Abstract
Miltefosine (hexadecylphosphocholine [HePC]) is the first orally active antileishmanial drug. Transient HePC treatment of Leishmania donovani promastigotes at 10 μM significantly reduced the phosphatidylcholine content and enhanced the phosphatidylethanolamine (PE) content in parasite membranes, suggesting a partial inactivation of PE-N-methyltransferase. Phospholipase D activity did not seem to be affected by HePC. In addition, the enhancement of the lysophosphatidylcholine content could be ascribed to phospholipase A2 activation. Moreover, transient HePC treatment had no effect on the fatty acid alkyl chain length or the fatty acid unsaturation rate. Concerning sterols, we found a strong reduction of the C24 alkylated sterol content, and the enhancement of the cholesterol content could be the result of the HePC condensation effect with sterols. Because some of the effects observed after transient HePC treatment were different from those previously observed in HePC-resistant parasites, it could be hypothesized that continuous in vitro drug pressure induces the mechanisms of regulation in Leishmania lipid metabolism.
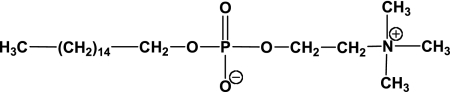
Leishmania is a protozoan parasite responsible for leishmaniasis, a complex of diseases classified according to the different clinical manifestations as cutaneous, invalidant mucocutaneous, and fatal visceral leishmaniases (11). Chemotherapy constitutes the real means of control of all forms of leishmaniasis (6). Unfortunately, the standard drugs administered parenterally, including pentavalent antimonials, amphotericin B, and paromomycin, are toxic and expensive. Moreover, the emergence of resistance to antimonials is now a widespread phenomenon, and almost all these standard treatments are relatively inefficient against leishmaniasis-human immunodeficiency virus coinfection (7). Therefore, the chemotherapy must be improved by developing new efficient, available, and less toxic drugs. Hexadecylphosphocholine (HePC), or miltefosine (Fig. 1), is an alkylphosphocholine initially used for its antitumoral properties, particularly against breast cancer metastases (31). HePC has also been developed for the treatment of visceral leishmaniasis (30). It proved to be the first drug orally active against visceral leishmaniasis, including antimony-resistant cases (12), and also against cutaneous leishmaniasis (29). Moreover, it is hoped that HePC can be used as a treatment for human immunodeficiency virus-Leishmania coinfection (20). HePC was registered in India in 2003 and in Germany in 2004 for the treatment of visceral leishmaniasis.
FIG. 1.
Chemical structure of miltefosine.
The parasite plasma membrane is the unique pharmacokinetic interface between the host and the parasite machineries, and various exchanges take place through this membrane. A defective inward translocation of miltefosine was demonstrated in Leishmania donovani (21), and the HePC transporter was cloned and characterized as a P-type ATPase (22). In addition, because HePC is an amphiphilic molecule, it could interact directly with the cell membrane. We have demonstrated that HePC can insert easily within a phospholipid monolayer, and we found a condensation effect with sterols (25). Moreover, HePC has been described to affect lipid metabolism in cancer cells (8, 9, 13, 14) and in rat neurons (23).
In the study described in this paper, we attempted to verify this assumption for Leishmania by determining the lipid compositions of HePC-treated parasites versus those of nontreated ones, because the finding of a difference in the membrane lipid composition of Leishmania might lead to the identification of an HePC target at the level of lipid metabolism.
MATERIALS AND METHODS
Chemical compounds.
HePC (miltefosine) was from Zentaris (Frankfurt, Germany). Bis(trimethylsilyl)trifluoroacetamide and boron trifluoride etherate were purchased from Sigma-Aldrich (Saint-Quentin Fallavier, France).
Parasite strains and culture.
Promastigote forms of wild-type (WT) Leishmania donovani LV9 (MHOM/ET/67/HU3) clone and L. donovani HePC-R, an HePC-resistant line able to grow up to a concentration of 40 μM HePC (28), were grown in medium 199 (Sigma-Aldrich, Saint-Quentin Fallavier, France) supplemented with 10% inactivated fetal calf serum (Invitrogen, Eragnie, France), 40 mM HEPES (VWR, Paisley, Scotland), 100 μM adenosine (Sigma-Aldrich), and 0.5 mg/liter hemin (Sigma-Aldrich) in the presence of 50 μg/ml gentamicin at 26°C in a dark environment. Drug pressure (40 μM HePC) was added for strain HePC-R.
For lipid analysis, L. donovani promastigotes were cultured in Erlenmeyer flasks at an initial density of 106 promastigotes/ml in 1 liter of the medium defined above. The flasks were placed in an orbital incubator under continuous shaking (150 rpm) at 26°C. HePC treatment was performed with the culture of the WT strain by adding 1 μM or 10 μM HePC aqueous solution 48 h before the promastigotes were harvested.
