Abstract
The rugged folding-energy landscapes of RNAs often display many competing minima. How do RNAs discriminate among competing conformations in their search for the native state? By using optical tweezers, we show that the folding-energy landscape can be manipulated to control the fate of an RNA: individual RNA molecules can be induced into either native or misfolding pathways by modulating the relaxation rate of applied force and even be redirected during the folding process to switch from misfolding to native folding pathways. Controlling folding pathways at the single-molecule level provides a way to survey the manifold of folding trajectories and intermediates, a capability that previously was available only to theoretical studies.
Keywords: mechanical force, misfolding, optical tweezers, RNA folding, single molecule
The folding of a macromolecule can be thought of as a biased diffusion over an energy surface that describes the thermodynamic and kinetic constraints of possible intramolecular interactions. RNAs differ from proteins in the nature, strength, specificity, and degeneracy of the interactions that stabilize their native structures. Whereas proteins are made of 20 amino acids, it takes only 4 nucleotides to build RNAs. This simpler composition, endowed with robust base-pairing rules, greatly increases the promiscuity of interactions between any given nucleotide and the rest of the RNA structure. These RNA–protein differences are reflected in the topography of the energy surface over which the molecules diffuse in their search for the native structure: the energy surfaces of RNAs are significantly more rugged than those of proteins, containing many competing local minima (1–4). Even relatively small RNAs, such as tRNA (4–6), tend to fold into stable alternate secondary structures with energies only a few kilocalories (1 kcal = 4.18 kJ on first use) per mole apart (4). Once trapped kinetically in a secondary structure with suboptimal folding energy, it is difficult for an RNA molecule to reach its native structure (1).
The promiscuous nature of secondary interactions in RNA begs answers for three questions: How do RNAs find their native structure? How do they avoid the kinetic traps on their rugged folding-energy landscape? And, how can RNAs switch between alternate structures in response to changes in the cellular environment, as happens, for example, during transcription attenuation in bacteria (7)? To address these questions, we need to follow the folding trajectories of RNA molecules as they diffuse over their energy landscape in search of stable conformations. Because of the structural polymorphism and manifold pathways of RNA, single-molecule approaches (8–12) are particularly useful for answering these questions by identifying folding intermediates and distinguishing native pathways from those that misfold.
Here we use optical tweezers to unfold and control the refolding of single RNA molecules by force (11, 13). Two micrometer-sized beads are attached to the ends of an RNA molecule and are manipulated by optical tweezers to exert force on the RNA (Fig. 1a). The unfolding and refolding of the RNA molecule, induced by controlled application of force, is monitored by nanometer changes in the end-to-end distance of the molecule. In a typical “pulling” experiment, force is increased or decreased at a fixed rate (the loading and unloading rate, respectively). Significantly, the applied tension slows the hairpin folding kinetics from an order of 106 s−1 at zero force (14) to a more measurable range from 102 to 10−2 s−1 at its transition force such that the process of the folding can be monitored in real time. The force (F)-dependent un/refolding rate constant (kF) can be described by an Arrhenius-type equation (15):
where k0 is the apparent rate constant of the mechanical unfolding process at zero force, and kBT is Boltzmann's constant times temperature in Kelvin. X‡, the distance to the transition state, is positive for unfolding and negative for refolding. Hence, we can control the un/refolding rate constants by manipulating the tension on a molecule.
Fig. 1.
Optical-tweezers assay for studying folding, misfolding, and rescue of TAR RNA. (a) Experimental design. RNA hairpin TAR, flanked by dsDNA/RNA handles, is tethered to two microspheres (11). One microsphere is held by a force-measuring trap, and the other is held on a micropipette. By moving the micropipette, tension (F) is exerted on the molecule, and the change in extension (X) is measured (13). (b) Native structure of TAR. (c) Three types of force-extension curves for TAR RNA. In each experiment, force was first relaxed from 20 to 1 pN (red) and then was raised back to 20 pN (blue). Unfolding is indicated by rips that increase the extension, whereas folding and rescue are indicated by zips that shorten the extension. The arrows point to the fluctuations/intermediates on refolding.
