Abstract
Cardiac hypertrophy is a common response to injury and hemodynamic stress and an important harbinger of heart failure and death. Herein, we identify the Kruppel-like factor 15 (KLF15) as an inhibitor of cardiac hypertrophy. Myocardial expression of KLF15 is reduced in rodent models of hypertrophy and in biopsy samples from patients with pressure-overload induced by chronic valvular aortic stenosis. Overexpression of KLF15 in neonatal rat ventricular cardiomyocytes inhibits cell size, protein synthesis and hypertrophic gene expression. KLF15-null mice are viable but, in response to pressure overload, develop an eccentric form of cardiac hypertrophy characterized by increased heart weight, exaggerated expression of hypertrophic genes, left ventricular cavity dilatation with increased myocyte size, and reduced left ventricular systolic function. Mechanistically, a combination of promoter analyses and gel-shift studies suggest that KLF15 can inhibit GATA4 and myocyte enhancer factor 2 function. These studies identify KLF15 as part of a heretofore unrecognized pathway regulating the cardiac response to hemodynamic stress.
Keywords: heart, transcription
Cardiac hypertrophy is a common adaptive response of the heart to injury and hemodynamic stress (1, 2). Hypertrophic remodeling can be concentric (characterized addition of sarcomeres in parallel and lateral growth of individual myocytes) or eccentric (characterized by addition of sarcomeres in series and longitudinal cell growth) (3). At the cellular level, hypertrophy is characterized by increased cell size/protein content and reactivation of fetal genes (e.g., ANF, BNP) (2, 4). Although initially compensatory, hypertrophy can eventually lead to decompensation characterized by heart failure, arrhythmias, and death (1, 2, 5). Although a number of therapies are now available, cardiac hypertrophy and its attendant consequences remain a source of considerable morbidity and mortality (3, 6). Thus, identification of novel molecular mechanisms underlying the development of cardiac hypertrophy is of considerable scientific and therapeutic interest (3, 4, 6).
Over the past two decades, there has been significant progress in our understanding of the molecular mechanisms that mediate cardiac hypertrophy. These studies have established that the hypertrophic response occurs through the activation of multiple cytosolic signaling pathways and nuclear factors (1, 2, 7). With respect to the latter, factors such as myocyte enhancer factor-2 (MEF2), GATA4, and nuclear factor of activated T cells (NFATs) have been implicated as key mediators of the hypertrophic transcriptional program (8–15). Prohypertrophic stimuli activate a series of intracellular signal transduction pathways that result in the induction of MEF2 and GATA4 DNA-binding activity and nuclear accumulation of NFATs. In vitro studies have demonstrated that overexpression of either MEF2 factors or GATA4 induces cardiomyocyte hypertrophy (10, 16). Cardiac-specific transgenic overexpression of either MEF2 (16, 17) or constitutively active calcineurin-A (18) cause cardiomyopathies characterized by increased cardiac mass, chamber dilation, and left-ventricular systolic dysfunction. High levels of GATA4 overexpression in the heart also result in a severe cardiomyopathy and premature death, and modest overexpression results in cardiac hypertrophy (10). Multiple lines of evidence indicate that these transcriptional pathways cooperate in the development of cardiac dysfunction (16). Despite the increasing body of knowledge describing the biology of transcriptional activators of cardiac hypertrophy, there is much less information regarding transcriptional repressors of hypertrophy (6).
Kruppel-like factors (KLFs) are a subclass of the zinc-finger family of transcriptional regulators (19, 20) homologous to the Drosophila gap gene Kruppel (21), a critical regulator of fly body patterning via its function as a transcriptional repressor (22). Previous studies from our group and others indicate that KLFs are also key regulators of gene expression, growth, and differentiation in a broad range of mammalian cell types (19, 20). A role for this family in cardiac fibroblast biology was highlighted by elegant studies involving the Kruppel-like factor 5 (KLF5), a factor expressed in cardiac fibroblasts (and not in cardiomyocytes) whose deficiency blunted cardiac hypertrophy and fibrosis in response to angiotensin II (23). Recently, we and others have identified a novel member of this family termed KLF15 that is expressed in cardiomyocytes (24, 25). Here, we provide evidence that KLF15 plays a critical role as a negative regulator of cardiac hypertrophy.
Results
KLF15 Expression in the Heart.
We identified KLF15 as being expressed in the rodent heart (24). As shown in Fig. 1A, KLF15 is expressed in the adult rat heart and several other tissues. Notably, KLF4 and KLF5 are expressed at very low levels (26, 27). Using isolated neonatal rat ventricular myocytes (NRVMs), we observed that KLF15 message was low under high-serum conditions and induced after serum deprivation, whereas an antiparallel expression pattern was observed for ANF/BNP (Fig. 1B). We also found that KLF15 was expressed at low levels in isolated neonatal rat ventricular fibroblasts in the presence of high serum and induced with serum starvation [supporting information (SI) Fig. 6C].
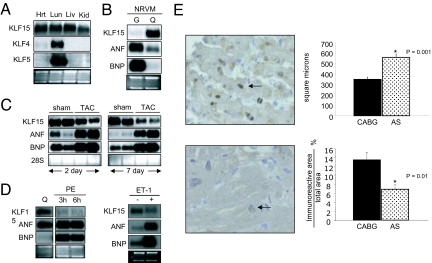
Fig. 1.
