Abstract
Clonal deviation is a mechanism by which immature thymocytes expressing a self-reactive T cell antigen receptor (TCR) are rescued from clonal deletion by adopting an alternative differentiation pathway resistant to apoptosis. Here, we confirm and generalize previous indications that genetic alleles in NOD mice condition ineffective clonal deviation toward the CD8αα lineage, a peculiar population of TCRαβ lymphocytes that electively colonizes the intraepithelial lymphocyte pool in the gut. Thymic selection of CD8αα cells was very age-dependent, occurring almost exclusively in the postnatal period. Fewer CD8αα cells were found in the thymus and intraepithelial lymphocytes of BDC2.5 TCR transgenic mice on the NOD than on the C57BL/6 (B6) background; this paucity extended to standard NOD mice, albeit to a lesser extent. CD8αα cells resided in the BDC2.5 pancreatic infiltrate, and they were more abundant on the B6 than the NOD background, correlating with aggressivity of the lesion. A (B6g7 × NOD)F2 intercross in agonist-challenged BDC2.5 fetal thymic organ cultures demonstrated the existence of a major quantitative trait locus on chromosome 3, coincident with an interval associated with resistance to clonal deletion. A replicate linkage confirmed these positions and showed that the same region also controls clonal deviation toward the CD4+FoxP3+ regulatory T cell lineage. That clonal deviation toward the CD8αα and regulatory T cell pathways share genetic control further highlights the similarities between these two “rescue lineages,” consistent with an immunoregulatory role for CD8αα cells.
Keywords: autoimmunity, FoxP3, tolerance
T cell antigen receptor (TCR)αβ CD8αα T cells constitute a peculiar subpopulation within the T cell lineage (1, 2). They are bona fide T cells, they express the classic αβTCR, and their global gene expression profile relates them quite closely to conventional CD8+ T cells (3), with which they share several basic functionalities. On the other hand, they have a unique localization, preferentially residing in the gut, where they form a sizeable fraction of intraepithelial lymphocytes (IELs), and their CD8αα homodimer, rather than performing the usual coreceptor role of the CD8αβ heterodimer, seems to serve primarily as a ligand for the MHC-like TL molecule (4), although this interaction is not exclusive and its functional relevance remains conjectural (5, 6).
The developmental origin of IELs was initially debated, with several early experiments suggesting that they may have an extrathymic origin; it now seems clear, however, that the thymus is the primary source of CD8αα IELs (reviewed in refs. 1 and 2). Lineage tracing and reconstitution experiments in fetal thymic organ cultures (FTOC) showed that the immature double-positive (DP) CD4+CD8+ compartment gives rise to CD8αα cells (3, 7). Their selection depends on β2-microglobulin and, thus, presumably on MHC class I-like molecules, but this is not solely restricted to the classical K and D molecules (8, 9). The differentiation into the CD8αα lineage is induced or enhanced by engagement of the TCR on immature thymocytes by a strong agonist ligand (3, 10–14). This clonal deviation provides a rescue pathway for self-reactive T cells, because signals from the TCR are blunted by the absence of an actively signaling coreceptor. The shutdown of CD8β is also accompanied by the activation of a distinctive gene-expression program, which includes FcεR through which they signal (15) and a number of molecules typical of cells of the innate immune system and of memory CD8+ cells (3). In this respect, CD8αα cells are conceptually similar to the FoxP3-dependent subpopulation of CD4+ regulatory T cells (Tregs) that also escape clonal deletion because of enhanced resistance to apoptotic signals (16–18).
In recent experiments, we used the BDC2.5 TCR transgenic (Tg) system to trace the genetic roots of the resistance to clonal deletion in the diabetes-prone NOD mouse; to our surprise, we also observed that BDC2.5 NOD FTOC lobes gave rise to very few CD8αα cells in response to agonist peptide (19). Here, we investigated the generality and genetic origins of this observation and found that the same genomic region that controls clonal deletion also conditions clonal deviation to both the CD8αα and Treg “rescue lineages.”
Results
Defective Generation of CD8αα Cells on the NOD Genetic Background.