When an RNA molecule is pulled and relaxed very slowly such that the process is thermodynamically reversible, simple hairpins are often seen to unfold and refold cooperatively in a single step (11, 16–21). One example is the 52-nt HIV transactivation response region (TAR) RNA (22), which folds into a 21-bp hairpin with a 3-nt bulge (Fig. 1b) (23). The equilibrium force (F1/2), at which its unfolding and refolding rates are equal, is 12.4 pN in 100 mM KCl, pH 8, at 22°C (19). When the force is held constant at or near the F1/2 value, an individual TAR molecule can be seen to transit between folded and unfolded states without detectable intermediates. At forces 1 pN below or above the F1/2, the molecule mainly stays in the folded or unfolded state. These observations are consistent with a two-step folding process in which the refolding occurs through a nucleation step: formation of the loop-closing first base pair followed by a helix-propagation process (24–26).
To explore the possibility of alternative folding pathways, individual TAR molecules were stretched and relaxed repeatedly at approximately fixed loading and unloading rates (pN/s). In a typical cycle, the force was first ramped down from 20 to <2 pN (Fig. 1c, red) before it was increased again to 20 pN (blue). These experiments revealed three different types of trajectories, each associated with distinctive refolding characteristics: single-step refolding, multiple-step refolding, and misfolding. The single-step refolding mostly occurs at force relaxation rates <1 pN/s. In this case the force-extension curve displays a single transition (zip) at 10–13 pN with a decrease in extension of ≈17 nm. This behavior is consistent with a single-step refolding process from a single strand to the native structure. When pulled, the natively folded TAR always displayed a sudden length increase of ≈18 nm that appeared as a single rip at ≈13–16 pN. In contrast, when the tension on the molecule was allowed to relax at a rate of 1.5 pN/s, >95% of the refolding curves show multiple-step refolding, displaying a decreasing extension with characteristic fluctuations (Fig. 1c, black arrows) followed by a small zip. This zip corresponds to the folding of an average of 26 nt, which is half the value for the refolding of the entire molecule. Subsequent pulling of such molecules displayed unfolding characteristics similar to those observed in the slow relaxation curves, indicating that these RNAs had folded into the native structure. The back-and-forth oscillations in the force-extension curve before the zip are likely successive refolding and unfolding events as the molecule moves into and out of alternative, competing folding pathways. Once an RNA molecule, following this search process, folds into intermediate structures that contain only native contacts, the remaining helix can form cooperatively, as manifested by the observed zip.
At unloading rates >2 pN/s, we observed another type of relaxation curve that showed no zipping at all; at low force the extension of the RNA never returned to that of the native hairpin (Fig. 1c, misfolding). When the force on the molecule was subsequently increased, the extension of the RNA increased monotonically, retracing the relaxation curve until a zip transition suddenly shortened the end-to-end distance of the molecule, against the increasing force. This transition, not observed previously, is a refolding event in that the extension of the molecule is shortened, yet it occurs as the force is increasing, which normally causes unfolding. The magnitude of this zip shows a broad distribution with a mean of ≈30 single-stranded nucleotides becoming paired (Fig. 2a), indicating that a range of partially folded structures existed before the transition. As the force was further raised, the force-extension curve always showed a rip similar to the unfolding of the native hairpin. We conclude that the structures at low force are misfolded, and that the application of force allowed the RNA molecules to form the native hairpin in the zip transition. Accordingly, we term this zip a “rescue” transition.
Fig. 2.