At the end of logarithmic phase, WT and HePC-treated promastigotes were harvested by centrifugation at 4,000 × g at 4°C and washed three times with large volumes of cold Tris-buffered saline (TBS; 10 mM Tris-HCl, 145 mM NaCl, pH 7). The pellet obtained was considered the total membrane fraction for sterol, fatty acid, and phospholipid determination.
Effect of HePC on promastigote growth.
The effect of HePC on the growth of the L. donovani promastigote culture was observed microscopically by counting the number of cells as a function of the incubation time (24, 48, and 72 h) and as a function of the HePC concentration (20, 10, 5, 2.5, and 1.25 μM).
Cell fractionation and identification of plasma membranes.
In order to obtain a fraction enriched with the plasma membrane, cell fractionation was performed by differential centrifugation by the method of Hasne and Lawrence (10). Briefly, promastigotes were grown in 1 liter of medium as described above, and the parasites were harvested by centrifugation at 4,000 × g for 5 min at 4°C and then washed three times with cold TBS and resuspended in 100 mM Tris/HCl, pH 7.5, containing 1 mM EDTA and 250 mM sucrose. The parasites were lysed by three cycles of freezing (−170°C) and thawing (37°C). Parasite breakage was assessed by phase-contrast microscopy. The lysate was centrifuged at 2,100 × g for 10 min, and the pellet contained the plasma membrane-rich fraction. Tartrate-resistant acid phosphatase was used as a plasma membrane marker (27). Membrane-bound and soluble acid phosphatases were measured at 37°C by using 5 mM p-nitrophenylphosphate in 50 mM acetate buffer, pH 5, in the presence and absence of 200 mM tartrate, respectively. Tartrate-resistant acid phosphatase was mainly recovered in the plasma membrane fraction.
Lipid extraction.
Promastigotes were harvested during the late logarithmic phase of growth by three centrifugations at 4,000 × g for 10 min each at 4°C and washing with cold TBS. The pellet obtained was resuspended in 1 ml TBS.
Total lipids were extracted from whole cells (total membranes), and the fraction was enriched for the plasma membrane by adding 2.50 ml of chloroform and 1.25 ml of methanol to the 1-ml parasite suspension. The mixture was left for at least 1 h. Five sonications for 30 s each were performed at 4°C with a sonifier cell disruptor. After centrifugation at 3,000 × g for 5 min, the lower phase containing total lipids was collected and evaporated to dryness at room temperature under a constant flow of nitrogen gas. The lipids were stored at −80°C.
A check that only one extraction was sufficient for Leishmania was performed, and it was found that a second extraction of the pellet gave no detectable quantity of lipids.
Phospholipid determination.
The phospholipid compositions of the total and the plasma membrane fractions were analyzed from the total lipid extracts by an improved high-pressure liquid chromatography method with a PVA-sil column and evaporative light scattering detector, as described by Gaudin et al. (7a).
Sterol determination.
The resulting pellet (total membranes) and the fraction enriched with the plasma membrane were resuspended in 20 ml of dichloromethane-methanol 2:1 (vol/vol) for about 24 h at 4°C. After centrifugation (11,000 × g, 1 h, 4°C), the extract was evaporated under vacuum. The residue was saponified with 30% KOH in methanol at 80°C for 2 h.
Sterols were extracted from the organic phases three times with hexane, and after evaporation, the residue was dissolved in dichloromethane. An aliquot of clear yellow sterol solution was added to 2 volumes of bis(trimethylsilyl)trifluoroacetamide, and the sealed tubes were heated at 80°C for 1 h. The trimethylsilyl ethers of the sterols were subjected to gas chromatography/mass spectrometry analysis.
Fatty acid determination.
Each aqueous phase of KOH methanolic extracts was acidified with 6 N sulfuric acid to pH 3. Fatty acids were extracted with hexane and transesterified with boron trifluoride-methanol at room temperature for 2 h. Methyl esters were extracted with hexane and redissolved in methanol-diethyl ether (1:1 [vol/vol]) prior to gas-liquid chromatography/mass spectrometry analysis. Gas-liquid chromatography was performed with a 5890 series II chromatograph (Hewlett-Packard) equipped with an Optima delta-3-0.2-μm column from Macherey-Nagel (Düren, Germany) (methyl/phenylsiloxane ratio, 95/5; dimensions, 25 m by 0.2 mm). The carrier gas was helium (1 ml/min). Analysis conditions were as follows: the column was kept at 270°C, the injector was kept at 300°C (splitless mode), and the transfer line was kept at 300°C. The linear gradient for the methyl esters was from 150 to 290°C at 10°C/min for 18 min and from 290 to 350°C at 20°C/min for the last 3 min. An HP 5989 mass spectrometer (Hewlett-Packard) was used in the electron impact mode (70 eV); the ion source was set at 280°C.
PLD assay.
