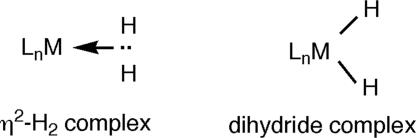
The binding of a dihydrogen molecule (H2) to a transition metal center in an organometallic complex was a major discovery because it changed the way chemists think about the reactivity of molecules with chemically “inert” strong bonds such as H H and C
H and C H. Before the seminal finding of side-on bonded H2 in W(CO)3(PR3)2(H2), it was generally believed that H2 could not bind to another atom in stable fashion and would split into two separate H atoms to form a metal dihydride before undergoing chemical reaction. Metal-bound saturated molecules such as H2, silanes, and alkanes (σ-complexes) have a chemistry of their own, with surprisingly varied structures, bonding, and dynamics. H2 complexes are of increased relevance for H2 production and storage in the hydrogen economy of the future.
H. Before the seminal finding of side-on bonded H2 in W(CO)3(PR3)2(H2), it was generally believed that H2 could not bind to another atom in stable fashion and would split into two separate H atoms to form a metal dihydride before undergoing chemical reaction. Metal-bound saturated molecules such as H2, silanes, and alkanes (σ-complexes) have a chemistry of their own, with surprisingly varied structures, bonding, and dynamics. H2 complexes are of increased relevance for H2 production and storage in the hydrogen economy of the future.
Dihydrogen (H2) and hydrocarbons are vital in chemical processes such as hydrogenation and conversions of organic compounds. Catalytic hydrogenations are the largest-volume chemical reactions: all crude oil is treated with H2 to remove sulfur/nitrogen, and >100 million tons of ammonia fertilizer are produced annually to support much of the world's population. The H2 molecule is married together by a very strong two-electron H H bond but is only useful chemically when the two H atoms divorce in controlled fashion. This also applies to other strong σ-bonds such as C
H bond but is only useful chemically when the two H atoms divorce in controlled fashion. This also applies to other strong σ-bonds such as C H in alkanes. However, the mechanism at the molecular level by which the union splits was established only relatively recently because such electronically saturated molecules were never caught in the act of chemically binding to a metal or other “third party,” usually the first step in breaking apart a strong bond. The discovery by Kubas and coworkers (1) in 1984 of coordination of a nearly intact H2 molecule to a metal complex (LnM; L = ligand) caught this in intimate detail and led to a new paradigm in chemistry (1–4) (see Sketch 1).
H in alkanes. However, the mechanism at the molecular level by which the union splits was established only relatively recently because such electronically saturated molecules were never caught in the act of chemically binding to a metal or other “third party,” usually the first step in breaking apart a strong bond. The discovery by Kubas and coworkers (1) in 1984 of coordination of a nearly intact H2 molecule to a metal complex (LnM; L = ligand) caught this in intimate detail and led to a new paradigm in chemistry (1–4) (see Sketch 1).
Sketch 1.
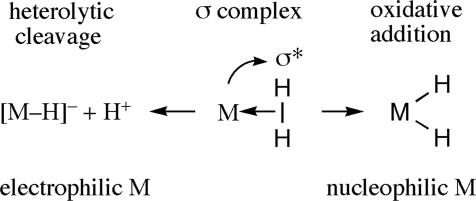
The H2 binds side-on (η2) to M primarily through donation of its two σ electrons to a vacant metal orbital to form a stable H2 complex. It is remarkable that the electrons already strongly bonded can donate to a metal to form a nonclassical 2-electron, 3-center bond, as in other “electron-deficient” molecules such as diborane (B2H6). M H2 and other “σ-complexes” (3, 5), encompassing interaction of any σ-bond (C
H2 and other “σ-complexes” (3, 5), encompassing interaction of any σ-bond (C H, Si
H, Si H, etc) with a metal center, are the major theme of this special feature.
H, etc) with a metal center, are the major theme of this special feature.
Introduction and Historical Perspective
Certain discoveries and how they came about are fascinating sagas, e.g., that for buckminsterfullerene (C60) (6). That its existence remained hidden for so long adds to the lore, and our unexpected revelation of metal–H2 complexes has some commonality. Metal dihydrides formed by oxidative addition (OA) of the H H bond to a metal center had been known early on to be a part of catalytic cycles (7), as documented in a 1980 retrospective on catalytic hydrogenation by a pioneer in the field, Jack Halpern (8). Although some form of metal–H2 interaction was assumed to participate in dihydride formation, it was thought to be unobservable. We were fortunate to observe it in the complex W(CO)3(PR3)2(H2), as detailed by this author (2, 3). This was the first molecular compound synthesized and isolated entirely under ambient conditions that contained the H2 molecule (albeit “stretched”) other than elemental H2 itself. The H
H bond to a metal center had been known early on to be a part of catalytic cycles (7), as documented in a 1980 retrospective on catalytic hydrogenation by a pioneer in the field, Jack Halpern (8). Although some form of metal–H2 interaction was assumed to participate in dihydride formation, it was thought to be unobservable. We were fortunate to observe it in the complex W(CO)3(PR3)2(H2), as detailed by this author (2, 3). This was the first molecular compound synthesized and isolated entirely under ambient conditions that contained the H2 molecule (albeit “stretched”) other than elemental H2 itself. The H H bond length in W(CO)3(PiPr3)2(H2) (0.89 Å) is stretched ≈20% over that in H2 (0.74 Å), showing that H2 is not physisorbed but rather chemisorbed, where the bond is “activated” toward rupture. Like H2, other saturated molecules such as alkanes were thought to be inert to such binding, although their C
H bond length in W(CO)3(PiPr3)2(H2) (0.89 Å) is stretched ≈20% over that in H2 (0.74 Å), showing that H2 is not physisorbed but rather chemisorbed, where the bond is “activated” toward rupture. Like H2, other saturated molecules such as alkanes were thought to be inert to such binding, although their C H bonds somehow could also be broken on metals. The “somehow” is why the finding of an H2 complex was important: it is the prototype for activation of all σ-bonds.
H bonds somehow could also be broken on metals. The “somehow” is why the finding of an H2 complex was important: it is the prototype for activation of all σ-bonds.
This discovery of W(CO)3(PiPr3)2(H2) ensued the serendipitous synthesis of its novel, “unsaturated” 16-e precursor, M(CO)3(PCy3)2 (M = Mo, W; Cy = cyclohexyl) (9). Its unusual purple color changed instantly and reversibly to yellow on exposure to N2 and H2 in both solution and solid states, signifying adduct formation (Eq. 1).
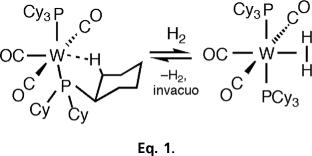 |
Crystallography later revealed a phosphine C H bond weakly occupying the sixth binding site in W(CO)3(PCy3)2 (10). This type of “agostic” interaction (11) relieves electronic unsaturation in coordinatively unsaturated complexes and is entropically favorable because it is “intramolecular.” “Intermolecular” binding of a C
H bond weakly occupying the sixth binding site in W(CO)3(PCy3)2 (10). This type of “agostic” interaction (11) relieves electronic unsaturation in coordinatively unsaturated complexes and is entropically favorable because it is “intramolecular.” “Intermolecular” binding of a C H bond as in an alkane σ-complex (often also termed “agostic”) is less stable. Irrefutable evidence for H2 binding in Eq. 1 came slowly because pinpointing H positions crystallographically is difficult, even by neutron diffraction. A consultant, Russ Drago, suggested an experiment elegant in its simplicity: synthesize the HD complex and look for a large HD coupling constant in the proton NMR that would show that the H
H bond as in an alkane σ-complex (often also termed “agostic”) is less stable. Irrefutable evidence for H2 binding in Eq. 1 came slowly because pinpointing H positions crystallographically is difficult, even by neutron diffraction. A consultant, Russ Drago, suggested an experiment elegant in its simplicity: synthesize the HD complex and look for a large HD coupling constant in the proton NMR that would show that the H D bond was mostly intact. It worked beautifully: the 1H NMR of W(CO)3(PiPr3)2(HD) showed a 1:1:1 triplet (deuterium spin = 1) with JHD = 33.5 Hz, nearly as high as in HD gas, 43.2 Hz. Observation of JHD higher than that for a dihydride complex (>2 Hz) became the premier criterion for an H2 complex.
D bond was mostly intact. It worked beautifully: the 1H NMR of W(CO)3(PiPr3)2(HD) showed a 1:1:1 triplet (deuterium spin = 1) with JHD = 33.5 Hz, nearly as high as in HD gas, 43.2 Hz. Observation of JHD higher than that for a dihydride complex (>2 Hz) became the premier criterion for an H2 complex.