Misfolding and rescue of TAR RNA. (a) Histogram of the decrease in end-to-end distance, ΔXrescue, in the rescue. ΔXrescue was converted to the number of single-stranded nucleotides that become paired in the rescue. (b) Fraction of misfolded trajectories as a function of unloading rates. Between 122 and 324 total trajectories were collected at each unloading rate. (c) Histogram of the rescue forces in the force-ramp experiment at an unloading rate of 11.2 pN/s followed by a loading rate of 2 pN/s. The rescue force was defined as the force at which the rescue transition starts. (d) The rescue-force distribution in c is fitted to Eq. 2, yielding an Xrescue1‡ of 6 ± 1 nm.
The faster the force is decreased, the more likely a molecule becomes trapped into a variety of stable misfolded structures with less base pairs and longer end-to-end distances than the native hairpin, as shown by the increase in frequency of misfolding with increasing force relaxation rates (Fig. 2b). Rescue occurred between 6 and 12 pN at a loading rate of 2 pN/s (Fig. 2c). The low threshold of the transition force argues against the idea that the prerescue structures are on-pathway folding intermediates. If they were so, one would expect them to fold quickly at <6 pN, given that the RNA folds into the native hairpin in seconds even at ≈12 pN (19). To further rule out this possibility, we kept the misfolded structures at zero tension for 30 min. Subsequent pulls showed the characteristic zip–rip signature (in that order), clearly indicating that these nonnative structures are stable at low forces and that forces >6 pN are required for their conversion to the native hairpin. Such forces were needed to break the nonnative interactions that trapped the RNA structure, allowing the molecule to search for its correct folding pathway and effectively rescuing it from the misfolded state.
Assuming that the rate-liming step in the rescue is disruption of nonnative base pairs, we can determine the kinetics for rescue. The distribution of rescue forces was fitted to the following equation (27),
 |
where [M(F, r)] is the fraction of misfolded molecule at each force (F), and the loading rate is (r); A is a factor reflecting both the rescue-rate constant at zero force and instrumental effects (11); and Xrescue1‡ represents the average distance to the transition state of the native fold from the misfolded structures. We obtained Xrescue1‡ of 6 ± 1 nm (Fig. 2d) from fitting the data shown in Fig. 2c to Eq. 2.
Which steps in the folding process of the native hairpin are perturbed by the fast force relaxation? High force favors the longer single-strand forms over base-paired structures. As the force is relaxed, new intramolecular contacts and partly folded structures become accessible to the molecule. The effect of relaxing the force, therefore, is to expand and change continuously the topography of the energy surface over which the molecules can diffuse. The folding of a hairpin involves nucleation and helix formation, both of which are influenced by force. Formation of the first base pair closing the native loop has to compete with many other possible loop-closing base pairs. When the molecule is allowed to equilibrate, either at constant force near its F1/2 or when the force is relaxed very slowly relative to the rate of forming the correct base pairs, the single strand folds into the native hairpin by sequentially base-pairing from the native loop; the refolding appears to be two-state. In contrast, when the force is relaxed fast, alternative loop closures and nonnative base pairings become accessible to the molecule before the native contacts form. As the force continues to decrease fast, some of these nonnative loop closures will not have sufficient time to open but instead lead to propagation of nonnative helices at lower forces. Such a structural evolution is reflected by the fluctuations displayed in the multiple-step refolding trajectories. Probabilities of both forming and unfolding of these misfolded structures depend on the instantaneous value of the force. When the force is decreased faster than the rate at which misfolded structures can unfold, the molecule becomes kinetically trapped in metastable states.
To reduce or avoid misfolding, the topography of the energy landscape needs to be modified in such a way that the channels leading to the native fold become more favorable than alternate pathways. This situation can be achieved by engineering the molecular structure such that the correct folding is speeded up and/or formation of the misfolded structures is slowed. To test these hypotheses, we measured misfolding of two mutant TAR hairpins. First, we tried to facilitate the correct folding. In the native structure (Fig. 1b), formation of the bulge-closing base pair is energetically unfavorable (28) and is a slow step in the helix formation. To eliminate this slow step, we constructed a mutant hairpin, TARΔb, in which the 3-nt bulge is deleted. As predicted, the bulgeless mutant is more stable than TAR, with an F1/2 of ≈16 pN. Moreover, TARΔb folds faster than TAR by four orders of magnitude (Fig. 3d) and shows no misfolding (Fig. 4).
