A new method of surface-selective laser sintering (SSLS) leads to the fabrication of three-dimensional (3D) composite scaffolds (spatial resolution ∼200 μm) that are both bioactive and biodegradable. Moreover, the scaffolds can have very precise dimensions and intricate structure. Conventionally, in selective laser sintering (SLS), the polymer absorbs infrared (λ=10.6 μm) radiation and this leads to a volumetric absorption by the whole polymer particle. In other words, each particle of polymer is completely melted and fuses to the next in order to form the desired morphology. In our experiments we have used near-infrared (λ=0.97 μm) laser radiation, which polymer particles do not absorb at all. To initiate the sintering process a small quantity (< 0.1 wt.-%) of carbon microparticles were homogeneously distributed on the surfaces of the polymer particles. Thus, the melting process was limited to only the surfaces of each particle. The carbon microparticles are strong absorbers of laser radiation, and this opens up the technique to a range of polymers that up till now could not be processed by laser sintering. More importantly, since the laser melts only the surfaces of the particles, delicate bioactive species trapped within each particle retain their activity throughout the processing. We have demonstrated the application of this technique by the incorporation of the enzyme ribonuclease A into particles of poly(d,l-lactic) acid (PLA) and the assembly of 3D matrices at three different laser intensities, using a 0.97 μm wavelength continuous wave (CW) diode laser.
Polymer composite structures, in which biologically active guest species are dispersed throughout a suitable porous polymer matrix to encourage formation of new tissue, have widespread biomedical applications as scaffolds for tissue engineering.[1,2] Conventional methods of preparing such composites normally use either organic liquid solvents (e.g., solvent casting, particulate leaching) or raised temperature (e.g., melt molding, thermally induced phase separation) to process the polymer. This often leads to solvent and thermally induced degradation and contamination or changes in molecular conformations of the bioactive species.[3] Moreover, none of these methods allow production of completely interconnected porous scaffolds with a regular and reproducible morphology. Rapid prototyping (RP) techniques are based on computer-assisted design (CAD) with computer-assisted manufacturing (CAM). These techniques are of particular interest to produce complex objects with desirable shape and internal structure.[4] However for tissue engineering, there are significant drawbacks in the use of existing RP technology to fabricate an ideal polymeric scaffold. For example, laser stereolithography is limited by the type of materials from which a 3D object with a reasonable size (e.g., larger than a few cubic millimeters) can be made.[5] Only a few acrylic and epoxy based monomers have the required characteristics to allow effective fabrication of such structures. Hence, the starting materials limit the range of biological responses that can be obtained in the final product. Nonlinear multiphoton crosslinking and polymerization of proteins and monomers (e.g., alkaline phosphatase and acrylamide) can be achieved using femtosecond laser systems and such[6] processes have been successfully exploited for fabrication of 3D sustained-release devices on the subcellular biological scale by microstereolithography.[7] However, the small size (ca. 100 μm×100 μm×10 μm) of these matrices precludes their use as an effective route for tissue-engineering-scaffold fabrication. Conventional selective laser sintering (SLS) can produce larger samples but exposes the polymer powders to temperatures which are prohibitively high for many biodegradable materials and bioactive species.[8] Fused deposition modeling (FDM) has only a narrow processing window for biomaterials applications that is limited to the melt processing of poly-ε-caprolactone.[9] In addition, FDM does not allow the use of fillers, and hence negates the addition of bioactive species. In the case of 3D printing, the use of organic solvents (e.g., chloroform) as binders for aliphatic polyesters (polylactic and polylactic-co-glycolic acids) is required. Finally, both FDM and 3D printing of biodegradable polyesters are associated with rapid formation of hydroxyacids which may lead to local cell toxicity.[10]