One reason that H2 complexes were so well hidden was the notion that such complexes could not be stable relative to classical dihydrides, as exemplified by the controversy over our initial findings. This paralleled the discovery of metal–dinitrogen complexes by Allen and Senoff, whose seminal paper was initially rejected (12). At the time of our finding, spectroscopic evidence for unstable M H2 interactions was found by photolysis of Cr(CO)6 in the presence of H2 at low T (13–16). Cr(CO)5(H2) was postulated based on IR CO stretching frequencies but could not be conclusively demonstrated; only recently has its 1H NMR spectrum been observed at low T (17, 18). Even theoretical bases for interaction of H2 and other σ-bonds with a metal was still in its infancy at the time of our discovery. Ironically, a computational paper by Saillard and Hoffmann (19) in 1984 on the bonding of H2 and CH4 to metal fragments such as Cr(CO)5 was published shortly after our publication (1) of the W–H2 complex, without mutual knowledge. Such interplay between theory and experiment has continued as one of the most valuable synergistic relations in all of chemistry (3, 4, 20). The innate simplicity of H2 was attractive computationally, but the structure/bonding/dynamics of H2 complexes turned out to be unimaginably complex and led to extensive study (>300 computational publications).
H2 interactions was found by photolysis of Cr(CO)6 in the presence of H2 at low T (13–16). Cr(CO)5(H2) was postulated based on IR CO stretching frequencies but could not be conclusively demonstrated; only recently has its 1H NMR spectrum been observed at low T (17, 18). Even theoretical bases for interaction of H2 and other σ-bonds with a metal was still in its infancy at the time of our discovery. Ironically, a computational paper by Saillard and Hoffmann (19) in 1984 on the bonding of H2 and CH4 to metal fragments such as Cr(CO)5 was published shortly after our publication (1) of the W–H2 complex, without mutual knowledge. Such interplay between theory and experiment has continued as one of the most valuable synergistic relations in all of chemistry (3, 4, 20). The innate simplicity of H2 was attractive computationally, but the structure/bonding/dynamics of H2 complexes turned out to be unimaginably complex and led to extensive study (>300 computational publications).
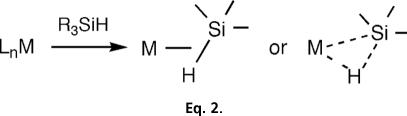
Initially, H2 binding in M(CO)3(PR3)2(H2) seemed unique because the bulky phosphines sterically inhibited formation of a 7-coordinate dihydride through OA. Kaesz and coworkers (21) viewed this as “arrested OA,” a descriptive term for the bonding in a silane complex, CpMn(CO)2(η2-HSiPh3). Silane complexes (22, 23) were among the first examples of σ-bond complexes but were initially unrecognized as such because the asymmetrically bound silane ligand (Eq. 2) lacked the superb clarity of the H2 ligand, which has electrons only in the H H bond.
H bond.
 |
The hundreds of H2 complexes synthesized after our discovery could not initially have been imagined, and it was difficult to know where to search for new ones. It would take more than a year before others were identified, notably by Morris, Crabtree, Chaudret, and Heinekey. This quartet has since performed elegant synthetic, reactivity, and NMR studies on H2 and silane complexes (5, 25–30) and was eventually joined by >100 investigators worldwide. Remarkably, several complexes initially believed to be hydrides were revealed to be H2 complexes by Crabtree and Hamilton in 1986 (5, 31), by using as criteria the short proton NMR relaxation times of H2 ligands (T1 < 100 msec). Particularly interesting was RuH2(H2)(PPh3)3 first reported in 1968 (32); it possessed unusual H2 lability that Singleton in 1976 commented was characteristic of “H2-like bonding” (33). However, attempts to prove H2 binding here was problematic, even long after H2 binding was established (34).‡
More than 600 H2 complexes are known (most of them stable) for nearly every transition metal and type of coligand and are the focus of 1,500 publications, dozens of reviews, and three monographs (2–5, 20, 25–30, 35–43). The view on H2 complexes has shifted from significance in basic science to a more practical bent, e.g., H2 fuel production and storage. Two frequent questions after their discovery were as follows. Are H2 complexes relevant in catalysis, i.e., does direct transfer of hydrogen from an H2 ligand to a substrate occur? And could methane bind to metal complexes? The answer to both is yes, and although a stable methane complex has not been isolated, alkane binding has been observed.
Synthesis and Diagnosis of H2 Complexes
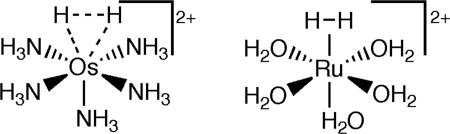
Most H2 complexes contain low-valent metals with d6 electronic configurations that favor side-on binding of σ-bonds. Reversibility of H2 binding is often a key feature, i.e., H2 can be removed simply upon exposure to vacuum and readded many times at ambient temperature/pressure, as in Eq. 1. Virtually all σ-complexes are diamagnetic, with one exception (44). σ-Ligands have not been definitively shown to bridge metals. Surprisingly, the coligands on H2 complexes can be simple classical nitrogen-donor ancillary ligands such as ammonia, as in [Os(NH3)5(H2)]2+ (45), which has a very long H H distance (dHH), ≈1.34 Å (46), more characteristic of a dihydride, which it was initially believed to be (47). Complexes containing only H2O (48) or CO (17, 18) coligands are also known, but are marginally stable (Scheme 1).
H distance (dHH), ≈1.34 Å (46), more characteristic of a dihydride, which it was initially believed to be (47). Complexes containing only H2O (48) or CO (17, 18) coligands are also known, but are marginally stable (Scheme 1).
Scheme 1.
Determining the presence of a H2 ligand and its dHH is nontrivial because even neutron diffraction has limited applicability and can give foreshortened dHH because of rapid H2 rotation/libration (49). 1JHD is the best criterion, and values determined in solution correlate well with dHH in the solid state through Eqs. 3 and 4 (50, 51).
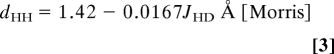 |
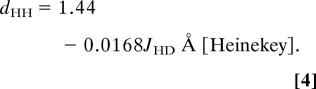 |
Data include dHH from crystallography and also solid-state NMR measurements by Zilm and Millar (52) that gave the most accurate dHH (direct measure of internuclear HH separation). For W(CO)3(PiPr3)2(H2), JHD = 34 Hz, giving dHH = 0.86–0.88 Å vs. 0.89 Å from solid-state NMR and 0.82(1) Å from neutron diffraction [uncorrected for H2 libration (49)]. Short T1 values for the H2 ligand (27) are also diagnostic (e.g., 4 msec for the W complex), although care must be exercised in interpretation (53–55). A powerful spectroscopic tool developed by a colleague at Los Alamos, Juergen Eckert, is inelastic neutron scattering studies of rapid H2 rotation in solid H2 complexes that provide unequivocal evidence for molecular H2 binding and also the presence of M–>H2 backdonation (56).
Several synthetic routes to H2 complexes are available; the simplest is reaction of H2 with an unsaturated complex such as W(CO)3(PR3)2 (Eq. 1). Displacement of weakly bound “solvento” ligands such as CH2Cl2 (57) or H2O from [Ru(H2O)6]2+ (Scheme 1) is also effective. Protonation of a hydride complex by acids is most often used (28, 38) and is widely applicable because it does not require an unsaturated precursor that may not be available. Neutral polyhydrides LnMHx are convenient targets for protonation to cationic H2 complexes, [LnM(H2)Hx-1]+, which can be more robust than complexes prepared from H2. Only a few stable solid bis-H2 complexes are known, e.g., [RhH2(H2)2(PCy3)2]+ (58), Tp*RuH(H2)2 (59), and RuH2(H2)2(PR3)2; R = Cy (30) and cyclopentyl (60), for which the neutron structure shows unstretched cis–H2 ligands.
Structure, Bonding, and Dynamics of H2 Complexes
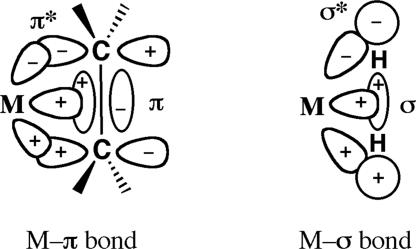
The 3-center metal–H2 interaction complements classical Werner-type coordination complexes where a ligand donates electron density through its nonbonding electron pair(s) and π-complexes such as olefin complexes in which electrons are donated from bonding π-electrons (Scheme 2). It is remarkable that the bonding electron pair in H2 can further interact with a metal center as strongly as a nonbonding pair in some cases. The resulting side-on bonding in M-H2 and other σ-complexes is “nonclassical,” by analogy to the 3-center, 2-electron bonding in carbocations and diborane. The M center may be considered to be isolobal with H+ and CH3+ (61), mimicking carbocation chemistry; i.e., a σ-complex such as M+–CH4 is related to CH5+, which is viewed as a highly dynamic H2 complex of CH3+ (62). H2 is thus a weak Lewis base that can bind to strong electrophiles, but transition metals are unique in stabilizing H2 and other σ-bond complexes by “backdonation” of electrons from a filled metal d orbital to the σ* antibonding orbital of H2 (Scheme 2), a critical interaction unavailable to main group atoms (3, 4, 20). The backdonation is analogous (4) to that in the Dewar–Chatt–Duncanson model (63, 64) for π-complexes, e.g., M–ethylene.