Regulation of KLF15 expression in the heart. (A) KLF15 mRNA expression in the heart. Lung (lu), liver (liv), kidney (Kid), and heart (Hrt) total RNA was probed with indicated cDNAs. (B) KLF15 is induced with serum starvation. NRVMs were cultured under growth (G; 10% FBS) or quiescent conditions (Q; 0% FBS and 1% insulin, transferrin and selenium). (C) KLF15 expression is reduced after AAC. Northern analysis of total ventricular RNA from 4-week-old SD rats 2 days or 7 days after TAC vs. sham-operation (Zivic, Pittsburgh, PA) was performed with indicated probes. (D) KLF15 expression is reduced in vitro by PE and ET-1. NRVM cultured in serum-free medium (Q) were treated with PE for 3 or 6 h or ET-1 for 24 h. Total RNA was isolated, and Northern blot analysis was performed by using indicated probes. (E) KLF15 expression is reduced in patients with AS. IHC was performed as described in Materials and Methods. Dark brown staining (arrow) indicates nuclear KLF15 expression.
In situ hybridization analyses revealed minimal cardiac expression during embryonic development (data not shown). Therefore, we next assessed whether KLF15 expression was induced postnatally (1, 28, 29) and found that KLF15 mRNA is barely detectable in the 3-day postnatal rat heart but induced to near adult levels by 30 days (SI Fig. 6A) in an antiparallel expression pattern to that of ANF and BNP. In addition, in situ hybridization revealed strong KLF15 mRNA expression in cardiomyocytes of adult rat hearts (SI Fig. 6B).
It is known that hypertrophic stimulation can reduce expression of certain postnatal genes with reinduction of fetal genes (2, 4, 28, 30, 31). To determine the effect of cardiac hypertrophy on KLF15 expression in the heart, transaortic constriction (TAC) studies were performed in adult rats. KLF15 expression is slightly reduced by 2 days and more significantly by 7 days after TAC, whereas ANF/BNP are induced at these same time points (Fig. 1C). Furthermore, treatment of NRVM with prohypertrophic stimuli such as phenylephrine (PE) and endothelin-1 also reduce KLF15 expression while inducing ANF/BNP expression (Fig. 1D).
To explore the significance of KLF15 in human disease, we assessed KLF15 protein expression in the myocardium of patients with left ventricular hypertrophy (LVH) induced by chronic valvular aortic stenosis (AS; n = 8) or control subjects undergoing coronary artery bypass graft (CABG) surgery but without LVH (control; n = 6) (32). KLF15 protein signal is detectable in the nuclei of myocytes from control CABG patients and is reduced in AS patients (Fig. 1E). Normalized KLF15 immunoreactivity was reduced ≈50% in the specimens obtained from patients with chronic AS vs. control CABG (Fig. 1E). Taken together, these data suggest that KLF15 is expressed in both rodent and human cardiomyocytes and that its expression is reduced by multiple prohypertrophic stimuli.
Effect of KLF15 Overexpression on Cardiomyocyte Gene Expression and Morphology.
The regulation of KLF15 expression by hypertrophic stimuli suggested that it may play a regulatory role in this process. Adenoviral overexpression of KLF15 (but not KLF2) in NRVM strongly reduced ANF and BNP message (Fig. 2A and SI Fig. 7). Consistent with this effect, KLF15 also strongly inhibited basal and PE-induced ANF and BNP promoter activity in NRVM (Fig. 2B). As shown in Fig. 2C, KLF15 strongly inhibited NRVM protein synthesis under both basal and PE-stimulated conditions (assessed by [3H]leucine incorporation). NRVM overexpressing KLF15 exhibited a marked reduction in cell size under both basal and PE-stimulated conditions (assessed by flow cytometry and α-actinin immunostaining) (Fig. 2D) (33). Taken together, these data show that KLF15 overexpression in cardiomyocytes can inhibit hallmark features of hypertrophy in vitro.
Fig. 2.
KLF15 inhibits cardiomyocyte hypertrophy in vitro. (A) KLF15 inhibits hypertrophic gene expression. NRVM were cultured for 24 h, infected with empty (Ad-GFP) or KLF15 adenovirus (Ad-GFP-KLF15) and total RNA was isolated for Northern blot analysis using indicated probes. (B) KLF15 inhibits basal and PE-induced ANF/BNP promoter activity. NRVMs overexpressing EV or KLF15 were transfected with −638 ANF-luc or −116 BNP-luc promoters. Luciferase activity (normalized to β-gal activity) was expressed as fold induction compared with empty vector (EV) (n = 9–12 per group). (C) KLF15 inhibits protein synthesis in NRVM as measured by leucine incorporation. Cells were treated with PE (50 μM) (+) or (−) [3H]leucine (2.5 μCi/ml) for 48 h (n = 9 per group). (D) KLF15 reduces myocyte size. NRVMs overexpressing EV or KLF15 were serum-starved for 48 h (+) or (−) PE (50 μM) and analyzed by using FACS in triplicate. The geometric mean of forward scatter (FSC) data were used as a correlate of cell size (Left). Representative micrographs of NRVM immunostained with anti-α-actinin antibodies (Right).