The Amplex Red phospholipase D (PLD) assay kit provides a sensitive method of measuring membrane PLD activity in vitro. In this enzyme-coupled assay, PLD activity is monitored indirectly by using 10-acetyl-3,7-dihydrophenoxasine, called the Amplex Red reagent. First, PLD cleaves the l-α-phosphatidylcholine (PC) substrate to yield choline and phosphatidic acid. Second, choline is oxidized by choline oxidase (choline oxidase from an Alcaligenes sp.) to betaine and H2O2. Finally, H2O2 in the presence of horseradish peroxidase reacts with the Amplex Red reagent to generate the highly fluorescent product resorufin (excitation and emission maxima, ∼563 and 587 nm, respectively), an H2O2 fluorogenic sonde (19, 37). The kit is specific for membrane PLD enzymes, with optimal activity at nearly neutral pH (pH 8). Assays for this isoform of PLD in total and plasma membranes were performed continuously in a fluorescence 96-well microplate with a final reaction volume of 200 μl per assay and a reaction time of 1 h. Purified PLD from Streptomyces chromofuscus and 10 μM H2O2 were used as positive controls.
To 100 μl of sample (total and plasma membranes) or controls in each well were added 100 μl of a reaction mixture containing the Amplex Red reagent at 100 μM, horseradish phosphatase at 2 U/ml, choline oxidase at 0.2 U/ml, and PC at 0.5 mM. After 1 h of incubation at 37°C, the fluorescence was measured with a fluorescence microplate reader (LS50 B; Perkin-Elmer, United Kingdom) at an excitation wavelength of 530 nm and an emission wavelength of 590 nm.
A standard curve was obtained under the same conditions with commercial Streptomyces chromofuscus PLD (Sigma). Enzyme dilutions were prepared in order to obtain a range of concentrations from 10 to 400 mU/ml.
The PLD assay kit can detect PLD levels as low as 10 mU/ml. One unit of PLD is defined as the amount of enzyme that liberates 1.0 μmol of choline from PC per minute at pH 8.0 at 30°C.
RESULTS
In the present study, we describe the biochemical modifications of the phospholipid, fatty acid, and sterol compositions in the membranes of Leishmania donovani WT and HePC-treated promastigotes in order to identify putative HePC targets in Leishmania lipid metabolism. Under our conditions, a 48-h incubation in the presence of 10 μM HePC inhibited 66% of the culture growth, whereas an HePC concentration of 1 μM had no significant effect on parasite growth (Fig. 2).
FIG. 2.
Effect of HePC on Leishmania donovani promastigote growth as a function of incubation time and HePC concentrations (n = 3).
Phospholipid determination.
Seven phospholipid classes were identified in the total and the plasma membranes from L. donovani samples. These phospholipids are phosphatidylglycerol, cardiolipin, phosphatidylinositol, phosphatidylethanolamine (PE), PC, sphingomyelin, and lysophosphatidylcholine (LPC).
The phospholipid composition of L. donovani total membranes is shown in Fig. 3. The major phospholipid was PC, which represented about 45% of the total phospholipid content. Whereas the phospholipid compositions of the WT strain and strain HePC-R were identical, treatment of the WT strain with 10 μM HePC for 48 h was responsible for a 42% diminution in the amount of PC and a 19% increase in the amount of PE.
FIG. 3.
Effects of HePC treatment (10 and 1 μM) on phospholipid composition of Leishmania donovani total membranes (n = 3). PG, phosphatidylglycerol; CL, cardiolipin; PI, phosphatidylinositol; SM, sphingomyelin; ND, not determined.
The phospholipid composition of L. donovani plasma membranes is shown in Fig. 4. The most abundant phospholipid was PE (about 35%), and the phospholipid compositions of the WT strain and strain HePC-R were identical. When cells were treated with 10 μM HePC for 48 h, we observed a 47% diminution in the amount of PC and a 25% increase in the relative amount of PE.
FIG. 4.
Effects of HePC treatment (10 and 1 μM) on phospholipid composition of L. donovani plasma membranes (n = 3). PG, phosphatidylglycerol; CL, cardiolipin; PI, phosphatidylinositol; SM, sphingomyelin; ND, not determined.
In summary, Fig. 2, 3, and 4 show a positive correlation between the effect of HePC on parasite growth and the phospholipid composition. Treatment of strain HePC-R with 1 and 10 μM HePC did not modify the phospholipid composition (data not shown).
Fatty acid determination.
In HePC-treated parasites, the C16 fatty acid content in the promastigote membranes was decreased by 41% compared to that for the nontreated parasites (Table 1). However, HePC did not exhibit a significant effect on the rate of unsaturation of fatty acid alkyl chains.
TABLE 1.
Composition of fatty acids in total membranes from HePC-treated (10 μM) and nontreated promastigotes of L. donovani WT
| Fatty acids | % Total fatty acids ± SD (n = 3)
|
|
|---|---|---|
| WT | HePC treated (10 μM) | |
| Dodecanoate | 1.71 ± 0.21 | 1.84 ± 0.24 |
| Tetradecanoate | 3.14 ± 0.42 | 3.85 ± 0.39 |
| 9-Hexadecenoate | 1.42 ± 0.17 | 1.56 ± 0.17 |
| Hexadecanoate | 9.45 ± 1.10 | 4.80 ± 0.58 |
| 9-Octadecenoate | 33.42 ± 4.21 | 34.22 ± 4.12 |
| Octadecanoate | 21.57 ± 3.76 | 22.33 ± 3.34 |
| Unsaturated fatty acids | 49 | 52 |
Sterol determination.
