Abstract
Eleven antifungal drugs were tested against representative isolates of the four phylogenetic clades of the Fusarium solani species complex obtained in a multilocus sequence analysis. They all showed very poor activity, with no differences among the clades. Amphotericin B was the most active drug.
Fusarium solani is a fungus that is widely distributed in nature and is able to produce many plant diseases with important economic impacts and, also, severe, usually fatal, human infections (7, 9, 15, 23). These infections can be characterized by their resistance to practically all available antifungal drugs (3, 21, 24). Primary therapy with voriconazole (VRC) or a lipid formulation of amphotericin B (AMB) is currently recommended (22). Although the response of Fusarium spp. to AMB is poor in general, it depends on the species involved, with F. solani being the most resistant species, at least in vitro (24). Recently, Zhang et al. (29) used a multilocus sequence analysis approach to demonstrate that under the generic name F. solani, at least 45 phylogenetically distinct species exist, most of which have not been described formally. It is unknown if antifungal susceptibility varies among these phylogenetic species. If such differences exist, knowing them could be useful for guiding clinical treatments. Due to the difficulties of testing representative strains of all the phylogenetic species, we tested isolates belonging to the four major clades, inferred from a combined phylogenetic analysis of fragments of three genes, i.e., the translation elongation factor 1α (EF-1α) and β-tubulin genes and the internal transcribed spacer (ITS) of the nuclear rRNA gene, against traditional and new antifungal drugs.
Fifty isolates from clinical or environmental sources, morphologically identified as F. solani (6), were included in the study (Table 1). Isolates were stored by lyophilization and submerged in slant cultures in mineral oil at room temperature. The procedures for DNA extraction, amplification, and sequencing of the different regions analyzed were described by Gilgado et al. (8). The annealing temperature was 55°C, and the primers used were EF-1H and EF-2T (16) for EF-1α, TUB-F (5) and T22 (17) for β-tubulin, and ITS5 and ITS4 (28) for the ITS. The phylogenetic analysis was performed using PAUP*, version 4.0b10 (27). Maximum parsimony trees were obtained after 100 heuristic searches with random sequence addition and tree bisection-reconnection branch-swapping algorithms, collapsing zero-length branches, and saving of all minimal-length trees (MulTrees). The results of the partition homogeneity test showed that the three locus sequence data sets were congruent (P = 0.2) and could therefore be combined. Sequences of the three genes were analyzed phylogenetically as separate (data not shown) and combined data sets.
TABLE 1.
Isolates included in this study
| Isolatea | Source | GenBank accession no.
|
||
|---|---|---|---|---|
| EF-1α | β-Tubulin | ITS | ||
| FMR 4389 | Human scrotum skin, United States | AM397191 | AM419414 | AM412634 |
| FMR 4391 | Human blood, United States | AM397184 | AM419413 | AM412635 |
| FMR 7140 | Aquarium sand, Spain | AM397227 | AM419415 | AM412636 |
| FMR 7141 | Aquarium sand, Spain | AM397228 | AM419416 | AM412637 |
| FMR 7238 | Human ulcer, Spain | AM397225 | AM419417 | AM412638 |
| FMR 7240 | Human skin lesion, Spain | AM397210 | AM419418 | AM412639 |
| FMR 7242 | Human skin lesion, Spain | AM397212 | AM419419 | AM412640 |
| FMR 7985 | Human keratitis, Brazil | AM397218 | AM419406 | AM412641 |
| FMR 7988 | Human keratitis, Brazil | AM397202 | AM419420 | AM412642 |
| FMR 7989 | Human keratitis, Brazil | AM397219 | AM419421 | AM412643 |
| FMR 7991 | Human keratitis, Brazil | AM397220 | AM419412 | AM412624 |
| FMR 7992 | Human keratitis, Brazil | AM397186 | AM419422 | AM412625 |
| FMR 7993 | Human keratitis, Brazil | AM397198 | AM419423 | AM412626 |
| FMR 7994 | Human keratitis, Brazil | AM397194 | AM419424 | AM412627 |
