Abstract
Boronic acid transition state inhibitors (BATSIs) with R1 side chains of cefotaxime and ceftazidime were assayed against SHV-1, SHV-2, SHV-5, D104K, and D104K G238S β-lactamases. The D104K variant was the most susceptible to inhibition by the ceftazidime BATSI (Ki, 730 ± 80 nM), while the D104K G238S variant was the most susceptible to the cefotaxime BATSI (Ki, 1.1 ± 0.2 μM).
Resistance to β-lactam antibiotics is a major public health threat resulting in failure of treatment of serious infections due to gram-negative pathogens. The principal mediator of resistance to penicillins and cephalosporins is the production of β-lactamase enzymes by bacteria. To maintain the effectiveness of β-lactam antibiotics, two strategies are employed: (i) to synthesize new β-lactam compounds that resist hydrolysis and (ii) to develop potent β-lactamase inhibitors (4).
The first strategy fostered the development of five “generations” of cephalosporins. When initially released into clinical practice, extended-spectrum cephalosporins resisted hydrolysis by the prevalent class-A β-lactamases, TEM-1 and SHV-1. Unfortunately, single amino acid substitutions in these related enzymes resulted in the emergence of the extended-spectrum β-lactamase (ESBL) phenotype. The premier mutation responsible for this phenotype in the SHV β-lactamase family is a G238S substitution (5, 6). The 1- to 3-Å binding pocket expansion caused by this substitution may be required for the bulky oxyimino-cephalosporins to bind in the active site (6, 7, 13).
Analysis of naturally occurring TEM and SHV ESBL variants has also identified lysine residues at Ambler positions 104 and 240 as contributing to cephalosporin resistance. These substitutions (104K and 240K), studied in TEM, and recently studied in SHV by our laboratory, are hypothesized to increase affinity via hydrogen bonding or ionic interactions with the R1 oxyimino side chains of the β-lactam (1, 8, 15). However, the multistep nature of cephalosporin hydrolysis, the inability to trap intermediates, and low turnover by SHV-1 limit an in-depth study of the effects of amino acid substitution on the ESBL phenotype.
The quest for novel inhibitors of class A β-lactamases led to the discovery of the boronic acid transition state inhibitors (BATSIs). These compounds are effective, reversible inhibitors of AmpC, TEM, and CTX-M β-lactamases, and they also are remarkable probes for studying active-site chemistry (2, 9, 10). Using our work with inhibitor-resistant SHV β-lactamases as a guide, we reasoned that BATSIs could also be used to investigate enzyme-substrate interactions that define the unique properties of the ESBL phenotype in SHV β-lactamase (14). To this end, we tested inhibitors that contain the R1 side chains of two extended-spectrum cephalosporins, cefotaxime and ceftazidime, attached to boronic acid (Fig. 1). We use these new compounds to gain fresh insight into the contributions of the single amino acid substitutions that confer resistance to the extended-spectrum cephalosporins. Additionally, we show that these molecules have lower inhibitor constant (Ki) values against some SHV enzymes displaying the ESBL phenotype.
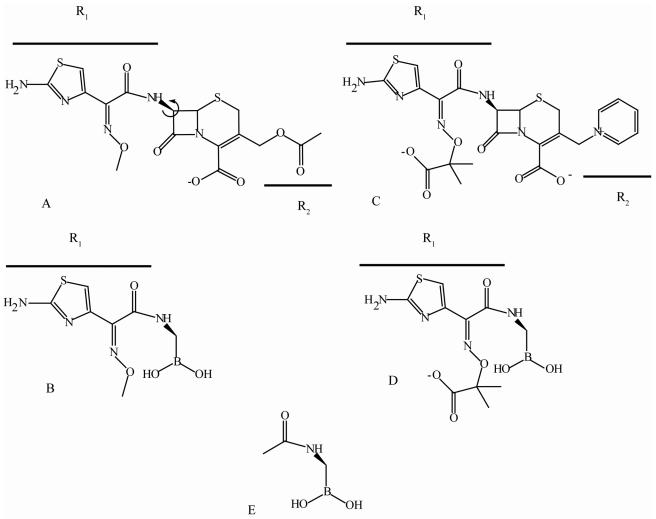
FIG. 1.
Structures of cefotaxime (A), the cefotaxime BATSI (B), ceftazidime (C), the ceftazidime BATSI (D), and a reference compound that lacks the oxyimino-cephalosporin R1 side chain (E). The arrow in the structure of compound A indicates the direction of proposed rotation in the binding pocket as discussed in the text.