The BDC2.5 transgene encodes a TCR with reactivity against a pancreatic islet self-antigen, and thus confers a strong potential for autoimmunity (20). Parallel FTOCs were set up with BDC2.5 day 15 embryos on the NOD and C57BL/6.H2g7 backgrounds. These backgrounds share the selecting H2g7 haplotype at the MHC but otherwise entail all of the genomic differences between the NOD and C57BL/6 (B6) strains. Reproducing our prior observations, far fewer CD8αα cells were generated in the BDC2.5/NOD cultures than in the BDC2.5/Bg7 cultures (Fig. 1a). [Note that we have demonstrated previously that this difference is not just a kinetic one (19).] The generation of CD8αα cells paralleled the intensity of clonal deletion, reflected as the proportion of clonotype positive DP cells over the range of peptide doses, with a 30-fold shift to higher values with BDC2.5/NOD (Fig. 1b). In BDC2.5/NOD lobes, CD8αα cells were comparatively rare, even when the profile was matched for the intensity of clonal deletion and when assessed by determining their proportion among CD8+ single-positive (SP) cells or by determining their total number per lobe.
Fig. 1.
CD8αα are T cells quantitatively induced by using agonist peptide in FTOC on the B6 but not the NOD background. (a) BDC2.5-Tg FTOCs on the NOD (Right) and B6g7 (Left) genetic backgrounds were incubated with 10 ng/ml mimotope peptide for 7 days and analyzed for expression of CD8α and CD8β (gated on BDC2.5+ thymocytes). (b) Absolute numbers of DP or CD8αα SP cells in these cultures are shown across a range of agonist peptide concentrations. (c) P14-Tg FTOCs on the NOD and B6g7 backgrounds were cultured with various concentrations of agonist peptide and analyzed as above (gated on Vα2+ thymocytes). Data are representative of three to five independent experiments.
To determine whether this difference was unique to cells displaying the BDC2.5 TCR, we studied FTOCs from P14 TCR Tg mice (Db-restricted and reactive to the GP33–41 peptide from lymphocytic choriomeningitis virus) crossed onto the NOD-H2b and B6 backgrounds. FTOCs were challenged with the GP peptide. Here again the NOD genome conferred relative resistance to clonal deletion and poor selection of CD8αα cells (Fig. 1c). Thus, the differential selection of CD8αα cells on the NOD and B6 backgrounds is not a quirk of the BDC2.5 TCR.
In the HY and OT-1 TCR Tg systems, the generation of CD8αα cells by agonist peptide presented by MHC class I molecules was accompanied by the activation of a particular cluster of genes, characteristic of cells of the innate immune system, in particular NK cells (3). To see whether the CD8αα cells differentiating in response to the BDC2.5 mimotope presented by MHC class II molecules were bona fide CD8αα cells, we used quantitative real-time PCR to measure the quantities of this set of transcripts in CD8αα cells from peptide-challenged BDC2.5/Bg7 FTOCs, relative to conventional CD8αβ T cells that developed in unmanipulated cultures. All of these transcripts showed the expected differential (Fig. 2), with overexpression ranging from 20- to 200-fold for transcripts encoding surface receptors, such as NKG2D, NK1.1, or 2B4, or the transcription factor Id2.
Fig. 2.
BDC2.5+ CD8αα cells express transcripts typical of innate immune system cells. RNA was isolated from CD8αα and CD8αβ BDC2.5-Tg thymocytes purified from peptide-supplemented and peptide-negative FTOCs, respectively. Selected transcripts previously shown to be up-regulated in thymic CD8αα cells (3) were quantitated by using real-time PCR. Results are presented as the ratio of transcript levels (normalized to hypoxanthine phosphoribosyl transferase) in CD8αα versus CD8αβ thymocytes. The data shown represent one of two experiments.
Genetic Control on the CD8αα Population in Vivo.
To generalize these FTOC findings, we surveyed the tissues of intact BDC2.5 mice on the NOD versus B6.H2g7 backgrounds. The BDC2.5 TCR recognizes an antigen specifically expressed in pancreatic islet β-cells, but early clonal deletion is detected in the thymus of neonatal BDC2.5 mice (19), suggesting that the BDC2.5 antigen is ectopically expressed in thymic stroma, as are a number of peripheral-tissue antigens (21). Indeed, CD8αα cells represented a major proportion of CD4−CD8+CD24lo mature SP cells in the thymus of 1-week-old BDC2.5/Bg7 mice (47.3 ± 10.9% on average), but were rare in thymi from their BDC2.5/NOD counterparts (4.5 ± 4.8% on average) (Fig. 3a Left). We have previously shown in the HY TCR Tg system that the selection of CD8αα cells in HY males occurs preferentially in the neonatal period, waning by a few weeks after birth (3). This temporal difference also was found in the BDC2.5 TCR Tg system, in which very few CD8αα T cells were detected in adult thymi on either genetic background (Fig. 3a Right).