In this paper we present a new method for bioactive and bioresorbable scaffold fabrication based on a modified and enhanced SLS process—surface-selective laser sintering (SSLS). The key difference in this technology is initiation of sintering by melting of only the polymer particle surface. In conventional SLS, volumetric absorption of the laser radiation by the polymer leads to melting of the whole particle and fusion. We have used PLA, which does not absorb near-infrared laser radiation. On the surface of the polymer particles we have added a small quantity (< 0.1 wt.-%) of homogeneously distributed biocompatible carbon microparticles.[11,12] Laser light is absorbed only by the carbon microparticles leading to localized surface heating. Optimization of the PLA/carbon-powder composite and of the processing parameters such as laser intensity and laser beam scanning speed has enabled reproducible fabrication of 3D polymer scaffolds with specific shape and internal structure. Because the bulk of each polymeric particle is not melted, this surface sintering facilitates the inclusion of bioactive species within the polymer particles. We demonstrate that the activity of a model enzyme (ribonuclease A) can be retained throughout the formation of a 3D scaffold by the SSLS process. This model enzyme has been used widely to demonstrate the effectiveness of processing of polymers for delivering bioactive species.[13]
The SLS experimental set-up consists of a CW diode laser (LS-097, "IRE-Polus Ltd", Moscow, Russia) emitting at λ=0.97 μm, with power of up to 20 W. This was delivered to the polymer/ribonuclease/carbon powder through a quartz fiber inserted into the pen holder of computer-controlled X–Y plotter. A 2 mm distance was maintained between the fiber end and the powder plane, casting a ∼200 μm diameter laser spot onto the polymer particles to enable their fusion during the laser-beam scanning. An initial powder layer of one monolayer thickness (200 μm) was laid down on the scanning surface.
Neither PLA nor ribonuclease has an absorbance at the laser wavelength (0.97 μm); only the carbon microparticles absorb to initiate surface melting and powder fusion. By optimization of the laser intensity, scan speed, and carbon-microparticle concentration in the mixture it was possible to achieve sintering, without significant temperature increase in the bulk of each particle. As the laser beam draws the contour of the image (straight lines separated by 500 μm, Fig. 1a, lines A), a thin layer (ca. 200 μm) of fused polymer particles is built up. A precision "elevator" then moves the floor of the cylinder down to allow the next powder layer to be prepared on top. The laser beam is then scanned again across the fresh powder, sintering the second contour (Fig. 1a, lines B) perpendicular to the first one. The process continues until the required layers have been laid down and sintered to produce a 3D object with an accuracy of 200 μm (Fig. 1c). In our experiments the threshold laser-beam intensity for melting the polymer surface at a scan speed of 1 mm s−1 was approximately ∼50 W cm−2. We used three different laser intensities 60, 125, and 180 W cm−2 to produce PLA/ribonuclease samples for further analysis.
Figure 1.
PLA/Ribonuclease/CAM structures fabricated by SSLS method. a) Showing preparation of perpendicular lines A and B. b) 3D structure built up from two such layers. c) Structure built up from fourteen layers. Total processing time is ca. 10 min and the structure has an accuracy of 100 μm and total size 5 mm×5 mm×5 mm.
Scanning electron microscopy (SEM) analysis (Fig. 2) demonstrates that this modified SLS technique can produce high-quality 3D structures from biodegradable polymers, in this case PLA. Clearly, in order to fuse the individual polymeric particles, melting of the polymer surface is initiated by laser radiation absorption by the carbon microparticles, allowing the growth of 3D structures of controlled geometry along the laser-beam scan trajectories (lines). Both the melting depth and the number of polymer particles involved in the sintering process can be seen to increase with laser intensity. Thus the mechanical strength and integrity of the scaffolds could be controlled and optimized by varying laser intensity.
Figure 2.
SEM images of the sintered sample. a) The paths along which the laser beam passes are indicated by the lines. b) Detailed view of the central area (square) of the structure. Note that the individual particles are clearly fused at their surfaces.