β-Lactamase genes encoding SHV-1, -2, -and 5, as well as the D104K and D104K G238S variants, were cloned into pB CSK(−) as previously described (1, 6, 12). β-Lactamases were expressed in Escherichia coli DH10B cells and were initially purified as described elsewhere (6). SHV enzymes were quantified, and purity was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The SHV-2 and D104K β-lactamases and the doubly substituted D104K G238S β-lactamase were further purified by size exclusion chromatography using a Waters high-performance liquid chromatography system (1).
Kinetic constants were measured by continuous assays at room temperature using an Agilent 8453 diode array spectrophotometer. Ki values were determined by competing increasing concentrations of BATSIs against the colorimetric substrate nitrocefin at 4 to 6 times the Michaelis constant (Km) for the enzyme. Data were analyzed using Origin 7.5 SR2 software and the equation
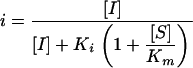 |
where i is percent inhibition, [S] is the nitrocefin concentration, [I] is the inhibitor concentration, and Km is the Km for nitrocefin (3). BATSIs were synthesized as previously reported (9). In addition, full progress curves were performed both in the absence of inhibitor and with two concentrations of each BATSI (5 μM and 10 μM for the ceftazidime BATSI; 10 μM and 20 μM for the cefotaxime BATSI), while the nitrocefin concentration was varied to determine the mode of inhibition. Results were graphed as Lineweaver-Burk plots (1/[S] versus 1/v, where v is the initial reaction velocity [μMs−1]), and points were fit by linear regression with Origin 7.5 SR2 software.
We found that BATSIs with the R1 side chains of two extended-spectrum cephalosporins bind SHV β-lactamases with micromolar affinity (Table 1). This was noted for both the ceftazidime BATSI and the cefotaxime BATSI. Both BATSIs inactivated SHV-1 in a time-independent fashion; the cefotaxime BATSI had a truly competitive mode of inhibition, while the ceftazidime BATSI exhibited mixed inhibition (data not shown). We compared these to a BATSI lacking an R1 side chain (reference compound [Fig. 1E]). As shown, the reference compound exhibited poor affinity for all enzymes (Table 1). This confirms the importance of R1 side chain contacts in defining substrate-enzyme interactions.
TABLE 1.
Affinities of BATSIs and a reference compound for β-lactamases
| β-Lactamase |
Ki (μM) of:
|
||
|---|---|---|---|
| Cefotaxime BATSI | Ceftazidime BATSI | Reference compound | |
| SHV-1 | 8.9 ± 0.9 | 2.2 ± 0.2 | 300 ± 15 |
| SHV-2 | 3.4 ± 0.7 | 6.8 ± 0.9 | 330 ± 10 |
| SHV-5 | 5.0 ± 0.5 | 4.5 ± 0.5 | 360 ± 20 |
| D104K | 5.0 ± 0.5 | 0.7 ± 0.1 | 190 ± 20 |
| G238S D104K | 1.1 ± 0.2 | 4.5 ± 0.3 | 300 ± 30 |
In general, the SHV ESBLs demonstrated greater affinity for the cefotaxime BATSI (Fig. 1B) than wild-type SHV-1. The substitutions at positions 104 and 238 lowered Ki values from 8.9 ± 0.9 μM for SHV-1 to 1.1 ± 0.2 μM for the D104K G238S variant (Table 1). This increase in affinity is in line with the observation that the G238S substitution is a “cefotaximase”-specific mutation in SHV (1, 6).
The ceftazidime BATSI had a similar range of binding affinities [6.8 ± 0.9 for SHV-2 (G238S) to 0.7 ± 0.1 μM for the D104K variant]. The relatively low Ki of the ceftazidime BATSI for SHV-1 was an unexpected finding. Taken together, these Ki measurements recall the observations made by Wang et al. in comparing TEM-1 and TEM-52 affinities for the same BATSI (15). They also describe a higher affinity of the ceftazidime BATSI for TEM-1 as compared to TEM ESBLs. This leads us to wonder whether the main impact of widening the active-site cavity with the G238S substitution on class A β-lactamases is on (i) the precovalent encounter complex or (ii) substrate reactivity postbinding. Clearly, the G→S substitution increases the kcat of TEM-19 and the MICs of SHV-2 for ceftazidime (6, 11).
These data support our previous studies of the importance of the D104K mutation for substrate hydrolysis by SHV (1). It is now evident that Lys at position104 plays a significant role in oxyimino-cephalosporin binding by SHV. In this analysis, the β-lactamase containing the 104K substitution demonstrates greater affinity for the BATSIs. Further studies are needed to investigate if the R1 side chain rotates in the binding pocket to align a carbonyl oxygen of the side chain with the Lys at position 104 (Fig. 1A) (1). BATSIs may be providing insights into affinity that are not possible with oxyimino-cephalosporin substrates because of poor turnover.
