Abstract
There is no consensus on when in the fish-tetrapod transition suction feeding, the primary method of prey capture in the aquatic realm, evolved into the direct biting on prey typical of terrestrial animals. Here, we show that differences in the morphology of selected cranial sutures between species that span the fish–tetrapod transition (the Devonian osteolepiform fish Eusthenopteron, the aquatic Devonian tetrapod Acanthostega, and the Permian terrestrial tetrapod Phonerpeton) can be used to infer when terrestrial feeding first appeared. Our approach consists of defining a sutural morphospace, assigning functional fields to that morphospace based on our previous measurements of suture function made during feeding in the living fish Polypterus, inferring the functions of the fossil sutures based on where they fall in the morphospace, and then using the correlation between feeding mode and the patterns of inferred suture function across the skull roof in taxa where feeding mode is unambiguous to infer the feeding mode practiced by Acanthostega. Using this procedure, we find that the suture morphologies of Acanthostega are inconsistent with the hypothesis that it captured prey primarily by means of suction, which suggests that it may have bitten directly on prey at or near the water's edge. Thus, our data strongly support the hypothesis that the terrestrial mode of feeding first emerged in aquatic taxa.
Keywords: Acanthostega, fish–tetrapod transition, suction feeding, Eusthenopteron
The origin of tetrapods and their invasion of terrestrial environments are major events in vertebrate evolution. Comparing early tetrapod taxa such as the Devonian tetrapods Acanthostega (1) and Ventastega (2) with the closely related osteolepiform fishes Eusthenopteron (3), Panderichthys (4), and Tiktaalik (5, 6) shows that the fish–tetrapod transition was defined by a suite of anatomical changes linked to changes in locomotion, respiration, reproduction, the sensory apparatus, and feeding (7–9).
Feeding in water presents organisms with different challenges than those experienced when feeding on land because water is 900 times as dense, and 80 times as viscous, as air (10). Because of these differences, suction feeding, the most widespread method of prey capture used by aquatic vertebrates, is impossible in air (10), so animals that capture prey in terrestrial settings use different techniques, such as overtaking prey items with the jaws and biting on them (11). Therefore, we assume that fish preceding the transition, such as Eusthenopteron, captured prey using suction, whereas later, fully terrestrial tetrapods captured prey items by biting on them (see also ref. 12). Transitional forms such as Acanthostega are thought to have captured prey in the water (12–14), but the exact type of prey capture (i.e., suction versus biting) used by Acanthostega and other early tetrapods is difficult to determine.
Stepwise morphological changes in the lower jaw, dentition, degree of ossification of the operculum, and relative size of the gill chamber in taxa that span the fish–tetrapod transition provide clues as to whether early tetrapods, including Acanthostega, captured prey using suction or biting (15). Specifically, the fishes Eusthenopteron, Panderichthys, and Tiktaalik, and the early tetrapod Ventastega, all possess large coronoid fangs, whereas these teeth are absent in the more derived Acanthostega. In addition, Eusthenopteron and Panderichthys both exhibit an ossified operculum, whereas the bony gill cover is lost in Tiktaalik, Ventastega, and Acanthostega. Finally, the glenoid fossa of the articular faces posteriordorsally in the fish taxa discussed here (Eusthenopteron, Panderichthys, and Tiktaalik) whereas, in the tetrapods Ventastega and Acanthostega, this fossa points dorsally, indicating that the lower jaw changed the nature of its articulation to the skull across the fish–tetrapod transition. (See ref. 15 for a discussion of these changes in all taxa save Tiktaalik; for Tiktaalik, see refs. 5 and 6.) These changes, along with the reduction of the gill chamber, are hypothesized to indicate a reduced reliance on suction feeding in early tetrapods compared with osteolepiform fishes (15).
However, it is unclear how definitive the morphological changes described above are in helping us understand when in the fish–tetrapod transition taxa were no longer dependent on suction feeding. For example, extant fish that capture prey by means of suction exhibit an incredible variety of tooth arrangements and jaw shapes (see ref. 16). In addition, the loss of the operculum observed in Acanthostega is reminiscent of the condition seen in the extant lungfish Neoceratodus (17), which nonetheless employs suction to capture prey (18). Therefore, whereas the changes noted above certainly indicate major changes in the details of the feeding mechanisms between taxa such as Eusthenopteron and Acanthostega, and may indicate a reduced ability of Acanthostega to develop a pressure drop within the buccal cavity compared with Eusthenopteron, these morphological changes do not enable us to determine how reliant Acanthostega might have been on suction feeding, per se.