Fig. 3.
CD8αα cells are preferentially selected on the B6 versus NOD backgrounds in vivo. (a) Thymocytes from BDC/Bg7 and BDC/NOD mice were isolated at 1 week (Left) and 5 weeks (Right) of age and analyzed by using cytometry (gated on BDC2.5+CD4−CD8α+ thymocytes). Representative data of at least two thymi per group are shown. (b) Pancreatic lymph node cells from 7- to 9-week-old BDC/Bg7 and BDC/NOD mice. (Left) The plots are gated on live cells. (Right) The plots are further gated on BDC2.5+B220−CD4−CD8α+ cells. (c) IELs were isolated from 9- to 14-week-old BDC/Bg7 and BDC/NOD mice and analyzed by using flow cytometry (gated on BDC2.5+ cells). Representative data from two experiments are shown.
In most peripheral lymphoid organs, CD8αα cells were quite rare, as illustrated in Fig. 3b for pancreatic lymph node cells, but there was a tendency to a higher representation on the B6g7 background. This trend was confirmed and amplified in the IEL populations from these mice (Fig. 3c). CD8αα cells made up a large fraction of the clonotype-positive IELs in BDC2.5/Bg7 mice, but they were essentially absent in BDC2.5/NOD mice.
Finally, we looked for CD8αα cells in the islet infiltrates of BDC2.5/NOD versus BDC2.5/Bg7 mice. In the BDC2.5 system, recognition of the β cell antigen begins in the pancreatic lymph node at ≈15 days of age, and infiltration of the islets ensues quickly. Insulitis is more aggressive on the B6.H2g7 background, leading to diabetes in 50% of BDC2.5/Bg7 animals by 6–10 weeks of age (22). In young mice at the onset of insulitis, CD8αα cells represented a large fraction of CD4−CD8+ T cells in the islet-infiltrating lymphocytes (Fig. 4a), an unusual observation given that CD8αα cells are normally confined to the gut. CD8αα cells were far fewer in the BDC2.5/NOD pancreas (Fig. 4a). Interestingly, CD8αα cells decreased in the more stable and “respectful” insulitis found at later times in both strains, which very rarely progresses to overt diabetes; the NOD versus B6g7 difference was still apparent, even after this decrease (Fig. 4b). Thus, the genetic control that modulates CD8αα selection in embryonic and neonatal thymi also seems reflected in the composition of gut- and pancreas-infiltrating CD8+ cells.
Fig. 4.
Fewer CD8αα cells on the NOD genetic background. (a and b) Lymphoid cells isolated from the pancreatic tissue from BDC2.5/Bg7 or BDC2.5/NOD mice at 3–4 weeks (a) and 8 weeks (b) of age. (a Left and b Left) The plots are gated on live cells. (a Right and b Right) The plots are further gated on BDC2.5+B220−CD4−CD8α+ cells (representative of two to four experiments). (c) Splenocytes from 7-week-old NOD and B6g7 mice (gated on TCRβ+CD4−CD8α+ cells, representative of four individual mice/strains). (d) (Left) IELs from 8- to 10-week-old B6g7 and NOD mice (gated on TCRβ+ IELs). (Right) Compilation of the percentage of CD8αα IELs among TCRβ+ IELs in B6g7 (n = 7) versus NOD (n = 7) mice.
The BDC2.5 TCR Tg system highlights the differentiation profile of T cells expressing a highly self-reactive TCR. To test the impact of NOD versus B6 genetic variation on CD8αα cells in the context of a polyclonal repertoire, we analyzed tissues from non-Tg NOD and B6.H2g7 mice. CD8αα cells were absent from spleens of both strains (Fig. 4c). In gut lymphocytes, a higher proportion of CD8αα cells were observed among IELs from B6.H2g7 than from NOD mice (35.2 vs. 21.5%, respectively on average; P = 0.02) (Fig. 4d). Thus the differential propensity for B6 and NOD mice to select CD8αα cells when triggered by the strongly self-reactive BDC2.5 TCR is also manifest, albeit in a more muted fashion, in normal polyclonal repertoires.
Genetic Mapping of Clonal Deviation.
