Abstract
The transcriptional coactivator p300 binds to and mediates the transcriptional functions of the tetrameric tumor suppressor p53. Both proteins consist of independently folded domains linked by intrinsically disordered sequences. A well studied short sequence of the p53 transactivation domain, p53(15–29), binds weakly to four folded domains of p300 [Taz1/cysteine–histidine-rich region 1 (CH1), Kix, Taz2/CH3, IBiD], with dissociation constants (KD) in the 100 μM region. However, we found that a longer N-terminal transactivation domain construct p53(1–57) bound tightly to each p300 domain. Taz2/CH3 had the greatest affinity (KD = 27 nM) and competes with the N-terminal domain of Mdm2 for the p53 N terminus. p300 thus can protect the N terminus of p53 against the binding of other proteins. Mutations of p53 that abrogate transactivation (L22Q/W23S, W53Q/F54S) greatly weakened binding to each p300 domain, linking phenotypic defects to weakened coactivator binding. We propose a complex between tetrameric p53 and p300 in which four domains of p300 wrap around the four transactivation domains of p53.
Keywords: AD1, cAMP response element binding protein-binding protein, IBiD homology domain, Mdm4, SRC-1
The p53 tumor suppressor protein is a transcription factor that is implicated in inducing apoptosis, cell cycle arrest, and DNA repair on activation by cellular stress (1). In unstressed cells, p53 is present at low levels, with a half-life between 5 and 40 min (2). p53 becomes phosphorylated on stress and its level increase. It induces transcription of cell cycle arrest- and apoptosis-inducing genes such as p21 and bax (1), as well as mdm2 (3), which participates in p53-negative regulation in concert with the homolog Mdm4 (for review, see ref. 4). Both Mdm2 and Mdm4 (or MdmX) bind to the N-terminal transactivation domain of p53, and both are required for p53 regulation and degradation. p53 is active as a homotetramer, with each of its subunits having a modular structure (Fig. 1A). The individual N-terminal core, tetramerization, and C-terminal domains have very similar NMR spectra as in the intact full-length protein (5).
Fig. 1.
Domain organization of p300 and p53. (A) Domain structure of human p53. TAD1/2 corresponds to the transactivation subdomains 1 and 2. Its precise boundaries are subject to debate. (B) Domain structure of human p300. Taz1, PHD, and ZZ-Taz2 are also described as CH1, CH2, and CH3, respectively. The approximate domain boundaries were taken from the p300 Pfam database entry (identifier Q094720) and sources from text.
The transcriptional functions of p53, as well as those of numerous unrelated transcription factors, are mediated via interaction with the transcriptional coactivators p300 and cAMP response element binding protein-binding protein (CBP). They are large, highly homologous multidomain proteins that possess histone acetyltransferase activity (Fig. 1B) (for review, see ref. 6). Numerous domains of p300 are reported to interact with p53. The cysteine–histidine-rich region 3 (CH3) domain, which is targeted by the adenoviral transforming protein E1a, interacts with an undefined segment of N-terminal transactivation domain of p53 (7, 8). E1a expression reduces p53-mediated p21 and bax induction through directly blocking the p53 N terminus/CH3 interaction (9, 10), underlining the importance of this domain in p53-dependent transcription. The CH1/Taz1 domain, which is homologous to Taz2, is proposed to interact with both the p53 N terminus (11) and the p53 core domain (12). There are further interactions of the p53 N terminus with Kix and IBiD (13, 14), as well as Ser20-phosphorylated p53 peptides with the IBiD homology domain (IHD) (15, 16). However, the role and importance of these domains in p53 activation are not well understood.
The p53 transactivation domain (TAD) (Fig. 1A) has been subdivided into two ill defined subdomains, TAD1 contained within residues 1–40, and TAD2 contained within 41–61 (17, 18). Their functions have been probed by deletions or mutations L22Q/W23S (QS1) and W53Q/F54S (QS2). Both mutants have reduced transcriptional activation of apoptosis and cell cycle arresting genes, and the quadruple p53 mutant QS1/QS2 lacks transcriptional activity (19–21). CBP/p300 binding is similarly affected by these mutations, implicating this interaction in p53-dependent transcription (7, 22). To date, the relationships between TAD1 and TAD2 functions are not clear, and target sites of TAD2 on p300 have not been identified.
Despite the importance of the CBP/p300–p53 interaction in p53-dependent transcription, little biochemical data are available on the details of this complex. The interaction between Taz2 (which is contained within CH3) and a peptide containing part of TAD1 is surprisingly weak (KD = 300 μM) (23), whereas the interaction between the same segment of p53 and the N-terminal domain of Mdm2 is very tight (KD ≈ 600 nM) (24–26). Some evidence suggests that damage-induced N-terminal phosphorylations may strengthen binding to CBP/p300, facilitating escape from Mdm2 degradation and activating subsequent transcription of p53-induced genes (27, 28). Here, we present a systematic quantitative analysis of the multiple interactions between the N-terminal transactivation domain of p53 and p300, and find implications for a structural basis of the stabilization of p53 by p300.
Results
p53(15–29) Interacts Weakly with Taz1, Kix, Taz2, and IBiD of p300.