| FMR 7995 | Human keratitis, Brazil | AM397206 | AM419425 | AM412628 |
| FMR 7996 | Human keratitis, Brazil | AM397199 | AM419376 | AM412629 |
| FMR 7997 | Human keratitis, Brazil | AM397209 | AM419377 | AM412630 |
| FMR 7998 | Human keratitis, Brazil | AM397204 | AM419378 | AM412631 |
| FMR 8000 | Human keratitis, Brazil | AM397207 | AM419379 | AM412632 |
| FMR 8013 | Human keratitis, Brazil | AM397189 | AM419380 | AM412633 |
| FMR 8014 | Human keratitis, Brazil | AM397205 | AM419381 | AM412604 |
| FMR 8016 | Human keratitis, Brazil | AM397226 | AM419382 | AM412605 |
| FMR 8017 | Human keratitis, Brazil | AM397208 | AM419383 | AM412606 |
| FMR 8019 | Human keratitis, Brazil | AM397197 | AM419384 | AM412607 |
| FMR 8021 | Human keratitis, Brazil | AM397195 | AM419385 | AM412608 |
| FMR 8023 | Human keratitis, Brazil | AM397201 | AM419386 | AM412609 |
| FMR 8024 | Human keratitis, Brazil | AM397223 | AM419409 | AM412610 |
| FMR 8027 | Human keratitis, Brazil | AM397203 | AM419387 | AM412611 |
| FMR 8028 | Human keratitis, Brazil | AM397196 | AM419388 | AM412612 |
| FMR 8030 | Human keratitis, Brazil | AM397229 | AM419389 | AM412613 |
| FMR 8031 | Human keratitis, Brazil | AM397213 | AM419407 | AM412614 |
| FMR 8032 | Human keratitis, Brazil | AM397222 | AM419410 | AM412615 |
| FMR 8036 | Human keratitis, Brazil | AM397192 | AM419390 | AM412616 |
| FMR 8037 | Human keratitis, Brazil | AM397188 | AM419391 | AM412617 |
| FMR 8038 | Human keratitis, Brazil | AM397230 | AM419392 | AM412618 |
| FMR 8039 | Human keratitis, Brazil | AM397231 | AM419393 | AM412619 |
| FMR 8040 | Human keratitis, Brazil | AM397193 | AM419394 | AM412620 |
| FMR 8207 | Nematode, Spain | AM397190 | AM419395 | AM412621 |
| FMR 8263 | Nematode, Spain | AM397187 | AM419396 | AM412622 |
| FMR 8281 | Nematode, Spain | AM397215 | AM419397 | AM412623 |
| FMR 8340 | Human fungemia, Qatar | AM397232 | AM419398 | AM412594 |
| FMR 8482 | Human clinical source, Qatar | AM397224 | AM419411 | AM412595 |
| FMR 8483 | Human clinical source, Qatar | AM397221 | AM419408 | AM412596 |
| FMR 8484 | Nematode, Spain | AM397200 | AM419399 | AM412597 |
| FMR 8631 | Human clinical source, Spain | AM397185 | AM419400 | AM412598 |
| FMR 8633 | Human nail, Venezuela | AM397214 | AM419401 | AM412599 |
| FMR 8634 | Human diabetic foot sore, Spain | AM397233 | AM419402 | AM412600 |
| FMR 8666 | Nematode, Spain | AM397211 | AM419403 | AM412601 |
| FMR 8673 | Nematode, Spain | AM397216 | AM419404 | AM412602 |
| FMR 8688 | Nematode, Spain | AM397217 | AM419405 | AM412603 |
FMR, Facultat de Medicina i Ciències de la Salut, Reus, Spain.
We evaluated the in vitro activities of 11 antifungal drugs against 27 representative strains (22 clinical and 5 environmental) randomly chosen from the main clades obtained in the phylogenetic analysis. The isolates were grown on potato dextrose agar plates and incubated at 25°C for 7 days. We used a microdilution reference method (14), with some modification. The inocula were adjusted to a final concentration of 4 × 103 to 5 × 104 conidia/ml with a hemocytometer and verified by quantitative colony counts on potato dextrose agar plates. Paecilomyces variotii ATCC 36257 was included in each batch of tests as a quality control strain. The antifungal agents tested were AMB, albaconazole, VRC, itraconazole, ravuconazole, terbinafine, ketoconazole (KTC), posaconazole (PSC), micafungin (MFG), fluconazole (FLC), and flucytosine (5-FC). MFG, FLC, and 5-FC were diluted in sterile distilled water, and the rest were diluted in dimethyl sulfoxide. Final drug concentrations ranged from 64 to 0.12 μg/ml for FLC and 5-FC, from 128 to 0.25 μg/ml for MFG, and from 16 to 0.03 μg/ml for the rest. The MIC end point for the triazoles and AMB was defined as the lowest concentration that produced complete inhibition of growth, and that for FLC, KTC, 5-FC, and MFG was defined as the lowest concentration that produced a 50% inhibition of growth.