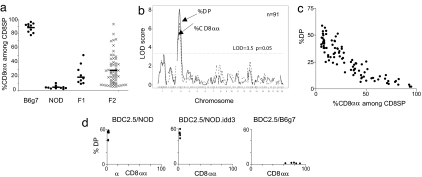
Because the genetic differences in clonal deviation to the CD8αα lineage observed in FTOC proved to have a strong correlate in vivo, it was of interest to map the location of the locus (or loci) that underlie(s) this difference. FTOCs were performed with lobes from parental, F1, and F2 intercrosses between BDC2.5/NOD and BDC2.5/Bg7 mice, all supplemented with a low dose of peptide to induce CD8αα cells. The primary quantitative traits assessed were the extent of clonal deletion (best reflected as the percentage of DP cells) (19) and of clonal deviation (best reflected as the percentage of CD8αα within clonotype-positive CD8+ SP cells). Lobes from F1 and F2 embryos showed an intermediate phenotype, skewed toward the NOD phenotype (Fig. 5a). The range of CD8αα phenotypes in the F2 offspring suggested multigenic control with a dominance of the NOD allele(s). A whole-genome scan was performed with 120 SNP markers, spaced at an average of 19.4 megabases (Mb); data were processed by using simple marker/trait association in R/qtl (23). A major quantitative trait locus (QTL) was found on chromosome 3, centered at ≈50 Mb, with a strongly significant logarithm of odds (LOD) score of 6.67 (permutation analysis showed a threshold LOD score of 3.5 for a genomic-wise corrected significance of P = 0.05) (Fig. 5b). A few weaker QTLs were observed in the suggestive range, not quite reaching full statistical significance, in particular on distal chromosome 1. Interestingly, these were the regions that showed the strongest association with resistance to clonal deletion in our previous analysis (19), and a strong QTL for clonal deletion (association with the percentage of DP) also was found in the same region in the present cohort. Not surprisingly, the two-parameter plot of Fig. 5c reveals a strong correlation between these two metrics among the F2 mice. Thus, the QTLs for resistance to clonal deletion and for ineffective clonal deviation to the CD8αα lineage are very tightly linked, if not identical.
Fig. 5.
The same genetic regions condition clonal deletion and clonal deviation to CD8αα. (a) BDC2.5-Tg FTOCs on the B6g7, NOD, and F1 or F2 intercrosses were incubated with 10 ng/ml peptide for 7 days and analyzed by using flow cytometry. Means are indicated by a horizontal bar. (b) LOD plots for the F2 cohort with the percentage of DP and CD8αα cells out of the total number of CD8 SP cells as quantitative traits (n = 91). (c) Two-parameter scatter plot of the percentage of DP and CD8αα cells (of the total number of CD8 SP cells) for the F2 cohort. (d) Comparison of BDC2.5-Tg FTOCs on the NOD, NOD.Idd3R450, and B6g7 backgrounds (supplemented with 10 ng/ml mimotope peptide). The two-parameter scatter plots show the percentage of DP (indicating clonal deletion) and the percentage of CD8αα cells among CD8 SP cells (clonal deviation).
These QTLs mapped quite closely to the position of the idd3 locus, an important component of susceptibility to autoimmune diabetes in the NOD mouse, one component of which is located between 36.3 and 37.2 Mb on chromosome 3 (24, 25). We tested whether the idd3 region might contain the clonal deletion/deviation QTL by crossing the BDC2.5 transgene onto the NOD.B6idd3R450 line and analyzing FTOC cultures prepared from such mice. As illustrated in Fig. 5d, lobes from NOD.idd3 mice were as resistant as those from NOD mice, indicating that the clonal deletion/deviation QTL maps outside of the 36–37 Mb interval and is distinct from the most tightly defined idd3 region, although it might correspond to other idd3 subloci.
As discussed above, the Treg subpopulation of CD4+ cells constitutes, as do CD8αα cells, a rescue lineage for self-reactive thymocytes. Whether Treg cell differentiation is actually induced by self-recognition, as suggested in some systems (17, 26), or simply results from a stronger resistance to clonal deletion (18, 27) may well be a function of the particular TCR and its affinity/avidity for self-MHC/peptide ligands. We have recently reported that the induction of Treg cells is also under strong genetic control, with a clear difference in responsiveness to induction by cognate peptide on the B6 and NOD backgrounds (M.F., unpublished observations). We asked whether the same regions might control diversion into the CD8αα and Treg pathways, employing a second cohort of FTOCs from BDC2.5/(B6g7 × NOD) F2 embryos. This time, the output of CD8αα and of FoxP3+ Treg cells were both recorded for the same thymic lobes in response to a fixed dose of peptide. As expected, a tight correlation was again observed between the degree of clonal deletion (inversely proportional to the percentage of DP) and the clonal deviation toward CD8αα cells (Fig. 6a Left). This correlation also extended to the proportion of FoxP3+ Treg cells among CD4+ SP cells (Fig. 6a Center and Right). The whole-genome SNP scan performed on these mice again brought up the main QTL on chromosome 3, essentially coincident for all three parameters (Fig. 6b). As might be expected from the data in Fig. 5d, the main peak is telomeric to the idd3 region but does encompass idd10 and idd17. Thus, the differential efficacy of apoptosis induction, CD8αα generation, and Treg induction encoded in the B6 and NOD genomes all map to the same chromosome and region thereof.