Five putative p53-binding domains (Taz1/CH1, IHD, Kix, Taz2/CH3, and IBiD) of human p300 were expressed and purified. NMR analysis showed that Taz1, Kix, Taz2, CH3, and IBiD were natively folded globular proteins. However, no folded structure could be detected in the IHD.
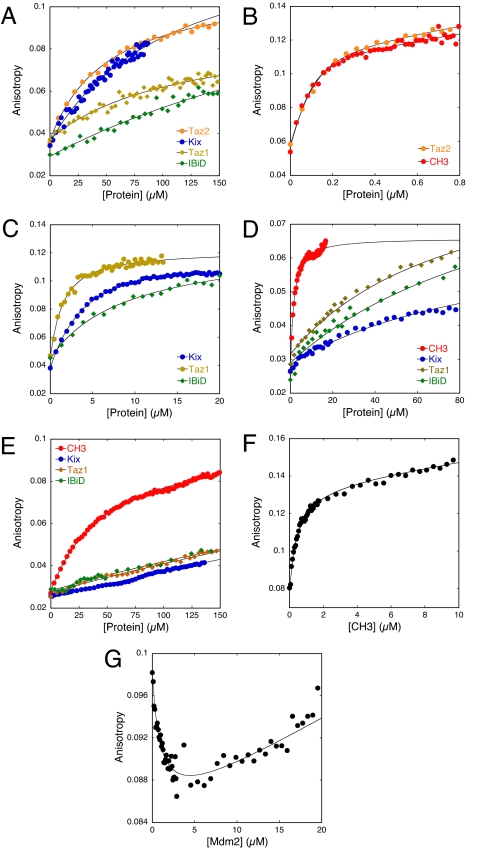
By using fluorescence anisotropy, we systematically assayed each domain for binding to a Lys-methoxy-coumarin-labeled p53(15–29) peptide at physiological ionic strength. The peptide corresponds to the optimal Mdm2-binding segment (26). Data were fitted to a 1:1 binding model. Taz2, which is contained within CH3, bound with KD = 52 μM, 6-fold tighter than reported (23). The Kix and Taz1 domains bound weakly (KD = 100 and 120 μM, respectively). The IBiD domain had KD > 200 μM (Table 1 and Fig. 2A). No interaction could be found with IHD.
Table 1.
Dissociation constants of various p53 N-terminal peptides binding to Taz2/CH3, Taz1, Kix, and IBiD domains of p300, and the N-terminal p53-binding domain of Mdm2
| p53 peptide |
KD, μM |
||||
|---|---|---|---|---|---|
| Taz2/CH3 | Taz1 | Kix | IBiD | Mdm2 | |
| 15–29 WT | 52 ± 4* | 119 ± 25 | 110 ± 30 | NE (>200) | 0.6 ± 0.1 |
| 1–29 WT | 4.1 ± 0.1* | 126 ± 34 | 43 ± 4 | 121 ± 37 | — |
| 1–57 WT | 0.027 ± 0.010 | 1.13 ± 0.2 | 3.0 ± 0.4 | 7.9 ± 1.6 | 0.16 ± 0.04 |
| 1–57 QS2 | 1.1 ± 0.1 | 26 ± 4.2 | 16 ± 3.4 | 27 ± 5.5 | — |
| 1–57 QS1/QS2 | 33 ± 3 | NE | NE | NE | NE |
All experiments were done at least in duplicate and represent averages. NE, KD was too high to be determined.
*Data refer to the interaction with Taz2. Because Taz2 and CH3 bind p53(1–57) at essentially identical affinities, the remaining dissociation constants refer to the more stable CH3 domain.
Fig. 2.
Binding of N-terminal fragments of p53 with p300 and Mdm2 domains. (A) Fluorescence anisotropy titrations of short p53(15–29) TAD1 peptide with Taz2, Taz1, Kix, and IBiD of p300. (B) Fluorescence anisotropy titrations of the full p53(1–57) peptide with CH3 and Taz2. (C) As above, but with Taz1, Kix, and IBiD, respectively. (D) Fluorescence anisotropy titrations of mutant p53(1–57)QS2 with CH3, Kix, Taz1, and IBiD of p300. (E) Titrations of mutant p53(1–57)QS1/QS2 with p300 domains. (F) Competition experiment of Mdm2 and CH3 for labeled p53(1–57). CH3 was titrated into a preformed complex of 100 nM WT p53(1–57) and 1,000 nM Mdm2. Note the higher anisotropy value at the beginning of the titration, which indicates the presence of the Mdm2–p53 complex. (G) Mdm2 was titrated into a mixture of 100 nM WT p53(1–57) and 300 nM CH3. The decrease in anisotropy indicates displacement of CH3 from p53. The upward slope that follows is because of linear drift.
The p53 N Terminus Interacts with p300 Domains Through an Extended Interface.