Thus, motivated by these uncertainties, we here provide an approach for evaluating whether transitional tetrapods, specifically Acanthostega, captured prey primarily using suction, or by biting directly on prey items. This work follows the suggestion that the shift from suction to biting prey capture should be reflected in the morphology of the cranial sutures of taxa that span the fish–tetrapod transition and the tetrapod invasion of land (13, 14, 19).
In vivo experiments demonstrate that cranial sutures are important indicators of skull function (20–23). However, no previous studies have documented quantitative changes in sutural morphology across the fish–tetrapod transition or linked specific sutural morphologies to specific feeding modes (e.g., suction feeding). Here, we assess where in the transition feeding changes occurred by (i) quantifying the three-dimensional morphology of selected sutures in taxa that span the fish–tetrapod transition and the tetrapod invasion of land; (ii) inferring the function of these sutures using correlations between suture morphology and deformation during feeding in the extant fish Polypterus (23); and (iii) associating specific fossil suture morphologies with aquatic (suction) feeding or terrestrial feeding (biting on prey).
Here, the fish–tetrapod transition is represented by the osteolepiform fish Eusthenopteron (3) and the Devonian tetrapod Acanthostega, the best-known early tetrapod (14). Although Panderichthys and Elpistostege are more closely related to tetrapods than Eusthenopteron is (8), we did not have access to any panderichthyid specimens so those taxa were not included. We selected the Permian terrestrial tetrapod Phonerpeton (Dissorophoidea) (24) to represent the invasion of terrestrial environments by tetrapods because of its terrestrial lifestyle (24), the fact that its small skull size falls within the range exhibited by Eusthenopteron and Acanthostega (24), and the excellent three-dimensional preservation exhibited by several specimens in the Museum of Comparative Zoology, Harvard University.
In this study, we are not concerned with the exact homologies between the skull roof bones of these taxa. Instead, we wish to compare bones that are similar in size, proportion (compared with the rest of the skull), and location in the skull, on the grounds that these bones experienced similar functional regimes. Traditionally, the large paired bones in the skull roof located between the orbits have been called “frontals.” (This terminology is still used in the fish literature; for example, see ref. 25.) However, these paired bones in tetrapods and in fishes are not homologous. Instead, fish do not possess true frontals; the large paired elements in Eusthenopteron and Polypterus are homologous to the parietals of tetrapods (see refs. 14 and 26, respectively). Similarly, the “parietals” of Eusthenopteron and Polypterus are not homologous to the parietals of Acanthostega and Phonerpeton. However, because we wish to evaluate the functional similarity of these bones, we refer to the largest midline skull roof bones of Eusthenopteron and Polypterus as frontals, and compare them with the true frontals of Acanthostega and Phonerpeton. In addition, we consider the bones the lie immediately posterior to these large bones (the parietals) of Eusthenopteron and Polypterus to be functionally comparable with the parietals of Acanthostega and Phonerpeton, given their similar shapes and positions in the skull.
Results
Quantifying Sutural Morphology.
Five metrics (27) were used to quantify the surface traces and cross-sectional morphologies of the interfrontal (IF), interparietal (IP), frontoparietal (FP), and nasofrontal (NF) sutures in Eusthenopteron, Acanthostega, and Phonerpeton: (i) interdigitation index: the length of the suture in cross-section divided by the distance between its ends (21); (ii) amount of overlap (= beveling) in cross-section (19); (iii) size of the largest interdigitation in the suture's cross-section; (iv) bone thickness at the suture; and (v) the index of sinuousity: length of the suture on the surface of the skull divided by the distance between its ends (equals the index of interdigitation in ref. 28). Metrics i–iv were measured at several locations across each suture analyzed (see Figs. 1–3 for locations and representative suture morphologies). All measurements of metrics i–v [see supporting information (SI) Table 1] were made by using ImageJ 1.32j (NIH, Bethesda, MD), and statistical analysis was conducted by using SPSS 12.0 (Chicago, IL). The mean values of the metrics used to quantify suture morphology in Eusthenopteron, Acanthostega, and Phonerpeton are provided in SI Table 1.
