Abstract
Gametophytic cytokinesis is essential for the development and function of the male and female gametophytes. We have previously described the isolation and characterisation of the gemini pollen 1 (gem1) that acts gametophytically to disturb asymmetric division and cytokinesis at pollen mitosis I in Arabidopsis. Here we describe the genetic and cytological analysis of an independent gametophytic mutant, gem2, with similar characteristics to gem1, but which maps to a different genetic locus. gem2 shows reduced genetic transmission through both male and female gametes and leads to the production of divided or twin-celled pollen. Developmental analysis revealed that gem2 does not affect karyokinesis at pollen mitosis I, but leads to repositioning of the cell plate and partial or complete failure of cytokinesis, resulting in symmetrical divisions or binucleate pollen grains respectively. Symmetrical divisions lead to altered pollen cell fate with both sister cells displaying vegetative cell fate. Moreover, we demonstrate that the predominant female defect in gem2 is a lack of cellularization of the embryo sac during megagametogenesis. GEM2 therefore defines an independent genetic locus that is involved in the correct specification of both male and female gametophytic cytokinesis.
Keywords: gemini pollen, Arabidopsis thaliana, pollen mitosis I, gametophytic cytokinesis, cellularization
Introduction
Cytokinesis results in the partitioning of the cytoplasm after nuclear division. In somatic plant cells this involves formation of the preprophase band of microtubules that mark the future division plane. Following anaphase of mitosis, golgi-derived vesicles that are guided by the phragmoplast to the center of the division plane, fuse to establish a tubular-vesicular network and a callosic cell plate. The cell plate expands centrifugally from the centre of the division plane until it reaches the parental walls. Subsequently, the cell plate undergoes maturation to produce a pectocellulosic cell wall. Thus, two cytoskeletal arrays, the preprophase band and the phragmoplast, play essential roles in somatic cell cytokinesis. Several cytokinesis-defective mutants affecting somatic cell division have been identified in Arabidopsis and some of the molecular mechanisms that control cytokinesis are being revealed using genetic approaches (see reviews: Assaad 2001; Nacry et al. 2000; Otegui and Staehelin 2000).
Gametophytic development provides an opportunity to study specialised types of cytokinesis such as asymmetric division and cellularization (Heese et al. 1998). Male gametophytic division at pollen mitosis I (PMI) is highly asymmetric and involves the production of a unique hemispherical cell wall that encloses the generative nucleus. This gives rise to two cells with different structures and fates, the vegetative cell (VC) and the generative cell (GC) (Tanaka 1997; Twell et al. 1998). Cytokinesis at PMI displays several unique features compared to somatic cytokinesis. First, a PPB is absent (Terasaka and Niitsu 1990; Van Lammeren et al. 1985), second, there is a crucial cell plate guidance step involving curved profiles of microtubules in the phragmoplast that appear to guide the centrifugal growth of cell plate. (Brown and Lemmon 1991b; Terasaka and Niitsu 1995). Third, the asymmetry of cytokinesis at PMI is critical for the determination of generative cell fate (Eady et al. 1995; Park et al. 1998).
The female gametophyte is a seven-celled structure consisting of the egg cell, two synergid cells, three antipodal cells and the central cell. After megasporogenesis the surviving megaspore undergoes three rounds of mitosis, producing an eight-nucleate syncytium. Following nuclear repositioning cellularization is initiated at the interfaces of overlapping arrays of microtubules that emanate radially from individual nuclei (Brown and Lemmon 1991a; Russell 1993). Regulation of cellularization in the embryo sac plays a critical role in the development of the embryo and the endosperm. Thus gametophytic cytokinesis is a key process in the development and function of the male and female gametophytes.
Gametophytic mutants have been described in Arabidopsis that affect various stages of pollen or embryo sac development (reviewed by Drews et al. 1998; Twell 2002; Yang and Sundaresan 2000; Drews and Yadegari 2002). However, only a small subset show defects in cytokinesis or cellularization during gametophytic development (Christensen et al. 1998; Park et al. 1998; Grini et al. 1999). We have previously described the gemini pollen1 (gem1) mutant that exhibits defects in gametophytic cytokinesis at PMI, resulting in altered cell division asymmetry and cell fate (Park et al. 1998; Park and Twell 2001). GEM1 was shown to be identical to MOR1 (Whittington et al. 2001), a member of the XMAP215/chTOG family of microtubule-associated proteins (Twell et al. 2002). In addition to its role in the organisation of cortical MT arrays, GEM1 was localised to areas of overlapping microtubules in the phragmoplast, where it appears to play an essential role in the organisation and/or stability of the cytokinetic phragmoplast.
Here we describe the characterisation of a second gametophytic mutant, gemini pollen2 (gem2), that also shows cytokinesis defects in both male and female gametophytic development, but maps to a different genetic locus from GEM1.