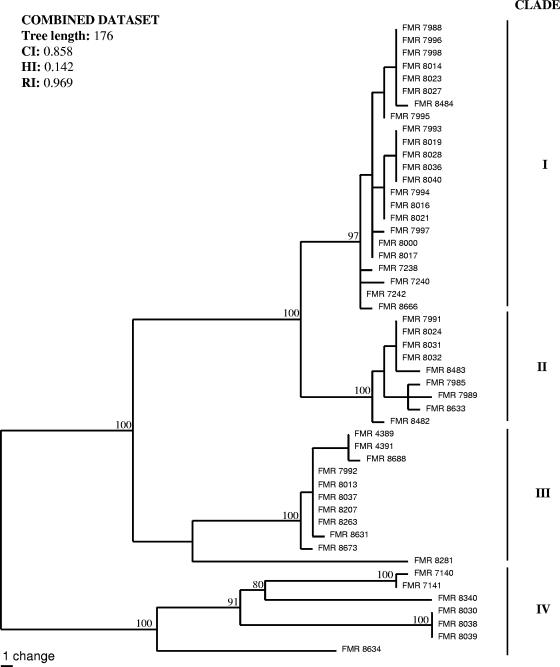
With the primers used, we were able to amplify and sequence 654, 461, and 573 bp of the EF-1α, β-tubulin, and ITS gene sequences, respectively. Parsimony analysis of the combined data set (1,688 bp) yielded three trees of 176 steps in length. Four main clades (I, II, III, and IV) with high bootstrap support were obtained, resulting in a total of 28 different haplotypes and numerous putative cryptic species (Fig. 1). We found no relationship between the biogeographical origins of the isolates and the molecular groups. In order to compare the topology of our trees with those obtained by Zhang et al. (29), we performed a new phylogenetic analysis based on ITS and EF-1α loci (data not shown). We included our isolates and several representative sequences, retrieved from GenBank, from Zhang et al.'s main groups. In general, our clades did not coincide with those of Zhang et al., which confirms the high genetic variability of this complex. However, several of our strains nested in their groups 3 and 4.
FIG. 1.
One of the three most parsimonious trees obtained from heuristic searches, based on a combined data set. Bootstrap support values above 70% are indicated at the nodes. CI, consistency index; RI, retention index; HI, homoplasy index.
Results of the in vitro susceptibility tests are shown in Table 2. In general, all drugs showed high MICs, with no remarkable differences among the clades. AMB was the most active drug, although in no case was the MIC lower than 1 μg/ml, followed by VRC. The latter drug showed the most variable results; sometimes the differences among MICs against different isolates in the same clade were >8-fold.
TABLE 2.
Distribution of 27 tested isolates from the four phylogenetic clades of F. solani, according to antifungal susceptibility
| Antifungala | Cladeb | No. of isolates with MIC (μg/ml)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | ||
| AMB | I | 1 | 8 | 2 | ||||||
| II | 4 | 1 | ||||||||
| III | 1 | 4 | 2 | |||||||
| IV | 1 | 3 | ||||||||
| VRC | I | 3 | 4 | 1 | 2 | |||||
| II | 1 | 4 | 1 | |||||||
| III | 1 | 1 | 4 | 1 | ||||||
| IV | 3 | 1 | ||||||||
| ABC | I to IV | 27 | ||||||||
| ITC | I to IV | 27 | ||||||||
| KTC | I to IV | 27 | ||||||||
| PSC | I to IV | 27 | ||||||||
| RVC | I to IV | 27 | ||||||||
| TBF | I to IV | 27 | ||||||||
| FLC | I to IV | 27 | ||||||||
| 5-FC | I to IV | 27 | ||||||||
| MFG | I to IV | 1 | 3 | 10 | 13 | |||||
AMB, amphotericin B; VRC, voriconazole; ABC, albaconazole; ITC, itraconazole; KTC, ketoconazole; PSC, posaconazole; RVC, ravuconazole; TBF, terbinafine; FLC, fluconazole; 5-FC, flucytosine; MFG, micafungin.
See Fig. 1.