The cholesterol content was two times higher in HePC-treated parasites than in the WT (Table 2 ). The amount of cholesta-5,7,24-trien-3β-ol was 32 times higher in HePC-treated parasites than in the WT, and the amounts of ergosta-7,24(28)-dien-3-β-ol and ergosta-5,7,24(28)-trien-3-β-ol in HePC-treated parasites were about 1/15 and 1/5 of those in the WT, respectively. Globally, the total C24 alkylated sterol content in HePC-treated parasites was reduced by 43%.
TABLE 2.
Composition of free sterols in total membranes from HePC-treated (10 μM) and nontreated promastigotes of L. donovani WT
| Sterol | RT (min)a | % Total sterol ± SD (n = 3)
|
|
|---|---|---|---|
| WT | HePC treated (10 μM) | ||
| Cholesterol | 1.00 | 32.0 ± 2.4 | 61.2 ± 5.9 |
| 14α-Methylcholesta-5,7,24-trien-3β-ol | 1.02 | 0.2 ± 0.1 | 0.1 ± 0.1 |
| 14α-Methylcholesta-8,24-dien-3β-ol | 1.04 | 0.1 ± 0.1 | 0.2 ± 0.1 |
| Cholesta-5,7,24-trien-3β-ol | 1.07 | 0.1 ± 0.1 | 3.2 ± 0.5 |
| Ergosterol | 1.10 | 25.8 ± 2.6 | 26.3 ± 2.8 |
| Ergosta-5,7,22,24(241)-tetraen-3β-ol | 1.13 | 1.9 ± 0.1 | 1.6 ± 0.2 |
| Ergosta-5,7,24(241)-trien-3β-ol | 1.17 | 25.4 ± 2.9 | 5.1 ± 0.6 |
| Stigmasta-7,24(241)-dien-3β-ol | 1.18 | 0.2 ± 0.1 | 0.3 ± 0.1 |
| Ergosta-7,22-dien-3β-ol | 1.19 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Ergosta-7,24(241)-dien-3β-ol | 1.20 | 6.1 ± 0.6 | 0.4 ± 0.1 |
| 14α-Methylergosta-5,7,24(241)-trien-3β-ol | 1.21 | 0.3 ± 0.1 | 0.3 ± 0.2 |
| 4,4α-Dimethylcholesta-8,24-dien-3β-ol | 1.22 | 0.3 ± 0.1 | 0.2 ± 0.1 |
| 4α,14α-Dimethylcholesta-8,24-dien-3β-ol | 1.23 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| 14α-Methylergosta-8,24(241)-dien-3β-ol | 1.25 | 0.2 ± 0.1 | 0.3 ± 0.1 |
| 4,4α-Dimethylergosta-8,24(241)-dien-3β-ol | 1.27 | 0.9 ± 0.1 | 0.7 ± 0.1 |
| 4α,14α-Dimethylergosta-8,24(241)-dien-3β-ol | 1.28 | 0.5 ± 0.1 | 0.2 ± 0.1 |
| 24-Methylenedihydrolanosterol | 1.45 | 1.9 ± 0.2 | 1.4 ± 0.1 |
| Total C24 alkyl sterols | 63.2 | 36.6 | |
RT, retention time relative to that of cholesterol by gas chromatography.
Effect of HePC on PLD activity.
The decrease in the PC content observed after treatment with 10 μM HePC could be the result of PC degradation involving phospholipases. We decided to check whether PLD was involved in this process. HePC could be a PLD pseudosubstrate because of its chemical structure. Moreover, a PLD stimulation was described in tumoral cells by Wieder et al. (36) and Lucas et al. (16) after HePC treatment.
PLD activity was found to be the same in all strains: the WT, strain HePC-R, and the HePC-treated WT (data not shown). Therefore, PLD activity was not affected by the transient and long-term action of HePC.
DISCUSSION
The effect of HePC on lipid metabolism in cancer cells has been described previously (13, 14, 33, 35), and it was interesting to investigate whether HePC has similar effects on Leishmania. Therefore, we report in this paper on an analysis of Leishmania donovani membrane lipids in parasites stressed with HePC at sublethal concentrations in order to know whether the mechanism of action of HePC in Leishmania promastigotes involves lipid metabolism. We confirmed that the main phospholipids in the total and plasma membranes of WT parasites were PC and PE, respectively, as described previously by Wassef et al. (34).
The phospholipid compositions of all membrane fractions were similar in WT and HePC-resistant Leishmania strains, as has been described in tumor cells (3).