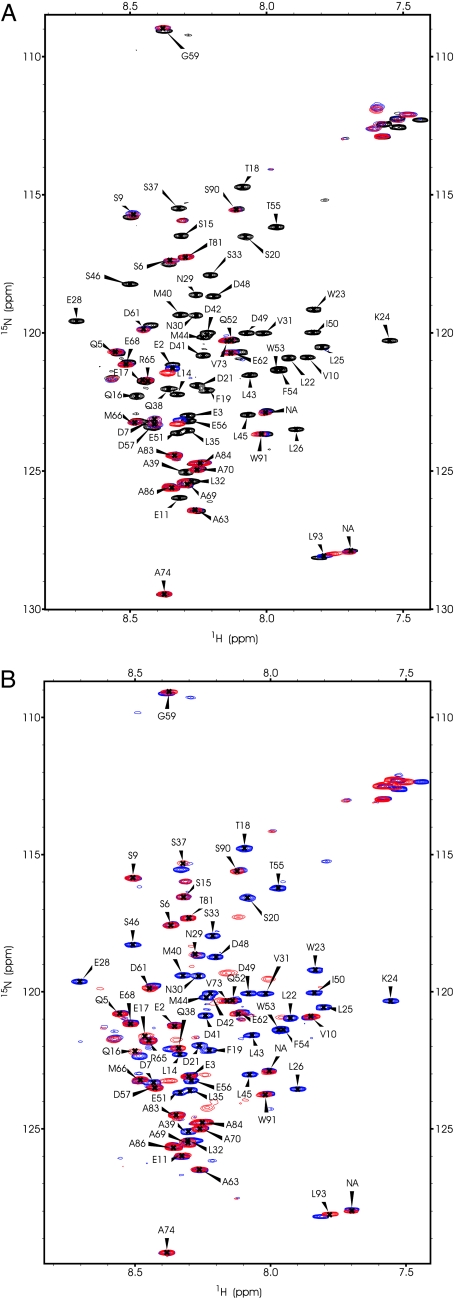
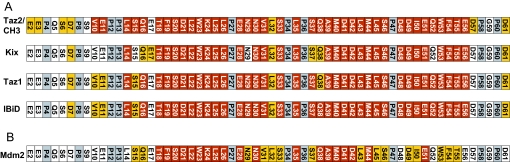
To determine whether the length of the p53 binding site affected p300 domain binding, we recorded heteronuclear single quantum coherence (HSQC) spectra of 15N-labeled p53(1–93) in the presence and absence of Taz2, CH3, Kix (Fig. 3 A and B), Taz1, and IBiD (data not shown). The spectra of p53(1–93) bound to Taz2 and to CH3 were essentially identical, suggesting that binding to Taz2 sufficiently accounts for the interaction. There were no further chemical shift perturbations on adding Taz2 to a higher concentration than p53, indicating a 1:1 stoichiometry of the complex. The HSQC spectra of p53 in complex with each of the p300 domains were very similar, because a largely identical subset of resonances of residues involved in binding disappeared or shifted (Figs. 3 and 4A). Resonances corresponding to residues L14 to E56 were absent, indicating an extended binding site involving both TAD1 and TAD2 for binding to all p300 domains. Taz2/CH3 appeared to affect several additional p53 residues (S6, D7, V10, E11), suggesting a slightly more extensive binding site (Figs. 3A and 4A). p53 residues beyond D57 were largely unaffected by any of the p300 domains, excluding the proline-rich region (residues 62–93) from the interactions (29).
Fig. 3.
NMR HSQC spectra of free 1H 15N-labeled p53(1–93) N terminus (150 μM) in the absence and presence of a 1.2-fold excess of unlabeled p300 domain. Resonances that display clearly assignable chemical shifts are labeled with the residue to which they correspond. Labels for resonances that are absent from the bound state remain at their original positions. (A) 15N p53(1–93) (black) overlaid with the Taz2-bound spectrum (blue) and with the CH3-bound spectrum (red). (B) 15N p53(1–93) (blue) overlaid with the Kix-bound spectrum (red).
Fig. 4.
Sequence-based chemical shift map of p53(1–61) in the presence of p300 domains (A) and Mdm2 (B). Residues 62–93 are not shown because few or no chemical shifts can be observed within that region. Peaks that remain unaffected in the bound state are shown as boxes in white. Prolines, which do not produce resonances in an HSQC, are shown in light gray. Resonances that shift >0.02 ppm in both dimensions are shown in orange. Resonances that disappear in their bound states are shown in red. The extended binding site on p53 is seen with all binding partners.
Incremental additions of Taz2 to 15N-labeled p53 N terminus (1–93) gave steadily decreasing but nonshifting peak intensities for interacting residues indicative of slow exchange (data not shown). It appears, therefore, that the absence of bound-state peaks is the result of conformational averaging in the p53–p300 domain complexes. Interestingly, signals also disappeared on the interaction of p53 N-terminal peptides with Mdm2 (see below and ref. 30) and replication protein A (31).
Both p53 Transactivation Subdomains Are Required for High-Affinity Binding.
