Abstract
The chimaerin family of Rac GTPase-activating proteins (GAPs) has been implicated in neural development and tumor progression, although the cellular mechanisms of their effects are poorly understood. To study their physiologic function, we used the Drosophila retina as a model system. Reduced expression of the fly chimaerin ortholog RhoGAP5a in the pupal eye led to an excess of interommatidial pigment cells, aberrant cell contacts, and an increase in activated ERK that localized specifically to the plasma membrane. Reducing RhoGAP5A levels suppressed the effects of disrupted EGF receptor signaling. Perturbation of Rac activity led to similar phenotypes, whereas coexpression of Rac and RhoGAP5A-dsRNAi resulted in the elimination of adherens junctions between interommatidial cells. Our results reveal a role for chimaerin in the regulation of ERK signaling and cell–cell adhesion and have implications for its participation in epithelial development and tumor progression.
Keywords: GTPase-activating protein, EGF
The chimaerins are a family of proteins that possess a characteristic tripartite structure: an N-terminal SH2 domain, a C1 domain that binds diacylglycerol (DAG) and tumor-promoting phorbol esters with high affinity (1, 2), and a C-terminal GAP domain that specifically inactivates Rac (3, 4). Mammalian genomes contain two chimaerin loci, each of which encodes both the full-length protein (α2- and β2-chimaerin) and at least one splice variant (α1 and β1) lacking the SH2 domain. The chimaerins, originally identified as proteins that display highly regulated expression patterns in developing brain (5), are thought to play a role in neurite outgrowth and collapse (6, 7). Work in zebrafish has highlighted the important role of chimaerins in development, identifying a critical role for chimaerin in gastrulation (8).
Recent work demonstrates a role for β2-chimaerin in cancer progression. β2-chimaerin expression is down-regulated in breast cancer and duodenal adenocarcinomas (9), and levels of β2-chimaerin decrease upon transformation of astrocytomas to malignant gliomas (10). Furthermore, overexpression of β2-chimaerin in breast cancer cell lines inhibits proliferation and metastasis in a Rac-dependent manner (9, 11). Rac itself shows increased activity in a variety of human carcinomas, including colorectal, breast, and pancreatic cancers (12, 13), although the cellular basis and function of increased Rac activity in many cancers remains unclear. Rac is active in a host of cellular processes including cell motility, adhesion, proliferation, apoptosis, and cytoskeletal organization, mediated through the actions of multiple downstream targets (14). This wide range of influence makes the spatial and temporal regulation of Rac activity critical to cellular physiology. Thus, cells employ a large number of regulators to activate (guanine nucleotide exchange factors) or inactivate (GAPs) Rac activity at distinct times and locations. Biochemical and cell culture experiments show compelling evidence that chimaerins behave as both regulators of Rac (3, 4) and targets of DAG signaling (1). However, much remains unknown about the physiologic functions of chimaerins in intact organisms.
The eye of Drosophila melanogaster has been used to dissect multiple signaling pathways and has been described as a model system for cancers (15, 16) such as medullary thyroid (17) and ovarian carcinomas (18), as well as proliferation (19), apoptosis (20, 21), and cell–cell adhesion in general (22, 23). Until late larval development, the fly eye exists as a simple, undifferentiated epithelium. By the mid-pupal stage, the eye has matured into a differentiated neuroepithelium in which all cell types are specified and present in the appropriate number. Reiterative use of a canonical EGFR/Ras/ERK MAP kinase pathway plays a central signaling role in organizing these cellular events (19).
In this study, we demonstrate that the fly ortholog of chimaerin, RhoGAP5A, is expressed in the pupal eye, and reduction of RhoGAP5A levels causes specific defects in eye development that implicate chimaerin in the regulation of cell number, cell–cell contacts, and the stability of adherens junctions. We extend previous work (24) by demonstrating that RhoGAP5A specifically modulates ERK activity and localization downstream of EGFR in the fly pupal eye. These studies provide insights into the cellular mechanism of the involvement of chimaerins and Rac in epithelial development and tumor progression.
Results
Reduction of RhoGAP5A Expression in the Eye Causes an Increase in Cell Number and Abnormal Cell Morphology.
The Drosophila genome contains 21 GAPs for the Rho family of G proteins (encompassing Rho, Rac, and Cdc42), but only a single member encodes the conserved three-domain structure characteristic of the chimaerin Rac GAPs (25). The fly ortholog of chimaerin, RhoGAP5A, is 37% identical to human β2-chimaerin, with higher degrees of conservation within each domain. Previous DNA microarray analysis indicated the presence of RhoGAP5A transcript in the pupal eye (26). Overexpression of RhoGAP5A in the eye showed no phenotype (data not shown). This was expected: the crystal structure of human β2-chimaerin suggests that the protein typically exists in an autoinhibited state, with its SH2 and GAP domains wrapped around the DAG-binding C1 domain (27).
To better explore the function of RhoGAP5A, we used RNA interference to reduce activity specifically in the eye [GMR>RhoGAP5A-dsRNAi], reducing the amount of RhoGAP5A transcript by 74% as measured by RT-PCR. Ubiquitous larval expression of the transgene (actin5c>RhoGAP5A-dsRNAi) reduced endogenous transcript levels 91% relative to controls. This knockdown was specific for RhoGAP5A: the amount of a similar RacGAP, DRacGAP, was unaffected (data not shown).