Materials and methods
Cytological analysis of pollen
Light and epifluorescence microscopy of DAPI stained microspores and pollen, including image capture and processing were performed as previously described (Park et al. 1998). Aniline blue staining and ultrastructural analysis of spores were carried out as described (Park and Twell 2001). VC fate was analysed using the nuclear-targeted VC-specific lat52 gus/nia marker according to Eady et al (1994).
Genetic analysis and mapping
To determine gametophytic transmission of gem2 reciprocal test crosses were performed between the wild-type (No-O) and heterozygous gem2 mutants and the pollen phenotype of progeny scored. The transmission efficiency of gem2 through male and female gametes represents the percentage of gametes carrying the mutant allele that successfully transmit the mutation compared with the wild type allele, and was calculated as the number of mutants/number of wild-type plants × 100 (Howden et al. 1998). Tetrad analysis was performed by analyzing the pollen phenotype of +/gem2; qrt1/qrt1 plants (Preuss et al. 1994). gem2 was mapped using simple sequence length polymorphic markers as previously described (Park et al. 1998).
Phenotypic characterization of female gametophytes
For phenotypic characterisation of mutant embryos, siliques of different lengths were dissected on a slide using syringe-needle (0.4 × 12mm) under a Zeiss STEMI SV8 dissecting microscope and embryos cleared in a drop of clearing solution (240 g chloral hydrate, 30 g glycerol, 90 ml water) for 30min at room temperature. Preparations were examined with an Olympus microscope (model BHS) equipped for differential interference contrast microscopy (DIC). Images were captured and processed as previously described (Park et al. 1998).
Results
gem2 disrupts division asymmetry and cytokinesis in developing pollen
gem2 showed a range of aberrant division phenotypes in mature pollen that were similar to those described in gem1 (Park et al. 1998). However, the frequency of aberrant pollen was lower at approximately 13% in heterozygotes (Table 1). The most frequent aberrant classes were divided pollen, uninucleate pollen and aborted pollen that occurred at similar frequencies (Table 1). The divided class exhibited a range of phenotypes that were classified as equal, unequal, or incomplete divisions and binucleate pollen with no internal walls (Fig. 1D, E, F, G). Pollen in this class showed two differentially, but diffusely stained, vegetative cell-like nuclei. A common phenotype observed in 30 % of the divided class was asymmetrically divided pollen in which a smaller compartment of cytoplasm was walled off from a larger cell. The larger cell compartments contained one (Fig. 1F) or two (Fig 1G) relatively diffuse nuclei. A further 27% showed incomplete cell walls containing one or two diffusely staining nuclei (data not shown). Uninucleate pollen was distinctly larger in size than mature wild-type tricellular pollen and contained a single, large diffusely staining nucleus (Fig. 1I). One rare mutant phenotypic class (∼2 %) consisted of undivided binucleate pollen with two differentially staining nuclei (Fig. 1H). This range of mature pollen phenotypes suggested that gem2 affects the positioning and completion of the cell plate at PMI and that GEM2 may play a role in the coordination of karyokinesis and cytokinesis.
Table 1.
Frequency of pollen phenotypic classes in gem2
| Normal | divided | uninucleate | aborted | |
|---|---|---|---|---|
| Wt (+/+) | 99.5 | - | - | 0.5 |
| gem2 (+/gem2) | 87.3 | 4.2 | 4.1 | 4.4 |
Counts were made on three heterozygous gem2 plants: Wt = No-O ecotype. Normal = trinucleate pollen with one vegetative and two sperm nuclei; divided = partially or completely divided pollen with one nucleus or two unequal nuclei (includes < 0.3 % undivided binucleate pollen); uninucleate = single large nucleus; aborted = collapsed pollen with no visible nuclei. Data was derived from >1000 spores.
Fig. 1.