Targeting of KLF15 by Homologous Recombination in Mice.
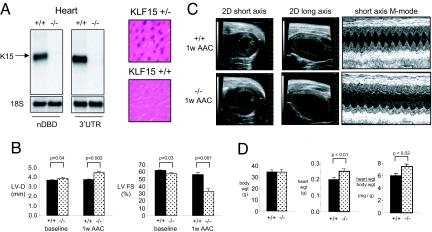
To assess the role of KLF15 in vivo, the KLF15 gene was targeted in mice by homologous recombination using the targeting strategy shown in SI Fig. 8A. Generation of targeted C57BL/6 ES cell clones (SI Fig. 8B) and germline transmission of the targeted construct (SI Fig. 8C) was verified. KLF15(−/−) mice were viable, fertile, and born in expected Mendelian ratios. Successful targeting was verified by Northern blot analysis using two distinct probes: an N-terminal probe homologous to the non-DNA binding domain of KLF15 and a probe homologous to the 3′ UTR. Both probes revealed the appropriate size message in wild-type heart tissue, although no transcript of any size was detected in knockout heart tissue (Fig. 3A Left). Consistent with this observation, myocardial β-galactosidase activity was detectable in the cardiomyocytes of KLF15(+/−) mice but not wild-type mice (Fig. 3A Right). KLF15(−/−) animals were backcrossed six generations onto a C57BL/6 background (≈97% pure) and used for further studies.
Fig. 3.
Effect of KLF15 deficiency on cardiac gene expression and pressure-overload hypertrophy. (A) Northern analysis (Left) of wild type and KLF15-null hearts using a cDNA probe coresponding to the non-DNA binding domain (nDBD) or the 3′ UTR of KLF15. β-galactosidase staining of ventricular sections from KLF15 +/+ and +/− mice indicates expression of endogenous KLF15 in cardiomyocytes (Right). (B) KLF15 −/− mice exhibit increased LV dimension and reduced LV systolic function at baseline and with pressure-overload. KLF15 +/+ and −/− mice were subject to AAC and LV diastolic dimension and fractional shortening assessed at 1 week after surgery. A marked reduction in LV function of KLF15 −/− mice is noted after AAC. (C) Representative 2D and M-mode echocardiograms from KLF15 +/+ and −/− animals that were used for the measurements in B and SI Table 1. (D) KLF15 −/− mice hearts (atria and ventricles) after AAC (1 week) are heavier.
Effect of KLF15 Deficiency on the Heart's Response to Pressure Overload.
Baseline transthoracic echocardiographic (TTE; n = 12–16 animals per group) studies in 12- to 16-week-old KLF15(+/+) vs. (−/−) mice revealed that KLF15(−/−) hearts had increased cavity size and reduced LV fractional shortening (Fig. 3B and SI Table 1). At baseline, there was a trend toward increased wall thickness in the KLF15(−/−) mice that did not reach statistical significance (SI Table 1). Animals were then subjected to ascending aortic constriction (AAC). In an initial study, we noticed that KLF15(−/−) mice were unable to tolerate AAC induced by ligation around a 27-guage needle. As such, we performed AAC in subsequent studies with milder constriction using a 25-guage needle. All sham-operated animals survived surgery and no significant differences in echocardiographic parameters or cardiac mass were noted between the two groups (SI Fig. 8E). All (+/+) mice survived AAC and, as expected, exhibited minimal change in LV cavity dimension and only modest reduction in LV systolic function (Fig. 3B and SI Table 1). In contrast, 13/21 (−/−) mice survived AAC with the majority of deaths occurring within 48 h postoperatively (SI Fig. 8D). In contrast to the (+/+) hearts, (−/−) hearts exhibited marked LV cavity dilation and reduction in systolic function in response to AAC (Fig. 3B and SI Table 1). Furthermore, by comparison to (+/+) hearts, KLF15(−/−) hearts did not augment wall thickness in response to AAC (SI Table 1). Sample echocardiographic images reflecting these observations are shown in Fig. 3C. Heart and body weight measurements taken one week after AAC confirmed increased cardiac mass in (−/−) mice (Fig. 3D).
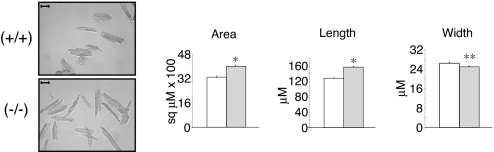
To understand the effect of KLF15 deficiency on cardiomyocytes, morphometric studies were undertaken on isolated myocytes. At baseline, no significant difference was observed between (+/+) and (−/−) myocyte area (data not shown). However, after AAC myocytes from the (−/−) animals had larger areas [4,000 ± 84 versus 3,292 ± 60 μm2, P < 10−10] and were longer [158 ± 2 versus 128 ± 2 μm, P < 10−20] than (+/+) myocytes (Fig. 4). There was a small but significant difference in cell width between the two groups [(−/−) = 25 ± 0.4 versus (+/+) = 26.4 ± 0.4 μm, P < 0.007]. Normal distribution of morphometric parameters across the entire isolated myocyte population are shown in histograms (SI Fig. 9A). Finally, to determine whether apoptosis contributed to the observed phenotype after AAC, TUNEL staining (SI Fig. 9B), immunohistochemistry for cleaved caspase-3 (data not shown) and apoptotic gene expression (SI Fig. 9B) were assessed and showed no significant differences between the genotypes (34).