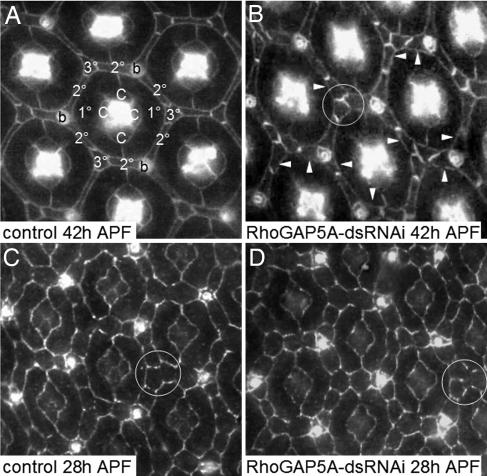
Apically, cells in the fly eye adhere to one another with Armadillo-containing adherens junctions that surround the cell perimeter. By 42 h after puparium formation (APF), cell positioning and determination of all cell types in the Drosophila eye is complete. These cells form an array of ≈750 ommatidia [consisting of primary (1°) pigment cells, cone cells, and photoreceptors], surrounded by a lattice of secondary (2°) and tertiary (3°) interommatidial pigment cells and bristles that interlock apically in a hexagonal shape around each ommatidium [Fig. 1A, (20)]. The precursors to the 2°s and 3°s are the interommatidial precursor cells (IPCs), which are the primary focus of our analysis.
Fig. 1.
Reduction of RhoGAP5A expression affects patterning of the interommatidial lattice in the pupal eye. (A and B) Pupal eyes were dissected 42 h APF and stained for Armadillo, highlighting adherens junctions. Apical focal plane is shown. (A) GMR>GFP fly eyes demonstrate wild-type ommatidia: a pair of primary pigment cells (1°) and four cone cells (C) separated and supported structurally by a framework of interommatidial pigment cells [3° for tertiary cells, which normally touch three other interommatidial cells, or 2° for secondary cells, which normally touch one 3° and either one bristle (b) or a second 3°]. (B) Expression of RhoGAP5A-dsRNAi (two copies) causes an increase in the number of 2°/3°s (see arrowheads) and inappropriate contact apically between three pigment cells at vertices of the interommatidial lattice (an example is circled). (C and D) Pupal eyes were dissected 28 h APF and stained for Armadillo. At 28 h APF, both control and GMR>RhoGAP5A-dsRNAi eyes exhibit extra interommatidial cells, because apoptotic pruning of the lattice is not yet complete, and many vertices have not yet fully matured: multiple IPCs are still competing for the 3° cell niche (examples are circled). No major phenotypic difference is observed between the two eyes at 28 h APF.
Reducing expression of RhoGAP5A throughout the retina (GMR>RhoGAP5A-dsRNAi) resulted in surplus 2°/3°s and aberrant cell–cell contacts between them [see supporting information (SI) Table 1 and Fig. 1B); no effect was observed on cone cells or photoreceptors. The phenotype was copy number-dependent, as two copies of GMR>RhoGAP5A-dsRNAi caused more severe defects including large regions of the eye that contained higher numbers of cells and aberrant contacts (see Fig. 1B) interspersed among regions that were more wild type in appearance. In addition, we often observed an increased strength of staining for adherens junctions between neighboring 2°/3°s relative to the 2°/3°–1° cell boundaries (as in Fig. 1B). The average number of 2°/3°s per ommatidium increased (from 15.1 in control to 16.5 with two copies of the dsRNAi), as did the range in cell number (control: 14–16.5 cells; dsRNAi: 14–20.5 cells; SI Table 1). In most cases of aberrant cell contacts, a 3° shifted to one side, permitting an abnormal contact between two 2°s (Fig. 1B). RNAi knockdown of DRacGAP did not phenocopy these effects (data not shown).
In general, the phenotype of GMR>RhoGAP5A-dsRNAi in the mature (42 h APF) pupal eye is reminiscent of the immature (28 h APF) pupal eye, suggesting that IPCs failed to complete their normal cell rearrangements. By 28 h APF, IPCs have normally arranged into a single row between ommatidia, but ectopic cells remain and some cells have yet to settle on their final position. For example, the selection of a single 3° to occupy the nonbristle interommatidial vertices is often incomplete as multiple cells still ‘compete' to occupy the corner niche [see Fig. 1 C and D and (23)]. At this stage, we detected no difference between control and GMR>RhoGAP5A-dsRNAi eyes, indicating that cells are especially sensitive to loss of RhoGAP5 as they complete movement into their final niches.
Consistent with its requirement in the IPC lattice, in situ analysis demonstrated that RhoGAP5A mRNA was expressed specifically in IPCs at 28 h APF. Low or no staining was observed in 1° or cone cells (data not shown).
Rac and RhoGAP5A Act Synergistically to Regulate the Development of Interommatidial Pigment Cells.
To further assess whether RhoGAP5A is a functional chimaerin family member, we examined its requirement for Rac activity; mammalian chimaerins act primarily as GAPs for Rac. In Drosophila, Rac activity is required for proper fly eye development: overexpression of wild-type or dominant-negative Rac1 leads to a rough eye phenotype in adults (28), and combining mutant loss-of-function alleles of the Drosophila Racs, Rac1, Rac2, and the more distantly related Mtl, leads to photoreceptor axon guidance defects (29). However, no effect on the interommatidial lattice has been described. Using FLP/FRT recombination (30), we induced small doubly-mutant clones (5–20 ommatidia) homozygous for Rac1J11 (strong hypomorph) plus Rac2Δ (null). In Rac1J11 Rac2Δ clones, we observed 2°/3° defects similar to those identified for GMR-RhoGAP5A-dsRNAi (Fig. 2A). For example, regions homozygous for Rac1J11 Rac2Δ often failed to resolve a single 3° at interommatidial vertices, and wild-type or heterozygous 3°s that bordered Rac1J11 Rac2Δ 2°s often extended inappropriately into niches normally reserved for the 2°s (Fig. 2A and SI Table 2).
Fig. 2.