Raman spectra of both initial PLA/ribonuclease/carbon powder and sintered structures were collected and analyzed. Two key spectroscopic regions were investigated in detail; 800–1800 cm−1 and 2900–3000 cm−1, corresponding to various vibrational modes of CH, CH3, COC, and COO.[14] The laser-spot diameter of the Raman microspectrometer (circa 25 μm) thus allowed the Raman signals across individual particles to be assessed at high resolution. During the analysis of over one hundred spectra from a very wide range of sintered particles (in the intensity range 50–180 W cm−2) no significant spectroscopic differences were found when compared to control samples. Only at very high laser intensity (>250 W cm−2) was any degradation observed. This was characterized by the appearance of very broad features across the Raman spectrum.
Gel permeation chromatography (GPC) analysis of the sintered scaffolds was performed at 30°C using chloroform as a solvent (LC-1120 pump and a PL-ELS 1000 light-scattering detector (Polymer Labs) calibrated using polystyrene narrow standard). As in the Raman microspectrometry study, there were no statistically significant differences in the PLA molecular weight or polydispersity when compared to control samples. At higher laser intensities, ca. 250 W cm−2, there was a distinct effect. These samples did not dissolve in chloroform, thus demonstrating the formation of crosslinks and implying polymeric degradation after the fusion process at such high laser intensities.
The ribonuclease activity of the enzyme-containing samples sintered at various laser intensities (60, 125, and 180 W cm−2) was assayed and compared to a ribonuclease activity standard curve. The amount of protein present in the samples was verified by bicinchoninic acid (BCA) protein assay. At all laser intensities we found that following the SLS processing, the enzyme retained substantial activity and demonstrated that the selective laser sintering can produce 3D composite structures that could be both bioactive and biodegradable.
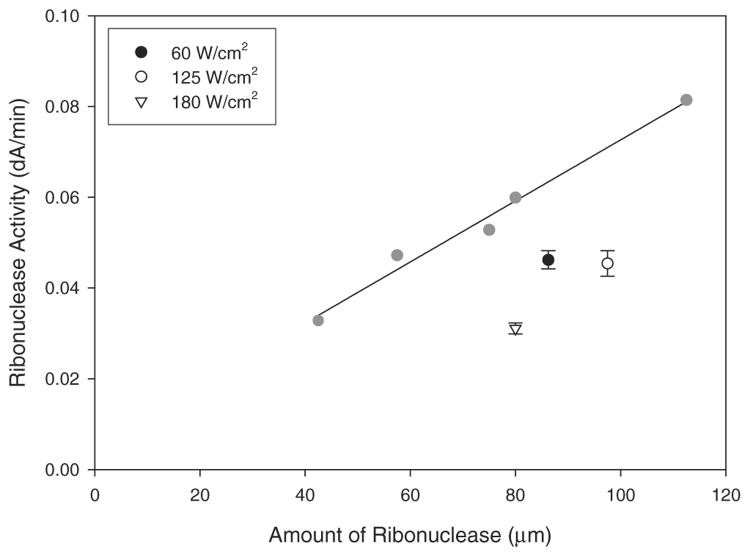
Since the laser absorption and fusion takes place at the surface of the particles, it is likely that the activity of the enzyme at the surface will be compromised by the laser-sintering process. However, the material in the bulk of the particles should remain unaffected. At lower laser intensities there is a relatively low loss of enzyme activity (at 60 W cm−2 ribonuclease activity was 79.4 ± 4.3% compared to the control) (Fig. 3), largely because the depth of melting of each particle is confined to close to the surface. At higher laser intensities, a greater proportion of the internal domain of each particle will melt, and this will in turn will lead to a greater proportion of denaturing of the enzyme within that domain (at 125 and 180 W cm−2 ribonuclease activity was 69.1 ± 6.2% and 60.6 ± 1.9% respectively, compared to the control).
Figure 3.
Ribonuclease activity. The graph shows the effect on ribonuclease activity following SLS processing at varying laser intensities (open and black symbols). The ribonuclease standard curve (grey symbols and line) was constructed from unprocessed ribonuclease. Error bars represent ± 1 standard deviation from the mean (n=6) and where error bars are not shown they lie within the area of the symbol.