Fig. 4.
Effect of KLF15 on cardiomyocyte cell size. KLF15-deficient myocytes exhibit increased cell area. Morphometric analysis was performed on isolated cardiomyocytes from KLF15 +/+ and −/− mice subjected to 1 week of AAC (representative photomicrograph on left; scale bar, 50 μM). For all measurements, at least 300 cells were analyzed from three animals per group (Right) (*, P < 0.001; **, P < 0.007).
KLF15 Inhibits Prohypertrophic Transcriptional Pathways.
To determine whether the marked cardiac dysfunction observed in KLF15(−/−) mice following AAC was associated with effects on hypertrophic gene expression, Northern analyses were undertaken. As shown in Fig. 5A, (−/−) hearts demonstrated enhanced induction of ANF/BNP mRNA in response to AAC. It is well established that the expression of these two classic markers of hypertrophy are regulated by pro-hypertrophic factors such as MEF2 and GATA4. This is thought to occur via direct DNA-binding which is enhanced in the setting of pressure overload states. As shown in Fig. 5B, the expression of GATA4 and MEF2 are similar in (+/+) and (−/−) heart protein extracts. However, gene reporter experiments reveal that KLF15 potently inhibits MEF2A and GATA4-mediated transcriptional activation of the ANF and BNP promoters, respectively (Fig. 5C). To further explore the basis for this repressive effect, nuclear extracts of NRVMs infected with either control (EV) or KLF15-adenovirus were subjected to gel-shift assays using consensus MEF and GATA4 binding sites. In EV infected cells, a strong singular DNA-protein complex containing MEF2 (Fig. 5D, upper gel shift) and GATA4 (Fig. 5D, lower gel shift) was observed and verified by competition and supershift studies. In contrast, MEF2 and GATA4 DNA-binding was markedly attenuated in nuclear extracts from KLF15 overexpressing myocytes. Conversely, by comparison to (+/+) hearts, we observed increased MEF2 and GATA4 binding in KLF15 (−/−) hearts after AAC (Fig. 5E). A reproduction of the GATA4 gelshift from Fig. 5E Lower exposed for a longer period is shown to highlight the supershifted GATA4 complex (SI Fig. 10). Taken together, these data suggest that KLF15 deficiency leads to eccentric cardiac hypertrophy and LV systolic function in response to pressure overload, as well as enhanced activation of well characterized prohypertrophic transcriptional pathways.
Fig. 5.
KLF15 inhibits GATA4 and MEF2 function. (A) Hearts from KLF15−/− mice exhibit enhanced hypertrophic gene expression. Northern blot analysis was performed for ANF/BNP in KLF15 +/+ and −/− mice after sham-operation and AAC. (B) KLF15 deficiency does not affect GATA4 and MEF2 protein expression. Total protein isolated from ventricular tissue of KLF15 +/+ and −/− mice was used in Western blot analysis. (C) KLF15 inhibits basal and MEF2-inducible activity on the ANF promoter (Upper) and also inhibits basal and GATA4-inducible activity of the BNP promoter (Lower). H9c2 cells were transfected with -638-ANF-luc or -112-BNP-luc reporter constructs, and with indicated combinations of expression plasmids for KLF15, MEF2A, GATA4, and pCDNA3.1 (n = 9 per group; P values < 0.05 were considered significant and are indicated in the figure). (D) KLF15 attenuates the ability of MEF2 and GATA4 to bind target DNA in NRVM. Nuclear extracts from NRVM overexpressing EV or KLF15 were used in binding with 32P-labeled DNA probes derived from the Atr promoter (top EMSA for MEF2) or BNP promoter (bottom EMSA for GATA4). Cold competition was performed by using 100× molar excess of unlabeled probe. Supershift studies were performed by preincubating reactions with α-MEF2 antibody (upper gel shift) or α-GATA4 antibody (lower gel shift). (E) KLF15 −/− hearts exhibit enhanced MEF2 and GATA4 DNA binding. Nuclear extracts were harvested from KLF15 +/+ and −/− mice after sham surgery vs. AAC. EMSA for MEF2 and GATA4 were performed by using the same probes as used in D.
Discussion
Despite significant progress in our understanding of the molecular mechanisms that promote cardiac hypertrophy, this disease process and its attendant consequences remain a leading cause of morbidity and mortality (6). This study identifies KLF15 as a novel inhibitor of the heart's response to pressure overload-induced hypertrophy. This conclusion is derived from several lines of evidence. First, in rodent models, KLF15 expression is reduced in response to pressure overload as well as prohypertrophic agonists (Fig. 1). These rodent data are strongly supported by the observation that the KLF15 protein level is reduced in the myocardium of human subjects with chronic aortic stenosis. Furthermore, overexpression of KLF15 potently inhibits PE-mediated increases in protein synthesis, cell size, and hypertrophic gene expression (Fig. 2). These in vitro overexpression data are complemented by studies in KLF15-deficient mice. In response to pressure-overload, KLF15 null animals exhibit increased heart weight and LV size, reduced LV systolic function, exaggerated expression of fetal genes, and increased myocyte size, all cardinal features of pathologic hypertrophic remodeling. Moreover, the increased cavity size and heart mass, minimal augmentation of wall thickness, and longitudinal growth of individual myocytes seen in the KLF15(−/−) mice is a typical manifestation of eccentric cardiac hypertrophy as observed in other mouse models (35).