This study shows that PC metabolism in L. donovani promastigotes was significantly affected by HePC since a diminution in the amount of PC was found after a temporary HePC treatment. In addition, there is a causal relationship between PC biosynthesis inhibition and growth arrest, since HePC was observed to have no effect on strain HePC-R. From these results we can hypothesize that long-term HePC treatment has different consequences on lipid metabolism, suggesting the establishment of a regulation process in lipid metabolism.
At the level of PC biosynthesis, the reductions in the amounts of PC in L. donovani total and plasma membranes could be ascribed to the HePC inhibition of PC synthesis via CDP-choline pathways by acting upon CTP-phosphocholinecytidylyltransferase activity. PC synthesis via the CDP-choline pathway requires transport of the choline precursor from the host. This choline transport was first characterized in L. major promastigotes (38). Those authors have reported that this transport is highly specific for choline and is inhibited by HePC and other molecules. This result is emphasized by observations obtained with other cells. Inhibition of exogenous choline incorporation to PC (de novo synthesis) by HePC was also observed in a large panel of cell lines (8, 9, 13, 23). This inhibitory effect is the consequence of an alteration in CTP-phosphocholinecytidylyltransferase activity in tumor cells (13, 14, 33, 35).
The increase in the relative amount of PE could be the result of the stimulation of CTP-PE cytidylyltransferase activity and/or the inhibition of PE-N-methyltransferase activity, which would also lead to a decrease in the amount of PC, as PC and PE are linked in the same biochemical pathway. As we observed a simultaneous decrease in the amount of PC and an increase in the amount PE, the inhibition of N-methyltransferase activity by HePC in L. donovani promastigotes is probably the more convincing hypothesis. Moreover, such a phenomenon induced by HePC has already been observed in the protozoan parasite Trypanosoma cruzi (15), and the inhibition of N-methyltransferase activity by HePC has also been described in tumor cells (14).
This study shows that transient HePC pressure did not exhibit consequences that were the same as those that we recently observed (26) after strong and durable HePC pressure. Thus, transient HePC pressure is responsible for a decrease in the amount of PC and an increase in the amount of PE, suggesting partial PE-N-methyltransferase inactivation, whereas such a phenomenon was not observed in strain HePC-R parasites. It seems that long-term pressure allows biochemical adaptation of the parasite.
In order to explain the PC degradation, we verified the possible involvement of PLD, as HePC has the chemical structure of a possible effector of PLD. Two isoforms of PLD with different subcellular localizations and optimal pH values were described in L. donovani promastigotes. PLD1 is localized in the perinuclear region with an optimal pH of 6; PLD2 is in the plasma membrane and has an optimal pH of 8 (4). We report in this study that PLD activity was not affected by HePC in L. donovani parasites. This result coincides with the observations of Berkovic et al. for a leukemic cell line (1). In contrast, it differs from those reported for other tumor cells, where HePC induced PLD stimulation (36) or modulation in the amount of PLD according to the HePC exposure frequency (16). However, our result concerning PLD should be considered preliminary, and a more extensive enzymology investigation of PLD in L. donovani must be run to draw conclusions about the effect of HePC on PLD activity.
The reduction in the amount of PC in membranes could also be ascribed to an enhanced specific activity of phospholipase C-PC that results in the release of second messenger lipids putatively involved in cell signaling. This assumption is contradictory to the observation of HePC inhibition of PLC-PC specific for leukemia cells (2).
The LPC content in the total membranes of the WT parasite and strain HePC-R is about 8.5%, and it was considerably increased when the cells were treated with HePC at 10 μM for 48 h. Classically, the presence of LPC is ascribed to PC degradation during sample preparation and/or assay. After the sonication process described in the experimental part of this study, no standard PC degradation was observed, suggesting that sonication was not responsible for the PC degradation. Since total membranes were directly obtained from the total cell extraction with organic solvents, creating conditions in which enzymes were inactivated, phospholipase A2 cannot be considered responsible for PC degradation.
Plasma membranes were found to contain an amount of LPC that represented about 30% of the total phospholipids, and treatment with HePC at 10 μM did not affect this percentage. Whereas the LPC content in plasma membranes was not modified by HePC treatment, the content in total membranes was enhanced three times, suggesting the internalization of HePC and the potential activation of phospholipase A2.
We were not able to measure the PS content variation potentially induced by HePC treatment since the PS contents were under the threshold of detection of our method. In tumor cells, the biosynthesis of this aminophospholipid, which is asymmetrically distributed within the membrane, is unaffected by HePC (14).
We used a saponification method to cleave phospholipids, releasing fatty acids whose alkyl chain lengths and unsaturation rates were analyzed by gas chromatography/mass spectrometry. The transient effect of HePC on fatty acid alkyl chains was completely different from those observed after continuous in vitro drug pressure. Whereas a transient action of HePC had no significant effect on the global C16 and C18 alkylated chain contents, continuous drug pressure was responsible for a significant increase in the C16 alkyl chain content and a diminution of the C18 alkyl chain contents (26). Furthermore, transient HePC treatment had no effect on the unsaturation rate of fatty acid alkyl chains, whereas continuous drug pressure diminished the unsaturated fatty acid content (26). These observations also suggest the possibility of the presence of a regulation system activated by a continuous drug pressure.
