Abstract
Long-term nonreplicating (dormant) Mycobacterium tuberculosis populations (26-day-old cells) were sterilized by metronidazole plus rifampin, but not by metronidazole or rifampin alone, after 7 and 11 days of exposure to the drugs. Lower or no drug activity was observed against 19- or 12-day-old dormant or 5-day-old actively replicating populations.
Two billion people are estimated to be latently infected with Mycobacterium tuberculosis (5, 7). In these individuals, M. tuberculosis is presumed to lie in a nonreplicating (NR) state (dormancy), particularly in the caseous nodules of the lungs known as tuberculomas, i.e., avascular lesions with little access to oxygen; noticeably, oxygen limitation is a signal for induction of M. tuberculosis dormancy (2, 10, 12). In about 10% of patients with latent tuberculosis (TB), infection reactivates, giving rise to an active and often fatal disease. Chemoprophylaxis can reduce the risk of reactivation by as much as 90% but does not eliminate the development of TB (5); thus, the search for drugs that kill dormant M. tuberculosis is an urgent need.
NR cell populations may be obtained by adaptation of stirred aerobic cultures to anaerobiosis through the self-generated formation of an oxygen gradient (Wayne model) (13-15). By this model we studied the bactericidal effect of rifampin (RMP) and metronidazole (MZ), a drug for anaerobes, against NR and actively replicating (AR) cells. M. tuberculosis strain H37Rv was grown in 20- by 125-mm screw-cap tubes containing Dubos Tween-albumin (DTA) broth and was stirred with 8-mm magnetic bars. For preparation of AR populations, mid-log-phase cultures (optical density at 600 nm [OD600], 0.4, corresponding to about 3 × 108 CFU/ml) were diluted in DTA broth to about 1 × 106 CFU/ml and transferred to tubes in 17-ml volumes. Tubes were incubated at 37°C with loosened screw caps and high-speed stirring (about 250 rpm). For preparation of NR populations, 17-ml tubes were inoculated with M. tuberculosis as described above, but in this case, to obtain anaerobic conditions, the caps were tightly screwed and tight-fitting rubber caps were put under the caps to permit addition of drugs by syringe. Tubes were incubated with slow stirring (about 120 rpm) at 37°C for 40 days; control tubes with 1.5 μg/ml methylene blue as an indicator of oxygen depletion were added in each experiment.
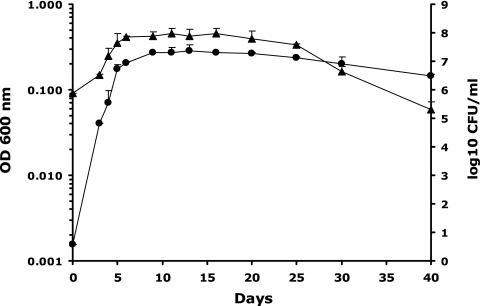
Bacterial growth was monitored by measuring the OD600 and CFU per milliliter on Middlebrook 7H10 agar (Difco) plates incubated at 37°C under 5% CO2 for 3 weeks. Both parameters increased rapidly up to day 5; then a slow increase in turbidity up to day 13 was seen (Fig. 1). Cultures showed slow decreases in the CFU count and OD600 up to about day 25, followed by an abrupt drop in the CFU count to day 40, which was not paralleled by a decrease in cell viability (broth counts were about 2 log10 units higher than CFU per milliliter on day 40 [unpublished data]).
FIG. 1.
Growth and survival of M. tuberculosis H37Rv in the Wayne dormancy culture model. Symbols: •, OD600; ▴, log10 CFU per milliliter. The oxygen indicator methylene blue was completely decolorized on day 13. Means and standard deviations from two independent experiments are shown.
To determine drug activity, MZ (8 μg/ml) and/or RMP (1 μg/ml) was added to AR and NR cultures. At various times, 200 μl of 1:10-diluted cultures was used for determination of CFU per milliliter (Fig. 2A to D) and measurement of the growth index (GI) by inoculation of BACTEC vials (BACTEC 460TB apparatus; Becton Dickinson, Sparks, MD) (Fig. 3A to N).
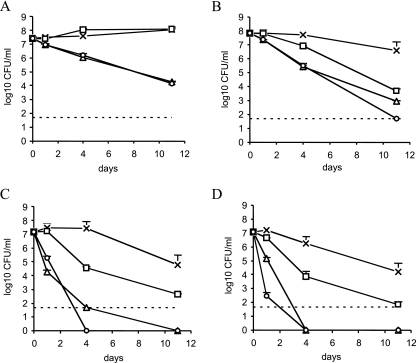
FIG. 2.
