Abstract
Miltefosine (hexadecylphosphocholine [HePC]) is currently on trial as a first-choice, orally active drug for the treatment of visceral leishmaniasis when resistance to organic pentavalent antimonials becomes epidemic. However, data on the targets involved in its leishmanicidal mechanism have, until now, been only fragmentary. We have carried out a systematic study of the alterations induced on the bioenergetic metabolism of Leishmania donovani promastigotes by HePC. Overnight incubation with HePC caused a significant decline in the intracellular ATP levels of the parasites, together with a reduction in the oxygen consumption rate and mitochondrial depolarization, while the integrity of the plasma membrane remained undamaged. In a further step, the effects of HePC on the respiratory chain were addressed in digitonized parasites. The inhibition of the oxygen consumption rate caused by HePC was not reverted either with the uncoupling agent carbonyl cyanide p-trifluoromethoxyphenylhydrazone or with tetramethyl-p-phenylenediamine plus ascorbate, which feeds the electron transport chain at the level of cytochrome c. These results suggest that cytochrome c oxidase is a likely target in the complex leishmanicidal mechanism of HePC. This was further confirmed from the finding that this enzyme was specifically inhibited in a dose-dependent manner by HePC, but not the cytochrome c reductase, ruling out an unspecific effect of HePC on the respiratory chain.
Leishmaniasis is a devastating human disease caused by infection with species of the intracellular protozoan parasite Leishmania. It threatens 350 million people worldwide, with an annual incidence of 2 million cases (http://www.who.int/leishmaniasis/disease_epidemiology/en/index.html). It shows a special relevance as a coinfection with human immunodeficiency virus, where Leishmania behaves as an opportunistic parasite (10). Chemotherapy is the only treatment currently available, with pentavalent antimonials as the first-line drugs (1), despite the fact that their clinical efficacies are severely jeopardized by both severe side effects (7) and the rising incidence of resistant parasites, all of which have led to their replacement by amphotericin B as a first-choice drug in northwest India (29). The lysophospholipid analogues (LPAs) edelfosine, miltefosine (hexadecylphosphocholine [HePC]), ilmofosine, and more recently, perifosine (38), formerly developed as antitumoral drugs (13, 18), have turned out to be good alternatives to classical treatments against Leishmania in recent years (for a review, see references 6 and 9). Indeed, in assays conducted in the field, HePC was the first successful oral drug against visceral leishmaniasis in India and the cutaneous form in Colombia (44). Its major advantage over other leishmanicidal drugs, namely, its oral activity, is complemented by mild side effects, limited to transitory gastrointestinal discomfort, as well as by its clinical efficacy in patients unresponsive to antimonial compound-based therapy, altogether a landmark improvement in therapeutics (6, 43).
The description of the leishmanicidal mechanism of HePC has, until now, been only fragmentary, quite likely because HePC possesses more than one site of action. Only two facts related to the mechanism of HePC have been firmly established: (i) the killing of the parasite occurs through an apoptosis-like process (31, 50), and (ii) its uptake by Leishmania is solely mediated by LdMT, a plasma membrane aminophospholipid translocase with ATPase activity (33, 34). Contrary to other leishmanicidal drugs, for which the analysis of resistance traits provided solid insights into the definition of their respective targets (30), the resistance obtained in the laboratory for HePC mapped exclusively to a faulty accumulation of the drug, produced either by mutation of LdMT (33), by mutation of its regulatory proteins (34), or by efflux pumps (35). In addition, these miltefosine resistance traits provided cross-resistance to other LPAs (41). HePC perturbs the biosynthesis of a wide variety of lipids. Thus, in Leishmania mexicana promastigotes, it inhibited the remodeling of ether-lipid by alkyl-specific acyl coenzyme A acyltransferase (26). Leishmania donovani promastigotes resistant to HePC showed changes in the length and the level of unsaturation of fatty acids, as well as a reduction in ergosterol levels (36). In Trypanosoma cruzi epimastigotes, treatment with HePC led to an inversion of the phosphatidylcholine/phosphatidylethanolamine ratio (22). The permeation of the plasma membrane by LPAs in the presence of serum was observed only at concentrations beyond full lethality, ruling out this effect as the ultimate reason for parasite killing (22).
The mitochondrion is another appealing target for LPAs in trypanosomatids; the mitochondrial membrane potential (ΔΨm) was substantially reduced after HePC treatment of Leishmania amazonensis promastigotes (38) or edelfosine-treated T. cruzi epimastigotes (37, 39). In fact, overexpression of HSP83 and SKCRP14.1, two proteins linked to the maintenance of ΔΨm, was shown to confer a slight resistance to HePC in L. donovani clinical field isolates (49). Furthermore, other noxious leishmanicidal agents, such as H2O2 (28), NO (16), and Sb3+ (42), which, like HePC, also induced apoptosis, cause mitochondrial dysfunction, stressing the importance of this organelle in this process.