The NMR data suggested that the p53 transactivation domain binds to the p300 domains with a more extended binding site than previously thought. Therefore, we synthesized N- and C-terminally extended peptides to assess the importance of residues ≈5–14 and 30–57 of p53 on p300 domain binding. p53(1–29) bound Taz2 10 times more tightly (KD = 4.1 μM), and p53(15–60) 100-fold tighter (KD = 0.4 μM) than p53(15–29) (Table 1, curves not shown). In contrast, p53(1–29) had a relatively unchanged affinity for Taz1, Kix, and IBiD. However, p53(15–60) did bind significantly tighter than p53(15–29) (data not shown). Both TAD1 and TAD2 are thus implicated in binding to all p300 domains.
A p53 peptide containing both transactivation subdomains (residues 1–57) bound to Taz2 and CH3 with KD values of 42 and 27 nM (Fig. 2B), respectively (Table 1). The longer peptide thus interacts >1,000-fold more strongly than p53(15–29). There were similar, but less dramatic, increases in affinity for the other domains. Taz1 bound p53(1–57) 100-fold tighter than p53(15–29) (KD = 1.1 μM), and Kix 40-fold tighter (KD = 3 μM). IBiD had a KD of 8 μM with p53(1–57) (Fig. 2C and Table 1). No binding was observed between the IHD and any of the long p53(1–57) peptides. Although Taz1 was reported to bind to the core domain of p53 (11, 12), we were unable to detect any interactions by HSQC NMR (data not shown). Taz2 was thus the highest affinity domain of p300 for p53, followed by Taz1, Kix, and IBiD.
The Transactivation-Deficient p53 Mutants QS1/QS2 Have Synergistically Impaired Binding.
Two mutants of p53, QS1 (L22Q/W23S) and QS2 (W53Q/F54S), have transcriptional deficiencies and weakened p300 binding (19, 22, 32). We analyzed the effects of QS2 and QS1/QS2 on p53(1–57) binding to each of the p300 domains. p53(1–57)QS2 bound more weakly to all domains: CH3 (Taz2) and Taz1 by factors of 41 and 23, respectively (KD = 1.1 and 26 μM); however, binding of Kix and IBiD was affected less (KD = 16 and 27 μM, respectively) (Table 1 and Fig. 2D). The QS1/QS2 quadruple mutant had a 1,200-fold lower affinity for CH3 (KD = 33 μM). The Taz1, Kix, and IBiD domains bound p53(1–57) QS1/QS2 too weakly for the KD values to be determined (Fig. 2E). Together, the QS1/2 quadruple mutations, which abrogate all transactivation activities of p53 (21), strongly impaired the individual interactions of p53 with Taz2/CH3, Taz1, Kix, and IBiD.
Taz2/CH3 Competes with Mdm2 for the p53 N Terminus.
The N-terminal domain of Mdm2(1–125) binds strongly to p53(15–29) with KD ≈ 600–700 nM (24–26). Under our conditions, the extended p53(1–57) peptide bound the Mdm2 domain four times more tightly (KD = 160 nM; Table 1; data not shown), implicating elements outside p53(15–29) in the interaction. NMR HSQC spectra of Mdm2-bound 15N p53(1–93) had chemical shift perturbations relative to unbound p53 from S15 to T55, with missing signals clustering between T18 and M44 (HSQC not shown) (Fig. 4B). The extended interaction site is in agreement with the findings of Chi et al. (30).
We investigated whether Taz2/CH3 could displace the N-terminal domain of Mdm2 from labeled p53(1–57) and vice versa by using appropriate concentrations of proteins to drive the reaction in either direction. On addition of CH3 at a concentration much greater than its dissociation constant to a preformed complex of p53(1–57) and the N-terminal domain of Mdm2, there was a rapid rise in anisotropy as the high concentration of CH3 displaced Mdm2 (Fig. 2F). Conversely, addition of a high concentration of Mdm2 to a preformed complex of p53(1–57) and CH3 caused a rapid decrease in anisotropy as CH3 was replaced by Mdm2 (Fig. 2G). KD values calculated from these data were in close agreement with those for the separate CH3:p53 and Mdm2:p53 interactions (Table 1). Thus, binding of Mdm2 and CH3 to p53 is mutually exclusive, and no ternary complex is formed.
Discussion
The p53 N-terminal binding proteins studied to date bind, in general, to only one of the two transactivation subdomains. Mdm2 (33) and its homolog Mdm4 (34), components of the transcriptional machinery such as TBP, the TFIIH subunits TAFII40 and TAFII60 (35), and human TAFII31 (17, 36), all interact mainly with TAD1. In contrast, replication protein A (37) and the p62/Tfb1 subunit of TFIIH (38, 39) bind to TAD2. Here, we found that both TAD1 and TAD2 of the p53 transactivation domain can simultaneously interact with three evolutionarily unrelated domains of p300 (Taz1/Taz2, Kix, and IBiD).
A precedent for such an extended interaction site spanning ≈40 residues is that of the related C-terminal activation domain of Hif 1-α, whose structure has been determined in complex with Taz1 of p300 (40, 41). The C-terminal activation domain contains a similar pattern of hydrophobic residues at the respective p53 QS1/QS2 sites that are directly involved in binding Taz1. Because of the homology of Taz1 and Taz2, and the sequence similarity between the C-terminal activation domain and p53 TAD, a similar structure of the Taz2:p53 complex is plausible.