Scheme 2.
A large variety of σ-bonds X H interact inter- or intramolecularly with metal centers (3, 42). In principle any X
H interact inter- or intramolecularly with metal centers (3, 42). In principle any X Y bond can coordinate to a metal center, providing substituents at X and Y do not interfere. Backdonation of electrons from M to H2 (or to σ* of any X
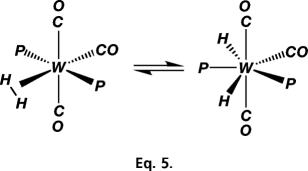
Y bond can coordinate to a metal center, providing substituents at X and Y do not interfere. Backdonation of electrons from M to H2 (or to σ* of any X Y bond) is crucial not only in stabilizing σ-bonding but also in activating the bond toward homolysis (3, 4, 20). If it becomes too strong, e.g., by increasing electron-donor strength of coligands on M, the σ-bond cleaves to form a dihydride because of overpopulation of the H2 σ* orbital. There is often a fine line between H2 and dihydride coordination, and in some cases equilibria exist in solution for W(CO)3(PR3)2(H2) (Eq. 5), showing that side-on coordination of H2 is the first step in H
Y bond) is crucial not only in stabilizing σ-bonding but also in activating the bond toward homolysis (3, 4, 20). If it becomes too strong, e.g., by increasing electron-donor strength of coligands on M, the σ-bond cleaves to form a dihydride because of overpopulation of the H2 σ* orbital. There is often a fine line between H2 and dihydride coordination, and in some cases equilibria exist in solution for W(CO)3(PR3)2(H2) (Eq. 5), showing that side-on coordination of H2 is the first step in H H cleavage (2).
H cleavage (2).
 |
Although electronic factors for OA are well established, the role of steric factors is less clear. Bulky phosphines can inhibit H2 splitting: for less bulky R = Me the equilibrium lies completely to the right, i.e., the complex is a “dihydride” (65). However, as shown above, H2 complexes are also stable with only small coligands L such as NH3 (Scheme 1), in some cases with greatly elongated dHH, two further paradigm shifts. This led to extensive efforts to vary M, L, and other factors to study stretching of the H H bond. Within the large regime of hundreds of LnM
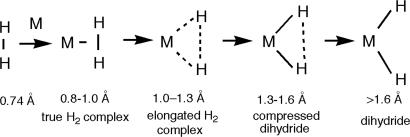
H bond. Within the large regime of hundreds of LnM H2 complexes, the reaction coordinate for the activation of H2 on a metal (Scheme 3) shows dHH varying enormously, from 0.82 to 1.5 Å (3, 18, 25–31, 35–38, 44–60, 65–68). This “arresting” of bond rupture along its entire reaction coordinate is unprecedented. Although the dHH ranges shown are arbitrary, each category of complexes has distinct properties. The dHH is relatively short (0.8–1.0 Å), and H2 is reversibly bound, in “true” H2 complexes best exemplified by W(CO)3(PR3)2(H2), much as in physisorbed H2 where dHH is <0.8 Å. Elongated H2 complexes (dHH = 1–1.3 Å) (29, 46, 66–69) were first clearly identified in 1991 in ReH5(H2)(PR3)2 where neutron diffraction showed a dHH of 1.357(7) Å (67). Complexes with dHH > 1.3 Å are now viewed as “compressed hydrides,” with NMR features differing from elongated H2 complexes, e.g., JHD increases with T for the former and decreases for the latter (69). These are terms because a near continuum of dHH has been observed.
H2 complexes, the reaction coordinate for the activation of H2 on a metal (Scheme 3) shows dHH varying enormously, from 0.82 to 1.5 Å (3, 18, 25–31, 35–38, 44–60, 65–68). This “arresting” of bond rupture along its entire reaction coordinate is unprecedented. Although the dHH ranges shown are arbitrary, each category of complexes has distinct properties. The dHH is relatively short (0.8–1.0 Å), and H2 is reversibly bound, in “true” H2 complexes best exemplified by W(CO)3(PR3)2(H2), much as in physisorbed H2 where dHH is <0.8 Å. Elongated H2 complexes (dHH = 1–1.3 Å) (29, 46, 66–69) were first clearly identified in 1991 in ReH5(H2)(PR3)2 where neutron diffraction showed a dHH of 1.357(7) Å (67). Complexes with dHH > 1.3 Å are now viewed as “compressed hydrides,” with NMR features differing from elongated H2 complexes, e.g., JHD increases with T for the former and decreases for the latter (69). These are terms because a near continuum of dHH has been observed.
Scheme 3.
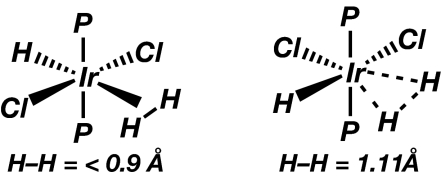
Activation of H2 is very sensitive to M, L, and charge, e.g., changing R from phenyl to alkyl in Mo(CO)(H2)(R2PC2H4PR2)2 leads to splitting of H2 (49). Strongly donating L, third-row M, and neutral charge favor elongation or splitting of H H, whereas first-row M, electron-withdrawing L, and positive charge (cationic complex) favor H2 binding and shorten dHH. The ligand trans to H2 has a powerful influence: strong π-acceptors such as CO (and also strong σ-donors such as H) greatly reduce backdonation and normally keep dHH < 0.9 Å. Thus one can favor a σ-complex by placing the potential σ-ligand trans to a strong π-acceptor. Conversely, mild σ-donors such as H2O or π-donors such as Cl trans to H2 elongate dHH (0.96–1.34 Å), as dramatically demonstrated by the isomers in Scheme 4(70). The cis-dichloro complex is actually a “compressed trihydride” (dHH ∼ 1.5 Å) in solution, but in the solid state it is an elongated H2 complex (dHH = 1.11 Å) due to Ir–Cl···H–Ir hydrogen bonding, illustrating the hypersensitivity of dHH to both intra- and intermolecular effects (71). Exceptions exist: the isomers of an “electron-poor” system, Cr(CO)4(PMe3)(H2), have similar JHD (≈34 Hz, hence dHH ≈ 0.86 Å) whether H2 is trans to CO or PMe3 (18).
H, whereas first-row M, electron-withdrawing L, and positive charge (cationic complex) favor H2 binding and shorten dHH. The ligand trans to H2 has a powerful influence: strong π-acceptors such as CO (and also strong σ-donors such as H) greatly reduce backdonation and normally keep dHH < 0.9 Å. Thus one can favor a σ-complex by placing the potential σ-ligand trans to a strong π-acceptor. Conversely, mild σ-donors such as H2O or π-donors such as Cl trans to H2 elongate dHH (0.96–1.34 Å), as dramatically demonstrated by the isomers in Scheme 4(70). The cis-dichloro complex is actually a “compressed trihydride” (dHH ∼ 1.5 Å) in solution, but in the solid state it is an elongated H2 complex (dHH = 1.11 Å) due to Ir–Cl···H–Ir hydrogen bonding, illustrating the hypersensitivity of dHH to both intra- and intermolecular effects (71). Exceptions exist: the isomers of an “electron-poor” system, Cr(CO)4(PMe3)(H2), have similar JHD (≈34 Hz, hence dHH ≈ 0.86 Å) whether H2 is trans to CO or PMe3 (18).
Scheme 4.
At what point is the H H bond “broken”? Theoretical analyses suggest 1.48 Å, i.e., twice the normal length (72), but little H
H bond “broken”? Theoretical analyses suggest 1.48 Å, i.e., twice the normal length (72), but little H H bonding interaction remains for dHH > 1.1 Å (29). In certain “elongated” H2 complexes, e.g., [OsCl(H2)(dppe)2]+, the energy barrier for stretching the H
H bonding interaction remains for dHH > 1.1 Å (29). In certain “elongated” H2 complexes, e.g., [OsCl(H2)(dppe)2]+, the energy barrier for stretching the H H bond from 0.85 Å all of the way to 1.6 Å is calculated (29, 69) to be astonishingly low, ≈1 kcal/mol. The H2 is highly delocalized: the H atoms undergo large amplitude vibrational motion along the reaction coordinate for H
H bond from 0.85 Å all of the way to 1.6 Å is calculated (29, 69) to be astonishingly low, ≈1 kcal/mol. The H2 is highly delocalized: the H atoms undergo large amplitude vibrational motion along the reaction coordinate for H H breaking. Remarkably, dHH is both temperature and isotope dependent in [CpM(diphosphine)(H2)]n+ (M = Ru, Ir; n = 1, 2) (73, 74). These phenomena illustrate the highly dynamic behavior of coordinated H2 (40), which can exhibit quantum-mechanical phenomena such as rotational tunneling (56) and exchange coupling (75). M
H breaking. Remarkably, dHH is both temperature and isotope dependent in [CpM(diphosphine)(H2)]n+ (M = Ru, Ir; n = 1, 2) (73, 74). These phenomena illustrate the highly dynamic behavior of coordinated H2 (40), which can exhibit quantum-mechanical phenomena such as rotational tunneling (56) and exchange coupling (75). M H2 and other σ-bond interactions are among the most dynamic, complex, and enigmatic chemical topologies known. The H2 ligand can bind/dissociate, reversibly split to dihydride, rapidly rotate, and exchange with cis hydrides, all on the same metal. Often these dynamics cannot be frozen out on the NMR time scale even at low T.