Rac and RhoGAP5A regulate adherens junction stability. At 42 h APF, eyes stained for adherens junctions with anti-Armadillo (A–G and I) or septate junctions with antidiscs large (H). (A) Rac1J11 Rac2Δ homozygous clones were induced by FLP/FRT recombination and examined for 2°/3° defects. Cells with at least one wild-type copy of each Rac stain green for β-galactosidase, whereas cells containing no wild-type copies of Rac1 and Rac2 do not stain green. White arrowheads point to extra 2°/3°s within the Rac mutant clone. Arrows point to two examples of heterozygous 3°s that border Rac mutant 2°s and exhibit an elongated morphology. (B and C) Pupal eyes in which Rac is overexpressed by “FLP-out.” Cells marked by GFP (green) overexpress Rac and typically display a smaller apical surface relative to neighboring wild-type cells. (E) Overexpression of wild-type Rac1 throughout the entire eye leads to a phenotype of extra 2°/3°s (see filled arrowheads) and inappropriate contact apically between three 2°/3°s at vertices of the hexagonal interommatidial lattice (an example is circled). (F) Coexpression of RhoGAP5A-dsRNAi with Rac1 (same induction as in E) causes widespread dismantling of adherens junctions between 2°/3°s. (G–I) Expression of Rac1N17 (dominant-negative) in the fly eye also specifically disrupts adherens junctions between 2°/3°s and additionally affects adherens junctions between 2°/3°s and 1°s [adherens junctions visualized by α-catenin-GFP (G)]. (H) Same field of view, septate junctions stained with antidiscs large. (I) A more mild heat shock-induction of Rac1N17 expression resulted in an intermediate phenotype, causing the partial loss of adherens junctions (open arrowheads).
Similarly, ectopic expression of the dominant-negative isoform Rac1N17 (by GMR-GAL4 or hs-GAL4 induction) led to 2°/3° patterning defects that included missing and misplaced 2°/3°s (Fig. 2 G and H). Interestingly, expression of this inactive isoform also resulted in the dismantling of adherens junctions between 2°/3°s throughout the eye. Three distinct readouts for adherens junctions confirmed this loss: anti-Armadillo staining, anti-DE-cadherin staining, and localization of GFP-tagged α-catenin (data not shown). The septate junction marker Discs Large and the adhesion molecule Roughest were unaffected by Rac1N17 (Fig. 2H and M. Seppa, personal communication), indicating that reducing Rac activity specifically disrupted adherens junctions. Of note, we found that expression of Rac1N17 led to the loss of adherens junctions only at time points after 28 h APF, which persisted until the last time point observed (42 h APF, SI Fig. 6). In contrast, others have shown that the transmembrane protein Crumbs is necessary for the early establishment of these junctions, but is dispensable for their maintenance at later time points (22).
Conversely, induction of Rac1 overexpression in individual cells by heat shock-induced “FLP out” resulted in smaller apical profiles when induced in pigment cells. For example, Rac1-overexpressing 2°s exhibited reduced apical profiles as neighboring wild-type 3°s extended into their niche (Fig. 2B). We observed a similarly reduced apical profile in 1°s that overexpressed Rac1 (Fig. 2 B and C).
Our results suggest Rac and RhoGAP5A may function together in a manner similar to their mammalian counterparts. To further assess this point, we examined the ability of RhoGAP5A mutants to hyperactivate Rac activity. We mildly induced Rac1 overexpression throughout the entire eye by heat-shock induction: hs>Rac1 yielded eyes characterized by surplus 2°/3°s and aberrant cell contacts within the interommatidial lattice (Fig. 2E), a phenotype similar to that of reducing RhoGAP5A activity (see above). At this level of Rac1 induction, we only occasionally observed a reduction or loss of adherens junction levels between individual 2°/3°s. Importantly, coexpression of hs>RhoGAP5A-dsRNAi strongly enhanced the hs>Rac1 phenotypes. We observed enhanced cell–cell contact defects: >50% of the eyes examined were missing most 2°/3° adherens junctions throughout the entire eye (Fig. 2F). Junctions between 2°/3°s and 1°s were intact, reflecting the low endogenous levels (by RNA in situ) of RhoGAP5A in 1°s: remaining junctional components in 2°/3°s may have been recruited to 1° contacts, which themselves would be unaffected by RhoGAP5A RNAi. Although cell number was often difficult to ascertain because of the loss of junctional staining, cell spacing was aberrant, suggesting enhanced patterning defects characteristic of both Rac1 and RhoGAP5A.
Together, these results indicate that Rac1 and RhoGAP5A act together to regulate IPC patterning. Reduction of either led to a failure of IPCs to complete movement into a final physical niche. This may be, in part, a reflection of the defects we also observed in 2°/3° junctions, which were often visibly disrupted. Interestingly, this disruption was observed with overexpression of either a wild-type or catalytically inactive Rac1 isoform, suggesting that tight regulation in the levels of physical protein are important.
RhoGAP5A Interacts Genetically with the EGFR/MAP Kinase Pathway in the Fly Eye.
We next examined whether RhoGAP5A exerted its effects on IPC development through known pupal eye signaling pathways such as Notch, Wingless, Roughest, and dEGFR. In both adult eyes and dissected pupal eyes, no genetic interactions were observed with alleles of roughest (rst3, rstCT) or wingless [wgcx4 (31)]. Similarly, the rough eye that resulted from expression of a truncated, dominant-negative form of Notch specifically in the eye (GMR>NΔICD) was not modified by GMR>RhoGAP5A-dsRNAi, although coexpression of GMR>RhoGAP5A-dsRNAi enhanced GMR>NΔICD-mediated pupal lethality (data not shown).