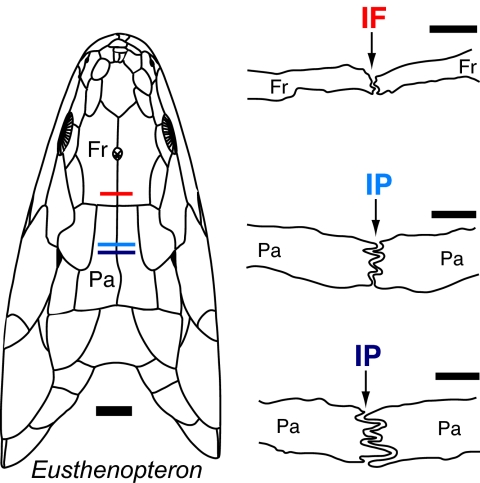
Fig. 1.
Cross-sections through the IF and IP sutures of the osteolepiform fish Eusthenopteron and their approximate positions through the skull roof (see Introduction for discussion of the terminology used for these bones). The cross-section drawings are modified after the original drawings in the literature (29). The color of the sutural label indicates the location of the slice through the skull. The dorsal reconstruction is modified from the literature (30) and was largely based on SMNH P222, the specimen used to generate the grinding series and cross-sectional drawings. [Scale bars: 1 mm (sutures) and 1 cm (skull).] Fr, frontal; Pa, parietal.
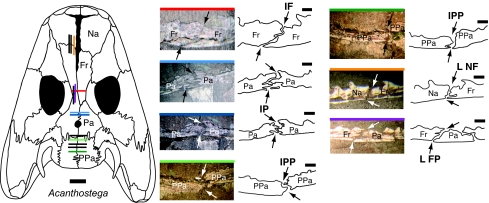
Fig. 2.
Photographs and camera lucida drawings of selected midline and coronal sutures in the aquatic Devonian tetrapod Acanthostega. The midline IF, IP, and IPP sutures were observed in coronal sections of MGUH f.n. 236. The NF and FP sutures were observed in MGUH f.n. 1305 (sectioned sagittally). The black arrows indicate the endocranial and ectocranial emergence of each suture. The colored bar on the top of each suture photograph indicates the approximate position of that slice through the skull roof of Acanthostega. Slices whose positions are shown in black on the dorsal reconstruction of Acanthostega were measured in this study but are not figured here. Note the dramatic shape changes within the IP and IPP sutures. [Scale bars: 1 mm (suture) and 1 cm (skull).] The dorsal reconstruction of the skull of Acanthostega is modified from the literature (31). Na, nasal; Fr, frontal; Pa, parietal; PPa, postparietal; L NF, left nasofrontal suture; L FP, left frontoparietal suture.
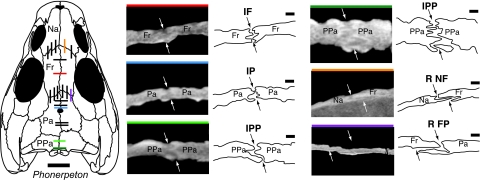
Fig. 3.
Cropped CT slices and line drawings of selected midline and coronal sutures in the Permian terrestrial tetrapod Phonerpeton and their positions in the skull roof. The white and black arrows indicate the ecto- and endocranial ends of each suture in cross-section. [Scale bars: 1 mm (suture) and 1 cm (skull).] The colored line across the top of each CT image indicates the position of that slice in the skull. Slices whose positions are shown in black on the dorsal reconstruction of Phonerpeton were measured in this study but are not figured here. The CT slices were obtained from Phonerpeton specimen MCZ 1414. Note the obvious shape change within the IPP suture. The dorsal reconstruction at left has been modified from the literature (24). Fr, frontal; Pa, parietal; PPa, postparietal; Na, nasal; R NF, right nasofrontal; R FP, right frontoparietal.