To better understand the mode of action of HePC, we undertook a characterization of the bioenergetic parameters in parasites treated with HePC, followed by a careful dissection of the interference of this drug in the respiratory chain. Overall, our results point toward an inhibition of cytochrome c oxidase (CcO) by HePC as an important target of its leishmanicidal mechanism.
MATERIALS AND METHODS
Cell lines.
Promastigotes from Leishmania donovani strain MHOM/SD/00/1S-2D were grown at 25°C in RPMI 1640 medium (Gibco, Paisley, United Kingdom) supplemented with 10% heat-inactivated fetal calf serum (RPMI-HIFCS), as described previously (23). The L. donovani 3-Luc strain was obtained from the aforementioned strain by transfection with the expression vector pX63NEO-3Luc, which encodes a cytoplasmic form of the Photinus pyralis luciferase mutated at its C-terminal tripeptide, as described previously (24). These promastigotes were grown as described above, except for the inclusion of 30 μg/ml Geneticin (G-418; Gibco) in the growth medium.
Chemicals.
Reagents of the highest quality available were obtained from Merck (Darmstadt, Germany) or Sigma (St. Louis, MO). Miltefosine was a kind gift from Zentaris (Frankfurt, Germany). Propidium iodide, SYTOX green, rhodamine 123 and the d-luciferin 1-(4, 5-dimethoxy-2-nitrophenyl)ethyl ester (DMNPE-luciferin) were obtained from Molecular Probes (Leiden, The Netherlands).
Cell proliferation measurements.
Parasites were harvested at late exponential phase, washed in RPMI-HIFCS, and resuspended in the same medium at a final concentration of 2 × 106 cells/ml. Unless otherwise stated, these conditions were maintained for the rest of the experiments.
Aliquots (100 μl) of this parasite suspension were incubated for 14 h with HePC at 25°C, washed with 1 ml of Hanks buffer supplemented with 10 mM d-glucose (Hanks-Glc) at 4°C to slow down the action of miltefosine, and resuspended in 100 μl of 0.5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) in Hanks-Glc. Reduction of MTT to formazan was allowed to proceed for 2 h, the formazan was solubilized by addition of 100 μl of 10% (wt/vol) sodium dodecyl sulfate solution, and the result was read in a 680 Bio-Rad microplate enzyme-linked immunosorbent assay reader equipped with a 595-nm filter (21).
The existence of a sub-G1 peak population in HePC-treated promastigotes, which was representative of apoptosis induction, was monitored by flow cytometry analysis of permeabilized parasites stained with propidium iodide in a FACSCalibur cytofluorometer (Becton Dickinson) (31).
Plasma membrane permeabilization.
The integrity of the plasma membrane was assessed by the entrance of the cationic vital dye SYTOX green into the cytoplasm, as described previously (5). Briefly, the parasite suspension was treated with increasing concentrations of HePC in RPMI-HIFCS and incubated for 14 h at 25°C. The cells were then washed twice with Hanks-Glc, SYTOX green was added at 1 μM (final concentration), and the parasites were incubated for 5 min in the dark and transferred into a 96-well microplate (100 μl/well). The increase in fluorescence, due to binding of the dye to intracellular nucleic acids, was monitored in a Polarstar Galaxy microplate reader (BMG Labotechnologies, Offenburg, Germany) equipped with 485- and 520-nm filters for the excitation and the emission wavelengths, respectively. Maximal permeabilization for each well was considered that achieved after the addition of 0.1% Triton X-100 (TX-100) as the final step.
Bioluminescence assays.
Parasites from the L. donovani 3-Luc strain were incubated according to the standard conditions with HePC from 4 to 14 h at 25°C. The cells were then washed twice with Hanks-Glc, the parasite suspension was adjusted to 4 × 107 cells/ml, and 50-μl aliquots of this suspension were transferred into a 96-well microplate. Then, an equal volume of a fresh solution of 50 μM DMNPE-luciferin in the same medium was added. Changes in luminescence, proportional to the intracellular ATP content, were recorded in a Polarstar Galaxy microplate reader fitted with luminescence optics, with the measurements averaged every 4 s (25).
Variation of the mitochondrial ΔΨm.