In the developing pupal eye, the fly EGFR (dEGFR) conveys a survival signal to IPCs through a canonical Ras/ERK MAP kinase pathway to regulate patterning and cell death (19). To explore the potential for a functional link, we examined the ability of reduced RhoGAP5A activity (GMR>RhoGAP5A-dsRNAi) to genetically modify the phenotype of the dEGFR allele dEGFREL-B1. Adult dEGFREL-B1 heterozygous eyes are rough (Fig. 3A) because of multiple ommatidial defects, including defects in 2°/3° number and contacts. dEGFREL-B1 is a hyperactive form of dEGFR that typically acts genetically as a loss-of-function allele because of feedback inhibition of the pathway (32, 33). Expression of GMR>RhoGAP5A-dsRNAi in a dEGFREL-B1 eye suppressed the rough eye phenotype (Fig. 3D). At the cellular level, dEGFREL-B1 pupal eyes showed a variety of defects: abnormal 2°/3° number and cell–cell contacts, disrupted shape and spacing of ommatidia, and aberrant numbers of cone cells. Expression of GMR>RhoGAP5A-dsRNAi rescued these phenotypes in patches, although other areas of the eyes appeared identical to dEGFREL-B1 controls (data not shown). Thus, GMR>RhoGAP5A-dsRNAi may rescue the effects of aberrant dEGFR signaling in multiple cell types in the fly eye.
Fig. 3.
RhoGAP5A interacts with the dEGFR signaling pathway in the fly eye. All flies depicted express the GMR-GAL4 driver. (A) dEGFREL-B1 causes a rough eye phenotype, which is suppressed by expression of RhoGAP5A-dsRNAi (D). (B) hs-Argos flies have a mildly rough eye because of Argos inhibition of dEGFR signaling. (E) Coexpression of RhoGAP5A-dsRNAi rescues the rough eye phenotype of hs-Argos. (C) A gain-of-function mutation in Drosophila ERK, rlSEM), causes a rough eye, which is suppressed by expression of RhoGAP5A-dsRNAi (F).
We extended these results using flies overexpressing Argos, a secreted dEGFR pathway target that interferes with ligand binding to provide feedback inhibition of the pathway (34). Mild induction of Argos expression by incubating hs-Argos flies at 25°C led to a mild rough eye phenotype (Fig. 3B). Coexpression of GMR>RhoGAP5A-dsRNAi completely rescued this phenotype, resulting in adult flies with eyes that were wild-type in appearance (Fig. 3E). GMR>RhoGAP5A-dsRNAi also suppressed (Fig. 3F) the rough eye phenotype of rlSEM (Fig. 3C), an activated allele of Rolled, a Drosophila ERK MAP kinase ortholog. Thus, reduction of RhoGAP5A expression suppresses the rough eye caused by the manipulation of three distinct components of the dEGFR/ERK signaling pathway. The rescue of both gain- and loss-of-function mutants reflects the complex nature of the pathway: hyperactivation of dEGFR signaling can cause feedback inhibition at the level of receptor, resulting in a loss-of-function phenotype. In either case, RhoGAP5A-dsRNAi could rescue by activating the pathway downstream of receptor. Also, gain or loss of signaling could both potentially be rescued by the relocalization of an active pathway component (see below).
RhoGAP5A could regulate dEGFR pathway activity at any of several points in the pathway. To understand whether it functions with dEGFR at the level of the downstream target ERK, we examined the activation state of ERKA/Rolled in the pupal eye using an antibody specific to the activated, phosphorylated isoform of ERK (dpERK). Densitometric analysis of Western blots for ERK levels in pupal eye lysates showed that the ratio of dpERK to total ERK was 5.6-fold higher (±0.8 SD, n = 2) in eyes expressing GMR>RhoGAP5A-dsRNAi (two copies) than in control eyes (Fig. 4N), indicating that activation of ERK is under the control of RhoGAP5A. Analysis of dpERK levels in situ at 28 h APF supported these findings: elevated levels were observed in GMR-RhoGAP5A-dsRNAi fly eyes compared with controls.
Fig. 4.
Reduction in RhoGAP5A expression causes an increase in activated ERK and up-regulation of Argos. (A–H) Images are from GMR-GAL4/+ control eyes or GMR>RhoGAP5A-dsRNAi. (A–D) Stained for dpERK. GMR>RhoGAP5A-dsRNAi eyes display elevated dpERK staining localized to the plasma membrane at 28 h APF. (E–H): stained for Argos. Up-regulation of Argos is seen at 41 h APF in GMR>RhoGAP5A-dsRNAi eyes. (I–M) Overexpression of the ERK phosphatase Mkp3 (heat shock at 24 h APF) results in missing cells, aberrant adherens junctions, and a failure of 2°/3°s to make appropriate contacts. (I–K) Eyes were dissected at 42 h APF. Filled arrowheads indicate a properly formed 2°/3° cell contact in the control (I) or a 2°/3° cell contact exhibiting weakened adherens junctions upon Mkp3 expression (J). Open arrowheads (K) indicate examples of areas that remain vacant of 2°/3°s because of the failure of 2°/3°s to extend appropriately and establish cell contacts. Points of contact between 2°/3°s often exhibit a “scalloped” shape, resulting from intercalation of a 1° cell (see arrow). Compare also to control in Fig. 1A. (L and M) Expression of Mkp3 (heat shock at 22 and 24 h APF) leads to reduced adherens junction staining by 28 h APF. (N) Western blot for dpERK (Upper) or total ERK (Lower). The blot was first probed for dpERK and then stripped and reprobed for total ERK. Left lane is control expression of GMR>GFP. Right lane is GMR>RhoGAP5A-dsRNAi.
Another readout of ERKA activity is expression of Argos, which is a target of the downstream ERKA target Pnt (35). Surprisingly, expression of Argos, normally an ERKA immediate early response gene, did not increase until 38 h APF with strongest levels at 41 h APF (Fig. 4 E–H); the importance of this late Argos expression is unclear. This suggests that the increased dpERK levels detected at earlier stages is either inactive or has different targets. ERK target specificity is regulated in part by targeting of the protein. We therefore examined the subcellular localization of dpERK more closely.