H2 and other σ-bond interactions are among the most dynamic, complex, and enigmatic chemical topologies known. The H2 ligand can bind/dissociate, reversibly split to dihydride, rapidly rotate, and exchange with cis hydrides, all on the same metal. Often these dynamics cannot be frozen out on the NMR time scale even at low T.
It is clear that H2 binding followed by OA serves as a prototype for other σ-bond activation processes, e.g., C H and Si
H and Si H. Silanes (HnSiR4-n) bind in η2-Si-H fashion (as in Eq. 2) (21–24, 42, 76). The η2-SiH4 structure in cis-Mo(CO)(SiH4)(Et2PC2H4PEt2)2, the first transition metal complex of SiH4, exists in equilibrium with its OA tautomer, MoH(SiH3)(CO)(Et2PC2H4PEt2)2, analogous to that for the W(η2-H2) system (Eq. 5), with similar structures and thermodynamic parameters (77). SiH4 binding and Si
H. Silanes (HnSiR4-n) bind in η2-Si-H fashion (as in Eq. 2) (21–24, 42, 76). The η2-SiH4 structure in cis-Mo(CO)(SiH4)(Et2PC2H4PEt2)2, the first transition metal complex of SiH4, exists in equilibrium with its OA tautomer, MoH(SiH3)(CO)(Et2PC2H4PEt2)2, analogous to that for the W(η2-H2) system (Eq. 5), with similar structures and thermodynamic parameters (77). SiH4 binding and Si H cleavage directly model that believed to occur for CH4 activation. Si
H cleavage directly model that believed to occur for CH4 activation. Si H distances in hydrosilane complexes vary widely, analogous to H2 complexes (3). A valuable yardstick for measuring activation in M(η2–X–H) bonds is the value of the NMR coupling constant JXH compared with that in the free ligand. There is typically a 50–80% reduction in JHD in unstretched HD complexes, a 74% reduction in J(13CH) for low-temperature cyclopentane coordination in CpRe(CO)2(C5H10) (78), and 65% in J(11BH) in complexes of neutral borane ligands (79). JSiH in M(η2–Si
H distances in hydrosilane complexes vary widely, analogous to H2 complexes (3). A valuable yardstick for measuring activation in M(η2–X–H) bonds is the value of the NMR coupling constant JXH compared with that in the free ligand. There is typically a 50–80% reduction in JHD in unstretched HD complexes, a 74% reduction in J(13CH) for low-temperature cyclopentane coordination in CpRe(CO)2(C5H10) (78), and 65% in J(11BH) in complexes of neutral borane ligands (79). JSiH in M(η2–Si H) are normally closer to those in OA products. η2–Ge
H) are normally closer to those in OA products. η2–Ge H bonds undergo OA much more easily than Si
H bonds undergo OA much more easily than Si H, and in general, the ease of OA of H2 lies between that of germanes and silanes (80). Backdonation is critical: silanes bind more strongly than alkanes and cleave much like H2 because the Si
H, and in general, the ease of OA of H2 lies between that of germanes and silanes (80). Backdonation is critical: silanes bind more strongly than alkanes and cleave much like H2 because the Si H bond is a good acceptor whereas C
H bond is a good acceptor whereas C H is not (the much higher energy of its σ* orbital reduces interaction with M d orbitals). However, the situation is more complex than for H2 activation because substituents at C or Si alter both electronics and sterics.
H is not (the much higher energy of its σ* orbital reduces interaction with M d orbitals). However, the situation is more complex than for H2 activation because substituents at C or Si alter both electronics and sterics.
Reactivity of σ-Complexes: Acidity and Heterolysis of X H Bonds
H Bonds
Aside from loss of H2, reactions of M H2 are dominated by homolytic cleavage of H2 (OA) and heterolytic cleavage, essentially deprotonation of bound H2 on electrophilic metal centers (Scheme 5) (25). σ-Complexes have several advantages in catalytic and other reactions. Foremost is that the formal oxidation state of M does not change on binding of H2, whereas formation of a dihydride formally increases the metal oxidation state by two. H2 ligands can also have far greater thermodynamic and kinetic acidity than hydrides, which is important in the ability of acidic H2 ligands to protonate substrates such as olefins and N2. In heterolytic cleavage (25, 40, 81, 82), the H2 ligand is deprotonated, and the remaining hydrogen ligates to the metal as a hydride. Both pathways have been identified in catalytic hydrogenation and also may be available for other σ-bond activations, e.g., C
H2 are dominated by homolytic cleavage of H2 (OA) and heterolytic cleavage, essentially deprotonation of bound H2 on electrophilic metal centers (Scheme 5) (25). σ-Complexes have several advantages in catalytic and other reactions. Foremost is that the formal oxidation state of M does not change on binding of H2, whereas formation of a dihydride formally increases the metal oxidation state by two. H2 ligands can also have far greater thermodynamic and kinetic acidity than hydrides, which is important in the ability of acidic H2 ligands to protonate substrates such as olefins and N2. In heterolytic cleavage (25, 40, 81, 82), the H2 ligand is deprotonated, and the remaining hydrogen ligates to the metal as a hydride. Both pathways have been identified in catalytic hydrogenation and also may be available for other σ-bond activations, e.g., C H cleavage. Heterolysis of X
H cleavage. Heterolysis of X H bonds through proton transfer to a basic site on a cis ligand or to an external base is a crucial step in many industrial and biological processes involving direct reaction of H2, silane, borane, and (possibly) alkane ligands.
H bonds through proton transfer to a basic site on a cis ligand or to an external base is a crucial step in many industrial and biological processes involving direct reaction of H2, silane, borane, and (possibly) alkane ligands.
Scheme 5.
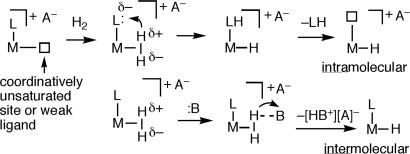
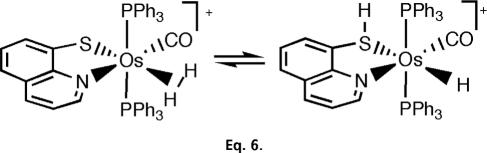
H2 complexes can undergo heterolysis in two distinct ways (Scheme 6). Intramolecular heterolysis is extremely facile for proton transfer to a cis ligand L (e.g., H or Cl) or to the counteranion of a cationic complex. The proton can also end up at a trans ligand (Eq. 6) (83).
Scheme 6.
 |
Intermolecular heterolysis involves protonation of an external base B, e.g., an ether solvent, to give a metal hydride (H− fragment) and the conjugate acid of the base, HB+. This is the reverse of protonation reactions used to synthesize H2 complexes (all reactions in Scheme 6 can be reversible), and the [HB]+ formed can relay the proton to internal or external sites (base-assisted heterolysis). Crabtree and Lavin (84) first demonstrated heterolysis of H2 by showing that the H2 in [IriH(H2)(LL)(PPh3)2]+ is deprotonated by LiR in preference to the hydride ligand. A milder base, NEt3, was shown by Heinekey and Chinn (85) to more rapidly deprotonate the η2-H2 tautomer in an equilibrium mixture of [CpRuH2(dmpe)]+ and [CpRu(H2)(dmpe)]+. The H2 ligand has greater kinetic acidity because deprotonation of an H2 complex involves no change in coordination number or oxidation state. Thus, H2 gas can be turned into a strong acid: free H2 is an extremely weak acid [pKa ∼ 35 in THF (86)], but binding it to an electrophilic cationic metal increases the acidity spectacularly, up to 40 orders of magnitude. The pKa can become as low as −6, i.e., η2–H2 can become more acidic than sulfuric acid as shown by Morris (25, 26, 82) and later Jia (36). Electron-deficient cationic H2 complexes with electron withdrawing ligands such as CO and short H H bonds (<0.9 Å), i.e., [Re(H2)(CO)4(PR3)]+ (87) are among the most acidic. Positive charge increases acidity: W(CO)3(PCy3)2(H2) is deprotonated only by strong bases (88), but on oxidation to [W(CO)3(PCy3)2(H2)]+ becomes acidic enough to protonate weakly basic ethers (89). Such ability is relevant to processes such as ionic hydrogenation and the function of metalloenzymes such as hydrogenases (H2ases).