Mechanistically, our studies provide evidence that KLF15 can affect hypertrophic signaling, at least in part, through inhibition of the MEF2 and GATA4 transcriptional pathways (10, 36). Members of the MEF2 family are well known to critically regulate heart development and recent studies strongly support a role for this pathway in the adult heart's response to stress. For example, transgenic overexpression of MEF2A or MEF2C leads to a dose-dependent dilated cardiomyopathy that is dramatically worsened following pressure overload (16, 17). Consistent with this role, overexpression of a dominant-negative MEF2 normalized ventricular dimensions and contractility in calcineurin transgenic mice (16). We note that the KLF15(−/−) mice are exquisitely sensitive to pressure-overload as they cannot survive “high afterload” AAC. Significant similarities exist between the phenotype of the KLF15-null mice and that of enhanced MEF activity in the heart. Although KLF15 mice show mild ventricular dilatation and reduced LV function at baseline, this is dramatically enhanced by mild afterload augmentation. Furthermore, at the cellular level, both overexpression of MEF2 factors and KLF15 deficiency lead to elongated myocytes (17). Interestingly, it is noteworthy that, although KLF15 deficiency does not affect MEF2 expression, it does lead to enhanced MEF2-DNA binding. This finding is consistent with the observation that MEF2-DNA (but not expression) is a primary downstream mechanism by which these factors carry out their prohypertrophic effects. Thus, on the basis of exaggerated MEF2 transcriptional activity it is not surprising that KLF15-null mice are exquisitely sensitive to even mild afterload augmentation.
Our data also indicate that KLF15 inhibits a second major transcriptional pathway: GATA4. This is an interesting observation because the importance of GATA and KLF family interactions has been previously appreciated in the context of erythropoiesis and β-globin gene synthesis (37, 38). In the context of cardiomyocyte biology, GATA4 is a known to increase cell size, protein synthetic capacity, and prohypertrophic gene expression (e.g., induction of ANF and BNP) (10). In addition, transgenic overexpression of GATA4 at high levels results in a severe cardiomyopathy and early death (10). Even modest overexpression of GATA4 results in a cardiomyopathy characterized by increased heart weight and hypertrophic gene expression (10). Similar to the observations related to MEF2, our studies show that overexpression of KLF15 inhibits GATA4 transcriptional activity and DNA-binding, whereas KLF15 deficiency results in enhanced GATA4 binding. Given that MEF2 and GATA4 factors can cooperate and serve as integrators for multiple hypertrophic signaling pathways, it is not surprising that heightened activity of these two factors leads to a marked decompensation in KLF15 null mice in response to stress. Indeed, we have observed a correlation between increased binding/activity of these prohypertrophic factors and the severity of the hypertrophic phenotype in KLF15 null mice. It is possible that, although the absence of KLF15 at baseline is permissive for binding of prohypertrophic factors to their target enhancer sequences, an additional stressful stimulus is required to achieve a level of factor binding/activity that will lead to a marked hypertrophic response. This may also explain why KLF15 null mice do not tolerate excessive afterload augmentation. In this setting, it can be anticipated that MEF2/GATA4 DNA-binding and net transcriptional activity may be dramatically enhanced, resulting in severe cardiac dilatation and reduction of contractility. Finally, the induction of KLF15 in the heart after birth constitutes an unusual expression pattern. Interestingly, this precise postnatal period is also characterized by a reduction in MEF2 (39) and GATA4 (J. D. Molkentin, University of Cincinnati, Cincinnati, OH, personal communication) DNA-binding activities. In light of our observations, it is interesting to speculate that KLF15 may serve as a “brake” to reduce unwanted MEF/GATA4 activity and target gene expression (e.g., ANF/BNP) during postnatal cardiomyocyte maturation.
There have been recent reports which describe the cardiac phenotype of mice with various levels of GATA-4 deficiency in vivo (40, 41). These studies find that GATA-4 deficiency results in abnormalities of cardiac morphology and function with a significantly increased rate of cardiomyocyte apoptosis. The phenotypes described in these two reports are intriguing in light of our current studies of KLF-deficiency and an earlier report describing the phenotype of the α-MHC/GATA-4 transgenic mouse (10). These seemingly contradictory phenotypes suggest that, although excessive GATA-4 activity may be deleterious, a precisely regulated level of GATA-4 activity is clearly important for myocyte homeostasis throughout the life of the organism. Improperly timed loss of GATA-4 function as well as excessive postnatal GATA-4 activity (as seen in our KLF15-null mice and in GATA4-transgenic mice) are both maladaptive. Thus, these studies collectively underscore the importance of timing and relative levels of expression of potent transcription factors during cardiac development, postnatal maturation and adulthood. Indeed, further studies using conditional gene targeting or inducible transgenesis will be necessary to dissect the precise roles of these critical transcription factors in the heart in vivo.