We examined localization of dpERK at 24, 26, 28, 30, and 41 h APF in both control and GMR>RhoGAP5A-dsRNAi eyes. During early time points, dpERK stain was characterized by a diffuse cytoplasmic background stain similar to controls, as others have recently reported (Fig. 4A and ref. 36). However, at 28 h APF in GMR>RhoGAP5A-dsRNAi eyes dpERK staining was observed in a surprising location: tightly localized to apical membrane boundaries of IPCs, 1° pigment cells, and cone cells (Fig. 4 A and B). Although very faint membrane localization of dpERK was infrequently observed in control flies, particularly between cone cells (as shown in Fig. 4A), bright membrane labeling (as in Fig. 4B) was observed only in GMR>RhoGAP5A-dsRNAi eyes (43%; n = 14). Maximal junction staining was observed at the 28 h time point and infrequently at 30 h APF.
Reduction in levels of RhoGAP5A, then, leads to increased ERKA signaling that corresponds with increased cell number and aberrant contacts in the pupal eye. However, (i) our detection of early dpERK localization primarily in the cytoplasm or at cell junctions and (ii) the failure to observe Argos expression at this early stage raises the question of whether dpERK is active at early stages of IPC patterning. For example, previous work (37) has suggested a mechanism of “cytoplasmic hold,” in which dpERKA is held out of the nucleus and is proposed to be inactive. To further explore this issue, we used the ERKA-specific phosphatase Mkp3 (38) to inhibit ERKA activity. To examine the role of Mkp3 in the interommatidial lattice, we induced expression by heat shock at 24 h APF. As expected, given the role of EGF signaling as a survival factor, this induction resulted in a reduction in the number of 2°/3°s (Fig. 4K). Furthermore, those 2°/3°s that survived to 42 h APF failed to make appropriate cell contacts (Fig. 4 J and K). In typical ommatidia, if a 2°/3° is missing, the remaining cells will stretch to contact one another, thereby filling the niche of the missing cell. In contrast, in pupal eyes in which Mkp3 was overexpressed, even in areas with a nearly normal complement of cells, 2°/3°s often failed to make contact with one another, instead merely retaining contact with 1° pigment cells despite close proximity to other 2°/3°s (see open arrowheads, Fig. 4K). Of note, 2°/3°s in Mkp3-overexpressing eyes appeared in many cases to exhibit lower levels of adherens junctions at points of contact with other 2°/3°s relative to the level of staining between 2°/3°s and 1° pigment cells (see Fig. 4 J, M). This is the opposite of what was observed for GMR>RhoGAP5A-dsRNAi eyes (see above) and supports the hypothesis that ERKA is involved in modulating the strength of adherens junctions in the pupal eye. Furthermore, we frequently observed “scalloping” of 2°/3°s in Mkp3-overexpressing eyes, reflecting an intrusion of 1° pigment cells into the apical space normally occupied by the 2°/3°s (see arrow, Fig. 4K). This may reflect the relative strength of adhesion between the two cell types: if the 1°s have stronger adherens junctions, their apical surfaces could be maximized to expand the domains of those junctions at the expense of cells, such as the 2°/3°s, which appear to have weakened adherens junctions. This scalloping is observed at earlier time points (27–36 h APF) in wild-type eyes as cell–cell adhesion is manipulated to permit the final patterning steps of the interommatidial lattice (23) but is seen less frequently at 42 h APF.
Discussion
To maintain a healthy epithelium, cells must establish intercellular structural contacts such as adherens junctions and propagate intracellular signals that permit selective proliferation and survival. The fly ortholog of the chimaerins, RhoGAP5A, plays a key role in both of these processes in the developing fly eye. We found that reduction of RhoGAP5A levels resulted in an increase of activated ERK levels at the plasma membrane, coupled to increased cell number and aberrant cell–cell contacts. Perturbation of Rac activity also disrupted cell–cell contacts, and this phenotype was exacerbated by reduction of RhoGAP5A levels. Based on these data, we propose that RhoGAP5A inactivates Rac to regulate its effects on cell contacts and adherens junctions and also regulates the activation of ERK, either directly or through modulation of Rac activity (Fig. 5).
Fig. 5.
A model for chimaerin signaling in epithelia. In healthy epithelial tissue, tightly regulated intracellular signaling ensures the formation of stable contacts between neighboring cells, regulated by Rac activity, and appropriate proliferation and survival, regulated by signaling through ERK MAP kinase and other pathways. In many cell types, Rac is required for full activation of ERK. Activated ERK phosphorylates distinct targets depending on its cellular location. Active Rac and chimaerin reside at the membrane. In the fly retina, both ERK and Rac are down-regulated by fly chimaerin (RhoGAP5A) at the plasma membrane, leading to effects on cell–cell contacts.
Rac is a well known participant in both EGFR signaling and the maintenance of adherens junctions. Growth factors are classical activators of ERK through the canonical Ras signaling pathway and also activate Rac. However, a connection between Rac and the activation of ERK has only recently been documented (12, 39). Our data suggest that chimaerin plays a key role in regulating Rac's interaction with ERK downstream of EGFR. Rac has also been shown to be important both upstream and downstream of adherens junction formation, and too much or too little Rac activity disrupts adherens junctions in cultured mammalian epithelial cells (40–42), a finding we have now verified in the fly eye. A key question remaining is whether the dismantling of adherens junctions is part of Rac's normal role in development or whether this occurs only when Rac is hyperactivated (as upon overexpression coupled with a decrease in RhoGAP5A levels) or mutated into a dominant-negative form. If the latter is the case, then our observation of Rac and RhoGAP5A-directed dismantling of adherens junctions may be more relevant as a model for tumor progression directed by activated Rac than as a model for typical epithelial development.