Effects of p53 Transactivation Mutations.
Transcription of p53-responsive genes largely depends on the p300–p53 interaction. Differential effects have been observed for various QS1/2 mutations and deletions on the transcription of cell cycle arrest and apoptotic genes (19–21, 32, 42, 43), and mouse studies showed QS1 to be more deleterious than QS2 (44, 45). Our finding that TAD1 and TAD2 are synergistically involved in the interaction with p300 and are not independent may imply that the transcriptional effects of QS1 and QS2 are simply the result of differentially weakened p300 binding. The more severe effect of QS1 is likely enhanced by reduced binding to components of the transcriptional machinery that participate in p53-mediated transcription (17, 35, 36).
Structural and Biological Implications.
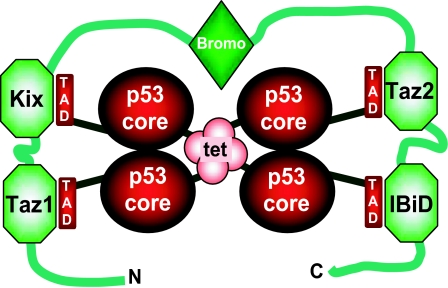
Because four domains of p300 can bind to p53, it is possible that a p300 monomer wraps around the p53 tetramer, with Taz2/CH3 binding the tightest (Fig. 5). Interestingly, the adenoviral protein E1a, which blocks p53-dependent transcription, targets the Taz2/CH3 domain. Synergistic simultaneous binding of the four p300 domains, along with interactions from the bromo and any other domains should lead to very tight binding between p53 and full-length p300/CBP, which will protect the N-terminal transactivation domains from binding to other proteins. The remaining p300 domains have lower affinities for the p53 transactivation domain. It is possible that individual domains could be competed off by other high-affinity p53 N-terminal binding proteins, leading to the formation of mixed complexes in which a p53 tetramer bridges p300 to other proteins. Indeed, complexes consisting of p53 tetramer, p300, and Mdm2 have been isolated from HeLa cells (46). It had been proposed that this complex resulted from direct binding between p300 and Mdm2 (11, 12), but the biological relevance of this interaction has been questioned (47, 48). Our model explains how these complexes could form in the absence of a direct p300–Mdm2 interaction.
Fig. 5.
Model of the p300–p53 tetramer interaction. Four TADs of a p53 tetramer tightly contact four p300 domains. The bromodomain of p300 may further stabilize this complex by weakly interacting with Lys-382-acetylated p53 (55). For clarity, the C-terminal regulatory domain of p53 and HAT, PHD, and ZZ of p300 are not shown.
The competition between Mdm2, Mdm4, and other proteins with p300 for binding to p53 will depend on the magnitudes of the individual dissociation constants of each of the full-length proteins with full-length p53 and their relative concentrations in the cell, which are as yet unknown. The validation of the binding in vivo will, of course, require the necessary biological controls, but the biophysical experiments provide a platform for exploring these.
Experimental Procedures
Plasmids.
Human p300 domains were cloned into the pminiRSET-GST vector, a derivative of pRSETA (Invitrogen, San Diego, CA) containing a GST expression tag and a thrombin cleavage site. The constructs used were p300 Taz1(313–433), Kix(564–658), Taz2(1717–1815), CH3(1660–1815), IBiD(2039–2141), and IHD(420–520 and 401–566), which were cloned by using the BamH1 and EcoR1 sites. The GST-Mdm2(1–125) plasmid was generated as described (26). The human p53 N terminus (1–93) plasmid was a gift from R. Weinberg (MRC Centre for Protein Engineering).
Peptide Synthesis and Purification.
Peptides were synthesized with the Pioneer Peptide Synthesizer (PerSeptive Biosystems, Framingham, MA), by using the Fmoc/tBu strategy as described (26). Fmoc-protected Fmoc-Lys-methoxycoumarin was obtained from NovaBiochem (San Diego, CA). All peptides were C-terminally labeled with Lys-methoxycoumarin. For the long p53(1–57) peptides, the standard protocol was modified to include a capping step after each coupling. Coupling times were increased to 60 min. For peptide purification, smaller fragments in the p53(1–57) crude peptide mixture were removed by gel filtration (Superdex 30; Amersham Biosciences, Piscataway, NJ) [50 mM potassium phosphate (KPi), pH 6.5]. Thereafter, purification was done as described by Schon et al. (26).
Protein Expression and Purification.
The pRGST plasmids containing the respective p300 or Mdm2 domains were transformed into Escherichia coli C41 cells, and grown at 37°C in 2xTY medium containing 100 mg/liter ampicillin until A600 = 0.8. Cells were induced with 0.3 mM isopropyl β-d-thiogalactoside, and grown for another ≈16 h at 23°C. After harvesting, the cells were resuspended in purification buffer (50 mM KPi/200 mM NaCl/10% glycerol/5 mM DTT, pH 7) containing 100 μg/liter DNase, 100 mg/liter lysozyme, and Roche (Gipf-Oberfrick, Switzerland) EDTA-free protease inhibitors. After sonication and centrifugation, the supernatant was loaded onto a GST affinity column (Amersham Biosciences). Fusion protein was eluted with purification buffer containing 10 mM reduced glutathione, and thrombin-digested overnight at 4°C. For CH3, Taz2, and Taz1, the solution was diluted 5-fold with 50 mM KPi (pH 7), and loaded onto a Resource S cation exchange column. Bound protein was eluted with an NaCl gradient. The correct fractions were pooled, and loaded on a HiLoad 26/60 Superdex 75 gel filtration column. Purity of the final protein product was assessed by SDS/PAGE, and the correct masses were confirmed by MALDI-TOF (Voyager DE Biospectrometry Workstation). The ion exchange steps were omitted with the Kix, IBiD, IHD, and Mdm2 purifications.