Surface Versus Cross-Sectional Sutural Shape.
Interestingly, our data show that the two major aspects of sutural shape [cross-sectional morphology and ectocranial (surface) trace] are not significantly correlated in the taxa examined here (Pearson correlation: r2 = 0.045; P = 0.464), a result anticipated by Clack (13) in her analysis of the skull roof sutures of Acanthostega. The fact that the cross-sectional complexity of a suture is not predictably reflected by its ectocranial trace, and that sutures with identical ectocranial traces can exhibit different cross-sectional shapes and perform different functions (23), means that the cross-sectional morphology of a suture is a better indicator of suture function than its surface appearance. Therefore, our efforts to quantify sutural morphologies across the fish–tetrapod transition and associate these morphologies with specific feeding styles focus on cross-sectional sutural morphology.
Unexpectedly, we observe no overall increase or decrease in cross-sectional sutural complexity (SI Table 1 and SI Fig. 6) across the transition from fish to terrestrial tetrapods, at least for the taxa examined here. Instead, certain sutures in Phonerpeton are more interlocking than in Eusthenopteron and Acanthostega, whereas other sutures are not (SI Fig. 6). In addition, whether a particular suture seems more or less complex across the transition also depends on the metric used to quantify it (SI Fig. 6).
Sutural Morphospace.
To fully describe the suture morphologies exhibited by the fossil taxa, we used a sutural morphospace, rather than considering each metric individually. We used three of the four cross-sectional sutural metrics to make the morphospace, omitting bone thickness because prior analysis of suture form and function during feeding in the extant fish Polypterus showed that bone thickness does not affect the deformation pattern exhibited by a suture (27). Therefore, we plotted only the index of interdigitation, beveling amount, and largest interdigitation size measured in the fossil sutures together with values from positionally comparable sutures in the extant fish Polypterus to create the sutural morphospace (Fig. 4) (27).
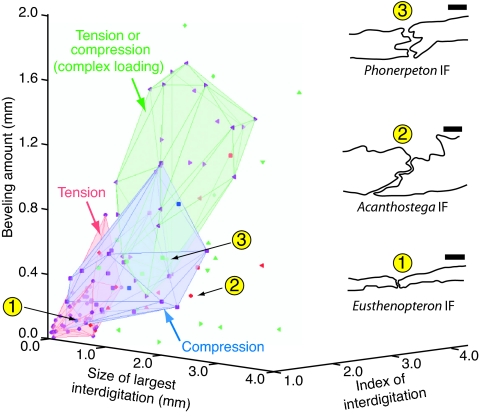
Fig. 4.
Sutural morphospace defined by cross-sectional sutural measurements in Eusthenopteron, Acanthostega, and Phonerpeton. The volumes defined by suture morphologies in the extant fish Polypterus known to experience tension, compression, or a combination of tension and compression that reflects more complex loading conditions (such as shearing or bending) are shown in pink, blue, and green, respectively. The locations of the fossil data points within these volumes are used to infer the loading conditions experienced by the fossil sutures. Taxa: Polypterus, purple; Eusthenopteron, blue; Acanthostega, red; Phonerpeton, green. Sutures: IF, ●; IP, ■; IPP, ♦; left frontoparietal (LFP), ◀; right frontoparietal (RFP), ▶; left nasofrontal (LNF), ▴; right nasofrontal (RNF), ▾. (Scale bars: 1 mm.)
Assigning Functions to the Sutural Morphospace.
The fact that we have previously measured the loading conditions experienced by these sutures in Polypterus during feeding (23) means that particular regions of the morphospace can be linked to particular strain polarities (i.e., tension or compression). Specifically, we found that the IF suture in Polypterus is tensed, whereas the IP suture is compressed during feeding (23). The FP suture of Polypterus may experience tension or compression (23), which probably indicates shearing or bending (27).