These findings are especially provocative in light of recent studies demonstrating that increased Rac activity (12) or decreased β2-chimaerin levels (9, 10) are linked to various cancers. In fact, overexpression of β2-chimaerin has recently been shown to suppress Rac-mediated activation of ERK in a breast cancer cell line (13). Our extension of these findings from cultured cell lines and tumors to a native epithelium supports the hypothesis that Rac and β2-chimaerin play important roles in the transformation of a healthy epithelium into cancerous tissue.
The tumor-promoting phorbol esters, which mimic DAG binding to C1 domains, are known to be potent activators of ERK signaling, a phenomenon that our data suggest is partly dependent on chimaerin activity. Our findings do not address the specific contribution of the C1 domain of RhoGAP5A to its actions in the fly eye, but others have shown that β2-chimaerin translocates from the cytosol to the plasma membrane upon activation by phorbol esters (1). In vivo, this translocation is mediated by phospholipase C-generated DAG in response to the activation of EGFR and limits the duration of Rac activity at the membrane (24). Thus, although phorbol esters and other C1 domain ligands have been investigated as therapeutic agents to activate PKC, they may also be useful in the therapeutic manipulation of chimaerins and Rac as their role in tumor progression continues to be elucidated.
The effects of RhoGAP5A in the fly eye include aspects of both ERK activation and localization and are consistent with the hypothesis that RhoGAP5A acts as a plasma membrane-localized suppressor of EGFR signaling through ERK. The localization of ERK is critical for its function, and dpERK has been shown to phosphorylate physiologically relevant targets in the cytoplasm and the nucleus, resulting in distinct cellular outcomes (ref. 43 and see Fig. 5). In the fly eye, the timed “hold” of dpERK in the cytoplasm appears to be critical for normal development (37). The function of dpERK at the plasma membrane, in contrast, is not well established. Recent data suggest that signaling of dpERK at the plasma membrane mediates negative selection in T cells (44). ERK can be activated at different membranes by binding specific scaffolds such as KSR, MT1, or β-arrestin, and disruption of ERK activation at specific membranes by the loss of scaffold activity or inhibition of endocytosis (45) can result in altered signaling amplitude or duration (46). Our data do not directly address whether RhoGAP5A regulates the inactivation of ERK at the plasma membrane or the transport (or release) of dpERK away from the plasma membrane once activated. Either mechanism could explain the buildup of dpERK at the membrane observed upon loss of RhoGAP5A expression. Plasma membrane localization could permit efficient coupling of ERK signaling to cellular events that occur at the membrane such as the maintenance or controlled turnover of adherens junctions. Interestingly, sustained activation of ERK can affect the expression and localization of adherens junctions in mammalian cells (47).
This article describes the Drosophila pupal eye as a model system for the study of chimaerin physiology. We have shown that the fly member of the chimaerin family (RhoGAP5A) and Rac coregulate cell number and cell–cell contacts in the neuroepithelium of the Drosophila eye. Further experiments will be necessary to explore the conservation of this cellular role for chimaerins in mammalian cells.
Materials and Methods
Fly Stocks and Conditions.
Flies were raised at 25°C on standard molasses/cornmeal food. Two transgenic fly strains expressing RhoGAP5A double-stranded RNA (dsRNAi) were used: UAS-RhoGAP5A-dsRNAi 1.3 (on chromosome II) and UAS-RhoGAP5A-dsRNAi 5.1 (on chromosome III); each displayed the same phenotype and were a gift from Liqun Luo (Stanford University, Stanford, CA) (48). GMR drives expression in all eye cells from late larval through midpupal development. When the GMR-GAL4 driver was used to express a UAS-dependent construct, “GMR>” is used to represent this induction, whereas “hs>” refers to hs-GAL4-driven expression of a transgene under control of the UAS promoter. When expression of two transgenes was driven by GMR-GAL4, a single copy of GMR-GAL4 was used. We obtained Rac1J11 Rac2Δ FRT80B/TM6UZ flies from Barry Dickson (Research Institute of Molecular Pathology, Vienna, Austria); wgCX4 from Amy Bejsovec (Duke University, Durham, NC); hs-Argos from H. Okano (Osaka University, Osaka, Japan); UAS-Mkp3 from Jose de Celis (Universidad Autonoma de Madrid, Madrid, Spain); UAS-α-catenin-GFP from the Drosophila Genetic Resource Center; GMR-GAL4 (on chromosome II), UAS-Rac1, UAS-Rac1L89, UAS-Rac1N17, rstCT, dEGFREllipse(B1), hs-GAL4(a), UAS-RasV12, and hsFLP22; arm-lacZ FRT80B from the Bloomington Stock Center; other strains used were Cagan laboratory stocks.
Clonal Analysis of Rac Mutants.
Rac1J11 Rac2Δ FRT 80B/TM6UZ males were crossed to hsFLP22; arm-lacZ FRT80 females, and flippase expression was induced by heat shock at 37°C for 1 h of 49- to 72-h larvae. Nontubby pupae were dissected and stained for β-gal and Armadillo. Cells carrying the nonmutant Rac chromosome were labeled by staining for β-gal, whereas cells homozygous for the Rac mutants were unlabeled. Mutant ommatidia were those containing at least one primary pigment cell homozygous for the Rac mutant chromosome; controls were those in which all cells in the ommatidium were positive for β-gal staining, without bordering any homozygous mutant ommatidia.
Expression of Rac1 in individual cells was induced by a 1-h heat shock at 37°C of white prepupae of the following genotype: yw hsFLP/+; Act5c>y>GAL4 UAS-GFP/UAS-Rac1. Heat shock induction of flippase leads to excision of a transcription termination cassette (<y<) in some cells, resulting in transcription of GAL4 and induction of transcription from UAS promoters (49). These cells are marked by GFP.
Immunostaining of Pupal Eyes.