The poor in vitro activities of different antifungal drugs against Fusarium have been reported by other authors many times, with F. solani being more resistant than the other species of the genus (12, 19, 24, 26). In this study, AMB was revealed as the most active drug, but only one isolate from clade I and another from clade III showed a MIC of 1 μg/ml, with all others showing higher values. Although this drug is recommended for the treatment of fusariosis, it has poor clinical success (7, 11, 15). In cases involving neutropenic patients receiving corticosteroids, the survival rate is practically nil, despite aggressive treatment (15). The poor efficacy of AMB was also demonstrated in animal studies (10). VRC, the other recommended drug, is the only agent indicated for treating refractory fusariosis (7). However, in our study, the VRC MICs were always higher than 2 μg/ml, which agrees with the results of other studies (12, 26). This drug was effective in a few clinical cases of fusariosis (2, 4), although in none of them was the species involved referred to as F. solani. This is probably linked to the fact that in animal studies F. solani was clearly more virulent and more difficult to treat than F. oxysporum and F. verticillioides, the two other common species of the genus (13, 18). In a recent clinical trial, 45.5% (5/11) of patients responded satisfactorily to VRC (20), although unfortunately the isolates were not identified to the species level. Arikan et al. (1) demonstrated that the combination of caspofungin plus AMB was synergistic against 100% of strains of F. oxysporum but against only 25% of F. solani strains. In the case of PSC, we also obtained high MICs, again agreeing with other studies (19, 26). However, in a recent retrospective analysis, PSC as an aggressive treatment for invasive fusariosis gave a 48% successful outcome (25), but the species involved were not listed. In conclusion, the F. solani species complex constitutes a group of genetically diverse fungi with poor in vitro and in vivo responses to different antifungal drugs.
Acknowledgments
We thank Félix Gilgado, Carol Serena, Rita Marimon, and Marçal Mariné for their contributions to this work.
Footnotes
Published ahead of print on 12 January 2007.
REFERENCES
- 1.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2002. In vitro synergy of caspofungin and amphotericin B against Aspergillus and Fusarium spp. Antimicrob. Agents Chemother. 46:245-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bigley, V. H., R. F. Duarte, R. D. Gosling, C. C. Kibbler, S. Seaton, and M. Potter. 2004. Fusarium dimerum infection in a stem cell transplant recipient treated successfully with voriconazole. Bone Marrow Transplant. 34:815-817. [DOI] [PubMed] [Google Scholar]
- 3.Capilla, J., M. Ortoneda, F. J. Pastor, and J. Guarro. 2001. In vitro antifungal activities of the new triazole UR-9825 against clinically important filamentous fungi. Antimicrob. Agents Chemother. 45:2635-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Consigny, S., N. Dhedin, A. Datry, S. Choquet, V. Leblond, and O. Chosidow. 2003. Successful voriconazole treatment of disseminated Fusarium infection in an immunocompromised patient. Clin. Infect. Dis. 37:311-313. [DOI] [PubMed] [Google Scholar]
- 5.Cruse, M., R. Telerant, T. Gallagher, T. Lee, and J. W. Taylor. 2002. Cryptic species in Stachybotrys chartarum. Mycologia 94:814-822. [PubMed] [Google Scholar]
- 6.de Hoog, G. S., J. Guarro, J. Gené, and M. J. Figueras. 2000. Atlas of clinical fungi, 2nd ed. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 7.Dignani, M. C., and E. J. Anaissie. 2004. Human fusariosis. Clin. Microbiol. Infect. 1:67-75. [DOI] [PubMed] [Google Scholar]
- 8.Gilgado, F., J. Cano, J. Gené, and J. Guarro. 2005. Molecular phylogeny of the Pseudallescheria boydii species complex: proposal of two new species. J. Clin. Microbiol. 43:4930-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guarro, J., and J. Gené. 1995. Opportunistic fusarial infections in humans. Eur. J. Clin. Microbiol. Infect. Dis. 14:741-754. [DOI] [PubMed] [Google Scholar]
- 10.Guarro, J., I. Pujol, and E. Mayayo. 1999. In vitro and in vivo experimental activities of antifungal agents against Fusarium solani. Antimicrob. Agents Chemother. 43:1256-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hennequin, C., V. Lavarde, J. L. Poirot, M. Rabodonirina, A. Datry, S. Aractingi, J. Dupouy-Camet, D. Caillot, F. Grange, L. Kures, O. Morin, B. Lebeau, S. Bretagne, C. Guigen, D. Basset, and R. Grillot. 1997. Invasive Fusarium infections: a retrospective survey of 31 cases. J. Med. Vet. Mycol. 35:107-114. [PubMed] [Google Scholar]