Although GATA-4 and MEF2 are central mediators of hypertrophic remodeling in the heart, the effects of KLF15 are certainly not limited to modulation of these two transcriptional pathways. KLF15 function may involve the regulation of other important transcriptional pathways and physiologic processes in the myocardium. We have previously reported that KLF15 regulates GLUT4 expression in adipose (24) and others have reported that KLF15 regulates mitochondrial acetyl-CoA synthase expression in skeletal muscle (42) and PEPCK expression in hepatocytes (43). In our initial evaluation we did not observe any significant difference in GLUT4 mRNA between KLF15 (+/+) vs. (−/−) hearts (data not shown). However, we cannot exclude the possibility that KLF15 deficiency may affect myocardial metabolism and energetics through yet unidentified pathways that may contribute to the observed phenotype.
Our observations, along with the recent studies from the Nagai laboratory (23), provide support for the KLFs as important regulators of cardiac biology. KLF5 is primarily expressed in cardiac fibroblasts (and not in cardiomyocytes), is induced by proliferative stimuli, and is an essential regulator of cardiac remodeling under stress. The current study provides the most cogent evidence to date implicating this family of factors in cardiomyocyte biology. Furthermore, our observations in rodents coupled with its altered expression in human disease states identify KLF15 as a potential target for therapeutic manipulation
Materials and Methods
Primary Cardiomyocyte Cultures.
Preparation of primary cultures of NRVMs and neonatal rat ventricular fibroblasts has been described (44). Quiescence was induced in NRVM by culturing for 24–48 h in serum-free DMEM supplemented with insulin, transferring, and selenium (1% ITS), after which pharmacologic stimulation was performed with 50 μM phenylephrine or 100 nM endothelin-1.
Adenoviral Infection and Transient Transfection Studies.
NRVM were infected 24 h after plating at 50 MOI for a period of 48 h (>90% infection efficiency). For transient transfections, NRVM or H9c2 cells were transfected by using FuGENE 6 (Roche Molecular Biochemicals) following manufacturer's protocol using luciferase-based reporters and assayed on a luminometer (see SI Text for further details).
Protein Synthesis Measurements.
Protein synthetic rate in NRVM was determined by [3H]leucine incorporation as described (45). NRVM in 24-well plates (600,000 cells per well) were infected with adenovirus in leucine-free DMEM. After 48 h, incubation cells were treated with fresh medium containing 2.5 μCi/ml [3H]leucine and incubated for an additional 24 h. For the stimulation, PE (50 μM) was added to either [3H]leucine- or leucine-free medium.
Electrophoretic Mobility Shift Assay (EMSA).
Nuclear extracts from NRVM were obtained as described (46). Nuclear extracts from mouse hearts were extracted with NE-PER extraction reagent (Pierce) according to the manufacturer's instructions. EMSAs were performed as described in SI Text.
KLF15 Immunohistochemistry in Human Subjects.
Biopsies of patients with pressure-overload and left ventricular (LV) hypertrophy due to severe aortic stenosis (n = 8) and control CABG patients (n = 6) without LV hypertrophy were collected and characterized as described (32). A primary antibody against KLF15 (generated by Zymed) was used for immunostaining. Myocyte cross-sectional area was determined, and the relative surface area of KLF15-immunoreactivity was expressed as percentage immunoreactive area over total area, using a Zeiss Axioplan2 microscope, a 3CCD video camera (DXC-93OP, Sony), and KS300 software as described (32).
KLF15 in Situ Hybridization.
See SI Text for details.
Immunocytochemistry and FACS Analysis.
For immunocytochemical analysis, NRVM were plated on laminin-coated six-well plates overnight. After 48 h of PE stimulation, the cells were washed twice with PBS, fixed, blocked, and incubated with monoclonal antibody against sarcomeric α-actinin (Sigma) at a dilution of 1:800 and anti-mouse tetramethylrhodamine β-isothiocyanate-conjugated secondary antibody (Pierce), as described (10). FACS analysis to assess cardiomyocyte hypertrophy was described (33) (see SI Text for details).
Aortic Banding and Transthoracic Echocardiography in Rodents.
In mice, AAC was performed in anesthetized (pentobarbital 15 mg/kg, IP) and ventilated animals (age 12–16 weeks) as described (47). After anterolateral thoracotomy, aortic constriction was induced by ligating the ascending aorta around a 25-guage needle using 7-0 silk suture. All mice were maintained in the same environment with regular lab chow and water ad libitum and killed 1 week after AAC. TTE was performed in mice as described using an Acuson Sequoia C-256 echocardiograph machine and a 15 MHz probe (50). Pressure-overload hypertrophy in adult male Sprague–Dawley rats was induced by transverse-aortic constriction (TAC) (performed by Zivic, Pittsburgh, PA). Rat ventricles were harvested 3 days and 30 days after TAC.
Targeting of KLF15 in Mice.
See SI Text for details.
Statistical Analysis.