Treatment with HePC was responsible for an enhancement in the amount of cholesterol within the membranes of two times. Since cholesterol is not biosynthesized by the parasite but is taken up from the external medium, we can hypothesize that HePC promotes cholesterol uptake in promastigotes perhaps by the condensation effect between HePC and sterols that we described previously (25). Such a phenomenon was recently described by using HePC-resistant L. donovani (30). In addition, we previously found similar results using amphotericin B-resistant L. donovani (18) and atovaquone-resistant Leishmania infantum promastigotes (5), suggesting that these drugs with an affinity for sterols could participate in cholesterol recruitment within the membranes. Further experiments should be carried out to explain the role of cholesterol as a consequence or a cause of drug effect.
We found a decrease in the amount of C24 alkyl sterols in HePC-treated membranes of about two times, even though the amount of the final C24 sterol alkylation product, ergosterol, was not changed. Since the amount of cholesta-5,7,24-trien-3β-ol was 32 times higher in HePC-treated parasites than in the WT and the amounts of ergosta-7,24(241)-dien-3-β-ol and ergosta-5,7,24(241)-trien-3-β-ol in HePC-treated parasites were about 1/15 and 1/5 of those in the WT, respectively, we can deduce that sterol alkylation occurs late in the pathway but has an efficient yield. The expression of the enzyme which catalyzes the incorporation of these alkyl groups, S-adenosyl-l-methionine-C24-delta-sterol-methyltransferase (SCMT), needs to be studied to understand this phenomenon. SCMT, which is an ideal chemotherapeutic target since C24 alkyl sterols are absent from the sterol metabolism of vertebrates (32), could be regulated by antileishmanial drugs. Thus, we previously described that SCMT is involved in the mechanism of amphotericin B resistance in Leishmania donovani (24). Besides the involvement of SCMT, the modest level of accumulation of cholesta-5,7,24-trien-3β-ol in treated cells cannot account for the marked reduction in the amount of mature sterols, suggesting a possible blockade at the prelanosterol level.
HePC was also described to inhibit the alkyl-specific acyl coenzyme A acyltransferase, an enzyme of the ether-lipid remodeling in Leishmania, at high concentrations (50 μM) (17). This study shows that transient HePC treatment significantly affects the lipid metabolism at the level of the polar head groups of phospholipids in L. donovani promastigotes. The effect of HePC on the phospholipid and sterol compositions of Leishmania membranes could be compared with the results obtained by Lira et al., who observed an inversion of the PC/PE ratio that was secondary to the altered sterol composition of the cell membranes in Trypanosoma cruzi (15).
These findings for L. donovani are similar to those described by Lira et al. (15) for Trypanosoma cruzi and suggest that the selective activity of HePC on the Kinetoplastida could result from its effect on the PC biosynthesis pathway present in these cells (the Greenberg or the PE-transmethylation route) compared with that used in mammalian cells (the Kennedy or the CDP-choline pathway).
Further studies will focus on the enzyme systems suspected to be affected by HePC, such as PE-N-methyltransferase and phospholipase A2.
Acknowledgments
This study was supported by an EC grant (grant QLRT-2000-01404).
We are grateful to the Zentaris Company (Frankfurt, Germany) for providing HePC and to Simon L. Croft (London, United Kingdom) for kindly providing us with the promastigote forms of WT Leishmania donovani and the derived HePC-R strain.
Footnotes
Published ahead of print on 22 January 2007.
REFERENCES
- 1.Berkovic, D., K. Berkovic, C. Binder, D. Haase, and E. A. Fleer. 2002. Hexadecylphosphocholine does not influence phospholipase D and sphingomyelinase activity in human leukemia cells. J. Exp. Ther. Oncol. 2:213-218. [DOI] [PubMed] [Google Scholar]
- 2.Berkovic, D., M. Goeckenjan, S. Lüders, W. Hiddemann, and E. A. Fleer. 1996. Hexadecylphosphocholine inhibits phosphatidylinositol and phosphatidylcholine phospholipase C in human leukemia cells. J. Exp. Ther. Oncol. 2:85-92. [PubMed] [Google Scholar]
- 3.Berkovic, D., D. Haase, W. Hiddemann, and E. A. Fleer. 1996. Induction of resistance in the human leukemia cell line HL60 towards hexadecylphosphoholine and other ether phospholipid analogues. J. Exp. Ther. Oncol. 1:368-376. [PubMed] [Google Scholar]
- 4.Blum, J. J., J. A. Lehman, J. M. Horn, and J. Gomez-Cambronero. 2001. Phospholipase D (PLD) is present in Leishmania donovani and its activity increases in response to acute osmotic stress. J. Eukaryot. Microbiol. 48:102-110. [DOI] [PubMed] [Google Scholar]
- 5.Cauchetier, E., P. M. Loiseau, J. Lehman, D. Rivollet, J. Fleury, A. Astier, M. Deniau, and M. Paul. 2002. Caracterisation of atovaquone resistance in Leishmania infantum promastigotes. Int. J. Parasitol. 32:1043-1051. [DOI] [PubMed] [Google Scholar]
- 6.Croft, S. L., K. Seifert, and V. Yardley. 2006. Current scenario of drug development for leismaniasis. Indian J. Med. Res. 123:399-410. [PubMed] [Google Scholar]
- 7.Croft, S. L., and V. Yardley. 2002. Chemotherapy of leishmaniasis. Curr. Pharm. Des. 8:319-342. [DOI] [PubMed] [Google Scholar]
- 7a.Gaudin, K., M. Rakotomanga, S. Blanc, A. Baillet, P. M. Loiseau, and P. Chaminade. 2004. Determination of the phospholipid composition of Leishmania donovani membranes by normal phase chromatography with a polyvinyl alcohol stationary phase and evaporative light scattering detection, abstr. B7, p. 118. 25th Int. Symp. Chromatogr., Paris, France, 4 to 8 October 2004.