For the NMR experiments, 15N-labeled p53(1–93) was expressed and purified as described (49). 15N-labeled p53 core domain was a gift from R. Weinberg.
NMR Experiments.
p53(1–93) was reassigned by using heteronuclear triple resonance experiments together with the existing assignment of 1–73 p53 (50). 1H-15N HSQC spectra of free or bound p53(1–93) were acquired on Bruker (Billerica, MA) 600- and 800-MHz spectrometers equipped with triple-resonance single-axis gradient probes, performing 32 scans, at 293 K. Buffer conditions were as in the titration buffer (see below), but 10% 2H2O was included. The concentration of free p53 was 150 μM, and bound samples contained 180 μM p300 domain or Mdm2. Data were analyzed with Sparky 3.0 (www.cgl.ucsf.edu/home/sparky) (51), and the extent of chemical shift perturbations was calculated for clearly reassignable peaks by using methodology described in ref. 52.
Fluorescence Anisotropy Titrations.
Titration experiments were run with equipment and an identical setup as used in refs. 49 and 53. The excitation and emission wavelengths were at 328 and 393 nm, respectively. Peptide concentrations were estimated from absorption readings by using OD324 = 12,000 M−1 cm−1 (54), and protein extinction coefficients were calculated from the amino acid sequence. The titration buffer was 50 mM Mes, 100 mM NaCl, 5 mM DTT (pH 6.8) at 23°C. Usually, 450 μl of protein of varying concentration (10–600 μM) were titrated into 1 ml of 0.5 μM peptide, in 9-μl steps. For the tight binding Taz2/CH3 domains, the peptide concentration of p53(1–57) had to be reduced to 0.1 μM to allow better estimation of KD values. Protein was dialysed, and peptides were dissolved in titration buffer before use.
Titration data were analyzed with a standard 1:1 binding model by using the quadratic solution to the equilibrium KD = [A][B]/[AB]. KD is the dissociation rate, and [A] and [B] refer to the concentrations of titrant (i.e., protein) and fluorescent peptide, respectively. The fitting equation contained an extra term to account for linear drift, which frequently occurs in anisotropy-based titrations (49).
Mdm2 Competition Experiment.
In the first experiment, CH3 was titrated as above into a preformed complex of Mdm2 (1,000 nM) and p53(1–57) (100 nM). For the second experiment, Mdm2 was titrated into a preformed complex of CH3 (300 nM) and p53(1–57) (100 nM). Data were fitted and analyzed with standard competition equations.
Acknowledgments
We thank Henning Tidow and Drs. Frank Boeckler, Sarah Burge, and Andreas Joerger for helpful discussion and critical reading of the manuscript. D.P.T. is supported by the MRC.
Abbreviations
- CBP
cAMP response element binding protein-binding protein
- CH
cysteine–histidine-rich region
- IHD
IBiD homology domain
- TAD
p53 transactivation domain
- HSQC
heteronuclear single quantum coherence.
Footnotes
The authors declare no conflict of interest.