Fig. 6.
The same genetic regions condition clonal deviation to CD8αα and Treg cells. (a) BDC2.5-Tg F2 FTOCs (n = 56) were incubated with 10 ng/ml mimotope peptide for 7 days and analyzed by using flow cytometry for the percentage of CD8αα (of total CD8 SP cells), percentage of DP cells, and percentage of FoxP3+ cells (of total CD4 SP cells). (b) LOD plot for the whole-genome scan in this F2 cohort, with the percentage of CD8αα cells among CD8 SP cells, the percentage of DP cells, and the percentage of FoxP3+ among CD4 SP cells as quantitative traits. (c) Higher resolution view of the chromosome 3 region for the same scan as b.
Discussion
The reactive potential and actual function of CD8αα cells remains controversial (1, 2). Whether CD8αα IELs mount protective responses to gut antigens or are primarily immunomodulatory is an open question. They do possess strong cytotoxic activity, suggesting active effector function (28, 29), but two reports indicate that they may also have regulatory roles, at least in colitis models (30, 31). The present results appear to weigh in favor of the latter function because they show both CD8αα cells and FoxP3+ Treg cells to be rescue lineages that share an important genetic control element. On the other hand, CD8αα cells are not Treg look-alikes because they do not express FoxP3, nor do they overexpress many of the genes of the well characterized “Treg signature” (data not shown).
The origin of CD8αα T cells was long debated, but the consensus is that they are of thymic origin (1, 2). Reaggregation chimera experiments (3) showed that CD8αα cells originate from immature DP when triggered by cognate antigen presented by MHC class I molecules. Our results confirm that selection to the CD8αα lineage is largely independent of the restricting molecule and can be induced by any strong agonist signal, irrespective of MHC class restriction.
There is an interesting temporal aspect to the selection of CD8αα cells. Our previous work (19) determined that their generation in the male HY TCR Tg system occurred predominantly in the fetal/neonatal stages and waned rapidly thereafter. The same kinetic behavior was observed with the BDC2.5 system. Because key elements distinguish the HY and BDC2.5 systems (restricted by MHC class I versus MHC class II molecules and by ubiquitous versus tissue-specific antigen), it is reasonable to speculate that this temporal variation may be a general phenomenon: CD8αα cells are selected among the autoreactive T cells present in the early stages of life, and this wave of rescued cells colonizes the gut. This view is consistent with the observation that thymectomy affects the CD8αα IEL pool only when performed in the neonatal period (32). The origin of this temporal difference is fully conjectural at this point: Different modes of antigen presentation in the perinatal thymus, differential activity of costimulatory pathways, or a different responsiveness of perinatal thymocytes could all be involved. The age-dependence of CD8αα selection does not mirror that of Treg cells, however, because the latter actually increase in frequency with the age of the individual (ref. 33 and our unpublished data).
CD8αα Cells and Diabetes.
Do CD8αα cells contribute to autoimmune diabetes, and is their relative paucity a component of susceptibility in NOD mice? The very presence of CD8αα cells in the lymphocytic infiltrate of BDC2.5 mice was quite surprising and suggestive because they were thought to be exclusive to IEL populations. It is quite striking that CD8αα cells are more abundant in the aggressive infiltrate, early in the course of disease, than in the more established and stable insulitic lesion after 8 weeks of age. This observation might be taken as an indication of a pathogenic role for CD8αα cells, but a counter interpretation in which they play a dampening role in the islets is equally valid. Their relative paucity in diabetes-prone NOD mice may also be taken as an argument for a protective role, but such clues can be misleading: There is clear precedent in the paradoxical pathogenic role of NK cells, which are underrepresented in NOD mice, allowing the animals to survive their autoimmune potential, at least until breeding age (34). Whether and how CD8αα cells are players in diabetes pathogenesis certainly warrants further exploration.
What Is the Locus (Loci)?