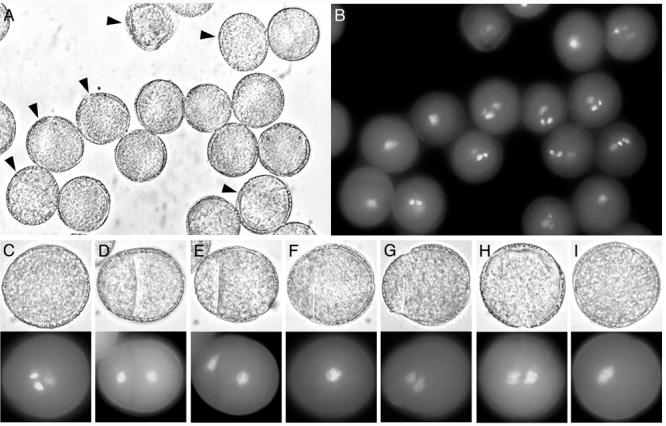
Mature pollen phenotypes of gem2. Panels show light micrographs and fluorescence images of DAPI stained material. A, gem2 population, with aberrant pollen indicated with arrowheads. B, DAPI staining of the same population. C-I, pollen phenotypic classes. C, morphologically normal pollen in gem2 with one diffusely-stained vegetative nucleus and two intensely-stained sperm nuclei. D, E, equal or unequally divided pollen. F, G, uninucleate and binucleate pollen with internal dividing walls. H, I, binucleate and uninucleate pollen showing failed cytokinesis
The ontogeny of aberrant cell division in gem2 pollen was investigated by examining spores released from DAPI stained anthers at four developmental stages. Detailed spore counts were carried out on anthers prior to PMI, and at bicellular, tricellular and mature pollen stages. No aberrant phenotypes were observed in stages prior to PMI (data not shown). However the most striking difference in development between gem2 and wild-type was first observed at the early bicellular stage. At this stage all aberrant spores were either binucleate or bicellular, and this percentage decreased to approximately 20% of all aberrant spores at the tricellular stage. Uninucleate pollen was observed during early tricellular stage with the percentage increasing from 29% to 41% all aberrant spores by late tricellular stage (Fig. 2). These data confirm that the aberrant divisions in gem2 occur as a result of defective cytokinesis at PMI and that uninucleate and divided uninucleate pollen phenotypes arise from nuclear fusion during pollen maturation.
Fig. 2.
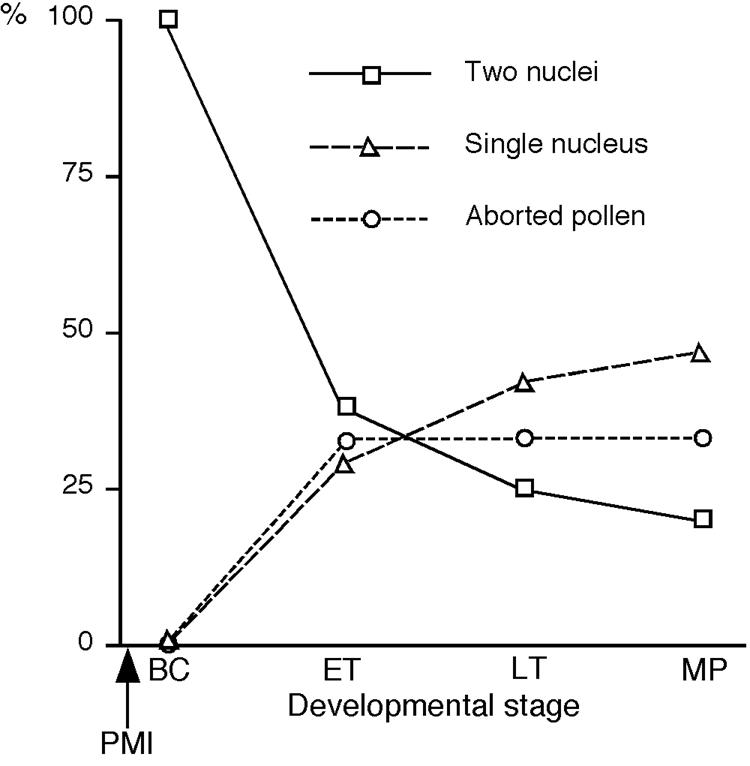
Summary of developmental analysis of phenotypically abnormal gem2pollen. Each abnormal pollen class is represented as a percentage of the total number of aberrant spores at each developmental stage. At bicellular (BC) pollen stage, all aberrant spores were either binucleate or bicellular, and this percentage decreased gradually during maturation. Uninucleate pollen was first observed at the early tricellular (ET) stage with the percentage increasing at late tricellular (LT) and mature pollen (MP) stages. Aborted pollen started to appear at ET-stage and thereafter the frequency remained constant
Genetic analysis of gem2
In contrast to gem1 that is located on chromosome 2 (Park et al. 1998), gem2 was mapped to a position on chromosome 5 between markers ciw9 and nga129. The gem2 mutation was partially transmitted through male and female backcross progeny, such that less than 50 % of the progeny showed the gem2 phenotype. Hence gem2 was isolated as a heterozygote and was predicted to act gametophytically. Reduced transmission of gem2 was evident as only 30% of self progeny showed the mutant phenotype. The transmission efficiency of gem2 through male and female gametes was determined by carrying out reciprocal test crosses. Only 11% of pollen and 23% of embryo sacs carrying the mutant gem2 allele successfully transmitted the mutation.