- 12.Lewis, R. E., N. P. Wiederhold, and M. E. Klepser. 2005. In vitro pharmacodynamics of amphotericin B, itraconazole, and voriconazole against Aspergillus, Fusarium, and Scedosporium spp. Antimicrob. Agents Chemother. 49:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayayo, E., I. Pujol, and J. Guarro. 1999. Experimental pathogenicity of four opportunist Fusarium species in a murine model. J. Med. Microbiol. 48:363-366. [DOI] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 15.Nucci, M., E. J. Anaissie, F. Queiroz-Telles, C. A. Martins, P. Trabasso, C. Solza, C. Mangini, B. P. Simoes, A. L. Colombo, J. Vaz, C. E. Levy, S. Costa, V. A. Moreira, J. S. Oliveira, N. Paraguay, G. Duboc, J. C. Voltarelli, A. Maiolino, R. Pasquín, and C. A. Souza. 2003. Outcome predictors of 84 patients with hematologic malignancies and Fusarium infection. Cancer 98:315-319. [DOI] [PubMed] [Google Scholar]
- 16.O'Donnell, K. 2000. Molecular phylogeny of the Nectria haematococca-Fusarium solani species complex. Mycologia 92:919-938. [Google Scholar]
- 17.O'Donnell, K., and E. Cigelnik. 1997. Two divergent intragenomic rADN ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 7:103-116. [DOI] [PubMed] [Google Scholar]
- 18.Ortoneda, M., J. Capilla, F. J. Pastor, I. Pujol, and J. Guarro. 2002. Efficacy of liposomal amphotericin B in treatment of systemic murine fusariosis. Antimicrob. Agents Chemother. 46:2273-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paphitou, N. I., L. Ostrosky-Zeichner, V. L. Paetznick, J. R. Rodríguez, E. Chen, and J. H. Rex. 2002. In vitro activities of investigational triazoles against Fusarium species: effects of inoculum size and incubation time on broth microdilution susceptibility test results. Antimicrob. Agents Chemother. 46:3298-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perfect, J. R., K. A. Marr, T. J. Walsh, R. N. Greenberg, B. DuPont, J. de la Torre-Cisneros, G. Just-Nubling, H. T. Schlamm, I. Lutsar, A. Espinel-Ingroff, and E. Johnson. 2003. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin. Infect. Dis. 36:1122-1131. [DOI] [PubMed] [Google Scholar]
- 21.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, and the Sentry Participants Group. 2002. Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: report from SENTRY Antimicrobial Surveillance Program. Antimicrob. Agents Chemother. 46:1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaller, M. A., P. G. Pappas, and J. R. Wingard. 2006. Invasive fungal pathogens: current epidemiological trends. Clin. Infect. Dis. 43:3-14. [Google Scholar]
- 23.Pontón, J., R. Rüchel, K. V. Clemons, D. C. Coleman, R. Grillot, J. Guarro, D. Aldebert, P. Ambroise-Thomas, J. Cano, A. J. Carrillo-Muñoz, J. Gené, C. Pinel, D. A. Stevens, and D. J. Sullivan. 2000. Emerging pathogens. Med. Mycol. 38:225-236. [DOI] [PubMed] [Google Scholar]
- 24.Pujol, I., J. Guarro, J. Gené, and J. Sala. 1997. In-vitro antifungal susceptibility of clinical and environmental Fusarium spp. strains. J. Antimicrob. Chemother. 39:163-167. [DOI] [PubMed] [Google Scholar]
- 25.Raad, I. I., R. Y. Hachem, R. Herbrecht, J. R. Graybill, R. Hare, G. Corcoran, and D. P. Kontoyiannis. 2006. Posaconazole as salvage treatment for invasive fusariosis in patients with underlying hematologic malignancy and other conditions. Clin. Infect. Dis. 42:1398-1403. [DOI] [PubMed] [Google Scholar]
- 26.Sabatelli, F., R. Patel, P. A. Mann, C. A. Mendrick, C. C. Norris, R. Hare, D. Loebenberg, T. A. Black, and P. M. McNicholas. 2006. In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob. Agents Chemother. 50:2009-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swofford, D. L. 2001. PAUP*. Phylogenetic analysis using parsimony (*and other methods), version 4.0. Sinauer Associates, Sunderland, MA.
- 28.White, T. J., T. Bruns, S. Lee, and J. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols: a guide to the methods and applications. Academic Press, New York, NY.
- 29.Zhang, N., K. O'Donnell, D. A. Sutton, F. A. Nalim, R. C. Summerbell, A. A. Padhye, and D. M. Geiser. 2006. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J. Clin. Microbiol. 44:2186-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]