Fig. 3.
Rescue at constant force. (a) The experiment took two steps: (1) the force was decreased from 20 to 9.5 pN at a rate of 11 pN/s and held constant (black) (the rescue is indicated by a zip), and (2) after the rescue occurred, the force was lowered to 1 pN (black) and then raised to 20 pN to check the folded structure (gray). (b) Time trace of the extension at the rescue force. (c) Histogram of ΔXrescue in the number of single-stranded nucleotides paired at rescue. (d) Rescue-rate constants (▴) as a function of force. Unfolding (■) and refolding (●) rates of TAR were measured by force jump (19). Unfolding (○) and refolding (□) rates of TARΔb were measured by constant force experiments. The standard deviation of each point is <10% of the mean. Solid lines are best least squares fit to Eq. 1.
Fig. 4.
The force-extension curve of TARΔb shows no misfolding at an unloading rate of 20 pN/s.
Next, we tried to reduce the alternative folding by destabilizing the misfolded structures. Among the nonnative structures predicted by the mfold program (29), several stable ones contain nonnative base pairs formed by the first 5 nt at the 5′ end (Fig. 5). We designed a mutant hairpin, TAR2, in which the second (G·C) and fourth (U·A) base pairs from the end of the native helix were interchanged to specifically destabilize the hypothetical misfolded structures. In addition, other alternative structures of this mutant are predicted to be less stable than those of TAR. Consistent with this prediction, this mutant RNA refolds without misfolding at unloading rates up to 21 pN/s. This observation strongly implicates the nucleotides at the 5′ end in the formation of the nonnative base-pairing in the misfolded structures. Thus, a combination of the bulge-closing slow step in the native helix formation and metastable misfolded structures allow alternative folding pathways to compete kinetically at fast force relaxation.
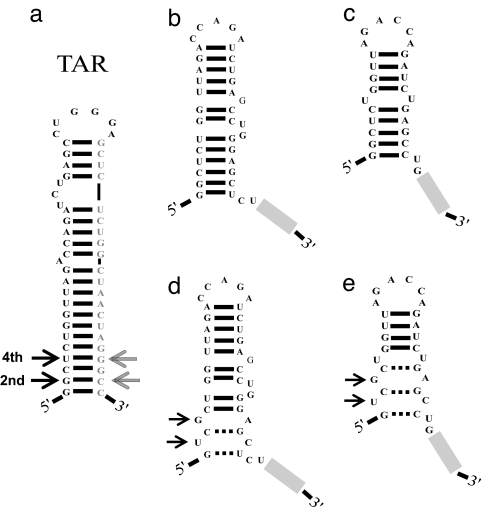
Fig. 5.
Hypothetical misfolded intermediates of TAR. (a) Native hairpin of TAR (−31.3 kcal/mol). In the TAR2 mutant, the second and fourth base pair from the bottom of the helix (arrows) were switched. (b and c) The 5′ end of TAR can form stable alternative structures [−11.4 (b) and −11.3 (c) kcal/mol]. The rest of the sequence is represented by a gray bar. (d and e) In TAR2, the same intermediate structures shown in b and c were greatly destabilized [−4.2 (d) and −0.5 (e) kcal/mol].
The three types of force-extension curves of TAR shown in Fig. 1c have also been observed in various concentrations of K+ and Mg2+. Transition forces, including unfolding/refolding, misfolding, and rescue, are affected by the type and concentration of the cation. As expected, increasing K+ and Mg2+ increases the stability and folding rates of the native hairpin and decreases the unfolding rates. The thermodynamic stability of TAR in 10 mM Mg2+ is similar to that in 1 M K+ (data not shown). We have not systematically investigated the effects of ionic conditions on the occurrence of misfolding, but specific effects of different cations are likely.