Based on the reduced male and female transmission of gem2, homozygous mutants were predicted to occur at a frequency of ∼2%, and heterozygotes at ∼25%, among self progeny. The expected frequency of heterozygotes was similar to that observed (30%). If homozygotes of gem2 were present among self-progeny, one would expect strong mutant phenotypes (>20% of aberrant pollen) or gem2 mutants with 100% mutant progeny. We tested the pollen phenotype of 10 progeny from ∼200 gem2 mutant plants, however we failed to identify homozygous individuals, suggesting that gem2 is zygotic lethal.
In a heterozygous plant with a fully penetrant, gametophytic mutation, a maximum of 50% of the pollen population are expected to show the mutant phenotype. Conversely gem2 heterozygotes showed only 13% aberrant pollen (Table 1). Therefore, the remaining 37% of pollen in gem2 heterozygotes that carry gem2, appear wild-type, demonstrating that gem2 is incompletely penetrant in pollen.
Tetrad analysis is possible in Arabidopsis using the quartet (qrt) mutants (Preuss et al. 1994). In a qrt/qrt background a maximum of two out of the four members of the tetrad show the phenotype in plants heterozygous for a gametophytic mutation. Whereas 99% of tetrads from homozygous qrt plants showed only wild-type pollen, in +/gem2;qrt1/qrt1 plants approximately 70% of tetrads contained aberrant pollen (Fig. 3A-C), with ∼40% possessing one and ∼ 30% two aberrant spores. The overall frequency of uninucleate, binucleate and divided pollen in +/gem2;qrt1/qrt1 plants was similar to that observed in +/gem2;QRT1/QRT1 (data not shown). These data further confirm that gem2 is an incompletely penetrant gametophytic mutation.
Fig. 3.
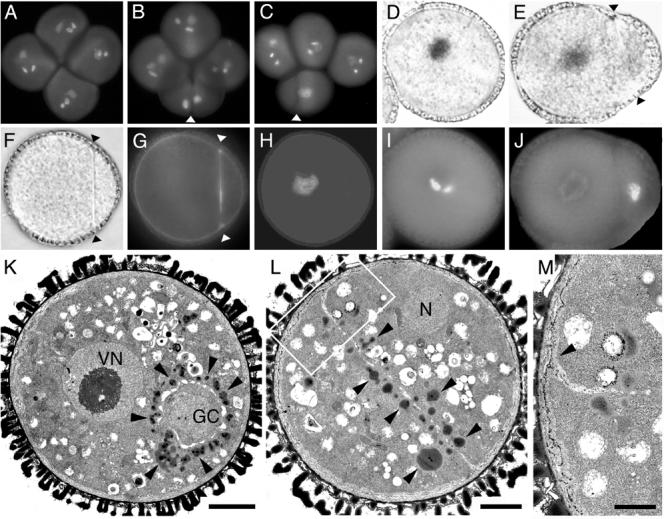
A-C Division axis, cell fate and ultrastructure of gem2 pollen. The division axis was analysed in DAPI stained tetrads of +/gem2; qrt1/qrt1. A, mature qrt1/qrt1 tetrad. B, C, +/gem2; qrt1/qrt1 tetrad showing the conserved plane of division orientated along the polar axis. Arrowheads indicate orientation of the internal wall. D, E, I, J, cell fate analysis in wild-type (D, I) and gem2 showing unequal division (Arrowheads in E, J). Pollen expressing lat52-gus/nia (D, E) and corresponding DAPI images (I, J). F-H, light (F) and fluorescence (G,H) images of a divided gem2 pollen grain showing aniline blue staining of callosic dividing wall (G) and corresponding DAPI image (H). K-M, ultrastructural analysis of wild-type pollen (K) the early bicellular stage. Vegetative nucleus (VN) is located at centrally and numerous lipid bodies (arrowheads) accumulate only in the VC cytoplasm around the generative cell (GC). L, divided gem2 pollen with an internal wall. Lipid bodies (arrowheads) are distributed in both daughter cells adjacent to the internal wall. M, magnified image of junction (arrowhead) region between the dividing wall and the intine shown in L (boxed area). Scale bars, K and L = 3μm, M = 0.5μm
gem2 shows similar defects in pollen cell fate and cytokinesis as gem1
gem2 pollen grains show altered division symmetry that ranges from highly asymmetric to nearly equal. The relationship between division symmetry and cell fate was examined using as criteria the intensity of the nuclear DAPI staining and repression of the lat52-gus/nia as markers for GC fate, and diffuse chromatin staining and activation of lat52-gus/nia as VC fate markers (Fig. 3D, I). Uninucleate pollen grains stained GUS positive and possessed a diffuse nucleus characteristic of VC fate (data not shown). However, in asymmetrically divided pollen, smaller daughter cells showed less intense GUS activity than their larger sister cells, and the intensity of DAPI staining showed the converse relationship (Fig. 3E, J). Therefore, cells with intermediate or mixed cell fate appear to result from aberrant unequal divisions similar to gem1 (Park et al. 1998).