H bonds (<0.9 Å), i.e., [Re(H2)(CO)4(PR3)]+ (87) are among the most acidic. Positive charge increases acidity: W(CO)3(PCy3)2(H2) is deprotonated only by strong bases (88), but on oxidation to [W(CO)3(PCy3)2(H2)]+ becomes acidic enough to protonate weakly basic ethers (89). Such ability is relevant to processes such as ionic hydrogenation and the function of metalloenzymes such as hydrogenases (H2ases).
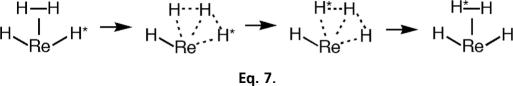
Complexes with H2 ligands are highly dynamic, and cis interactions, which are hydrogen-bonding-like interactions between η2–H2 and a cis hydride observable in the solid state (3, 20, 42, 90), facilitate solution exchange processes (Eq. 7). The intermediate is a “trihydrogen” complex (91, 92). Although not isolated, evidence exists for its intermediacy in facile H-atom exchange in ReH2(H2)(CO)(PR3)3 (93), which can be exceedingly fast even at −140°C in hydrido(H2) complexes (94–99). The barrier for hydrogen exchange in IrClH2(H2)(PiPr3)2 is only 1.5 kcal/mol even in the solid state (95, 96).
 |
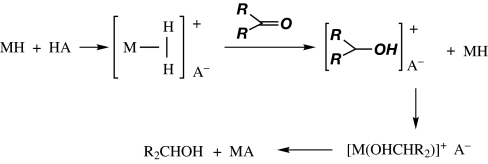
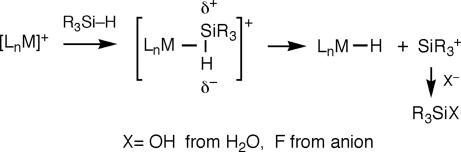
Can direct transfer of hydrogens from an H2 ligand occur in catalytic hydrogenation? Although difficult to prove conclusively, there is evidence in ionic hydrogenation where an organometallic hydride, e.g., CpMoH(CO)3, plus a strong acid, e.g., HO3SCF3, reduce ketones (100, 101). An acidic H2 complex is involved in proton transfer (Scheme 7). An impressive example of catalysis employing heterolysis of H2 is the asymmetric hydrogenation of ketones to alcohols catalyzed by the ruthenium system of Nobel Laureate Ryoji Noyori (102, 103). Other σ-bonds can be cleaved heterolytically, particularly on electrophilic metals (3, 40, 42). For coordinated Si H bonds, the bond becomes polarized Si(δ+)–H(δ−), i.e., the Si becomes positively charged (Scheme 8). Very reactive silylium ions are eliminated; they scavenge nucleophiles such as water or abstract fluoride from anions such as B(C6F5)4− (104). Similarly, a coordinated B
H bonds, the bond becomes polarized Si(δ+)–H(δ−), i.e., the Si becomes positively charged (Scheme 8). Very reactive silylium ions are eliminated; they scavenge nucleophiles such as water or abstract fluoride from anions such as B(C6F5)4− (104). Similarly, a coordinated B H bond in a BH3·PMe3 ligand in [Mn(CO)4(PR3)(BH3·PMe3)]+ cleaves to give H− (forming MnH(CO)4(PR3)) and “[BH2·PMe3]+” (105).
H bond in a BH3·PMe3 ligand in [Mn(CO)4(PR3)(BH3·PMe3)]+ cleaves to give H− (forming MnH(CO)4(PR3)) and “[BH2·PMe3]+” (105).
Scheme 7.
Scheme 8.
Can C H bonds in alkanes bind to electrophilic M to form a σ-alkane complex that can be split heterolytically? Proton transfer to a cis ligand (or anion) could take place followed by functionalization of the resultant methyl complex. Increased acidity of C
H bonds in alkanes bind to electrophilic M to form a σ-alkane complex that can be split heterolytically? Proton transfer to a cis ligand (or anion) could take place followed by functionalization of the resultant methyl complex. Increased acidity of C H bonds in transient alkane complexes analogous to that for coordinated H
H bonds in transient alkane complexes analogous to that for coordinated H H bonds may be important in alkane activation such as conversion of methane to methanol, a holy grail in chemistry well addressed in this special feature and the prolific work of Bercaw, Periana, and Bergman. In 1965, Chatt discovered OA of an arene C
H bonds may be important in alkane activation such as conversion of methane to methanol, a holy grail in chemistry well addressed in this special feature and the prolific work of Bercaw, Periana, and Bergman. In 1965, Chatt discovered OA of an arene C H bond to a metal complex and in 1976 predicted that “in 25 years methane will be the most popular ligand in coordination chemistry,” as noted by Shilov (106). As can be seen, this prediction has become true. As in H2 activation, alkane σ-complexes should be intermediates, astonishingly even in reaction media as harsh as sulfuric acid at 200°C in PtII-catalyzed methane to methanol conversions (107, 108), despite the weak binding energy of CH4 to metals [≈10 kcal/mol (109)].
H bond to a metal complex and in 1976 predicted that “in 25 years methane will be the most popular ligand in coordination chemistry,” as noted by Shilov (106). As can be seen, this prediction has become true. As in H2 activation, alkane σ-complexes should be intermediates, astonishingly even in reaction media as harsh as sulfuric acid at 200°C in PtII-catalyzed methane to methanol conversions (107, 108), despite the weak binding energy of CH4 to metals [≈10 kcal/mol (109)].
Molecular binding and heterolysis of H2 on metal surfaces and small metal clusters is rarely observed because formation of hydrides is favored. H2 binding to a stepped Ni(510) surface containing unsaturated sites was seen by electron energy-loss spectroscopy (110) and is the first step in hydriding other surfaces (111, 112). H2 also ligates at low T in small clusters such as Cu3(H2) (113), Pd(H2) (114), and similar species (115). Oxides adsorb and activate H2, including Cr2O3, MgO, and ZnO even at 25°C; some of these could involve molecular binding. (η2–H2)CrO2 has been prepared by cocondensation of CrO2 molecules with H2 in Ar at 11 K and photoisomerized to HCrO(OH), ostensibly through H2 heterolysis (116). RuO2 (111) has also been found to bind H2 at 85 K (νHH = 2960 cm−1; calcd dHH = 0.89 Å) (117) suggesting that, as for H2 on Ni surfaces, the binding of H2 is similar to that in organometallics. Zeolites can bind H2 (118, 119), notably to the extraframework iron in Fe–ZSM5 at 110 K. Research at the interface between heterogeneous and homogeneous catalysis (41) includes employing H2 interactions as probes for the catalytic sites in both regimes (120–122). An elegant example is the demonstration that Ir(CO)Cl(PPh3)2 catalyzes hydrogenation of unsaturated compounds both in solution and solid state through an H2 complex (123).
Activation of H2 on Biological and Nonmetal Systems
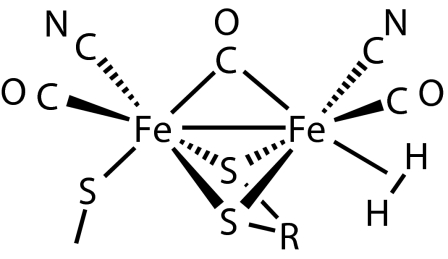
H2ases are redox enzymes in microorganisms that catalyze H2 ⇌ 2H+ + 2e− to either use H2 as an energy source or dispose of excess electrons as H2 (124–127). Biologically unprecedented CO and CN ligands are present in the dinuclear active site of iron-only H2ases (128) that are remarkably organometallic-like and have been extensively modeled for biomimetic H2 production (126, 127, 129–132) (see Sketch 2).
Sketch 2.
This site presumably transiently binds and heterolytically splits H2, most likely at a site trans to bridging CO, where a proton transfers to a thiolate ligand as in Eq. 6 or other Lewis-basic site (127). Such heterolysis has recently been shown to occur on a mononuclear Fe complex with a pendant nitrogen base (132). Nature apparently designed these enzymes billions of years ago to use the CO ligand, whose strong trans influence favors reversible H2 binding and heterolysis (3, 40). An H2 complex of a H-ase model, [Ru2(μ-H)(μ-S2C3H6)2(H2)(CO)3(PCy3)2]+, is known, albeit with Ru instead of Fe (133).
H2 can also be activated at nonmetals, e.g., the bridging sulfides in Cp2Mo2S4 that react with H2 to form SH ligands perhaps via a 4-center S2H2 transition state (134). Metal-free hydrogenation of ketones on strong bases such as t-BuOK occurs under harsh conditions, apparently through base-assisted heterolysis of H2 (135, 136). Thus, H2 is a very weak acceptor (Lewis acid) through electron donation to its σ* orbital and can interact with the O in alkoxide or metal oxides and undergo heterolysis (3). Significantly, the first example of reversible splitting of H2 on a nonmetal center has been found (137). The phosphine borane in Scheme 9 has a strong Lewis acidic center (boron) linked to a Lewis basic site (phosphorus). It is likely that H2 heterolysis takes place at boron where proton transfer from an H2-like complex to the basic phosphorus site occurs to form a phosphenium–borate.