How can a misfolded RNA molecule escape from the kinetic trap and find its minimum energy structure? To gain insight into this question, we monitored the structural evolution of misfolded RNAs to the native fold. First, force was decreased on an unfolded molecule from 20 pN to between 7 and 9.5 pN at a rate of ≈11 pN/s to induce misfolding; in nearly half of the trajectories, the molecule was not completely folded when the set force was reached. Then, the force was held constant until the native fold was reached, which was indicated by a decrease in extension (Fig. 3a). Typically, the extension of the misfolded structures remained relatively constant for a few seconds until a zipping event occurred (Fig. 3b). The decrease in extension of this zip, ΔXrescue2, shows a broad distribution (Fig. 3c), indicating that many different partially folded structures, with an average of 10 ± 3 bp, had formed after the fast force relaxation. At the rescue forces, the single strand would fold into the native hairpin in milliseconds or less, yet lifetimes of these partially folded structures are at least tens of seconds (Fig. 3d), indicating that these structures must be off-pathway, misfolded species. After the zipping, the extension varied little up to 5 min, indicating that the native hairpin is stable at this force and that the mechanical rescue of the native structure from the metastable structures is irreversible. Subsequent pulling verified the native folding (Fig. 3a, gray curve).
The lifetimes of the misfolded structures at each force fit a single exponential distribution, from which we calculated the rescue-rate constant at force F, krescue2(F). The force dependence of the rescue-rate constant is fitted to Eq. 1, in which Xrescue2‡ represents the average distance to the transition state of the native fold from the misfolded structures. The rescue rate increases with the force, yielding a positive value of Xrescue2‡ (4.9 ± 0.8 nm; Fig. 3d), characteristic of a helix-unfolding transition (30). This value also agrees with the Xrescue1‡ obtained from pulling experiments (Fig. 2d). Rescue below 7 pN took at least a few minutes and was rarely observed, consistent with the rescue-force distribution observed in the force-ramp experiments (Fig. 2b). However, between 9.5 and 11 pN, both the native folding rate and the rescue rate are so fast that misfolded structures are practically not observable.
In a classic study of RNA misfolding, the activity of a misfolded tRNA was restored by heating the sample in the presence of Mg2+ (6). Presumably, raising the temperature, just as increasing the force, disrupts the nonnative conformation and thereby allows correct folding. Here, the rescue rate was raised by increasing the force applied to the molecule, confirming that the rate-limiting step in the rescue is the disruption of incorrect, trapped structures. An important question, then, is: Must the trapped molecule unfold completely to gain access to the native fold? If, during rescue, the misfolded RNAs were first unfolded into a single strand, the extension would increase by ≈10 nm on average. However, such an unfolding step was not observed before the rescue zip (Fig. 3). To further investigate the nature of the molecular rearrangements that take place during rescue, we characterized the fluctuations of the molecular extension in the constant-force rescue experiments. The root-mean-square deviation of the molecular extension before the appearance of the zip is 0.7 ± 0.2 nm, just slightly higher than the value observed after the native hairpin formed (0.5 ± 0.1 nm). The lack of a distinctive unfolding step suggests that a series of partial unfolding and refolding events in small steps are responsible for gradually converting the RNA into an on-pathway intermediate, at which time the zipping transition occurred. It is notable that in the multiple-step refolding observed at high relaxation rates, many small-step unfolding/refolding transitions occur before the final zip (Fig. 1c). Taken together these observations suggest that the structural rearrangements that lead the molecule from misfolded structures to the native state occur through pathways made up of multiple small unfolding/refolding steps and not through a path that requires the complete unfolding of the RNA. A similar mechanism has been proposed for the conformational switch of a ribozyme (31).