Cell walls in divided gem2 pollen grains were analysed for the presence of callose. All internal walls in twin-celled pollen, whether partial or complete, showed positive callose staining with aniline blue (Fig. 3F-H). Ultrastructural analysis was carried out on anthers at late bicellular pollen stage. In wild-type, the GC was located in a cortical position and was surrounded by numerous lipid bodies (Fig. 3K), that provide an ultrastructural marker of VC fate (Park and Twell 2001). In contrast, gem2 exhibited well-developed internal walls with increased thickness and intense callose staining at the junction with the intine (Fig. 3G, M). Internal walls divided the cytoplasm into twin compartments with a similar cytoplasmic constitution (Fig. 3L). These data and the presence of lipid bodies, is consistent with both compartments adopt VC fate.
gem2 disrupts cellularization of the embryo sac
The genetic transmission of gem2 through the female was reduced to only 23% suggesting that gem2 affects megagametogenesis or post-fertilisation events. We analysed the ovule and seed phenotypes in heterozygous gem2 plants. Under our growth conditions, approximately 36% of the ovules in gem2 heterozygotes were phenotypically abnormal (Table 2), appearing as small white masses typical of ovules containing defective female gametophytes (Meinke and Sussex 1979).
Table 2.
Summary of phenotypic analysis of female gametophytes and mature seed in gem2
| Developmental stage | No-O |
gem2 |
||
|---|---|---|---|---|
| Normal | Abnormal (%) | Normal | Abnormal (%) | |
| 1 Pre-fertilization | 55 | 3 (5) | 37 | 15 (29) |
| 2 2-4 cell embryo | 99 | 5 (5) | 71 | 36 (34) |
| 3 Globular embryo | 90 | 1 (1) | 74 | 40 (35) |
| 4 Mature seed | 1042 | 23 (2) | 878 | 497 (36) |
Data presented in this table are obtained using Nomarski optics 1, 2, 3 and from dissecting mature siliques 4 at comparable developmental stages. At mature seed stage aborted ovules could easily be distinguished from aborted seeds. The frequency of aborted seeds in wild type and gem2 was approximately 2%. It is possible that aborted seed could include gem2 homozygotes
The phenotypes of embryo sacs at eight-nucleate stages, FG5 to FG7 (Christensen et al. 1998), were examined using Nomarski optics. In wild-type embryo sacs cellularization begins immediately after the third round of mitosis and is completed before polar nuclei fusion (Fig. 4A, B). In gem2 heterozygotes approximately 29% of the ovules showed morphological abnormalities in the embryo sac at FG5 to FG7. Although antipodal cell nuclei were not clearly observed, five nuclei were always present at the micropylar pole (Fig. 4E). Moreover, in these abnormal embryo sacs, cell boundaries were generally not present between nuclei (Fig. 4E). However, some embryo sacs were observed with evidence of partial cellularization that was associated with a discrete nucleus at the micropylar pole (Fig. 4F). During the terminal FG7 stage, mutant embryo sacs usually contained one or two extremely large nuclei, presumably arising from fusion of free nuclei at the micropylar pole (Fig. 4G, H).
Fig. 4.
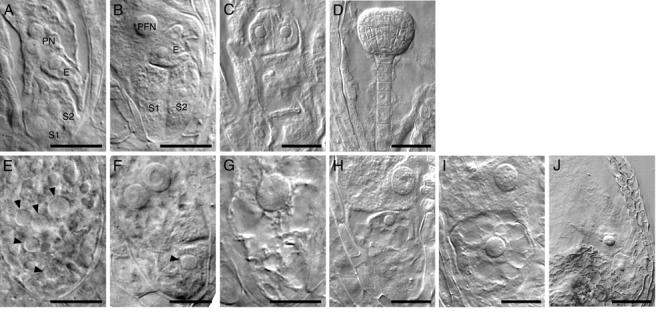
Megagametophyte development in gem2. Nomarski images of wild-type (A-D) and gem2 (E-J) at comparable stages. A, E, eight-cell stage. B, F, G, terminal stage. C, H, I, four-celled embryo stage. D, I, J, late globular embryo stage. A, Wild-type embryo sac with unfused polar nuclei (PN), egg cell (E) and two synergid cells (S1 and S2). B Polar nucleus fused to form large diploid polar fusion nucleus (PFN). C, wild-type embryo showing four-celled embryo proper. D, wild-type globular embryo. E, mutant embryo sacs of gem2 are uncellularized, but contained five nuclei, with various size of nucleoli at the at the micropylar end. F-G Terminal phenotype of a gem2 female gametophyte. Occasionally a mutant embryo sac exhibited a partial wall (F) or a single larger nucleus (G) which is likely to be caused by aberrant nuclear fusion. H-J Mutant female gametophyte of gem2 is unable to form embryo and endosperm as a result of failure of fertilization. gem2 ovule usually contain two nuclei at the micropylar end that is desiccated eventually. Arrowheads (E-J) indicate mutant nucleoli with large size. Scale bars: A-C, E-I = 20μm, D and J = 50μm
During post-fertilisation development mutant embryo sacs were smaller and clearly distinct from wild-type at the four-celled embryo proper stage (Fig 4C). Approximately 34% of embryo sacs contained only two nuclei of different sizes (Table 2; Fig 4H, I). The smaller nucleus, always located at micropylar pole, was often isolated by a visible cell boundary. This micropylar nucleus was surrounded by a number of vacuoles, a unique feature of the egg cell after degeneration of the synergids (Fig. 4I; Faure et al. 2002). This indicates that egg cell fate may be maintained even in embryo sacs showing incomplete cellularization. The morphology of mutant embryo sacs did not change in siliques at globular embryo stage (Fig. 4D, J). Mutant embryo sacs subsequently gave rise to desiccated ovules at a frequency of 36% (Table 2). Taken together these data demonstrate that the predominant female defect in gem2 is a lack of cellularization of the embryo sac.