Pupal eyes were dissected as described (36). The following primary antibodies were used: mouse α−Argos (1:10), mouse α−Armadillo (3:10), rat α−DE-cadherin (1:20), and mouse α−Discs Large (1:10), all from the Developmental Studies Hybridoma Bank; rabbit α−β-galactosidase (1:2,500), from Cappel; and rabbit α−dpERK and α−ERK (1:500), from Cell Signaling Technology (Beverly, MA). Secondary antibodies used were Alexa Fluor 488-conjugated α−rabbit and α−rat, and Alexa Fluor 568-conjugated α−mouse (1:1,000; Molecular Probes, Eugene, OR).
Western Blot Analysis.
Eyes were dissected and transferred immediately to 3× Laemmli sample buffer with 7.5% 2-mercaptoethanol and 0.15 M NaVO3. Samples were boiled for 10 min and centrifuged for 10 min, and volumes equivalent to 20 eyes were electrophoresed on a 12% polyacrylamide gel, transferred to PVDF membrane, stained with rabbit α−dpERK, and detected by using α−rabbit-HRP (1:10,000) and the Lumi-LightPLUS Western Blotting Substrate (Roche, Indianapolis, IN). Membrane was then stripped [2% SDS/50 mM Tris (pH 6.8)/0.1 M 2-mercaptoethanol for 30 min] and reprobed with rabbit α−ERK as above. Bands were quantified by densitometry (QuantityOne).
Quantification of 2°/3° Number.
Interommatidial cells were counted as described (20). Bristles were included to standardize counts because, in some portions of the eye, they are replaced by 3° cells.
Supplementary Material
Acknowledgments
We thank Dennis Poehling and Scot Portman for injecting fly embryos; Liqun Luo, Barry Dickson, and Jose de Celis for fly strains, Tanya Wolff and Aaron DiAntonio for advice; the Cagan and Baranski labs for reagents and discussion, and the Developmental Studies Hybridoma Bank and the Bloomington Stock Center for reagents. This work was supported by National Institutes of Health Grant GM63720-01 (to T.J.B.), the Culpeper Award (to T.J.B.), the Monsanto–Searle Biomedical Program Grant (to T.J.B.), and the PhRMA Foundation (S.P.B.).
Abbreviations
- GAP
GTPase-activating protein
- DAG
diacylglycerol
- IPCs
interommatidial precursor cells
- 1°
primary pigment cell
- 2°
secondary pigment cell
- 3°
tertiary pigment cell
- APF
after puparium formation
- EGFR
EGF receptor.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701686104/DC1.
References
- 1.Caloca MJ, Garcia-Bermejo ML, Blumberg PM, Lewin NE, Kremmer E, Mischak H, Wang S, Nacro K, Bienfait B, Marquez VE, Kazanietz MG. Proc Natl Acad Sci USA. 1999;96:11854–11859. doi: 10.1073/pnas.96.21.11854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Areces LB., Kazanietz MG, Blumberg PM. J Biol Chem. 1994;269:19553–19558. [PubMed] [Google Scholar]
- 3.Hall C, Sin WC, Teo M, Michael GJ, Smith P, Dong JM, Lim HH, Manser E, Spurr NK, Jones TA, et al. Mol Cell Biol. 1993;13:4986–4998. doi: 10.1128/mcb.13.8.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leung T, How BE, Manser E, Lim L. J Biol Chem. 1994;269:12888–12892. [PubMed] [Google Scholar]
- 5.Hall C, Monfries C, Smith P, Lim HH, Kozma R, Ahmed S, Vanniasingham V, Leung T, Lim L. J Mol Biol. 1990;211:11–16. doi: 10.1016/0022-2836(90)90006-8. [DOI] [PubMed] [Google Scholar]
- 6.Brown M, Jacobs T, Eickholt B, Ferrari G, Teo M, Monfries C, Qi RZ, Leung T, Lim L, Hall C. J Neurosci. 2004;24:8994–9004. doi: 10.1523/JNEUROSCI.3184-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van de Ven TJ, VanDongen HM, VanDongen AM. J Neurosci. 2005;25:9488–9496. doi: 10.1523/JNEUROSCI.2450-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leskow FC, Holloway BA, Wang H, Mullins MC, Kazanietz MG. Proc Natl Acad Sci USA. 2006;103:5373–5378. doi: 10.1073/pnas.0508585103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang C, Liu Y, Coluccio Leskow F, Weaver VM, Kazanietz MG. J Biol Chem. 2005;280:24363–24370. doi: 10.1074/jbc.M411629200. [DOI] [PubMed] [Google Scholar]
- 10.Yuan S, Miller DW, Barnett GH, Hahn JF, Williams BR. Cancer Res. 1995;55:3456–3461. [PubMed] [Google Scholar]
- 11.Menna PL, Skilton G, Leskow FC, Alonso DF, Gomez DE, Kazanietz MG. Cancer Res. 2003;63:2284–2291. [PubMed] [Google Scholar]
- 12.Sahai E, Marshall CJ. Nat Rev Cancer. 2002;2:133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 13.Yang C, Liu Y, Lemmon MA, Kazanietz MG. Mol Cell Biol. 2006;26:831–842. doi: 10.1128/MCB.26.3.831-842.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etienne-Manneville S, Hall A. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 15.Brumby AM, Richardson HE. Nat Rev Cancer. 2005;5:626–639. doi: 10.1038/nrc1671. [DOI] [PubMed] [Google Scholar]
- 16.Vidal M, Larson DE, Cagan RL. Dev Cell. 2006;10:33–44. doi: 10.1016/j.devcel.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Vidal M, Wells S, Ryan A, Cagan R. Cancer Res. 2005;65:3538–3541. doi: 10.1158/0008-5472.CAN-04-4561. [DOI] [PubMed] [Google Scholar]