Scheme 9.
H2 Storage and Production: A Glance to the Future
H2 is a fuel of the future, but vexing challenges exist. Materials for H2 storage are difficult to design because, although H2 can readily be extruded from a variety of compounds, it can be difficult to add back. The materials also must be light and contain >6% by weight H2, reducing prospects for known facile reversible systems such as metal–H2 or hydride complexes. Amine borane, H3NBH3, is a popular candidate and also combines both Lewis acidic (B) and basic (N) centers. Here, however, these centers are directly bonded, whereas the acidic and basic sites are separated by linkers in the phosphine-borane in Scheme 9. The metal-free aspect is relevant because precious metals such as platinum are often used in catalysis and can be environmentally unfriendly as well as costly or in short supply. Materials such as metal-organic frameworks (MOFS) (138–140) are now being examined for H2 storage and have huge surface area capable of binding large numbers of H2 molecules. Neutron scattering studies by Eckert are critical in determining whether H2 binds to unsaturated metal centers as in organometallics and/or is physisorbed in the framework. Calculations indicate complexes with multiple H2, i.e., Cr(H2)6 may be stable (141), and species such as [M(H2)n]+ have a fleeting gas phase existence (142), but isolation in condensed phases will be problematic.
Production of H2 fuel from water by means of solar energy is of high interest (143). Catalysis may involve H2 complexes at least as intermediates, and H2 complexes have been implicated in solar energy conversion schemes based on photoreduction of water (144). Industrially important water gas shift and related H2-producing reactions undoubtedly proceed through transient H2 complexes (145). Biomimetic H2 production, particularly solar driven (photocatalysis), is also a challenge and may take a cue from models of the active site of H2ase coupled with models of nature's photosystems (129–131, 143). Here the formation of H H bonds from protons and electrons, the microscopic reverse of H2 heterolysis, will be crucial in leading to formation of H2 and is very rapid at the Fe sites in H2-ases. Coupling model catalysts with photochemical water splitting will require fine-tuning of electrochemical potentials for tandem catalysis schemes.
H bonds from protons and electrons, the microscopic reverse of H2 heterolysis, will be crucial in leading to formation of H2 and is very rapid at the Fe sites in H2-ases. Coupling model catalysts with photochemical water splitting will require fine-tuning of electrochemical potentials for tandem catalysis schemes.
Acknowledgments
I am grateful to funding by the Department of Energy, Basic Energy Sciences, Chemical Sciences that allowed me to carry out the basic research leading to the discovery of H2 complexes and Los Alamos National Laboratory for Laboratory Directed Research and Development funding.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission. J.A.L. is a guest editor invited by the Editorial Board.
In 1993 Zilm obtained solid-state 1H NMR evidence for H2 coordination (dHH= 0.93 Å) on a sample we prepared.
References
- 1.Kubas GJ, Ryan RR, Swanson BI, Vergamini PJ, Wasserman HJ. J Am Chem Soc. 1984;106:451–452. [Google Scholar]
- 2.Kubas GJ. Acc Chem Res. 1988;21:120–128. [Google Scholar]
- 3.Kubas GJ. Metal Dihydrogen and σ-Bond Complexes. New York: Kluwer Academic/Plenum; 2001. [Google Scholar]
- 4.Kubas GJ. J Organometal Chem. 2001;635:37–68. [Google Scholar]
- 5.Crabtree RH. Angew Chem Int Ed Engl. 1993;32:789–805. [Google Scholar]
- 6.Baggott J. Perfect Symmetry: The Accidental Discovery of Buckminsterfullerene. Oxford: Oxford Univ Press; 1995. [Google Scholar]
- 7.James BR. Homogeneous Hydrogenation. New York: Wiley; 1973. [Google Scholar]
- 8.Halpern J. J Organometal Chem. 1980;200:133. [Google Scholar]
- 9.Kubas GJ. Chem Commun. 1980:61–62. [Google Scholar]
- 10.Wasserman HJ, Kubas GJ, Ryan RR. J Am Chem Soc. 1986;108:2294–2301. doi: 10.1021/ja00269a027. [DOI] [PubMed] [Google Scholar]
- 11.Brookhart M, Green MLH, Wong L-L. Prog Inorg Chem. 1988;36:1–124. [Google Scholar]
- 12.Allen AD, Senoff CV. J Chem Soc Chem Commun. 1965:621–622. [Google Scholar]
- 13.Perutz RN, Turner JJ. J Am Chem Soc. 1975;97:4791–4800. [Google Scholar]
- 14.Sweany RL. J Am Chem Soc. 1985;107:2374–2379. [Google Scholar]
- 15.Upmacis RK, Gadd GE, Poliakoff M, Simpson MB, Turner JJ, Whyman R, Simpson AF. J Chem Soc Chem Commun. 1985:27–30. [Google Scholar]
- 16.Church SP, Grevels F-W, Hermann H, Shaffner K. J Chem Soc Chem Commun. 1985:30–31. [Google Scholar]
- 17.Matthews SL, Pons V, Heinekey DM. J Am Chem Soc. 2005;127:850–851. doi: 10.1021/ja0433370. [DOI] [PubMed] [Google Scholar]
- 18.Matthews SL, Heinekey DM. J Am Chem Soc. 2006;128:2615–2620. doi: 10.1021/ja057912r. [DOI] [PubMed] [Google Scholar]
- 19.Saillard J-Y, Hoffmann R. J Am Chem Soc. 1984;106:2006–2026. [Google Scholar]
- 20.Maseras F, Lledós A, Clot E, Eisenstein O. Chem Rev. 2000;100:601–636. doi: 10.1021/cr980397d. [DOI] [PubMed] [Google Scholar]
- 21.Andrews MA, Kirtley SW, Kaesz HD. Adv Chem Ser. 1978;167:229–245. [Google Scholar]
- 22.Schubert U. Adv Organomet Chem. 1990;30:151–187. [Google Scholar]
- 23.Corey JY, Braddock-Wilking J. Chem Rev. 1999;99:175–292. doi: 10.1021/cr9701086. [DOI] [PubMed] [Google Scholar]
- 24.Nikonov GI. Adv Organomet Chem. 2005;51:217. [Google Scholar]
- 25.Jessop PG, Morris RH. Coord Chem Rev. 1992;121:155–284. [Google Scholar]
- 26.Morris RH. Can J Chem. 1996;74:1907–1915. [Google Scholar]
- 27.Crabtree RH. Acc Chem Res. 1990;23:95–101. [Google Scholar]
- 28.Heinekey DM, Oldham WJ., Jr Chem Rev. 1993;93:913–926. [Google Scholar]
- 29.Heinekey DM, Lledós A, Lluch JM. Chem Soc Rev. 2004;33:175–182. doi: 10.1039/b304879a. [DOI] [PubMed] [Google Scholar]
- 30.Sabo-Etienne S, Chaudret B. Coord Chem Rev. 1998;178–180:381–407. [Google Scholar]
- 31.Crabtree RH, Hamilton DG. J Am Chem Soc. 1986;108:3124–3125. [Google Scholar]
- 32.Knoth WH. J Am Chem Soc. 1968;90:7172–7173. [Google Scholar]
- 33.Ashworth TV, Singleton E. J Chem Soc Chem Commun. 1976:705–706. [Google Scholar]
- 34.Gusev DG, Vymenits AB, Bakhmutov VI. Inorg Chim Acta. 1991;179:195–201. [Google Scholar]
- 35.Esteruelas MA, Oro LA. Chem Rev. 1998;98:577–588. doi: 10.1021/cr970322u. [DOI] [PubMed] [Google Scholar]