Survival of M. tuberculosis H37Rv in the Wayne dormancy culture model after 0, 1, 4, and 11 days of exposure to drugs, as estimated by CFU counts on Middlebrook 7H10 plates. Symbols: ×, drug-free control; □, 8 μg/ml of MZ; ▵, 1 μg/ml of RMP; ○, 8 μg/ml of MZ plus 1 μg/ml of RMP. (A) Five-day-old AR cultures were incubated aerobically with drugs. Twelve-day-old (B), 19-day-old (C), and 26-day-old (D) NR cultures were incubated anaerobically with drugs. Dashed lines indicate the limit of log10 CFU per milliliter detected on Middlebrook 7H10 plates (50 CFU/ml). Error bars, standard deviations.
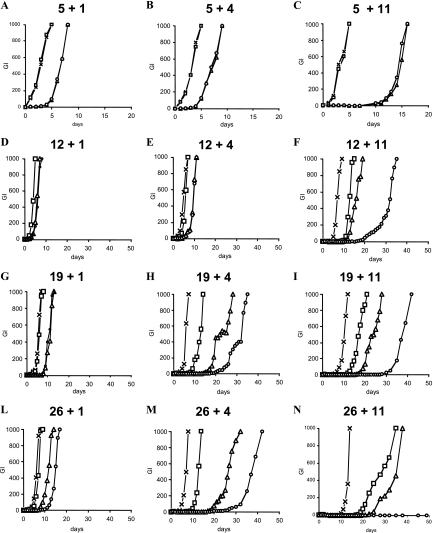
FIG. 3.
Survival of M. tuberculosis H37Rv in the Wayne dormancy model after 1, 4, and 11 days of exposure to drugs, as estimated by regrowth in liquid medium (GI) using the radiometric BACTEC 460TB system. Symbols: ×, drug-free control; □, 8 μg/ml of MZ; ▵, 1 μg/ml of RMP; ○, 8 μg/ml of MZ plus 1 μg/ml of RMP. The first number at the top of each panel indicates the age (in days) of the culture, and the second number indicates the days of drug exposure. For panel N, the GI of MZ-RMP was 0 up to day 150.
On 5-day-old AR populations (Fig. 2A), MZ was ineffective while RMP was bactericidal after 1, 4, or 11 days of exposure; MZ did not enhance the effect of RMP. In contrast, on 12 (Fig. 2B)-, 19 (Fig. 2C)-, or 26 (Fig. 2D)-day-old NR cells, MZ and RMP were effective after 1, 4, or 11 days, and MZ-RMP was more active than MZ or RMP alone. No colonies were seen after exposure of 19- or 26-day-old cells to MZ-RMP or RMP for 4 or 11 days. Since dormant M. tuberculosis may not form colonies on agar (2), the samples for which results are shown in Fig. 2 were also inoculated in BACTEC vials in order to provide a more qualitative viability test. Again, as seen on agar, MZ, but not RMP, was ineffective after 1, 4, or 11 days of exposure against 5-day-old AR cells (Fig. 3A to C). On 12 (Fig. 3D to F)-, 19 (Fig. 3G to I)-, or 26 (Fig. 3L and N)-day-old NR populations, MZ-RMP was more bactericidal than MZ and RMP after at least 23 days of total anaerobic incubation (Fig. 3F, H, I, L, and M); 26-day-old NR cells exposed to MZ-RMP for 11 days failed to regrow (Fig. 3N).
In another experiment, 5-, 19-, and 26-day-old cells were exposed to 8 μg/ml of MZ and/or 8 μg/ml of RMP for 7 and 11 days, and 10 times more cells than were used in the experiment described above (200 μl of 1-ml cultures washed and resuspended in 1 ml of DTA broth) were inoculated in BACTEC and agar plates; 26-day-old populations were sterilized by MZ-RMP after 7 and 11 days (GI, 0 up to day 100, and no CFU, respectively), while 19-day-old populations were sterilized after 11 days only. No sterilization was observed for 5-day-old AR populations or for MZ- or RMP-treated groups.
In M. tuberculosis, a putative pyruvate:ferredoxin oxidoreductase, a key enzyme for MZ activation in anaerobic organisms (1, 2, 11), is encoded by the Rv2454c and Rv2455c genes. Quantitative real-time PCR was performed using primers SigA-S (5′-AGTCGGAGGCCCTGCGTCAA-3′), SigA-AS (5′-GCCAGCCTCGATCCGCTTGG-3′), Rv2454-S (5′-TACGTCATCCTCAACACCATCC-3′), Rv2454-AS (5′-GCTGGAGCATCCGATACCG-3′), Rv2455-S (5′-CAGCCTTATGCCCGTGACC-3′), and Rv2455-AS (5′-AACATGGATACCGTCGATCTTGG-3′). Compared to those of sigA, mRNA levels of both the Rv2454c and Rv2455c genes increased 3 to 5 times from day 3 to 40, with a peak on day 25.