Based on these correlations between suture form and function, we assume the following: that fossil sutures that plot in the morphospace volume defined by the Polypterus IF suture measurements (pink) experienced tension; that sutures that lie in the Polypterus IP volume (blue) experienced compression; and that fossil suture morphologies that fall in the Polyperus FP volume (green) of the morphospace experienced more complex loading conditions that would have been manifested as a combination of tension and compression (Fig. 4). For example, we infer that the IF suture of Eusthenopteron was loaded in tension, whereas this suture was compressed in Phonerpeton (Fig. 4). In contrast, the IF suture in Acanthostega plots outside all three volumes of known deformation; therefore, it probably did not experience any of the specific loading conditions we observed in Polypterus (Fig. 4). The inferred deformation types (where they could be made) for the IF, IP, interpostparietal (IPP), NF, and FP sutures in all three fossil taxa, as well as the known sutural deformation patterns in Polypterus, are shown in Fig. 5.
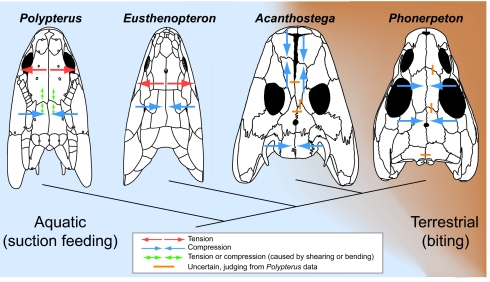
Fig. 5.
Inferred strain patterns in the skulls of Eusthenopteron, Acanthostega, and Phonerpeton based on sutural morphology. Strain patterns observed in the extant fish Polypterus (23) are included for comparison. The strain pattern inferred for Acanthostega suggests that it may have been capable of feeding on land, although Acanthostega may also have occasionally captured prey by means of suction in the aquatic realm. Dorsal view reconstructions are modified from published illustrations: Polypterus (26), Eusthenopteron (30), Phonerpeton (24), and Acanthostega (31). See Figs. 1–3 for suture morphologies and bone labels.
Discussion
Linking Patterns of Sutural Morphology to Feeding Modes.
At the outset of this work, we hypothesized that taxa that practice aquatic feeding should exhibit similar sutural morphologies, and that at least some of these sutures would be quantitatively different from those found in terrestrial taxa. Our measurements support these hypotheses. Eusthenopteron and Polypterus are both characterized by tension anteriorly across the IF suture and compression posteriorly at the IP suture (Fig. 5), despite the taxonomic distance between these “fish” (Eusthenopteron is a sarcopterygian, whereas Polypterus is a basal actinopterygian). One or more common activities among fish (e.g., swimming, gill breathing, and suction feeding) could result in this strain pattern (Fig. 5). However, our previous work shows that, in Polypterus, pumping water across the gills and steady swimming do not cause appreciable sutural deformation; instead, the sutures are strained by feeding activity, most prominently by sucking a prey item into the mouth (23). Therefore, the “tension anteriorly, compression posteriorly” strain pattern that we observed in Polypterus, and have inferred for Eusthenopteron, probably results from suction feeding, which is widely used by aquatic vertebrates (10).
In contrast to the “tension anteriorly, compression posteriorly” strain pattern found in the unequivocally aquatic Polypterus and Eusthenopteron, the terrestrial tetrapod Phonerpeton exhibits compression between the frontals and parietals and a possible tension-compression shift denoting complex loading between the postparietals (Fig. 5). No clear evidence of tensile strain in isolation was found at any suture in Phonerpeton. Although the specific method Phonerpeton used to obtain food cannot be determined here, we can at least be certain that this terrestrial tetrapod did not capture prey by means of suction. Instead, Phonerpeton probably captured prey by biting. Therefore, we suggest that the inferred compression of the IF and IP sutures in this terrestrial taxon (Fig. 5) resulted from biting on prey items.
Feeding in Acanthostega.
Based on its paddle-like limb morphology, internal gills, and broad tail fin bearing fin rays, Acanthostega is inferred to have been a largely aquatic animal (1, 14) that probably fed in the water (13, 14). Therefore, if tension anteriorly and compression posteriorly in the skull is a signature of suction feeding, then this strain pattern would be exhibited by Acanthostega if this primarily aquatic tetrapod captured prey by means of suction. However, although the posterior region of the skull of Acanthostega was probably loaded in compression (Fig. 5), our measurements of sutural morphology do not support the occurrence of simple tension anteriorly at the midline of the skull (see Acanthostega IF suture location in Fig. 4). Therefore, Acanthostega probably did not routinely capture prey using suction feeding. In fact, the distant location of the IF suture of Acanthostega from the regions of known strain in the sutural morphospace (Fig. 4) suggests that this suture experienced very different loading conditions than the IF of Eusthenopteron and Polypterus, although the specific conditions cannot be inferred here.