- 36.Jia G, Lau C-P. Coord Chem Rev. 1999;190–192:83–108. [Google Scholar]
- 37.Esteruelas MA, Oro LA. Adv Organomet Chem. 2001;47:1–59. [Google Scholar]
- 38.Kuhlman R. Coord Chem Rev. 1997;167:205–232. [Google Scholar]
- 39.McGrady GS, Guilera G. Chem Soc Rev. 2003;32:383–392. doi: 10.1039/b207999m. [DOI] [PubMed] [Google Scholar]
- 40.Kubas GJ. Adv Inorg Chem. 2004;56:127–178. [Google Scholar]
- 41.Kubas G. Catal Lett. 2005;104:79–101. [Google Scholar]
- 42.Perutz RN, Sabo-Etienne S. Angew Chem Int Ed. 2007;46:2578–2592. doi: 10.1002/anie.200603224. [DOI] [PubMed] [Google Scholar]
- 43.Peruzzini M, Poli R. Recent Advances in Hydride Chemistry. Amsterdam: Elsevier Science; 2001. [Google Scholar]
- 44.Bart SC, Lobkovsky E, Chirik PJ. J Am Chem Soc. 2004;126:13794–13807. doi: 10.1021/ja046753t. [DOI] [PubMed] [Google Scholar]
- 45.Harman WD, Taube H. J Am Chem Soc. 1990;112:2261–2263. [Google Scholar]
- 46.Hasegawa T, Li Z, Parkin S, Hope H, McMullan RK, Koetzle TF, Taube H. J Am Chem Soc. 1994;116:4352–4365. [Google Scholar]
- 47.Malin J, Taube H. Inorg Chem. 1971;10:2403–2406. [Google Scholar]
- 48.Aebischer N, Frey U, Merbach AE. Chem Comm. 1998:2303–2304. [Google Scholar]
- 49.Kubas GJ, Burns CJ, Eckert J, Johnson S, Larson AC, Vergamini PJ, Unkefer CJ, Khalsa GRK, Jackson SA, Eisenstein O. J Am Chem Soc. 1993;115:569–581. [Google Scholar]
- 50.Maltby PA, Schlaf M, Steinbeck M, Lough AJ, Morris RH, Klooster WT, Koetzle TF, Srivastava RC. J Am Chem Soc. 1996;118:5396–5407. [Google Scholar]
- 51.Luther TA, Heinekey DM. Inorg Chem. 1998;37:127–132. doi: 10.1021/ic970975t. [DOI] [PubMed] [Google Scholar]
- 52.Zilm KW, Millar JM. Adv Mag Opt Reson. 1990;15:163–199. [Google Scholar]
- 53.Desrosiers PJ, Cai L, Lin Z, Richards R, Halpern J. J Am Chem Soc. 1991;113:4173–4184. [Google Scholar]
- 54.Gusev DG, Kuhlman RL, Renkema KH, Eisenstein O, Caulton KG. Inorg Chem. 1996;35:6775–6783. doi: 10.1021/ic960693d. [DOI] [PubMed] [Google Scholar]
- 55.Morris RH, Wittebort RJ. Mag Res Chem. 1997;35:243–250. [Google Scholar]
- 56.Eckert J, Kubas GJ. J Chem Phys. 1993;97:2378–2384. [Google Scholar]
- 57.Fang X, Huhmann-Vincent J, Scott BL, Kubas GJ. J Organometal Chem. 2000;609:95–103. [Google Scholar]
- 58.Ingleson MJ, Brayshaw SK, Mahon MF, Ruggiero GD, Weller AS. Inorg Chem. 2005;44:3162–3171. doi: 10.1021/ic0482739. [DOI] [PubMed] [Google Scholar]
- 59.Moreno B, Sabo-Etienne S, Chaudret B, Rodriguez A, Jalon F, Trofimenko S. J Am Chem Soc. 1995;117:7441–7451. [Google Scholar]
- 60.Grellier M, Vendier L, Chaudret B, Albinati A, Rizzato S, Mason S, Sabo-Etienne S. J Am Chem Soc. 2005;127:17592–17593. doi: 10.1021/ja055126g. [DOI] [PubMed] [Google Scholar]
- 61.Elian M, Chen MML, Mingos DMP, Hoffmann R. Inorg Chem. 1976;15:1148–1155. [Google Scholar]
- 62.Thompson KC, Crittenden DL, Jordan MJT. J Am Chem Soc. 2005;127:4954–4958. doi: 10.1021/ja0482280. [DOI] [PubMed] [Google Scholar]
- 63.Dewar MJS. Bull Soc Chim Fr. 1951;18:C79. [Google Scholar]
- 64.Chatt J, Duncanson LA. J Chem Soc. 1953:2929. [Google Scholar]
- 65.Heinekey DM, Law JK, Schultz SM. J Am Chem Soc. 2001;123:12728–12729. doi: 10.1021/ja016766w. [DOI] [PubMed] [Google Scholar]
- 66.Yousufuddin M, Wen TB, Mason SA, McIntyre GJ, Jia G, Bau R. Angew Chem Int Ed. 2005;44:7227–7230. doi: 10.1002/anie.200502297. [DOI] [PubMed] [Google Scholar]
- 67.Brammer L, Howard JA, Johnson O, Koetzle TF, Spencer JL, Stringer AM. J Chem Soc Chem Commun. 1991:241–243. [Google Scholar]
- 68.Johnson TJ, Albinati A, Koetzle TF, Ricci J, Eisenstein O, Huffman JC, Caulton KG. Inorg Chem. 1994;33:4966–4976. [Google Scholar]
- 69.Gelabert R, Moreno M, Lluch JM. Chem Eur J. 2005;11:6315–6325. doi: 10.1002/chem.200500287. [DOI] [PubMed] [Google Scholar]
- 70.Albinati A, Bakhmutov VI, Caulton KG, Clot E, Eckert J, Eisenstein O, Gusev DG, Grushin VV, Hauger BE, Klooster WT, et al. J Am Chem Soc. 1993;115:7300–7312. [Google Scholar]
- 71.Gusev DG. J Am Chem Soc. 2004;126:14249–14257. doi: 10.1021/ja0465956. [DOI] [PubMed] [Google Scholar]
- 72.Hush NS. J Am Chem Soc. 1997;119:1717. [Google Scholar]
- 73.Law JK, Mellows H, Heinekey DM. J Am Chem Soc. 2002;124:1024–1030. doi: 10.1021/ja0118284. [DOI] [PubMed] [Google Scholar]
- 74.Gelabert R, Moreno M, Lluch JM, Lledós A, Heinekey DM. J Am Chem Soc. 2005;127:5632–5640. doi: 10.1021/ja043011r. [DOI] [PubMed] [Google Scholar]
- 75.Sabo-Etienne S, Chaudret B. Chem Rev. 1998;98:2077–2091. doi: 10.1021/cr9601066. [DOI] [PubMed] [Google Scholar]
- 76.Lin Z. Chem Soc Rev. 2002;31:239–245. doi: 10.1039/b106620j. [DOI] [PubMed] [Google Scholar]
- 77.Luo X-L, Kubas GJ, Burns CJ, Bryan JC, Unkefer CJ. J Am Chem Soc. 1995;117:1159–1160. [Google Scholar]
- 78.Geftakis S, Ball GE. J Am Chem Soc. 1998;120:9953–9954. [Google Scholar]
- 79.Merle N, Koicok-Kohn G, Mahon MF, Frost CG, Ruggerio GD, Weller AS, Willis MC. J Chem Soc Dalton. 2004:3883–3892. doi: 10.1039/b413650k. [DOI] [PubMed] [Google Scholar]
- 80.Huhmann-Vincent J, Scott BL, Butcher R, Luo S, Unkefer CJ, Kubas GJ, Lledós A, Maseras F, Tomas J. Organometallics. 2003;22:5307–5323. [Google Scholar]
- 81.Brothers PJ. Prog Inorg Chem. 1981;28:1–61. [Google Scholar]
- 82.Morris RH. In: Recent Advances in Hydride Chemistry. Peruzzini M, Poli R, editors. Amsterdam: Elsevier Science; 2001. pp. 1–38. [Google Scholar]
- 83.Schlaf M, Lough AJ, Morris RH. Organometallics. 1996;15:4423–4436. [Google Scholar]
- 84.Crabtree RH, Lavin M. J Chem Soc Chem Commun. 1985:794–795. [Google Scholar]
- 85.Chinn MS, Heinekey DM. J Am Chem Soc. 1987;109:5865–5867. [Google Scholar]
- 86.Buncel E, Menon B. J Am Chem Soc. 1977;99:4457–4461. [Google Scholar]
- 87.Huhmann-Vincent J, Scott BL, Kubas GJ. J Am Chem Soc. 1998;120:6808–6809. [Google Scholar]
- 88.Van Der Sluys LS, Miller MM, Kubas GJ, Caulton KG. J Am Chem Soc. 1991;113:2513–2520. [Google Scholar]
- 89.Bruns W, Kaim W, Waldhor E, Krejcik M. Inorg Chem. 1995;34:663–672. [Google Scholar]
- 90.Van Der Sluys LS, Eckert J, Eisenstein O, Hall JH, Huffman JC, Jackson SA, Koetzle TF, Kubas GJ, Vergamini PJ, Caulton KG. J Am Chem Soc. 1990;112:4831–4841. [Google Scholar]
- 91.Brintzinger HH. J Organomet Chem. 1979;171:337–344. [Google Scholar]
- 92.Burdett JK, Phillips JR, Pourian MR, Poliakoff M, Turner JJ, Upmacis R. Inorg Chem. 1987;26:3054–3063. [Google Scholar]
- 93.Luo X-L, Crabtree RH. J Am Chem Soc. 1990;112:6912–6918. [Google Scholar]