- 8.Geilen, C. C., T. Wieder, and W. Reutter. 1992. Hexadecylphosphocholine inhibits translocation of CTP-choline-phosphate cytidylyltransferase in Madin-Darby canine kidney cells. J. Biol. Chem. 267:6719-6724. [PubMed] [Google Scholar]
- 9.Haase, R., T. Wieder, C. C. Geilen, and W. Reutter. 1991. The phospholipid analogue hexadecylphosphocholine inhibits phosphatidylcholine biosynthesis in Madin-Darby canine kidney cells. FEBS Lett. 288:129. [DOI] [PubMed] [Google Scholar]
- 10.Hasne, M. P., and F. Lawrence. 1999. Characterization of prenylated protein methyltransferase in Leishmania. Biochem. J. 342:513-518. [PMC free article] [PubMed] [Google Scholar]
- 11.Herwaldt, B. L. 1999. Leishmaniasis. Lancet 354:1191-1199. [DOI] [PubMed] [Google Scholar]
- 12.Jha, T. K., S. Sundar, C. P. Thakur, P. Bachmann, J. Karbwang, C. Fischer, A. Voss, and J. Berman. 1999. Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis. N. Engl. J. Med. 341:1795-1800. [DOI] [PubMed] [Google Scholar]
- 13.Jimenez-Lopez, J. M., M. P. Carrasco, J. L. Segovia, and C. Marco. 2002. Hexadecylphosphocholine inhibits phosphatidylcholine biosynthesis and the proliferation of HepG2 cells. Eur. J. Biochem. 269:4649-4655. [DOI] [PubMed] [Google Scholar]
- 14.Jimenez-Lopez, J. M., M. P. Carrasco, J. L. Segovia, and C. Marco. 2003. Hexadecylphosphocholine inhibits phosphatidylcholine synthesis via both the methylation of phosphatidylethanolamine and CDPcholine pathways in HePG2 cells. Int. J. Biochem. Cell Biol. 36:153-161. [DOI] [PubMed] [Google Scholar]
- 15.Lira, R., L. M. Contreras, R. M. Santa Rita, and J. A. Urbina. 2001. Mechanism of action of anti-proliferative lysophospholipid analogues against the protozoan parasite Trypanosoma cruzi: potentiation of in vitro activity by the sterol biosynthesis inhibitor ketoconazole. Antimicrob. Agents Chemother. 47:537-546. [DOI] [PubMed] [Google Scholar]
- 16.Lucas, L., R. Hernadez-Alcoceba, V. Panalva, and J. C. Lacal. 2001. Modulation of phospholipase D by hexadecylphosphocholine: a putative novel mechanism for its antitumoral activity. Oncogene 20:1110-1117. [DOI] [PubMed] [Google Scholar]
- 17.Lux, H., N. Heise, T. Klenner, D. Hart, and F. R. Opperdoes. 2000. Ether-lipid (alkyl-phospholipid) metabolism and the mechanism of action of ether-lipid analogues in Leishmania. Mol. Biochem. Parasitol. 111:1-14. [DOI] [PubMed] [Google Scholar]
- 18.Mbongo, N., P. M. Loiseau, M. A. Billion, and M. Robert-Gero. 1998. Mechanism of amphotericin B resistance in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 42:352-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohanty, J. G., J. S. Jaffe, E. S. Schulman, and D. G. Raible. 1997. A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. J. Immunol. Methods 202:133-141. [DOI] [PubMed] [Google Scholar]
- 20.Murray, H. W. 2000. Suppression of post treatment recurrence of experimental visceral leishmaniasis in T-cell-deficient mice by oral miltefosine. Antimicrob. Agents Chemother. 44:3235-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez-Victoria, F. J., S. Castanys, and F. Gamarro. 2003. Leishmania donovani resistance to miltefosine involves a defective inward translocation of the drug. Antimicrob. Agents Chemother. 47:2397-2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez-Victoria, F. J., F. Gamarro, M. Ouellette, and S. Castanys. 2003. Functional cloning of the miltefosine transporter, a novel P-type phospholipid translocase from Leishmania involved in drug resistance. J. Biol. Chem. 278:49965-49971. [DOI] [PubMed] [Google Scholar]