The variation of the accumulation of rhodamine 123 in parasites, which is directly related to the electrochemical potential of the mitochondrion, was used to assess changes in the mitochondrion due to HePC, as described previously (11). Parasites were incubated for 14 h with HePC under standard conditions. The free drug was removed by two washings with Hanks-Glc at 4°C; and the parasites were loaded with rhodamine 123 (0.3 μg/ml, 5 min, 37°C), washed, and resuspended at 2 × 106 cells/ml. Rhodamine 123 accumulation was monitored in a FACSCalibur cytofluorometer (Becton Dickinson) (excitation and emission wavelengths, 488 and 525 nm, respectively). A total of 20,000 events were acquired in the region previously established as that corresponding to the parasites. Promastigotes with a depolarized mitochondrion poisoned with 10 mM KCN were taken as negative controls.
Determination of oxygen consumption rates.
Oxygen consumption rates were measured in a Clark oxygen electrode (Hansatech, KingsLynn, United Kingdom) at 25°C by using 1 ml of a parasite suspension (108 cells/ml) in respiration buffer supplemented with 5 mM succinate and 1 mg/ml bovine serum albumin, as described previously (2). Cells were permeabilized with 60 μM digitonin, as it allows selective permeation of the plasma membrane but not the inner mitochondrial membrane (48). Afterwards, 100 μM ADP was added to restore state 3; and once a steady rate was reached, HePC was added, followed by the addition of the following selective set of substrates and inhibitors of the respiratory chain at the indicated final concentrations: 0.1 mM tetramethyl-p-phenylenediamine plus 1.7 mM ascorbate (TMPD-ascorbate), 1 μM antimycin A, 12.6 μM oligomycin, 10 μM carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP), and 10 mM KCN.
To test the long-term effect of miltefosine on parasites, promastigotes were incubated for 14 h with different HePC concentrations, harvested, washed, and adjusted in respiration buffer to 108 cells/ml. Oxygen consumption rates were measured in a Clark oxygen electrode, as mentioned above.
Retrieval of mitochondrial fraction.
A mitochondrion-enriched fraction was obtained as described by Chen et al. (4). The promastigotes were washed twice in Hanks buffer, resuspended in hypoosmotic 5 mM Tris-HCl (pH 7.4) buffer for 10 min at 25°C, and homogenized in a Potter-Elvehjem homogenizer on ice. Cellular debris was removed by centrifugation (1,000 × g, 10 min, 4°C). The membrane fraction was obtained by centrifugation (13,000 × g, 20 min, 4°C). The pellet, which contained the mitochondrial fraction, was resuspended in 75 mM sodium phosphate (pH 7.4); and the protein content was adjusted to 2 mg/ml, as measured by use of the Bradford reagent (Bio-Rad).
Measurement of CcO and cytochrome c reductase activities.
Determination of CcO activity was carried out as described by Sottocasa et al. (45), based on the oxidation of reduced cytochrome c measured at 550 nm. Briefly, the incubation mixture contained 200 μg/ml of mitochondrial fraction, 0.02% TX-100, and 32 μM reduced cytochrome c in 75 mM sodium phosphate (pH 7.4). HePC was added at the corresponding concentration, and changes in the absorbance at 550 nm were monitored for 50 min at 37°C. The spontaneous oxidation background was determined by using samples previously incubated with 10 mM KCN. For the determination of cytochrome c reductase activity, a similar protocol was followed, except for a previous inhibition of CcO by addition of 10 mM KCN and the use of oxidized cytochrome c. The reaction was started by the addition of 5 mM succinate, and changes in the absorbance at 550 nm were monitored. Samples without succinate and samples with 2 μM antimycin A, an inhibitor of cytochrome c reductase, were taken as controls.
Statistical analysis.
Data represent the mean of triplicates ± the standard deviation. The experiments were repeated at least twice. The 50% effective concentrations were calculated by the procedure of Litchfield and Wilcoxon.
RESULTS
Loss of promastigote viability after HePC treatment.
The reduction of MTT by living parasites is a proper parameter that can be used to check for parasite viability (21). As such, its variation was measured in L. donovani promastigotes after incubation with HePC. As shown in Fig. 1, this process underwent a dose-dependent inhibition, with a 50% effective concentration of 10.2 ± 0.5 μM and full inhibition at 40 μM.
FIG. 1.