- 94.Gusev DG, Hubener R, Burger P, Orama O, Berke H. J Am Chem Soc. 1997;119:3716–3731. [Google Scholar]
- 95.Wisniewski LL, Mediati M, Jensen CM, Zilm KW. J Am Chem Soc. 1993;115:7533–7534. [Google Scholar]
- 96.Li S, Hall MB, Eckert J, Jensen CM, Albinati J Am Chem Soc. 2000;122:2903–2910. [Google Scholar]
- 97.Gusev DG, Berke H. Chem Ber. 1996;129:1143–1155. [Google Scholar]
- 98.Pons V, Conway SLJ, Green MLH, Green JC, Herbert BJ, Heinekey DM. Inorg Chem. 2004;43:3475–3483. doi: 10.1021/ic0496875. [DOI] [PubMed] [Google Scholar]
- 99.Janak KE, Shin JH, Parkin G. J Am Chem Soc. 2004;126:13054–13070. doi: 10.1021/ja047554c. [DOI] [PubMed] [Google Scholar]
- 100.Bullock RM, Song J-S, Szalda DJ. Organometallics. 1996;15:2504–2516. [Google Scholar]
- 101.Guan H, Iimura M, Magee MP, Norton JR, Zhu G. J Am Chem Soc. 2005;127:7805–7814. doi: 10.1021/ja0506861. [DOI] [PubMed] [Google Scholar]
- 102.Noyori R. Angew Chem Int Ed. 2002;41:2008–2022. [PubMed] [Google Scholar]
- 103.Ohkuma T, Noyori R. J Am Chem Soc. 2003;125:13490–13503. doi: 10.1021/ja030272c. [DOI] [PubMed] [Google Scholar]
- 104.Luo X-L, Crabtree RH. J Am Chem Soc. 1989;111:2527–2535. [Google Scholar]
- 105.Yasue T, Kawano Y, Shimoi M. Angew Chem Int Ed. 2003;42:1727. doi: 10.1002/anie.200219992. [DOI] [PubMed] [Google Scholar]
- 106.Shilov AE. Metal Complexes in Biomimetic Chemical Reactions. Boca Raton, FL: CRC; 1997. Chap 2. [Google Scholar]
- 107.Periana RA, Taube DJ, Evitt ER, Löffler DG, Wentrcek PR, Voss G, Masuda T. Science. 1993;259:340–343. doi: 10.1126/science.259.5093.340. [DOI] [PubMed] [Google Scholar]
- 108.Periana RA, Taube DJ, Gamble S, Taube H, Satoh T, Fujii H. Science. 1998;280:560–564. doi: 10.1126/science.280.5363.560. [DOI] [PubMed] [Google Scholar]
- 109.Hall C, Perutz RN. Chem Rev. 1996;96:3125–3146. doi: 10.1021/cr9502615. [DOI] [PubMed] [Google Scholar]
- 110.Martensson A-S, Nyberg C, Andersson S. Phys Rev Lett. 1986;57:2045–2048. doi: 10.1103/PhysRevLett.57.2045. [DOI] [PubMed] [Google Scholar]
- 111.Kresse G. Phys Rev B. 2000;62:8295–8305. [Google Scholar]
- 112.Schmidt PK, Christman K, Kresse G, Hafner J, Lischka M, Gross A. Phys Rev Lett. 2001;87 doi: 10.1103/PhysRevLett.87.096103. 096103. [DOI] [PubMed] [Google Scholar]
- 113.Hauge RH, Margrave JL, Kafafi ZH. NATO ASI Ser Ser B. 1987;158:787. [Google Scholar]
- 114.Ozin GA, Garcia-Prieto J. J Am Chem Soc. 1986;108:3099–3100. [Google Scholar]
- 115.Andrews L. Chem Soc Rev. 2004;33:123–132. doi: 10.1039/b210547k. [DOI] [PubMed] [Google Scholar]
- 116.Zhou M, Zhang L, Shao L, Wang W, Fan K, Qin Q. J Phys Chem A. 2001;105:10747–10752. [Google Scholar]
- 117.Wang J, Fan CY, Sun Q, Reuter K, Jacobi K, Scheffler M, Ertl G. Angew Chem Int Ed Engl. 2003;42:2151–2154. doi: 10.1002/anie.200250659. [DOI] [PubMed] [Google Scholar]
- 118.Mojet BL, Eckert J, van Santen RA, Albinati A, Lechner RE. J Am Chem Soc. 2001;123:8147–8148. doi: 10.1021/ja016078c. [DOI] [PubMed] [Google Scholar]
- 119.Eckert J, Nicol JM, Howard J, Trouw FR. J Phys Chem. 1996;100:10646–10651. [Google Scholar]
- 120.Bianchini C, Burnaby DG, Evans J, Frediani P, Meli A, Oberhauser W, Psaro R, Sordelli L, Vizza F. J Am Chem Soc. 1999;121:5961–5971. [Google Scholar]
- 121.Matthes J, Pery T, Grundemann S, Buntkowsky G, Sabo-Etienne S, Chaudret B, Limbach H-H. J Am Chem Soc. 2004;126:8366–8367. doi: 10.1021/ja0475961. [DOI] [PubMed] [Google Scholar]
- 122.Casty GL, Matturro MG, Myers GR, Reynolds RP, Hall RB. Organometallics. 2001;20:2246–2249. [Google Scholar]
- 123.Matthes J, Pery T, Grundemann S, Buntkowsky G, Sabo-Etienne S, Chaudret B, Limbach H-H. J Am Chem Soc. 2004;126:8366–8367. doi: 10.1021/ja0475961. [DOI] [PubMed] [Google Scholar]
- 124.Armstrong FA. Curr Opin Chem Biol. 2004;8:133–140. doi: 10.1016/j.cbpa.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 125.Volbeda A, Fonticella-Camps JC. Coord Chem Rev. 2005:1609–1619. [Google Scholar]
- 126.Liu X, Ibrahim SK, Tard C, Pickett CJ. Coord Chem Rev. 2005:1641–1652. [Google Scholar]
- 127.Darensbourg MY, Lyon EJ, Zhao Z, Georgakaki IP. Proc Natl Acad Sci USA. 2003;100:3683–3688. doi: 10.1073/pnas.0536955100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Peters JW, Lanzilotta WN, Lemon BJ, Seefeldt LC. Science. 1998;282:1853–1858. doi: 10.1126/science.282.5395.1853. [DOI] [PubMed] [Google Scholar]
- 129.Capon J-F, Gloagen F, Schollhammer P, Talarmin J. Coord Chem Rev. 2005:1664–1676. [Google Scholar]
- 130.Sun L, Akermark B, Ott S. Coord Chem Rev. 2005:1653–1663. [Google Scholar]
- 131.Alper J. Science. 2003;299:1686–1687. doi: 10.1126/science.299.5613.1686. [DOI] [PubMed] [Google Scholar]
- 132.Henry RM, Shoemaker RK, Newell RH, Jacobsen GM, DuBois DL, Rakowski DuBois M. Organometallics. 2005;24:2481–2491. [Google Scholar]
- 133.Justice AK, Linck RC, Rauchfuss TB, Wilson SR. J Am Chem Soc. 2004;126:13214–13215. doi: 10.1021/ja0455594. [DOI] [PubMed] [Google Scholar]
- 134.Rakowski DuBois M. Chem Rev. 1989;89:1–9. [Google Scholar]
- 135.Berkessel A, Schubert TJS, Muller TN. J Am Chem Soc. 2002;124:8693–8698. doi: 10.1021/ja016152r. [DOI] [PubMed] [Google Scholar]
- 136.Chan B, Radom L. J Am Chem Soc. 2005;127:2443–2454. doi: 10.1021/ja0450253. [DOI] [PubMed] [Google Scholar]
- 137.Welch GC, San Juan RR, Masuda JD, Stephan DW. Science. 2006;314:1124–1126. doi: 10.1126/science.1134230. [DOI] [PubMed] [Google Scholar]
- 138.Rosi NL, Eckert J, Eddaoudi M, Vodak DT, Kim J, O'Keeffe M, Yaghi OM. Science. 2003;300:1127–1129. doi: 10.1126/science.1083440. [DOI] [PubMed] [Google Scholar]
- 139.Rowsell JLC, Eckert J, Yaghi OM. J Am Chem Soc. 2005;127:14904–14910. doi: 10.1021/ja0542690. [DOI] [PubMed] [Google Scholar]
- 140.Forster PM, Eckert J, Heiken BD, Parise JB, Yon JW, Jhung SW, Chang J-S, Cheetham AK. J Am Chem Soc. 2006;128:16846–16850. doi: 10.1021/ja0649217. [DOI] [PubMed] [Google Scholar]
- 141.Gagliardi L, Pyykko P. J Am Chem Soc. 2004;126:15014–15015. doi: 10.1021/ja045991l. [DOI] [PubMed] [Google Scholar]
- 142.Weisshaar JC. Acc Chem Res. 1993;26:213–219. [Google Scholar]
- 143.Lewis NS, Nocera DG. Proc Natl Acad Sci USA. 2006;103:15729–15735. doi: 10.1073/pnas.0603395103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sutin N, Creutz C, Fujita E. Comments Inorg Chem. 1997;19:67–92. [Google Scholar]
- 145.Torrent M, Solà M, Frenking G. Chem Rev. 2000;100:439–493. doi: 10.1021/cr980452i. [DOI] [PubMed] [Google Scholar]