We have shown how direct mechanical manipulation can be used to control the folding pathway of a single molecule. In particular, we showed that decreasing the force applied to the molecule can be used to change, in real time, the number of configurations accessible to the molecule; lowering the force increases the number of structures and interactions accessible to the molecule. Moreover, by controlling the rate at which the force is relaxed, we effectively modify the folding-energy landscape (15, 21, 32) and the alternative pathways over which the molecule diffuses in its search for the native state. The relaxation rate, then, is the control parameter that determines how many and how fast these pathways become accessible to the molecule, the degree of kinetic competition between these pathways, and, just as importantly, how long the molecule is allowed to follow a particular pathway before other competing paths become available.
As shown here in the case of TAR, by direct manipulation and modification of the force applied to the molecule, it is possible to suddenly “tilt” the energy landscape of the molecule, increasing its chance to attain off-pathway states and even allow the molecule to switch back to the on-pathway amid the folding. We know of no other experimental method that permits such control over the detailed kinetics of refolding of a molecule in real time. Combined with mutagenesis, we have characterized here what the crucial elements are in TAR that favor misfolding (i.e., the three-base bulge and the sequence at the 5′ end). More elaborate schemes of force relaxation interspersed with constant force events could be used to characterize the molecular processes leading to various intermediates, to trapping, and to structural rearrangement of the RNA.
To maintain cellular homeostasis, living cells must ensure the proper folding of millions of copies of RNAs. Deletion of RNA chaperones has been implicated in cell death after UV irradiation and in autoimmune disease (33). Mounting evidence, accumulated in recent years, suggests that RNA folding in vivo is an assisted process through the participation of specialized proteins (34–40). The mechanism by which RNA chaperone proteins facilitate correct RNA folding remains unclear (3, 41). The results presented above suggest two possible scenarios. Proteins may simply facilitate the attainment of the folded structure by binding to specific regions of the unfolded structure and sterically interfering with the formation of alternative competing pathways on the folding-energy surface. Alternatively, motor proteins, such as helicases (17), generate and apply force to actively induce the mechanical disruption of RNA molecules trapped in nonnative folds in the cell. The methods described here make it possible to investigate the mechanisms used by proteins or other molecular components to steer and facilitate the attainment of the native fold through the rugged RNA landscapes.
Materials and Methods
RNA Preparation.
The RNA samples were prepared as described previously (19). The entire RNA, the hairpin with flanking handles (500 nt each), was transcribed from plasmid template by T7 RNA polymerase. Two ≈500-bp dsDNA handles corresponding to the regions flanking the hairpin were amplified by PCRs and modified by either biotin or digoxigenin at each of the termini. The DNA handles were annealed to the RNA. Properly annealed molecules can be tethered between two microspheres coated with streptavidin and anti-digoxigenin antibody, respectively (11) (Fig. 1a).
Optical Tweezers.
Dual-beam optical tweezers (13) were used to study the RNA folding. The streptavidin-coated bead was placed on a micropipette mounted on a piezoelectric flexture stage. The anti-digoxigenin antibody-coated bead was held by the optical trap. Force is determined by the displacement of the trapped bead from the center of the trap. The change in the extension of the molecule results from the movement of both the trapped bead and the bead on the micropipette.
Manipulation of Single Molecules.
In the force-ramp experiments, the piezoelectric stage moved the micropipette at a constant rate (nm/s), which changed the tension on the RNA with an approximately constant (un)loading rate (pN/s) between 3 and 20 pN. When constant force was implemented, a feedback mechanism using a proportional, integrative, and differential algorithm was used to maintain the tension. In all experiments, force and extension were recorded at 100 Hz.
Folding Condition.
The folding was studied in 10 mM Hepes (pH 8.0), 100 mM KCl, and 0.1 mM EDTA; 0.05% NaN3 was added to prevent the growth of bacteria. All folding reactions were studied at 22°C.
Conversion of ΔX.