The same genetic region appears to condition the defective handling of T cells displaying strongly self-reactive TCRs by using three different modes of tolerance induction. At the level of resolution afforded by the present genetic analysis, one cannot state that the very same gene is involved in all three defects, but this does make an attractive hypothesis. For instance, a signaling defect downstream of the TCR in NOD thymocytes might result in globally weaker signals and thus dampen the activation of apoptosis and of alternative modes of differentiation. The NOD phenotype appears mostly dominant in F1 mice, which might suggest that the NOD allele encodes an overactive inhibitor, such as an inhibitory phosphatase. These defects map broadly to the idd3–idd17 region and may thus correspond to a QTL for general susceptibility to diabetes but not to the idd3 subregion centered around the Il2 and Il21 genes. This coincidental mapping supports the notion that the selection defects do partake in the susceptibility to autoimmune diabetes in NOD mice.
Whatever the nature of the locus (or loci) turns out to be, the present results indicate that the T cell repertoire in NOD mice must have a peculiarly biased composition. Cells whose reactivity to self would destine them to deletion or to clonal deviation into harmless Treg or CD8αα phenotypes on the B6g7 background are allowed to mature and populate lymphoid compartments in the NOD mouse. It is conceivable that some adaptation or tuning takes place and that the genetic variation that affects thymocyte maturation also influences signals in peripheral T cells and thus prevents or inhibits their activation. Nevertheless, a dangerous situation is created, one that may promote the emergence of fully autoreactive cells should this damper become inactivated. One could draw here a parallel between NOD mice and the scurfy mutant. The FoxP3 deficiency in the latter prevents the sequestration of autoreactive TCRs into the Treg compartment, and these TCRs instead appear on standard T cells, where they generate uncontrolled lymphoproliferation and autoimmunity (35).
In summary, we show that the defective tolerance induction in the NOD thymus encompasses three distinct aspects of the adaptation of the T cell repertoire to self under the control of the same genetic region if not of the same gene. It will be important to further explore the impact of this shift in repertoires. The partial deficit of CD8αα cells in the NOD mouse adds another potential player in the cast of characters that mediate autoimmune diabetes.
Materials and Methods
Mice.
NOD/LtDOI (NOD), C57BL/6.H2g7 (B6g7), and BDC2.5 TCR Tg mice (20) on the NOD and B6.H2g7 backgrounds (BDC2.5/NOD and BDC2.5/Bg7, respectively) were bred in our specific-pathogen-free facility at the Joslin Diabetes Center. F2 embryos were generated by intercrossing F1 mice (BDC2.5 Tg × non-Tg) and genotyping for the BDC2.5 transgene and H2g7 haplotype (19). NOD.Idd3R450 congenic mice (Taconic Farms, Germantown, NY) were intercrossed with BDC2.5/NOD mice, genotyped at the Idd3 interval with D3Mit21. P14 TCR Tg mice on the NOD and B6 backgrounds were from The Jackson Laboratory (Bar Harbor, ME).
FTOCs.
Fetal thymus lobes were dissected from embryonic-day-15.5 embryos and cultured as described (19). When indicated, they were supplemented with a BDC2.5-specific peptide mimotope (BDCmi, peptide 1040-63) (36) or a P14-specific peptide, p33 (KAVYNFATM) (37). F2 FTOCs were carried out in the presence of 10 ng/ml BDCmi peptide. Medium and peptide were changed every other day. For most analyses of BDC2.5+ CD8αα cells, thymocytes were stained with anti-BDC2.5 clonotype (38), anti-CD4, anti-CD8α, and anti-CD8β [H35–17.2, which recognizes both the NOD (CD8β.1) and B6 (CD8β.2) allelic forms of CD8β] (BD Pharmingen, San Diego, CA).
Analysis of Lymphoid Populations.
Single-cell suspensions of lymph node, spleen, or pancreas were generated by using mechanical disruption with frosted glass slides. For IELs, the small intestine was cut into small pieces (≈5 mm) and extensively washed in cold PBS; then IEL was extracted by using shaking in 1× Hanks's balanced salt solution/10% FCS/10 mM EDTA at 37°C for 20 min (three repeats). Released IEL were purified by using Percoll gradient centrifugation (600 × g) at the interphase between 67% and 44% fractions. Vβ4+CD4−CD8α+β− or CD8α+β+ thymocytes were sorted from peptide-supplemented or control FTOCs, RNA was isolated by using TRIzol, and cDNAs were quantitated by using SYBR Green quantitative real-time PCR as described (3).
Genetic Mapping.