Data were expressed as mean ± SEM. For in vitro data, differences between experimental groups were evaluated for statistical significance by using the Student's t test for unpaired data. For mouse echocardiographic measurements, differences between the KLF15 (+/+) and (−/−) hearts were compared by two-tailed Student's t test. Survival data were assessed by Kaplan–Meier analysis. P values <0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Veronica Herias for help with in situ hybridizations. This work was supported by National Institutes of Health Grants HL-72952 (to M.K.J.), HL-75427 (to M.K.J.), HL-76754 (to M.K.J.), P01 HL48743 (to M.K.J.), F32 HL077052 (to S.F.), DK064950 (to S.G.), and F32 HL78183 (to A.K.), American Diabetes Association Grant 1-02-JF-40 (to M.K.J.), American Heart Association Grant 0250030N (to M.K.J.), and Postdoctoral Fellowship 0425789T (to Z.L.).
Abbreviations
- MEF
myocyte enhancer factor
- KLF
Kruppel-like factor
- NRVM
neonatal rat ventricular myocyte
- PE
phenylephrine
- AS
aortic stenosis
- CABG
coronary artery bypass graft
- AAC
ascending aortic constriction.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701981104/DC1.
References
- 1.Olson EN, Schneider MD. Genes Dev. 2003;17:1937–1956. doi: 10.1101/gad.1110103. [DOI] [PubMed] [Google Scholar]
- 2.Dorn GW, Jr, Hahn HS. Ann NY Acad Sci. 2004;1015:225–237. doi: 10.1196/annals.1302.019. [DOI] [PubMed] [Google Scholar]
- 3.Frey N, Katus HA, Olson EN, Hill JA. Circulation. 2004;109:1580–1589. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- 4.MacLellan WR, Schneider MD. Annu Rev Physiol. 2000;62:289–319. doi: 10.1146/annurev.physiol.62.1.289. [DOI] [PubMed] [Google Scholar]
- 5.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 6.Hardt SE, Sadoshima J. Cardiovasc Res. 2004;63:500–509. doi: 10.1016/j.cardiores.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Akazawa H, Komuro I. Circ Res. 2003;92:1079–1088. doi: 10.1161/01.RES.0000072977.86706.23. [DOI] [PubMed] [Google Scholar]
- 8.Molkentin JD. J Biol Chem. 2000;275:38949–38952. doi: 10.1074/jbc.R000029200. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa K, Lee SJ, Jobe SM, Markham BE, Kitsis RN. Circulation. 1997;96:3943–3953. doi: 10.1161/01.cir.96.11.3943. [DOI] [PubMed] [Google Scholar]
- 10.Liang Q, De Windt LJ, Witt SA, Kimball TR, Markham BE, Molkentin JD. J Biol Chem. 2001;276:30245–30253. doi: 10.1074/jbc.M102174200. [DOI] [PubMed] [Google Scholar]
- 11.Pikkarainen S, Tokola H, Kerkela R, Ruskoaho H. Cardiovasc Res. 2004;63:196–207. doi: 10.1016/j.cardiores.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 12.Black BL, Olson EN. Annu Rev Cell Dev Biol. 1998;14:167–196. doi: 10.1146/annurev.cellbio.14.1.167. [DOI] [PubMed] [Google Scholar]
- 13.McKinsey TA, Zhang CL, Olson EN. Trends Biochem Sci. 2002;27:40–47. doi: 10.1016/s0968-0004(01)02031-x. [DOI] [PubMed] [Google Scholar]
- 14.Molkentin JD. Cardiovasc Res. 2004;63:467–475. doi: 10.1016/j.cardiores.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Wilkins BJ, Molkentin JD. Biochem Biophys Res Commun. 2004;322:1178–1191. doi: 10.1016/j.bbrc.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 16.van Oort RJ, van Rooij E, Bourajjaj M, Schimmel J, Jansen MA, van der Nagel R, Doevendans PA, Schneider MD, van Echteld CJ, De Windt LJ. Circulation. 2006;114:298–308. doi: 10.1161/CIRCULATIONAHA.105.608968. [DOI] [PubMed] [Google Scholar]
- 17.Xu J, Gong NL, Bodi I, Aronow BJ, Backx PH, Molkentin JD. J Biol Chem. 2006;281:9152–9162. doi: 10.1074/jbc.M510217200. [DOI] [PubMed] [Google Scholar]
- 18.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bieker JJ. J Biol Chem. 2001;276:34355–34358. doi: 10.1074/jbc.R100043200. [DOI] [PubMed] [Google Scholar]
- 20.Feinberg MW, Lin Z, Fisch S, Jain MK. Trends Cardiovasc Med. 2004;14:241–246. doi: 10.1016/j.tcm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Stanojevic D, Hoey T, Levine M. Nature. 1989;341:331–335. doi: 10.1038/341331a0. [DOI] [PubMed] [Google Scholar]
- 22.Zuo P, Stanojevic D, Colgan J, Han K, Levine M, Manley JL. Genes Dev. 1991;5:254–264. doi: 10.1101/gad.5.2.254. [DOI] [PubMed] [Google Scholar]