Discussion
We have characterised an independent gametophytic mutant, gem2 that shows very similar genetic and phenotypic characteristic to the cytokinesis-defective mutant, gem1. This finding is significant for two reasons. First, gem2 maps to an independent genetic locus, therefore the cloning of GEM2 would extend our understanding of the molecular mechanisms of gametophytic cytokinesis in Arabidopsis. Second, we demonstrate that gem2 acts during both male and female gametophytic development, such that GEM2 may be considered to specify essential gametophytic functions.
GEM2 is required for male and female gametophyte development
In common with gem1, gem2 showed a range of pollen division phenotypes and reduced transmission through male and female gametes. Both mutants showed incomplete phenotypic penetrance in pollen and a stronger reduction in transmission through the male. gem2 heterozygotes showed only ∼13% aberrant pollen, therefore 37% of pollen was genotypically mutant but morphologically normal. Even still the transmission efficiency of gem2 was only 11%, a significant fraction of this ‘normal’ gem2 pollen fails during the progamic phase. This suggests that the role of GEM2 in pollen extends from division at pollen mitosis I to progamic development. In contrast the transmission efficiency of gem2 through the female was 23%, which indicated that 38.5% of ovules in gem2 heterozygotes failed to transmit gem2. This was similar to the proportion of infertile ovules (34-36%) that were observed in gem2 heterozygotes.
Despite the significant transmission of gem2 through male and female gametes we were unable to isolate gem2 homozygotes. Thus, the gem2 homozygote is to likely be zygotic lethal. GEM2 is therefore involved in functions required for the development of both gametophytes and appears to function during progamic and post-fertilisation development.
gem2 disturbs cytokinesis and cell fate during male and female gametophytic development
The earliest detectable mutant phenotypes in gem2 were apparent at PMI. Since karyokinesis was unaffected, gem2 does not appear to affect mitosis or spindle function. A common phenotype was the uncoupling of nuclear division and cytokinesis, suggesting that GEM2 may be required for the co-ordination of these events at PMI. Cell walls were mis-positioned, partially formed and sometimes absent, which could reflect disturbances in phragmoplast assembly or dynamic stability. The importance of microtubules in cytokinesis has been demonstrated by microtubule destabilisation at the phragmoplast, such that cytokinesis is initiated only at the periphery resulting in incomplete cell walls (McIntosh et al. 1995). The similar cytokinetic defects observed in gem1 and gem2 together with the role of GEM1 as an essential microtubule binding protein located at the phragmoplast midzone, suggest that GEM2 may also have a role in phragmoplast organisation. We speculate that GEM2 could represent a potential interacting partner of GEM1 that could be mediated through the multiple HEAT repeats possessed by GEM1 (Twell et al 2002).