DNA was isolated from BDC2.5 (B6g7 × NOD) F2 embryos and was genotyped for SNP markers distinguishing B6 and NOD alleles by using fluorogenic PCR. SNP markers covered all 19 autosomes and Idd loci with an average spacing of ≈19.4 Mb (see full list of primer/probe sets and genomic locations in ref. 19). Analyses used various mapping and statistical packages (S+, Mapmaker/Exp, Mapmaker/QTL, and R/qtl) (39). Data were checked for genotyping errors by using R/qtl. Linkage peaks were identified by using simple marker regression (S+) or interval mapping (R/qtl), with normalized quantitative traits (divided by the experimental mean to control for between-experiment variability). Empirical P values for experiments were established by using permutation tests (10,000 permutations, R/qtl). Tests for dominant/additive/recessive effects and calculations of variance explained were performed by using Mapmaker/QTL and S+.
Acknowledgments
We thank E. Hyatt and K. Hattori for assistance with mice, J. LaVecchio and G. Buruzala for cytometry, and W. Besse and C. Campbell for SNP genotyping. This work was supported by Juvenile Diabetes Research Foundation Grant 4-2004-368, National Institutes of Health Grant DK60027, Young Chair funds, and the Joslin Diabetes Center's National Institute of Diabetes and Digestive and Kidney Diseases/Diabetes and Endocrinology Research Center core facilities. P.D.H., T.Y., W.J., and M.F. received postdoctoral fellowships from the Cancer Research Institute, from the Uehara Memorial Foundation and the Iacocca Foundation, from the American Diabetes Association (a mentor-based fellowship), and from the German Research Foundation (Emmy–Noether Fellowship FE 801/1-1), respectively.
Abbreviations
- B6
C57BL/6
- DP
double-positive
- FTOC
fetal thymic organ culture
- IEL
intraepithelial lymphocyte
- LOD
logarithm of odds
- QTL
quantitative trait locus
- SP
single-positive
- TCR
T cell antigen receptor
- Tg
transgenic
- Treg
regulatory T cell.
Footnotes
The authors declare no conflict of interest.
References
- 1.Guy-Grand D, Vassalli P. Curr Opin Immunol. 2002;14:255–259. doi: 10.1016/s0952-7915(02)00330-8. [DOI] [PubMed] [Google Scholar]
- 2.Cheroutre H. Immunol Rev. 2005;206:114–131. doi: 10.1111/j.0105-2896.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 3.Yamagata T, Mathis D, Benoist C. Nat Immunol. 2004;5:597–605. doi: 10.1038/ni1070. [DOI] [PubMed] [Google Scholar]
- 4.Leishman AJ, Naidenko OV, Attinger A, Koning F, Lena CJ, Xiong Y, Chang HC, Reinherz E, Kronenberg M, Cheroutre H. Science. 2001;294:1936–1939. doi: 10.1126/science.1063564. [DOI] [PubMed] [Google Scholar]
- 5.Kern PS, Teng MK, Smolyar A, Liu JH, Liu J, Hussey RE, Spoerl R, Chang HC, Reinherz EL, Wang JH. Immunity. 1998;9:519–530. doi: 10.1016/s1074-7613(00)80635-4. [DOI] [PubMed] [Google Scholar]
- 6.Pardigon N, Darche S, Kelsall B, Bennink JR, Yewdell JW. Int Immunol. 2004;16:1305–1313. doi: 10.1093/intimm/dxh133. [DOI] [PubMed] [Google Scholar]
- 7.Eberl G, Littman DR. Science. 2004;305:248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- 8.Gapin L, Cheroutre H, Kronenberg M. J Immunol. 1999;163:4100–4104. [PubMed] [Google Scholar]
- 9.Park SH, Guy-Grand D, Lemonnier FA, Wang CR, Bendelac A, Jabri B. J Exp Med. 1999;190:885–890. doi: 10.1084/jem.190.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocha B, von Boehmer H, Guy-Grand D. Proc Natl Acad Sci USA. 1992;89:5336–5340. doi: 10.1073/pnas.89.12.5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guehler SR, Bluestone JA, Barrett TA. J Exp Med. 1996;184:493–503. doi: 10.1084/jem.184.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruz D, Sydora BC, Hetzel K, Yakoub G, Kronenberg M, Cheroutre H. J Exp Med. 1998;188:255–265. doi: 10.1084/jem.188.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leishman AJ, Gapin L, Capone M, Palmer E, MacDonald HR, Kronenberg M, Cheroutre H. Immunity. 2002;16:355–364. doi: 10.1016/s1074-7613(02)00284-4. [DOI] [PubMed] [Google Scholar]