However, the increased melting at the higher laser intensities also leads better fusion of one particle to its neighbor. Our observations demonstrate that at the lowest laser intensity, the smaller fused contact area between the individual particles did indeed lead to a fragile sintered structure. Thus there is a very delicate interplay between all of the key factors required to produce an optimized bioactive composite with good mechanical integrity. In these examples, the samples prepared at 125 W cm−2 were found to show sufficient mechanical strength for manipulation during analysis whilst the sample also retained a substantial portion of enzyme activity. In future work we will report on the optimization of spatial resolution and mechanical properties with respect to laser power.
A new method of selective laser sintering of polymer powders is presented. A major advance is that 3D polymeric scaffolds can now be fabricated from a very wide range of biodegradable polymers. Moreover, this "gentle" processing route opens up the possibility of incorporating any bioactive species such as enzymes or growth factors and retaining activity. We have produced biodegradable composite scaffolds of controlled architecture with a spatial resolution in the region of 100 μm. The sintering process is triggered not by volumetric polymer particle absorption of the laser radiation, but by specific and controlled melting of only each particle surface. This was facilitated by incorporation of a small amount of biocompatible carbon powder homogeneously distributed on the polymer surface to absorb the laser energy. In these demonstration experiments ribonuclease A was incorporated into poly(d,l)-lactide matrix scaffolds fabricated at three different laser intensities. Assays of this model enzyme after laser processing demonstrate that ribonuclease activity is substantially retained, and moreover there are no significant changes in the molecular weight or polydispersity of the polymer.
Experimental
Sample Preparation
All materials were purchased from Sigma (UK) unless stated. Ribonuclease A powder (5 wt.-%) was mixed with poly (d,l-lactic acid) powder (Alkermes Medisorb, low inherent viscosity (IV), Mw = 84 kDa (1 Da=1.66053×10−24 g), polydispersity = 1.4) and processed using supercritical carbon dioxide to ensure homogeneous mixing of protein and polymer as described previously [15,16]. Briefly, the high-pressure vessel was pressurized to 173 bar (1 bar = 1 × 105 Pa) and heated to 35°C for 20 min. Upon depressurization a porous polymer monolith containing ribonuclease was formed. This was ground to a fine powder using a pestle and mortar. The particles had a broad size distribution and a mean particle diameter of around 200 μm (determined by SEM). To this PLA/enzyme powder mixture a small amount (< 0.1 wt.-%) of furnace black carbon microparticles (synthesized by Gubkin Oil and Gas Institute, Moscow, Russia) with surface area ca. 100 m2g−1 was added. By mixing carefully, a low concentration of carbon microparticles was homogeneously distributed across the surfaces of the larger PLA/enzyme particles.
PLA/Ribonuclease Characterization
The PLA/ribonuclease sintered products were analyzed by scanning electron microscopy, Fourier-transform (FT) microRaman spectroscopy (Nicolet, Almega Dispersive Raman) and gel permeation chromatography (Polymer Labs). The activity of the ribonuclease prior to and after laser treatment was tested using a specific activity assay (vide infra). SEM was used to determine particle morphology. Samples were mounted on aluminum SEM stubs using double-sided carbon tape (Agar Scientific, UK). The samples were sputter-coated with gold for 4 min under an argon atmosphere in a Blazers SCD 030 sputter-coating unit. Coated samples were examined with a Phillips 505 scanning electron microscope operating at an accelerating voltage of 25 kV. Image analysis was carried out using a Semicaps 2000A (version 8.2) digital imaging system.