References
- 1.Vogelstein B, Lane D, Levine AJ. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 2.Reihsaus E, Kohler M, Kraiss S, Oren M, Montenarh M. Oncogene. 1990;5:137–145. [PubMed] [Google Scholar]
- 3.Bond GL, Hu W, Levine AJ. Curr Cancer Drug Targets. 2005;5:3–8. doi: 10.2174/1568009053332627. [DOI] [PubMed] [Google Scholar]
- 4.Toledo F, Wahl GM. Nat Rev Cancer. 2006;6:909–923. doi: 10.1038/nrc2012. [DOI] [PubMed] [Google Scholar]
- 5.Veprintsev DB, Freund SM, Andreeva A, Rutledge SE, Tidow H, Canadillas JM, Blair CM, Fersht AR. Proc Natl Acad Sci USA. 2006;103:2115–2119. doi: 10.1073/pnas.0511130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodman RH, Smolik S. Genes Dev. 2000;14:1553–1577. [PubMed] [Google Scholar]
- 7.Gu W, Shi XL, Roeder RG. Nature. 1997;387:819–823. doi: 10.1038/42972. [DOI] [PubMed] [Google Scholar]
- 8.Avantaggiati ML, Ogryzko V, Gardner K, Giordano A, Levine AS, Kelly K. Cell. 1997;89:1175–1184. doi: 10.1016/s0092-8674(00)80304-9. [DOI] [PubMed] [Google Scholar]
- 9.Lill NL, Grossman SR, Ginsberg D, DeCaprio J, Livingston DM. Nature. 1997;387:823–827. doi: 10.1038/42981. [DOI] [PubMed] [Google Scholar]
- 10.Steegenga WT, van Laar T, Riteco N, Mandarino A, Shvarts A, van der Eb AJ, Jochemsen AG. Mol Cell Biol. 1996;16:2101–2109. doi: 10.1128/mcb.16.5.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wadgaonkar R, Collins T. J Biol Chem. 1999;274:13760–13767. doi: 10.1074/jbc.274.20.13760. [DOI] [PubMed] [Google Scholar]
- 12.Grossman SR, Perez M, Kung AL, Joseph M, Mansur C, Xiao ZX, Kumar S, Howley PM, Livingston DM. Mol Cell. 1998;2:405–415. doi: 10.1016/s1097-2765(00)80140-9. [DOI] [PubMed] [Google Scholar]
- 13.Van Orden K, Giebler HA, Lemasson I, Gonzales M, Nyborg JK. J Biol Chem. 1999;274:26321–26328. doi: 10.1074/jbc.274.37.26321. [DOI] [PubMed] [Google Scholar]
- 14.Livengood JA, Scoggin KE, Van Orden K, McBryant SJ, Edayathumangalam RS, Laybourn PJ, Nyborg JK. J Biol Chem. 2002;277:9054–9061. doi: 10.1074/jbc.M108870200. [DOI] [PubMed] [Google Scholar]
- 15.Dornan D, Shimizu H, Perkins ND, Hupp TR. J Biol Chem. 2003;278:13431–13441. doi: 10.1074/jbc.M211460200. [DOI] [PubMed] [Google Scholar]
- 16.Finlan L, Hupp TR. J Biol Chem. 2004;279:49395–49405. doi: 10.1074/jbc.M405974200. [DOI] [PubMed] [Google Scholar]
- 17.Chang J, Kim DH, Lee SW, Choi KY, Sung YC. J Biol Chem. 1995;270:25014–25019. doi: 10.1074/jbc.270.42.25014. [DOI] [PubMed] [Google Scholar]
- 18.Walker KK, Levine AJ. Proc Natl Acad Sci USA. 1996;93:15335–15340. doi: 10.1073/pnas.93.26.15335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu J, Zhou W, Jiang J, Chen X. J Biol Chem. 1998;273:13030–13036. doi: 10.1074/jbc.273.21.13030. [DOI] [PubMed] [Google Scholar]
- 20.Candau R, Scolnick DM, Darpino P, Ying CY, Halazonetis TD, Berger SL. Oncogene. 1997;15:807–816. doi: 10.1038/sj.onc.1201244. [DOI] [PubMed] [Google Scholar]
- 21.Venot C, Maratrat M, Sierra V, Conseiller E, Debussche L. Oncogene. 1999;18:2405–2410. doi: 10.1038/sj.onc.1202539. [DOI] [PubMed] [Google Scholar]
- 22.Liu G, Xia T, Chen X. J Biol Chem. 2003;278:17557–17565. doi: 10.1074/jbc.M210696200. [DOI] [PubMed] [Google Scholar]
- 23.De Guzman RN, Liu HY, Martinez-Yamout M, Dyson HJ, Wright PE. J Mol Biol. 2000;303:243–253. doi: 10.1006/jmbi.2000.4141. [DOI] [PubMed] [Google Scholar]
- 24.Lai Z, Auger KR, Manubay CM, Copeland RA. Arch Biochem Biophys. 2000;381:278–284. doi: 10.1006/abbi.2000.1998. [DOI] [PubMed] [Google Scholar]
- 25.Sakaguchi K, Herrera JE, Saito S, Miki T, Bustin M, Vassilev A, Anderson CW, Appella E. Genes Dev. 1998;12:2831–2841. doi: 10.1101/gad.12.18.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schon O, Friedler A, Bycroft M, Freund SM, Fersht AR. J Mol Biol. 2002;323:491–501. doi: 10.1016/s0022-2836(02)00852-5. [DOI] [PubMed] [Google Scholar]
- 27.Dornan D, Hupp TR. EMBO Rep. 2001;2:139–144. doi: 10.1093/embo-reports/kve025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambert PF, Kashanchi F, Radonovich MF, Shiekhattar R, Brady JN. J Biol Chem. 1998;273:33048–33053. doi: 10.1074/jbc.273.49.33048. [DOI] [PubMed] [Google Scholar]