To compare the increase in end-to-end extension, ΔX, at different forces, each ΔX is converted to the number of single-stranded ribonucleotides at the corresponding force. The conversion is calculated by using the worm-like-chain interpolation formula (42) with a single-stranded RNA contour length of 0.59 nm/nt and persistent length of 1 nm.
Acknowledgments
We thank Ms. Maria Manosas, Mr. Jeff Vieregg, Dr. Gang Chen, and Dr. Felix Ritort for critical reading of the manuscript. We thank Dr. Laurent Lacroix for sharing thermal melting data. This work is supported by National Institutes of Health Grants GM-10840 (to I.T.) and GM-32543 (to C.B.).
Abbreviation
- TAR
transactivation response region.
Footnotes
The authors declare no conflict of interest.
References
- 1.Turner DH. In: Nucleic acids. Bloomfield V, Crothers D, Tinoco I Jr, editors. Sausalito, CA: University Science Books; 2000. pp. 111–164. [Google Scholar]
- 2.Treiber DK, Rook MS, Zarrinkar PP, Williamson JR. Science. 1998;279:1943–1946. doi: 10.1126/science.279.5358.1943. [DOI] [PubMed] [Google Scholar]
- 3.Herschlag D. J Biol Chem. 1995;270:20871–20874. doi: 10.1074/jbc.270.36.20871. [DOI] [PubMed] [Google Scholar]
- 4.Gralla J, DeLisi C. Nature. 1974;248:330–332. doi: 10.1038/248330a0. [DOI] [PubMed] [Google Scholar]
- 5.Chakshusmathi G, Kim SD, Rubinson DA, Wolin SL. EMBO J. 2003;22:6562–6572. doi: 10.1093/emboj/cdg625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindahl T, Adams A, Fresco JR. Proc Natl Acad Sci USA. 1966;55:941–948. doi: 10.1073/pnas.55.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merino E, Yanofsky C. Trends Genet. 2005;21:260–264. doi: 10.1016/j.tig.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Tan E, Wilson TJ, Nahas MK, Clegg RM, Lilley DM, Ha T. Proc Natl Acad Sci USA. 2003;100:9308–9313. doi: 10.1073/pnas.1233536100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhuang X, Bartley LE, Babcock HP, Russell R, Ha T, Herschlag D, Chu S. Science. 2000;288:2048–2051. doi: 10.1126/science.288.5473.2048. [DOI] [PubMed] [Google Scholar]
- 10.Onoa B, Dumont S, Liphardt J, Smith SB, Tinoco I, Jr, Bustamante C. Science. 2003;299:1892–1895. doi: 10.1126/science.1081338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liphardt J, Onoa B, Smith S, Tinoco I, Jr, Bustamante C. Science. 2001;292:733–737. doi: 10.1126/science.1058498. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez JM, Li H. Science. 2004;303:1674–1678. doi: 10.1126/science.1092497. [DOI] [PubMed] [Google Scholar]
- 13.Smith SB, Cui Y, Bustamante C. Methods Enzymol. 2003;361:134–162. doi: 10.1016/s0076-6879(03)61009-8. [DOI] [PubMed] [Google Scholar]
- 14.Pörschke D. In: Chemical Relaxation in Molecular Biology. Pecht I, Rigler R, editors. New York: Springer; 1977. pp. 191–218. [Google Scholar]
- 15.Bell GI. Science. 1978;200:618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- 16.Collin D, Ritort F, Jarzynski C, Smith SB, Tinoco I, Jr, Bustamante C. Nature. 2005;437:231–234. doi: 10.1038/nature04061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumont S, Cheng W, Serebrov V, Beran RK, Tinoco I, Jr, Pyle AM, Bustamante C. Nature. 