Protein Assays
To assay concentration and activity of the ribonuclease, the processed samples were placed in 2 mL of pre-warmed phosphate-buffered saline (pH7.4) for 15 min at 37°C. The samples were then vortexed and filtered through a 0.22 μm filter to remove any polymer and carbon microparticles before further analysis. Sample ribonuclease content was determined using a colorimetric assay based on bicinchoninic acid (BCA) [17] and was used in a kit form (Sigma, UK) using ribonuclease as standard. The specific activity of ribonuclease was determined by the rate of hydrolysis of cytidine 2′:3′-monophosphate and adapted from the method of Crook [18].
Footnotes
The authors are grateful to Dr. Daniel Bratton for his technical advice. The authors also acknowledge the financial support of the Engineering and Physical Sciences Research Council (EPSRC) and the Wellcome Trust (Collaborative Research Initiative Grants No. 062760 and No. 073913). S. M. H is a Royal Society–Wolfson Research Merit Award Holder.
References
- 1.Stock UA, Vacanti JP. Annu. Rev. Med. 2001;52:443. doi: 10.1146/annurev.med.52.1.443. [DOI] [PubMed] [Google Scholar]
- 2.Murphy WL, Peters MC, Kohn DH, Mooney DJ. Biomaterials. 2000;21:2521. doi: 10.1016/s0142-9612(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 3.Fu K, Klibanov AM, Langer R. Nat. Biotechnol. 2000;18:24. doi: 10.1038/71875. [DOI] [PubMed] [Google Scholar]
- 4.Hutmacher DW. Biomaterials. 2000;21:2529. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 5.a) http://www.3Dsystems.com.; b) Antonov EN, Evseev AV, Markov MA, Panchenko VY, Popov VK, Topol'nitsky OZ, Volozhin AI, Doktorov AA, Kurdyumov SG. Opt. Tekh. 1998;13:55. [Google Scholar]
- 6.Pitts JD, Campagnola PJ, Epling GA, Goodman SL. Macromolecules. 2000;33:1514. [Google Scholar]
- 7.Basu S, Campagnola PJ. Biomacromolecules. 2004;5:572. doi: 10.1021/bm0344194. [DOI] [PubMed] [Google Scholar]
- 8.Landers R, Hübner U, Schmelzeisen R, Mülhaupt R. Biomaterials. 2002;23:4437. doi: 10.1016/s0142-9612(02)00139-4. [DOI] [PubMed] [Google Scholar]
- 9.Zein I, Hutmacher DW, Tan KC, Teoh SH. Biomaterials. 2002;23:1169. doi: 10.1016/s0142-9612(01)00232-0. [DOI] [PubMed] [Google Scholar]
- 10.Liew CL, Leong KF, Chua CK, Du Z. Int. J. Adv. Manuf. Technol. 2001;18:717. [Google Scholar]
- 11.Price RL, Waid MC, Haberstroh KM, Webster TJ. Biomaterials. 2003;24:1877. doi: 10.1016/s0142-9612(02)00609-9. [DOI] [PubMed] [Google Scholar]
- 12.Elias KL, Price RL, Webster TJ. Biomaterials. 2002;23:3279. doi: 10.1016/s0142-9612(02)00087-x. [DOI] [PubMed] [Google Scholar]
- 13.Volkin DB, Staubli A, Langer R, Klibanov AM. Biotechnol. Bioeng. 1991;37:843. doi: 10.1002/bit.260370908. [DOI] [PubMed] [Google Scholar]
- 14.Kister G, Cassanas G, Vert M. Polymer. 1998;39:267. [Google Scholar]
- 15.Howdle SM, Watson MS, Whitaker MJ, Popov VK, Davies MC, Mandel FS, Wang JD, Shakesheff KM. Chem. Commun. 2001:109. [Google Scholar]
- 16.Watson MS, Whitaker MJ, Shakesheff KM, Howdle SM. Adv. Mater. 2002;14:1802. [Google Scholar]
- 17.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Anal. Biochem. 1985;150:76. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 18.Crook EM, Mathias AP, Rabin BR. Biochem. J. 1960;74:234. doi: 10.1042/bj0740234. [DOI] [PMC free article] [PubMed] [Google Scholar]