Intriguingly, Acanthostega exhibits compression at both the anterior and posterior margins of the skull, a strain pattern more similar to the widespread compression inferred for Phonerpeton than to either of the two fish taxa (Fig. 5). Therefore, the feeding mode used by Acanthostega was probably more similar to the terrestrial biting prey capture practiced by Phonerpeton. Thus, our data suggest that Acanthostega bit on prey items in the water or even lurked in shallow water and preyed on terrestrial animals (such as arthropods or possibly other tetrapods whose fossil record is unknown) that approached the water's edge. Therefore, our experimental and morphological data support the hypothesis of Ahlberg and Clack (15) that Acanthostega exhibited a reduced reliance on suction feeding to capture prey.
Clack has suggested that the complex morphology of several sutures in Acanthostega may be linked to this animal lifting captured prey out of the water during feeding (13); however, our previous work on the extant fish Polypterus suggests that prey capture, rather than subsequent prey processing, has a greater influence on sutural morphology in fish (23) (and possibly in tetrapods) that capture prey in the water. Therefore, we favor the hypothesis that the inferred strain pattern in the skull of Acanthostega (Fig. 5) may be linked to the initial capture of prey by biting in the water or near the water's edge, rather than lifting prey out of the water during prey processing.
Despite the numerous adaptations to an aquatic lifestyle that Acanthostega exhibited (1, 14, 17), our analysis of its cranial suture morphologies coupled with experimental data from living taxa indicates that this early tetrapod most likely used a feeding strategy typical of terrestrial organisms (i.e., biting directly on prey items). Thus, it would seem that the terrestrial mode of feeding, like the tetrapod mode of locomotion, first emerged in an aquatic environment. In this context, it will be interesting to examine sutural morphologies exhibited by taxa that lie phylogenetically between Eusthenopteron and Acanthostega; the recently discovered tetrapod-like fish Tiktaalik (5, 6) would be an ideal candidate. In light of these data, it will also be interesting to revisit the detailed differences in the lower jaw, dentition, and operculum of these taxa (15) in the hope of elucidating the details of the functional changes associated with the shift from suction feeding to biting.
Materials and Methods
Taxa.
Although several Eusthenopteron specimens housed at the Swedish Museum of Natural History (SMNH), Stockholm, were used as a guide in our analysis of suture morphology in this taxon (specifically SMNH P33, SMNH P236c, SMNH P246a, and SMNH P2609), cross-sectional measurements of the sutures of interest could not be gathered from these fossils. Instead, we measured selected sutures from drawings of the primary specimen (SMNH P222) destructively sampled by Erik Jarvik (using Sollas's grinding method) in his definitive study of Eusthenopteron (3). Jarvik abraded away the Eusthenopteron specimen at 200-μm increments and photographed the newly revealed cross-sections; therefore, subsequent analyses must rely on the photographs made during the grinding process and on drawings based on the photographs. Our data were collected from the drawings available in the literature (3, 29) (slices 234, 240, and 185) and not the original photographs because they currently cannot be located at the SMNH.
In addition, the morphology of selected sutures was quantified in one specimen of Phonerpeton (24) [MCZ 1414; Museum of Comparative Zoology (MCZ), Harvard University, Cambridge, Massachusetts] and in two specimens of Acanthostega [Museum Geologicum Universitatis Hafniensis, Copenhagen, Denmark (MGUH) field number 236; MGUH field number 1305] (figured in refs. 13, 14, and 32, respectively). The Phonerpeton specimen MCZ 1414 is a small, well preserved, uncrushed skull. The morphology of the sutures in Phonerpeton was assessed by using computed tomography (CT) scanning (see CT Scanning). In contrast, no CT scan of Acanthostega was available for our use. Instead, thick sections of two Acanthostega specimens made by J. Clack were used to measure suture morphology in this species. Clack sectioned MGUH f.n. 236 in the coronal plane, whereas MGUH f.n. 1305 was sectioned in the sagittal plane. These Acanthostega specimens are currently housed at the University Museum of Zoology, Cambridge, U.K. All data used in this study were collected from M.J.M.'s camera lucida drawings and photographs of specimens MGUH f.n. 1305 and MGUH f.n. 236, not from previous figures (13, 14, 32) of these specimens.