In gem2 embryo sacs, defects in cellularization were revealed as partial or absent cell boundaries after third mitosis. Such defects could result from effects on microtubule configurations involved in cellularization. Webb and Gunning (1994) described distinct and varied arrangements of microtubules in the constituent cells of the mature seven-celled embryo sac. At the coenocytic stage phragmoplast arrays were established between sister and non-sister nuclei leading to cellularization with conspicuous cell boundaries. Moreover, differential cell fate appears to be established only following cellularization. Mutant gem2 embryo sacs exhibited multinucleate cells caused by lack of cellularization. Ultimately the egg, synergids and central cell in gem2 embryo sacs were unable to differentiate to form the female germ unit required for double fertilization. Partial cellularization of a fusion nucleus at the micropylar pole of embryo sacs in gem2 produced large cells with vacuolation characteristic of egg cells. This suggests that egg cell differentiation may occur even in embryo sacs showing incomplete cellularization, and that previously established gradients within the coenocytic embryo sac could be reinforced by local cellularization. Compared to gem2, we have observed that gem1 exhibits even more severe embryo sac phenotypes, with failed and partial cellularization of embryo sacs before fertilisation (Park and Twell, unpublished data). Since GEM1 is a microtubule binding protein, it is likely to be involved in organization of internuclear microtubules that are essential for normal cellularization of the embryo sac. We have also identified a novel gametophytic mutant, two in one (tio), in which cytokinesis fails at PMI leading to the production of binucleate pollen grains (Twell 2002; Oh and Twell unpublished data). TIO also affects megagametogenesis and results in failed cellualrization of the embryo sac (Twell 2002; Oh and Twell unpublished data). Thus, tio, together with gem1 and gem2 will be useful for studying the relationship between microtubule organization, cellularization and cell differentiation during megagametogenesis.
A number of genes required for cytokinesis in sporophytic cells have been identified through mutational analysis in Arabidopsis (Assaad 2001). Genetic and molecular analyses do not suggest a role for these genes in gametophytic development. Thus, genes required for somatic cytokinesis seem to be partially distinct from those required for gametophytic cytokinesis (Otegui and Staehelin 2000). Nevertheless, the isolation of gametophytic cytokinesis-defective mutants is of value in identifying genes that function in both gametophytic and sporophytic cytokinesis, such as GEM1, GEM2 and TIO. Such cytokinesis-related mutants have not been identified by a specific mutant phenotype in the sporophyte, but from their phenotype first expressed during haploid development. Well-characterized gametophytic mutations will accelerate the dissection of molecular mechanisms regulating gametophytic cytokinesis. Moreover, directed morphological screening of insertion lines informed by male gametophytic transcriptome data (Honys and Twell 2003, and unpublished) provides an additional route for the identification of components of the cytokinetic machinery during gametophytic development.
Acknowledgements
We gratefully acknowledge the Biotechnology and Biological Sciences Research Council for financial support.
References
- Assaad FF. Plant cytokinesis. Exploring the links. Plant Physiol. 2001;126:509–516. doi: 10.1104/pp.126.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RC, Lemmon BE. Pollen development in orchids. 1. Cytoskeleton and the control of division plane in irregular patterns of cytokinesis. Protoplasma. 1991a;163:9–18. [Google Scholar]
- Brown RC, Lemmon BE. Pollen mitosis in orchids: 5. A generative cell domain involved in spatial control of the hemispherical cell plate. J Cell Sci. 1991b;100:559–565. [Google Scholar]
- Christensen CA, King EJ, Jordan JR, Drews GN. Megagametogenesis in Arabidopsis wild type and the Gf mutant. Sex Plant Reprod. 1997;10:49–64. [Google Scholar]
- Christensen CA, Subramanian S, Drews GN. identification of gametophytic mutations affecting female gametophyte development in Arabidopsis. Dev Biol. 1998;202:136–151. doi: 10.1006/dbio.1998.8980. [DOI] [PubMed] [Google Scholar]
- Drews GN, Yadegari R. Development and function of the angiosperm female gametophyte. Annu Rev Genet. 2002;36:99–124. doi: 10.1146/annurev.genet.36.040102.131941. [DOI] [PubMed] [Google Scholar]
- Eady C, Lindsey K, Twell D. Differential activation and conserved vegetative-cell-specific activity of a late pollen promoter in species with bi- and tricellular pollen. Plant J. 1994;5:543–550. [Google Scholar]