Acknowledgments
This work was supported in part by the Veterans Affairs Medical Center Merit Review Program and National Institutes of Health (NIH) grant 1R01 A1063517-01. J.M.T. was supported in part by NIH grant T32 GM07250 and the Case Medical Scientist Training Program. F.P. was supported by the Fondazione Cassa di Risparmio di Modena.
Footnotes
Published ahead of print on 12 January 2007.
REFERENCES
- 1.Bethel, C. R., A. M. Hujer, K. M. Hujer, J. M. Thomson, M. W. Ruszczycky, V. E. Anderson, M. Pusztai-Carey, M. Taracila, M. S. Helfand, and R. A. Bonomo. 2006. Defining the role of Asp104 in the SHV beta-lactamase. Antimicrob. Agents Chemother. 50:4124-4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, Y., B. Shoichet, and R. Bonnet. 2005. Structure, function, and inhibition along the reaction coordinate of CTX-M beta-lactamases. J. Am. Chem. Soc. 127:5423-5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng, Y., and W. H. Prusoff. 1973. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 22:3099-3108. [DOI] [PubMed] [Google Scholar]
- 4.Fisher, J. F., S. O. Meroueh, and S. Mobashery. 2005. Bacterial resistance to beta-lactam antibiotics: compelling opportunism, compelling opportunity. Chem. Rev. 105:395-424. [DOI] [PubMed] [Google Scholar]
- 5.Hujer, A. M., K. M. Hujer, and R. A. Bonomo. 2001. Mutagenesis of amino acid residues in the SHV-1 beta-lactamase: the premier role of Gly238Ser in penicillin and cephalosporin resistance. Biochim. Biophys. Acta 1547:37-50. [DOI] [PubMed] [Google Scholar]
- 6.Hujer, A. M., K. M. Hujer, M. S. Helfand, V. E. Anderson, and R. A. Bonomo. 2002. Amino acid substitutions at Ambler position Gly238 in the SHV-1 beta-lactamase: exploring sequence requirements for resistance to penicillins and cephalosporins. Antimicrob. Agents Chemother. 46:3971-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orencia, M. C., J. S. Yoon, J. E. Ness, W. P. Stemmer, and R. C. Stevens. 2001. Predicting the emergence of antibiotic resistance by directed evolution and structural analysis. Nat. Struct. Biol. 8:238-242. [DOI] [PubMed] [Google Scholar]
- 8.Petit, A., L. Maveyraud, F. Lenfant, J. P. Samama, R. Labia, and J. M. Masson. 1995. Multiple substitutions at position 104 of beta-lactamase TEM-1: assessing the role of this residue in substrate specificity. Biochem. J. 305:33-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powers, R. A., E. Caselli, P. J. Focia, F. Prati, and B. K. Shoichet. 2001. Structures of ceftazidime and its transition-state analogue in complex with AmpC beta-lactamase: implications for resistance mutations and inhibitor design. Biochemistry 40:9207-9214. [DOI] [PubMed] [Google Scholar]
- 10.Powers, R. A., and B. K. Shoichet. 2002. Structure-based approach for binding site identification on AmpC beta-lactamase. J. Med. Chem. 45:3222-3234. [DOI] [PubMed] [Google Scholar]
- 11.Raquet, X., J. Lamotte-Brasseur, E. Fonze, S. Goussard, P. Courvalin, and J. M. Frere. 1994. TEM beta-lactamase mutants hydrolysing third-generation cephalosporins. A kinetic and molecular modelling analysis. J. Mol. Biol. 244:625-639. [DOI] [PubMed] [Google Scholar]
- 12.Rice, L. B., L. L. Carias, A. M. Hujer, M. Bonafede, R. Hutton, C. Hoyen, and R. A. Bonomo. 2000. High-level expression of chromosomally encoded SHV-1 beta-lactamase and an outer membrane protein change confer resistance to ceftazidime and piperacillin-tazobactam in a clinical isolate of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 44:362-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun, T., M. Nukaga, K. Mayama, E. H. Braswell, and J. R. Knox. 2003. Comparison of beta-lactamases of classes A and D: 1.5- Å crystallographic structure of the class D OXA-1 oxacillinase. Protein Sci. 12:82-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomson, J. M., A. M. Distler, F. Prati, and R. A. Bonomo. 2006. Probing active site chemistry in SHV beta-lactamase variants at Ambler position 244. Understanding unique properties of inhibitor resistance. J. Biol. Chem. 281:26734-26744. [DOI] [PubMed] [Google Scholar]
- 15.Wang, X., G. Minasov, J. Blazquez, E. Caselli, F. Prati, and B. K. Shoichet. 2003. Recognition and resistance in TEM beta-lactamase. Biochemistry 42:8434-8444. [DOI] [PubMed] [Google Scholar]