Overall, these observations indicated that the combination of RMP and MZ, an efficacious drug against anaerobes, killed NR populations adapted to oxygen deprivation. The up-regulation of the Rv2454c and Rv2455c genes, particularly on day 25, i.e., when NR cells became susceptible to MZ, suggests that these genes may have a role as MZ activators in M. tuberculosis. The concentrations of MZ (8 μg/ml) and RMP (1 and 8 μg/ml) used here are reached in humans, with doses of 400 mg of MZ and 600 mg of RMP yielding serum drug levels of 13 ± 2.8 and 8.9 ± 2.3 μg/ml for MZ and RMP, respectively (6, 8). In experimental murine TB, MZ-RMP (9), but not MZ alone or other MZ-containing combinations (3, 4), was active against persisting organisms. On 28-day-old unagitated cultures, RMP (0.1 μg/ml) enhanced the bactericidal effect of MZ (8 μg/ml) after 8 days of anaerobic exposure (13). Overall, our data confirmed these observations in the classical Wayne model of dormancy with gentle stirring (14, 15) and extended them, demonstrating sterilization of long-term NR populations by 8 μg/ml MZ plus 8 μg/ml of RMP after 7 and 11 days by using a broth method to assess M. tuberculosis viability. These observations can be important for the treatment of latent TB.
Acknowledgments
This work was supported in part by Italian grants M5B (Ricerca Finalizzata 2004/126), 50G/1 (AIDS project), and N1C (MIUR project) and by European grant LSHP-CT-2003-503240 (MUVAPRED project).
We thank Antonio Cassone for critical review of the manuscript.
Footnotes
Published ahead of print on 22 January 2007.
REFERENCES
- 1.Barry, C. E., III, H. I. Boshoff, and C. S. Dowd. 2004. Prospects for clinical introduction of nitroimidazole antibiotics for the treatment of tuberculosis. Curr. Pharm. Des. 10:3239-3262. [DOI] [PubMed] [Google Scholar]
- 2.Boshoff, H. I., and C. E. Barry III. 2005. Tuberculosis—metabolism and respiration in the absence of growth. Nat. Rev. Microbiol. 3:70-80. [DOI] [PubMed] [Google Scholar]
- 3.Brooks, J. V., S. K. Furney, and I. M. Orme. 1999. Metronidazole therapy in mice infected with tuberculosis. Antimicrob. Agents Chemother. 43:1285-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhillon, J., B. W. Allen, Y. M. Hu, A. R. Coates, and D. A. Mitchison. 1998. Metronidazole has no antibacterial effect in Cornell model murine tuberculosis. Int. J. Tuberc. Lung Dis. 2:736-742. [PubMed] [Google Scholar]
- 5.Frothingham, R., J. E. Stout, and C. D. Hamilton. 2005. Current issues in global tuberculosis control. Int. J. Infect. Dis. 9:297-311. [DOI] [PubMed] [Google Scholar]
- 6.Gerding, D. N., L. R. Peterson, C. E. Hughes, and D. M. Bamberger. 1986. Extravascular antimicrobial distribution in man, p. 938-994. In V. Lorian (ed.), Antibiotics in laboratory medicine. Williams & Wilkins, Baltimore, MD.
- 7.Gomez, J. E., and J. D. McKinney. 2004. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis 84:29-44. [DOI] [PubMed] [Google Scholar]
- 8.Lamp, K. C., C. D. Freeman, N. E. Klutman, and M. K. Lacy. 1999. Pharmacokinetics and pharmacodynamics of the nitroimidazole antimicrobials. Clin. Pharmacokinet. 36:353-373. [DOI] [PubMed] [Google Scholar]
- 9.Paramasivan, C. N., G. Kubendiran, and D. Herbert. 1998. Action of metronidazole in combination with isoniazid and rifampicin on persisting organisms in experimental murine tuberculosis. Indian J. Med. Res. 108:115-119. [PubMed] [Google Scholar]
- 10.Ulrichs, T., and S. H. Kaufmann. 2006. New insights into the function of granulomas in human tuberculosis. J. Pathol. 208:261-269. [DOI] [PubMed] [Google Scholar]
- 11.Upcroft, P., and J. A. Upcroft. 2001. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin. Microbiol. Rev. 14:150-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voskuil, M. I., D. Schnappinger, K. C. Visconti, M. I. Harrell, G. M. Dolganov, D. R. Sherman, and G. K. Schoolnik. 2003. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 198:705-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wayne, L. G., and H. A. Sramek. 1994. Metronidazole is bactericidal to dormant cells of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 38:2054-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wayne, L. G., and L. G. Hayes. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64:2062-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wayne, L. G. 2001. In vitro model of hypoxically induced nonreplicating persistence of Mycobacterium tuberculosis, p. 247-269. In T. Parish and N. Stoker (ed.), Methods in molecular medicine. Mycobacterium tuberculosis protocols. Humana Press, Totowa, NJ. [DOI] [PubMed]