- 29.Dornan D, Shimizu H, Burch L, Smith AJ, Hupp TR. Mol Cell Biol. 2003;23:8846–8861. doi: 10.1128/MCB.23.23.8846-8861.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chi SW, Lee SH, Kim DH, Ahn MJ, Kim JS, Woo JY, Torizawa T, Kainosho M, Han KH. J Biol Chem. 2005;280:38795–38802. doi: 10.1074/jbc.M508578200. [DOI] [PubMed] [Google Scholar]
- 31.Vise PD, Baral B, Latos AJ, Daughdrill GW. Nucleic Acids Res. 2005;33:2061–2077. doi: 10.1093/nar/gki336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu J, Zhang S, Jiang J, Chen X. J Biol Chem. 2000;275:39927–39934. doi: 10.1074/jbc.M005676200. [DOI] [PubMed] [Google Scholar]
- 33.Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, Pavletich NP. Science. 1996;274:948–953. doi: 10.1126/science.274.5289.948. [DOI] [PubMed] [Google Scholar]
- 34.Shvarts A, Steegenga WT, Riteco N, van Laar T, Dekker P, Bazuine M, van Ham RC, van der Houven van Oordt W, Hateboer G, van der Eb AJ, et al. EMBO J. 1996;15:5349–5357. [PMC free article] [PubMed] [Google Scholar]
- 35.Thut CJ, Chen JL, Klemm R, Tjian R. Science. 1995;267:100–104. doi: 10.1126/science.7809597. [DOI] [PubMed] [Google Scholar]
- 36.Lu H, Levine AJ. Proc Natl Acad Sci USA. 1995;92:5154–5158. doi: 10.1073/pnas.92.11.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bochkareva E, Kaustov L, Ayed A, Yi GS, Lu Y, Pineda-Lucena A, Liao JC, Okorokov AL, Milner J, Arrowsmith CH, et al. Proc Natl Acad Sci USA. 2005;102:15412–15417. doi: 10.1073/pnas.0504614102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Lello P, Jenkins LM, Jones TN, Nguyen BD, Hara T, Yamaguchi H, Dikeakos JD, Appella E, Legault P, Omichinski JG. Mol Cell. 2006;22:731–740. doi: 10.1016/j.molcel.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Xiao H, Pearson A, Coulombe B, Truant R, Zhang S, Regier JL, Triezenberg SJ, Reinberg D, Flores O, Ingles CJ, et al. Mol Cell Biol. 1994;14:7013–7024. doi: 10.1128/mcb.14.10.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dames SA, Martinez-Yamout M, De Guzman RN, Dyson HJ, Wright PE. Proc Natl Acad Sci USA. 2002;99:5271–5276. doi: 10.1073/pnas.082121399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freedman SJ, Sun ZY, Poy F, Kung AL, Livingston DM, Wagner G, Eck MJ. Proc Natl Acad Sci USA. 2002;99:5367–5372. doi: 10.1073/pnas.082117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chao C, Saito S, Kang J, Anderson CW, Appella E, Xu Y. EMBO J. 2000;19:4967–4975. doi: 10.1093/emboj/19.18.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roemer K, Mueller-Lantzsch N. Oncogene. 1996;12:2069–2079. [PubMed] [Google Scholar]
- 44.Johnson TM, Hammond EM, Giaccia A, Attardi LD. Nat Genet. 2005;37:145–152. doi: 10.1038/ng1498. [DOI] [PubMed] [Google Scholar]
- 45.Tang M, Wahl GM, Nister M. Nat Genet. 2006;38:395–397. doi: 10.1038/ng0406-395. [DOI] [PubMed] [Google Scholar]
- 46.Kobet E, Zeng X, Zhu Y, Keller D, Lu H. Proc Natl Acad Sci USA. 2000;97:12547–12552. doi: 10.1073/pnas.97.23.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matt T, Martinez-Yamout MA, Dyson HJ, Wright PE. Biochem J. 2004;381:685–691. doi: 10.1042/BJ20040564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nyborg JK, Peersen OB. Biochem J. 2004;381:e3–e4. doi: 10.1042/BJ20041096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weinberg RL, Freund SM, Veprintsev DB, Bycroft M, Fersht AR. J Mol Biol. 2004;342:801–811. doi: 10.1016/j.jmb.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 50.Lee H, Mok KH, Muhandiram R, Park KH, Suk JE, Kim DH, Chang J, Sung YC, Choi KY, Han KH. J Biol Chem. 2000;275:29426–29432. doi: 10.1074/jbc.M003107200. [DOI] [PubMed] [Google Scholar]
- 51.Goddard TD, Kneller DG. Sparky, NMR Assignment and Integration Software. San Francisco: Univ of California; 2004. Version 3.0. [Google Scholar]
- 52.Ayed A, Mulder FA, Yi GS, Lu Y, Kay LE, Arrowsmith CH. Nat Struct Biol. 2001;8:756–760. doi: 10.1038/nsb0901-756. [DOI] [PubMed] [Google Scholar]
- 53.Weinberg RL, Veprintsev DB, Fersht AR. J Mol Biol. 2004;341:1145–1159. doi: 10.1016/j.jmb.2004.06.071. [DOI] [PubMed] [Google Scholar]
- 54.Knight CG, Willenbrock F, Murphy G. FEBS Lett. 1992;296:263–266. doi: 10.1016/0014-5793(92)80300-6. [DOI] [PubMed] [Google Scholar]
- 55.Mujtaba S, He Y, Zeng L, Yan S, Plotnikova O, Sachchidanand, Sanchez R, Zeleznik-Le NJ, Ronai Z, Zhou MM. Mol Cell. 2004;13:251–263. doi: 10.1016/s1097-2765(03)00528-8. [DOI] [PubMed] [Google Scholar]