CT Scanning.
The skull and cranial sutures of Phonerpeton were visualized by using high-resolution x-ray CT scanning. The specimen (MCZ 1414) was scanned by M. Colbert at the University of Texas High-Resolution X-Ray CT Facility (UTCT). The original coronal data set consisted of 583 slices. Each slice is 133 μm thick, 61.0 mm wide by 61.0 mm tall, at an image resolution of 16.8 pixels per mm. To visualize sutures positioned in the coronal plane of the skull, the original CT data set was digitally resliced in the sagittal plane to create a sagittal data set of 942 slices. All digital reslicing was performed at the UTCT facility by using a custom Interactive Data Language (IDL) routine (IDL; Research Systems Inc., Boulder, CO). Each new sagittal slice measures 61.0 mm wide by 61.0 mm tall, and is 52.6 μm thick, at an image resolution of 21.3 pixels per mm. The software program ImageJ 1.32j (NIH, Bethesda, MD) was used to examine all CT slices.
Sutures Included in This Analysis.
For ease of comparison between Eusthenopteron, Acanthostega, and Phonerpeton, which possess different skull shapes and proportions, only midline and coronal sutures in approximately equivalent locations were used in this study (see Figs. 1–3). In addition, the relatively small number of slices available for Acanthostega and Eusthenopteron limited the range of sutures we could measure. Therefore, our analysis of Eusthenopteron includes only the IF and IP sutures (Fig. 1) (see introduction for a note on the terminology used for these bones). For Acanthostega, we gathered data from the IF and IP, as well as from the IPP, left frontoparietal (L FP), and left nasofrontal (L NF) sutures (Fig. 2). Finally, in Phonerpeton we chose to measure the same set of sutures (i.e., IF, IP, IPP, L FP, and L NF) as we measured in Acanthostega, plus the right frontoparietal (R FP), and right nasofrontal (R NF) sutures (Fig. 3).
Supplementary Material
Acknowledgments
We thank Jennifer Clack for access to Acanthostega specimens at the University Museum of Zoology (Cambridge, U.K.). We also thank Thomas Mörs and Jonas Hagström (Swedish Museum of Natural History) for help during M.J.M's visit, and Matthew Colbert of UTCT for scanning our Phonerpeton specimen. We appreciate Mehul Sampat's assistance with Matlab. Finally, we thank Farish Jenkins, George Lauder, Andrew Knoll, Russell Main, and Corwin Sullivan for their help and advice during the writing of this manuscript, as well as thorough reviews by Per Ahlberg, Jennifer Clack, and an anonymous reviewer.
Abbreviations
- IF
interfrontal suture
- IP
interparietal suture
- IPP
interpostparietal suture
- FP
frontoparietal suture
- NF
nasofrontal suture
- SMNH
Swedish Museum of Natural History
- MCZ
Harvard Museum of Comparative Zoology
- CT
computed tomography.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701706104/DC1.