- 14.Guehler SR, Finch RJ, Bluestone JA, Barrett TA. J Immunol. 1998;160:5341–5346. [PubMed] [Google Scholar]
- 15.Guy-Grand D, Rocha B, Mintz P, Malassis-Seris M, Selz F, Malissen B, Vassalli P. J Exp Med. 1994;180:673–679. doi: 10.1084/jem.180.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papiernik M, de Moraes ML, Pontoux C, Vasseur F, Penit C. Int Immunol. 1998;10:371–378. doi: 10.1093/intimm/10.4.371. [DOI] [PubMed] [Google Scholar]
- 17.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Nat Immunol. 2001;2:283–284. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 18.Van Santen HM, Benoist C, Mathis D. J Exp Med. 2004;200:1221–1230. doi: 10.1084/jem.20041022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zucchelli S, Holler P, Yamagata T, Roy M, Benoist C, Mathis D. Immunity. 2005;22:385–396. doi: 10.1016/j.immuni.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 21.Kyewski B, Derbinski J. Nat Rev Immunol. 2004;4:688–698. doi: 10.1038/nri1436. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez A, Katz JD, Mattei MG, Kikutani H, Benoist C, Mathis D. Immunity. 1997;7:873–883. doi: 10.1016/s1074-7613(00)80405-7. [DOI] [PubMed] [Google Scholar]
- 23.Broman KW, Wu H, Sen S, Churchill GA. Bioinformatics. 2003;19:889–890. doi: 10.1093/bioinformatics/btg112. [DOI] [PubMed] [Google Scholar]
- 24.Wicker LS, Todd JA, Prins J-B, Podolin PL, Renjikian RJ, Peterson LB. J Exp Med. 1994;180:1705–1713. doi: 10.1084/jem.180.5.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyons PA, Armitage N, Argentina F, Denny P, Hill NJ, Lord CJ, Wilusz MB, Peterson LB, Wicker LS, Todd JA. Genome Res. 2000;10:446–453. doi: 10.1101/gr.10.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Apostolou I, von Boehmer H. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liston A, Gray DH, Lesage S, Fletcher AL, Wilson J, Webster KE, Scott HS, Boyd RL, Peltonen L, Goodnow CC. J Exp Med. 2004;200:1015–1026. doi: 10.1084/jem.20040581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guy-Grand D, Malassis-Seris M, Briottet C, Vassalli P. J Exp Med. 1991;173:1549–1552. doi: 10.1084/jem.173.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawamoto Y, Sasaki K, Kato Y, Kojima K, Tsuji T, Miyama A. Eur J Immunol. 1996;26:653–658. doi: 10.1002/eji.1830260322. [DOI] [PubMed] [Google Scholar]
- 30.Poussier P, Ning T, Banerjee D, Julius M. J Exp Med. 2002;195:1491–1497. doi: 10.1084/jem.20011793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saurer L, Seibold I, Rihs S, Vallan C, Dumrese T, Mueller C. J Immunol. 2004;172:4176–4183. doi: 10.4049/jimmunol.172.7.4176. [DOI] [PubMed] [Google Scholar]
- 32.Bandeira A, Itohara S, Bonneville M, Burlen-Defranoux O, Mota-Santos T, Coutinho A, Tonegawa S. Proc Natl Acad Sci USA. 1991;88:43–47. doi: 10.1073/pnas.88.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S. J Immunol. 2006;176:6586–6593. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- 34.Poirot L, Benoist C, Mathis D. Proc Natl Acad Sci USA. 2004;101:8102–8107. doi: 10.1073/pnas.0402065101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. Nat Immunol. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 36.Judkowski V, Pinilla C, Schroder K, Tucker L, Sarvetnick N, Wilson DB. J Immunol. 2001;166:908–917. doi: 10.4049/jimmunol.166.2.908. [DOI] [PubMed] [Google Scholar]
- 37.Sebzda E, Wallace VA, Mayer J, Yeung RSM, Mak TW, Ohashi PS. Science. 1994;263:1615–1618. doi: 10.1126/science.8128249. [DOI] [PubMed] [Google Scholar]
- 38.Kanagawa O, Militech A, Vaupel BA. J Immunol. 2002;168:6159–6164. doi: 10.4049/jimmunol.168.12.6159. [DOI] [PubMed] [Google Scholar]
- 39.Doerge RW, Churchill GA. Genetics. 1996;142:285–294. doi: 10.1093/genetics/142.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]