- 23.Shindo T, Manabe I, Fukushima Y, Tobe K, Aizawa K, Miyamoto S, Kawai-Kowase K, Moriyama N, Imai Y, Kawakami H, et al. Nat Med. 2002;8:856–863. doi: 10.1038/nm738. [DOI] [PubMed] [Google Scholar]
- 24.Gray S, Feinberg MW, Hull S, Kuo CT, Watanabe M, Sen-Banerjee S, DePina A, Haspel R, Jain MK. J Biol Chem. 2002;277:34322–34328. doi: 10.1074/jbc.M201304200. [DOI] [PubMed] [Google Scholar]
- 25.Uchida S, Tanaka Y, Ito H, Saitoh-Ohara F, Inazawa J, Yokoyama KK, Sasaki S, Marumo F. Mol Cell Biol. 2000;20:7319–7331. doi: 10.1128/mcb.20.19.7319-7331.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shields JM, Christy RJ, Yang VW. J Biol Chem. 1996;271:20009–20017. doi: 10.1074/jbc.271.33.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawai-Kowase K, Kurabayashi M, Hoshino Y, Ohyama Y, Nagai R. Circ Res. 1999;85:787–795. doi: 10.1161/01.res.85.9.787. [DOI] [PubMed] [Google Scholar]
- 28.Chien KR, Zhu H, Knowlton KU, Miller-Hance W, van-Bilsen M, O'Brien TX, Evans SM. Annu Rev Physiol. 1993;55:77–95. doi: 10.1146/annurev.ph.55.030193.000453. [DOI] [PubMed] [Google Scholar]
- 29.Sadoshima J, Izumo S. Annu Rev Physiol. 1997;59:551–571. doi: 10.1146/annurev.physiol.59.1.551. [DOI] [PubMed] [Google Scholar]
- 30.Parker TG, Schneider MD. Annu Rev Physiol. 1991;53:179–200. doi: 10.1146/annurev.ph.53.030191.001143. [DOI] [PubMed] [Google Scholar]
- 31.Knowlton KU, Rockman HA, Itani M, Vovan A, Seidman CE, Chien KR. J Clin Invest. 1995;96:1311–1318. doi: 10.1172/JCI118166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heymans S, Schroen B, Vermeersch P, Milting H, Gao F, Kassner A, Gillijns H, Herijgers P, Flameng W, Carmeliet P, et al. Circulation. 2005;112:1136–1144. doi: 10.1161/CIRCULATIONAHA.104.516963. [DOI] [PubMed] [Google Scholar]
- 33.Petersen CA, Burleigh BA. Infect Immun. 2003;71:4441–4447. doi: 10.1128/IAI.71.8.4441-4447.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chandrashekhar Y. Curr Heart Fail Rep. 2005;2:18–22. doi: 10.1007/s11897-005-0003-5. [DOI] [PubMed] [Google Scholar]
- 35.Peng X, Kraus MS, Wei H, Shen TL, Pariaut R, Alcaraz A, Ji G, Cheng L, Yang Q, Kotlikoff MI, et al. J Clin Invest. 2006;116:217–227. doi: 10.1172/JCI24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molkentin JD, Lin Q, Duncan SA, Olson EN. Genes Dev. 1997;11:1061–1072. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- 37.Merika M, Orkin SH. Mol Cell Biol. 1995;15:2437–2447. doi: 10.1128/mcb.15.5.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gregory RC, Taxman DJ, Seshasayee D, Kensinger MH, Bieker JJ, Wojchowski DM. Blood. 1996;87:1793–1801. [PubMed] [Google Scholar]
- 39.Molkentin JD, Markham BE. J Biol Chem. 1993;268:19512–19520. [PubMed] [Google Scholar]
- 40.Oka T, Maillet M, Watt AJ, Schwartz RJ, Aronow BJ, Duncan SA, Molkentin JD. Circ Res. 2006;98:837–845. doi: 10.1161/01.RES.0000215985.18538.c4. [DOI] [PubMed] [Google Scholar]
- 41.Bisping E, Ikeda S, Kong SW, Tarnavski O, Bodyak N, McMullen JR, Rajagopal S, Son JK, Ma Q, Springer Z, et al. Proc Natl Acad Sci USA. 2006;103:14471–14476. doi: 10.1073/pnas.0602543103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto J, Ikeda Y, Iguchi H, Fujino T, Tanaka T, Asaba H, Iwasaki S, Ioka RX, Kaneko IW, Magoori K, et al. J Biol Chem. 2004;279:16954–16962. doi: 10.1074/jbc.M312079200. [DOI] [PubMed] [Google Scholar]
- 43.Teshigawara K, Ogawa W, Mori T, Matsuki Y, Watanabe E, Hiramatsu R, Inoue H, Miyake K, Sakaue H, Kasuga M. Biochem Biophys Res Commun. 2005;327:920–926. doi: 10.1016/j.bbrc.2004.12.096. [DOI] [PubMed] [Google Scholar]
- 44.Sadoshima J, Jahn L, Takahashi T, Kulik TJ, Izumo S. J Biol Chem. 1992;267:10551–10560. [PubMed] [Google Scholar]
- 45.Choukroun G, Hajjar R, Kyriakis JM, Bonventre JV, Rosenzweig A, Force T. J Clin Invest. 1998;102:1311–1320. doi: 10.1172/JCI3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schluter KD, Wollert KC. Cardiovasc Res. 2004;63:367–372. doi: 10.1016/j.cardiores.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 47.Liao R, Jain M, Cui L, D'Agostino J, Aiello F, Luptak I, Ngoy S, Mortensen RM, Tian R. Circulation. 2002;106:2125–2131. doi: 10.1161/01.cir.0000034049.61181.f3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.