References
- 1.Clack JA, Coates MI. B Mus Natl Hist Nat Paris. 1995;17:359–372. [Google Scholar]
- 2.Ahlberg PE, Luksevics E, Lebedev O. Philos Trans R Soc London B. 1994;343:303–328. [Google Scholar]
- 3.Jarvik E. Basic Structure and Evolution of the Vertebrates. London: Academic; 1980. [Google Scholar]
- 4.Vorobyeva EI, Schultze H-P. In: Origins of the Higher Groups of Tetrapods: Controversy and Consensus. Schultze H-P, Treub L, editors. Ithaca, NY: Cornell Univ Press; 1991. pp. 68–109. [Google Scholar]
- 5.Daeschler EB, Shubin NH, Jenkins FA., Jr Nature. 2006;440:757–763. doi: 10.1038/nature04639. [DOI] [PubMed] [Google Scholar]
- 6.Shubin NH, Daeschler EB, Jenkins FA., Jr Nature. 2006;440:764–771. doi: 10.1038/nature04637. [DOI] [PubMed] [Google Scholar]
- 7.Carroll RL. J Paleontol. 2001;75:1202–1213. [Google Scholar]
- 8.Clack JA. In: Amphibian Biology, Palaeontology. Heatwole H, Carroll RL, editors. Vol 4. Australia: Surrey Beatty and Sons, Chipping Norton NSW; 2000. pp. 979–1029. [Google Scholar]
- 9.Clack JA. Palaeogeogr Palaeoclimatol Palaeoecol. 2006;232:167–189. [Google Scholar]
- 10.Lauder GV. In: Functional Vertebrate Morphology. Hildebrand M, Bramble DM, Liem KF, Wake DB, editors. Cambridge, MA: Harvard Univ Press; 1985. pp. 210–229. [Google Scholar]
- 11.Bramble DM, Wake DB. In: Functional Vertebrate Morphology. Hildebrand M, Bramble DM, Liem KF, Wake DB, editors. Cambridge, MA: Harvard Univ Press; 1985. pp. 230–261. [Google Scholar]
- 12.Long JA, Gordon MS. Physiol Biochem Zool. 2004;77:700–719. doi: 10.1086/425183. [DOI] [PubMed] [Google Scholar]
- 13.Clack JA. Trans R Soc Edinburgh Earth Sci. 2002;93:17–33. [Google Scholar]
- 14.Clack JA. Gaining Ground: The Origin and Evolution of Tetrapods. Bloomington, IN: Indiana Univ Press; 2002. [Google Scholar]
- 15.Ahlberg PE, Clack JA. Trans R Soc Edinburgh Earth Sci. 1998;89:11–46. [Google Scholar]
- 16.Helfman GS, Collette BB, Facey DE. The Diversity of Fishes. London: Blackwell Science; 1997. [Google Scholar]
- 17.Coates MI, Clack JA. Nature. 1991;352:234–236. [Google Scholar]
- 18.Bemis WE. J Morphol. 1986;1(Suppl):249–275. [Google Scholar]
- 19.Kathe W. Zool J Linn Soc London. 1999;126:1–39. [Google Scholar]
- 20.Herring SW, Mucci RJ. J Morphol. 1991;207:225–239. doi: 10.1002/jmor.1052070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rafferty KL, Herring SW. J Morphol. 1999;242:167–179. doi: 10.1002/(SICI)1097-4687(199911)242:2<167::AID-JMOR8>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herring SW, Teng S. Am J Phys Anthropol. 2000;112:575–593. doi: 10.1002/1096-8644(200008)112:4<575::AID-AJPA10>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markey MJ, Main RP, Marshall CR. J Exp Biol. 2006;209:2085–2102. doi: 10.1242/jeb.02266. [DOI] [PubMed] [Google Scholar]
- 24.Dilkes DW. J Vert Paleontol. 1990;10:222–243. [Google Scholar]
- 25.Grande L, Bemis WE. Soc Vertebr Paleontol Mem. 1998;4:1–690. (suppl to J Vertebr Paleontol 18) [Google Scholar]
- 26.Allis EP. J Anat. 1922;56:189–292. [PMC free article] [PubMed] [Google Scholar]
- 27.Markey MJ, Marshall CR. J Morphol. 2007;268:89–102. doi: 10.1002/jmor.10504. [DOI] [PubMed] [Google Scholar]
- 28.Jaslow CR. J Biomech. 1990;23:313–321. doi: 10.1016/0021-9290(90)90059-c. [DOI] [PubMed] [Google Scholar]
- 29.Jarvik E. K Svenska Vetensk Akad Handl. 1954;5:1–104. [Google Scholar]
- 30.Jarvik E. K Svenska Vetensk Akad Handl. 1944;21:1–48. [Google Scholar]
- 31.Clack JA. Trans R Soc Edinburgh Earth Sci. 2003;93:163–165. [Google Scholar]
- 32.Clack JA. Medd Groen Geosci. 1994;31:1–24. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.