- 18.Moberg KH, Bell DW, Wahrer DC, Haber DA, Hariharan IK. Nature. 2001;413:311–316. doi: 10.1038/35095068. [DOI] [PubMed] [Google Scholar]
- 19.Baker NE. Semin Cell Dev Biol. 2001;12:499–507. doi: 10.1006/scdb.2001.0274. [DOI] [PubMed] [Google Scholar]
- 20.Wolff T, Ready DF. Development (Cambridge, UK) 1991;113:825–839. doi: 10.1242/dev.113.3.825. [DOI] [PubMed] [Google Scholar]
- 21.Cagan RL, Ready DF. Dev Biol. 1989;136:346–362. doi: 10.1016/0012-1606(89)90261-3. [DOI] [PubMed] [Google Scholar]
- 22.Grzeschik NA, Knust E. Development (Cambridge, UK) 2005;132:2035–2045. doi: 10.1242/dev.01800. [DOI] [PubMed] [Google Scholar]
- 23.Bao S, Cagan R. Dev Cell. 2005;8:925–935. doi: 10.1016/j.devcel.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Yang C, Leskow FC, Sun J, Canagarajah B, Hurley JH, Kazanietz MG. EMBO J. 2006;25:2062–2074. doi: 10.1038/sj.emboj.7601098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernards A. Biochim Biophys Acta. 2003;1603:47–82. doi: 10.1016/s0304-419x(02)00082-3. [DOI] [PubMed] [Google Scholar]
- 26.Jassim OW, Fink JL, Cagan RL. EMBO J. 2003;22:5622–5632. doi: 10.1093/emboj/cdg543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Canagarajah B, Leskow FC, Ho JY, Mischak H, Saidi LF, Kazanietz MG, Hurley JH. Cell. 2004;119:407–418. doi: 10.1016/j.cell.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Nolan KM, Barrett K, Lu Y, Hu KQ, Vincent S, Settleman J. Genes Dev. 1998;12:3337–3342. doi: 10.1101/gad.12.21.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hakeda-Suzuki S, Ng J, Tzu J, Dietzl G, Sun Y, Harms M, Nardine T, Luo L, Dickson BJ. Nature. 2002;416:438–442. doi: 10.1038/416438a. [DOI] [PubMed] [Google Scholar]
- 30.Xu T, Rubin GM. Development (Cambridge, UK) 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 31.Cordero J, Jassim O, Bao S, Cagan R. Mech Dev. 2004;121:1523–1530. doi: 10.1016/j.mod.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Lesokhin AM, Yu SY, Katz J, Baker NE. Dev Biol. 1999;205:129–144. doi: 10.1006/dbio.1998.9121. [DOI] [PubMed] [Google Scholar]
- 33.Spencer SA, Powell PA, Miller DT, Cagan RL. Development (Cambridge, UK) 1998;125:4777–4790. doi: 10.1242/dev.125.23.4777. [DOI] [PubMed] [Google Scholar]
- 34.Klein DE, Nappi VM, Reeves GT, Shvartsman SY, Lemmon MA. Nature. 2004;430:1040–1044. doi: 10.1038/nature02840. [DOI] [PubMed] [Google Scholar]
- 35.Shilo BZ. Exp Cell Res. 2003;284:140–149. doi: 10.1016/s0014-4827(02)00094-0. [DOI] [PubMed] [Google Scholar]
- 36.Monserrate JP, Baker Brachmann C. Cell Death Differ. 2006;14:209–217. doi: 10.1038/sj.cdd.4401947. [DOI] [PubMed] [Google Scholar]
- 37.Kumar JP, Hsiung F, Powers MA, Moses K. Development (Cambridge, UK) 2003;130:3703–3714. doi: 10.1242/dev.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomez AR, Lopez-Varea A, Molnar C, de la Calle-Mustienes E, Ruiz-Gomez M, Gomez-Skarmeta JL, de Celis JF. Dev Dyn. 2005;232:695–708. doi: 10.1002/dvdy.20227. [DOI] [PubMed] [Google Scholar]
- 39.Eblen ST, Slack JK, Weber MJ, Catling AD. Mol Cell Biol. 2002;22:6023–6033. doi: 10.1128/MCB.22.17.6023-6033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Betson M, Lozano E, Zhang J, Braga VM. J Biol Chem. 2002;277:36962–36969. doi: 10.1074/jbc.M207358200. [DOI] [PubMed] [Google Scholar]
- 41.Braga VM, Betson M, Li X, Lamarche-Vane N. Mol Biol Cell. 2000;11:3703–3721. doi: 10.1091/mbc.11.11.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kovacs EM, Ali RG, McCormack AJ, Yap AS. J Biol Chem. 2002;277:6708–6718. doi: 10.1074/jbc.M109640200. [DOI] [PubMed] [Google Scholar]
- 43.Marenda DR, Vrailas AD, Rodrigues AB, Cook S, Powers MA, Lorenzen JA, Perkins LA, Moses K. Development (Cambridge, UK) 2006;133:43–51. doi: 10.1242/dev.02168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daniels MA, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, Werlen G, Hollander GA, Gascoigne NR, Palmer E. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 45.Lua BL, Low BC. J Cell Sci. 2005;118:2707–2721. doi: 10.1242/jcs.02383. [DOI] [PubMed] [Google Scholar]
- 46.Dard N, Peter M. BioEssays. 2006;28:146–156. doi: 10.1002/bies.20351. [DOI] [PubMed] [Google Scholar]
- 47.Wu J, Sheibani N. J Cell Biochem. 2003;90:121–137. doi: 10.1002/jcb.10600. [DOI] [PubMed] [Google Scholar]
- 48.Billuart P, Winter CG, Maresh A, Zhao X, Luo L. Cell. 2001;107:195–207. doi: 10.1016/s0092-8674(01)00522-0. [DOI] [PubMed] [Google Scholar]
- 49.Ito K, Awano W, Suzuki K, Hiromi Y, Yamamoto D. Development (Cambridge, UK) 1997;124:761–771. doi: 10.1242/dev.124.4.761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.