- 23.Posse de Chaves, E., D. E. Vance, R. B. Campenot, and J. E. Vance. 1995. Alkylphosphocholines inhibit choline uptake and phosphatidylcholine biosynthesis in rat sympathetic neurons and axonal extension. Biochem. J. 312:411-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pourshafie, M., S. Morand, A. Virion, M. Rakotomanga, C. Dupuy, and P. M. Loiseau. 2004. Cloning of S-adenosyl-l-methionine:C-24-delta-sterol-methyltransferase (ERG6) from Leishmania donovani and characterization of mRNAs in wild-type and amphotericin B-resistant promastigotes. Antimicrob. Agents Chemother. 48:2409-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rakotomanga, M., P. M. Loiseau, and M. Saint-Pierre-Chazalet. 2004. Hexadecylphosphocholine interaction with lipid monolayers. Biochim. Biophys. Acta 1661:212-218. [DOI] [PubMed] [Google Scholar]
- 26.Rakotomanga, M., M. Saint-Pierre-Chazalet, and P. M. Loiseau. 2005. Alteration of fatty acid and sterol metabolism in miltefosine-resistant Leishmania donovani promastigotes and consequences for drug membrane interactions. Antimicrob. Agents Chemother. 49:2677-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Remelay, A. T., S. Das, P. I. Campbell, G. M. LaRocca, M. Pope, and R. H. Glew. 1985. Characterization of Leishmania donovani acid phosphatases. J. Biol. Chem. 260:880-886. [PubMed] [Google Scholar]
- 28.Seifert, K., S. Matu, F. J. Perez-Victoria, S. Castanys, F. Gamarro, and S. L. Croft. 2003. Characterization of Leishmania donovani promastigotes resistant to hexadecylphosphocholine (miltefosine). Int. J. Antimicrob. Agents 22:380-387. [DOI] [PubMed] [Google Scholar]
- 29.Soto, J., J. Toledo, P. Gutierez, R. S. Nicholls, J. Padilla, J. Engel, C. Fischer, A. Voss, and J. Berman. 2001. Treatment of American cutaneous leishmaniasis with miltefosine, an oral agent. Clin. Infect. Dis. 33:57-61. [DOI] [PubMed] [Google Scholar]
- 30.Sundar, S., A. Makharia, D. K. More, G. Agrawal, A. Voss, C. Fischer, P. Bachmann, and H. W. Murray. 2000. Short-course of oral miltefosine for treatment of visceral leishmaniasis. Clin. Infect. Dis. 31:1110-1113. [DOI] [PubMed] [Google Scholar]
- 31.Unger, C., M. Peukert, H. Sindermann, P. Hilgard, G. Nagel, and H. Eibl. 1992. Hexadecylphosphocholine in the topical treatment of skin metastases in breast cancer patients. Prog. Exp. Tumor Res. 34:153-159. [DOI] [PubMed] [Google Scholar]
- 32.Urbina, J. A. 1997. Lipid biosynthesis pathways as chemotherapeutic targets in kinetoplastid parasites. Parasitology 114(Suppl.):S91-S99. [PubMed] [Google Scholar]
- 33.Vogler, W. R., M. Shoji, D. J. Hayzer, Y. P. Xie, and M. Renshaw. 1996. The effect of edelfosine on CTP:cholinephosphate cytidylyltransferase activity in leukemic cell lines. Leuk. Res. 20:947-951. [DOI] [PubMed] [Google Scholar]
- 34.Wassef, M. K., T. B. Fioretti, and D. M. Dwyer. 1985. Lipid analysis of isolated surface membranes of Leishmania donovani promastigotes. Lipids 20:108-115. [DOI] [PubMed] [Google Scholar]
- 35.Wieder, T., A. Haase, C. C. Geilen, and C. E. Orfanos. 1995. The effect of two synthetic phospholipids on cell proliferation and phosphatidylcholine biosynthesis in Madin-Darby canine kidney cells. Lipids 30:389-393. [DOI] [PubMed] [Google Scholar]
- 36.Wieder, T., Z. Zhang, C. C. Geilen, C. E. Orfanos, A. E. Giuliano, and M. C. Cabot. 1996. The antitumor phospholipid analog, hexadecylphosphocholine, activates phospholipase D. Cancer Lett. 100:71-79. [DOI] [PubMed] [Google Scholar]
- 37.Zhou, M., Z. Diwu, N. Panchuk-Voloshina, and R. P. Haugland. 1997. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal. Biochem. 253:162-168. [DOI] [PubMed] [Google Scholar]
- 38.Zufferey, R., and C. Ben Mamoun. 2002. Choline transport in Leishmania major promastigotes and its inhibition by choline and phosphocholine analogs. Mol. Biochem. Parasitol. 125:127-134. [DOI] [PubMed] [Google Scholar]