- Eady C, Lindsey K, Twell D. The significance of microspore division and division symmetry for vegetative cell-specific transcription and generative cell differentiation. Plant Cell. 1995;7:65–74. doi: 10.1105/tpc.7.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure J-E, Rotman N, Fortuné P, Dumas C. Fertilization in Arabidopsis thaliana wild type: developmental stages and time course. Plant J. 2002;30:481–488. doi: 10.1046/j.1365-313x.2002.01305.x. [DOI] [PubMed] [Google Scholar]
- Grini PE, Schnittger A, Schwarz H, Zimmermann I, Schwab B, Jürgens G, Hülskamp M. Isolation of ethyl methanesulfonate-induced gametophytic mutants in Arabidopsis thaliana by a segregation distortion assay using the multimarker chromosome 1. Genetics. 1999;151:849–863. doi: 10.1093/genetics/151.2.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heese M, Mayer U, Jürgens G. Cytokinesis in flowering plants: cellular process and developmental integration. Curr Opin Plant Biol. 1998;1:486–491. doi: 10.1016/s1369-5266(98)80040-x. [DOI] [PubMed] [Google Scholar]
- Honys D, Twell D. Comparative analysis of the Arabidopsis pollen transcriptome. Plant Physiol. 2003;132:640–652. doi: 10.1104/pp.103.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden R, Park SK, Moore JM, Orme J, Grossniklaus U, Twell D. Selection of T-DNA-tagged male and female gametophytic mutants by segregation distortion in Arabidopsis. Genetics. 1998;149:621–631. doi: 10.1093/genetics/149.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K, Pickett-Heaps JD, Gunning BES. Cytokinesis in Spirogyra: integration of cleavage and cell plate formation. Int J Plant Sci. 1995;156:1–8. [Google Scholar]
- Meinke DW, Sussex IM. Embryo-lethal mutants of Arabidopsis thaliana. A model system for genetic analysis of plant embryo development. Dev Biol. 1979;72:50–61. doi: 10.1016/0012-1606(79)90097-6. [DOI] [PubMed] [Google Scholar]
- Nacry P, Mayer U, Jürgens G. Genetic dissection of cytokinesis. Plant Mol Biol. 2000;43:719–733. doi: 10.1023/a:1006457723760. [DOI] [PubMed] [Google Scholar]
- Otegui M, Staehein LA. Cytokinesis in flowering plants: more than one way to divide a cell. Curr Opin Plant Biol. 2000;3:493–502. doi: 10.1016/s1369-5266(00)00119-9. [DOI] [PubMed] [Google Scholar]
- Park SK, Howden R, Twell D. The Arabidopsis thaliana gametophytic mutation gemini pollen1 disrupts microspore polarity, division asymmetry and pollen cell fate. Development. 1998;125:3789–3799. doi: 10.1242/dev.125.19.3789. [DOI] [PubMed] [Google Scholar]
- Park SK, Twell D. Novel patterns of ectopic cell plate growth and lipid body distribution in the Arabidopsis gemini pollen1 mutant. Plant Physiol. 2001;126:899–909. doi: 10.1104/pp.126.2.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss D, Rhee SY, Davis RW. Tetrad analysis possible in Arabidopsis with mutation of the QUARTET (QRT) genes. Science. 1994;264:1458–1460. doi: 10.1126/science.8197459. [DOI] [PubMed] [Google Scholar]
- Russell SD. The egg cell: development and role in fertilization and early embryogenesis. Plant Cell. 1993;5:1349–1359. doi: 10.1105/tpc.5.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka I. Differentiation of generative and vegetative cells in angiosperm pollen. Sex Plant Reprod. 1997;10:1–7. [Google Scholar]
- Terasaka O, Niitsu T. Unequal cell division and chromatin differentiation in pollen grain cells. II. Microtubule dynamics associated with the unequal cell division. Bot Mag Tokyo. 1990;103:133–142. [Google Scholar]
- Terasaka O, Niitsu T. The mitotic apparatus during microspore division observed by confocal laser scanning microscopy. Protoplasma. 1995;189:187–93. [Google Scholar]
- Twell D. Pollen developmental biology. In: O'Neill SD, Roberts JA, editors. Plant Reproduction. Vol. 6. Sheffield: Sheffield Academic Press; 2002. pp. 86–153. Annual Plant Reviews. [Google Scholar]
- Twell D, Park SK, Lalanne E. Asymmetric division and cell fate determination in developing pollen. Trends Plant Sci. 1998;3:305–310. [Google Scholar]
- Twell D, Park SK, Hawkins TJ, Schubert D, Schmidt R, Smertenko A, Hussey PJ. MOR1/GEM1 has an essential role in the plant-specific cytokinetic phragmoplast. Nat Cell Biol. 2002;4:711–714. doi: 10.1038/ncb844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lammeren A, Keijzer C, Willemse M, Kieft H. Structure and function of the microtubular cytoskeleton during pollen development in Gasteria verrucosa (Mill) H. Duval. Planta. 1985;165:1–11. doi: 10.1007/BF00392205. [DOI] [PubMed] [Google Scholar]
- Webb MC, Gunning BES. Embryo sac development in Arabidopsis thaliana II. The cytoskeleton during megagametogenesis. Sex Plant Reprod. 1994;7:153–163. [Google Scholar]
- Whittington AT, Vugrek O, Wei KJ, Hasenbein NG, Sugimoto K, Rashbrooke MC, Wasteneys GO. MOR1 is essential for organizing cortical microtubules in plants. Nature. 2002;411:610–613. doi: 10.1038/35079128. [DOI] [PubMed] [Google Scholar]
- Yang W-C, Sundaresan V. Genetics of gametophyte biogenesis in Arabidopsis. Curr Opin Plant Biol. 2000;3:53–57. doi: 10.1016/s1369-5266(99)00037-0. [DOI] [PubMed] [Google Scholar]