2006;439:105–108. doi: 10.1038/nature04331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li PTX, Bustamante C, Tinoco I., Jr Proc Natl Acad Sci USA. 2006;103:15847–15852. doi: 10.1073/pnas.0607202103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li PTX, Collin D, Smith SB, Bustamante C, Tinoco I., Jr Biophys J. 2006;90:250–260. doi: 10.1529/biophysj.105.068049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodside MT, Behnke-Parks WM, Larizadeh K, Travers K, Herschlag D, Block SM. Proc Natl Acad Sci USA. 2006;103:6190–6195. doi: 10.1073/pnas.0511048103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodside MT, Anthony PC, Behnke-Parks WM, Larizadeh K, Herschlag D, Block SM. Science. 2006;314:1001–1004. doi: 10.1126/science.1133601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy S, Delling U, Chen CH, Rosen CA, Sonenberg N. Genes Dev. 1990;4:1365–1373. doi: 10.1101/gad.4.8.1365. [DOI] [PubMed] [Google Scholar]
- 23.Puglisi JD, Tan R, Calnan BJ, Frankel AD, Williamson JR. Science. 1992;257:76–80. doi: 10.1126/science.1621097. [DOI] [PubMed] [Google Scholar]
- 24.Poland D. Cooperative Equilibria in Physical Biochemistry. Oxford: Clarendon; 1978. [Google Scholar]
- 25.Cantor C, Schimmel PR. Biophys Chem. San Francisco: Freeman; 1980. [Google Scholar]
- 26.Bloomfield VA, Crothers DM, Tinoco I., Jr 2000:13–43. [Google Scholar]
- 27.Evans E, Ritchie K. Biophys J. 1997;72:1541–1555. doi: 10.1016/S0006-3495(97)78802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathews DH, Sabina J, Zuker M, Turner DH. J Mol Biol. 1999;288:911–940. doi: 10.1006/jmbi.1999.2700. [DOI] [PubMed] [Google Scholar]
- 29.Zuker M. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tinoco I., Jr Annu Rev Biophys Biomol Struct. 2004;33:363–385. doi: 10.1146/annurev.biophys.33.110502.140418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LeCuyer KA, Crothers DM. Proc Natl Acad Sci USA. 1994;91:3373–3377. doi: 10.1073/pnas.91.8.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hyeon C, Thirumalai D. Proc Natl Acad Sci USA. 2005;102:6789–6794. doi: 10.1073/pnas.0408314102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Wolin SL. J Mol Med. 2004;82:232–239. doi: 10.1007/s00109-004-0529-0. [DOI] [PubMed] [Google Scholar]
- 34.Zhang F, Ramsay ES, Woodson SA. RNA. 1995;1:284–292. [PMC free article] [PubMed] [Google Scholar]
- 35.Nikolcheva T, Woodson SA. J Mol Biol. 1999;292:557–567. doi: 10.1006/jmbi.1999.3083. [DOI] [PubMed] [Google Scholar]
- 36.Mahen EM, Harger JW, Calderon EM, Fedor MJ. Mol Cell. 2005;19:27–37. doi: 10.1016/j.molcel.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 37.MacRae IJ, Doudna JA. Cell. 2005;121:495–501. doi: 10.1016/j.cell.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Brion P, Schroeder R, Michel F, Westhof E. RNA. 1999;5:947–958. doi: 10.1017/s1355838299990477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donahue CP, Yadava RS, Nesbitt SM, Fedor MJ. J Mol Biol. 2000;295:693–707. doi: 10.1006/jmbi.1999.3380. [DOI] [PubMed] [Google Scholar]
- 40.Schroeder R, Barta A, Semrad K. Nat Rev Mol Cell Biol. 2004;5:908–919. doi: 10.1038/nrm1497. [DOI] [PubMed] [Google Scholar]
- 41.Stein AJ, Fuchs G, Fu C, Wolin SL, Reinisch KM. Cell. 2005;121:529–539. doi: 10.1016/j.cell.2005.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bustamante C, Marko JF, Siggia ED, Smith S. Science. 1994;265:1599–1600. doi: 10.1126/science.8079175. [DOI] [PubMed] [Google Scholar]