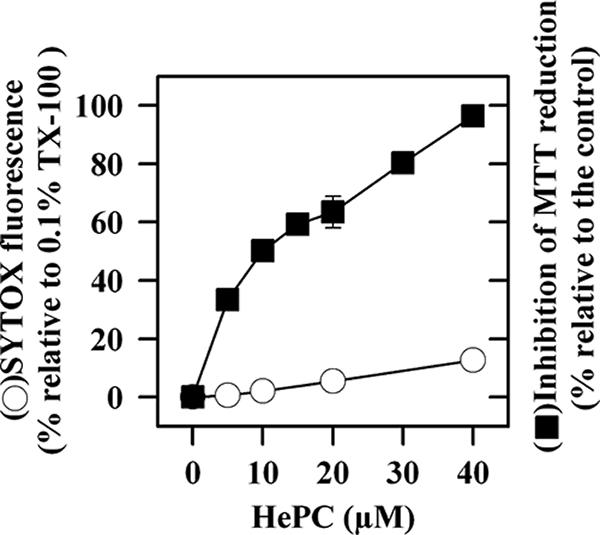
Plasma membrane permeabilization and viability of L. donovani promastigotes after incubation for 14 h with HePC. The entry of SYTOX green after incubation with HePC was monitored by determination of the increase in fluorescence (excitation λ, 485 nm; emission λ, 520 nm). The results were normalized as the percentage of fluorescence relative to that obtained by maximal parasite permeabilization with 0.1% TX-100. The inhibition of parasite viability was assessed by determination of the inhibition of MTT reduction.
In order to discern the processes responsible for this loss of viability, the extent of plasma membrane permeabilization and the extent of apoptosis induction were first examined. Although HePC and other LPAs cause a fast detergent-like permeabilizing effect on parasites, this effect was virtually abolished in the presence of serum (38). Nevertheless, since under these conditions structural damage to the membrane has been reported for trypanosomatids upon long-term incubation with LPAs (37, 38), the integrity of the plasma membrane under our current assay conditions was assessed by the entrance of the vital dye SYTOX green into the HePC-treated parasites (25). After 14 h at the highest HePC concentration tested (40 μM), at which promastigote viability was fully abrogated, the level of SYTOX green fluorescence attained was only 10%; as a reference, the parasites were fully permeabilized with 0.1% TX-100 (Fig. 1).
The DNA fragmentation that occurred in apoptotic cells was translated into a decrease in the amount of propidium iodide bound to DNA in permeabilized parasites. This appears as a sub-G1 population peak when the cells are monitored by flow cytometry (31). The proportion of cells inside this region amounted to 38% after 24 h of exposure to 20 μM HePC, while for the control promastigotes, this proportion was reduced to 6% (data not shown).
Variation in intracellular levels of ATP by HePC.
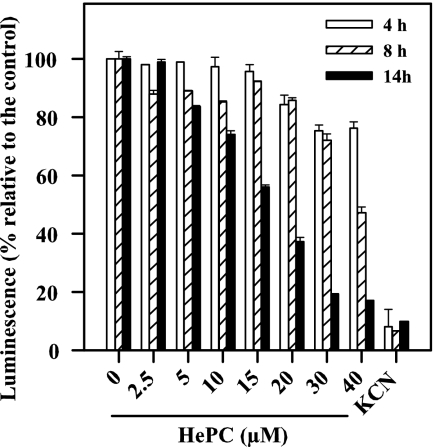
The in vivo luminescence of strain 3-Luc promastigotes affords the real-time monitoring of changes in the cytoplasmic level of ATP (24). In promastigotes exposed to HePC, dose- and time-dependent decreases in luminescence and, hence, of the intracellular ATP pool were observed (Fig. 2). In parasites exposed to 15 μM HePC for 14 h, the luminescence was slashed by half.
FIG. 2.
Variation of in vivo luminescence of strain 3-Luc L. donovani promastigotes treated with HePC. Promastigotes incubated for different times with HePC at the indicated concentrations were loaded with 25 μM DMNPE-luciferin. The variation in luminescence was normalized relative to that for the untreated parasites. Maximal inhibition of luminescence was achieved with 10 mM KCN.
HePC causes mitochondrial dysfunction in L. donovani promastigotes.
After incubation of promastigotes with HePC, a drop in their intracellular ATP levels was observed. This might reflect an overall deterioration of parasite homeostasis; or it may be due to the specific effects of HePC on processes directly involved in ATP synthesis, as described for other leishmanicidal drugs (24), or to accelerated ATP decay, as described for other leishmanicidal agents that induce plasma membrane permeability (25). The latter was discarded by the scarce intracellular accumulation of SYTOX green observed. To get insight into the mechanism for the impairment of ATP synthesis, we investigated oxidative phosphorylation as a plausible target for HePC, since in Leishmania this process is known to be the main source of ATP (47). The HePC-induced variation of two functional mitochondrial parameters, ΔΨm and respiration rate, was gauged in living parasites from the level of rhodamine 123 accumulation and the oxygen consumption rate, respectively.
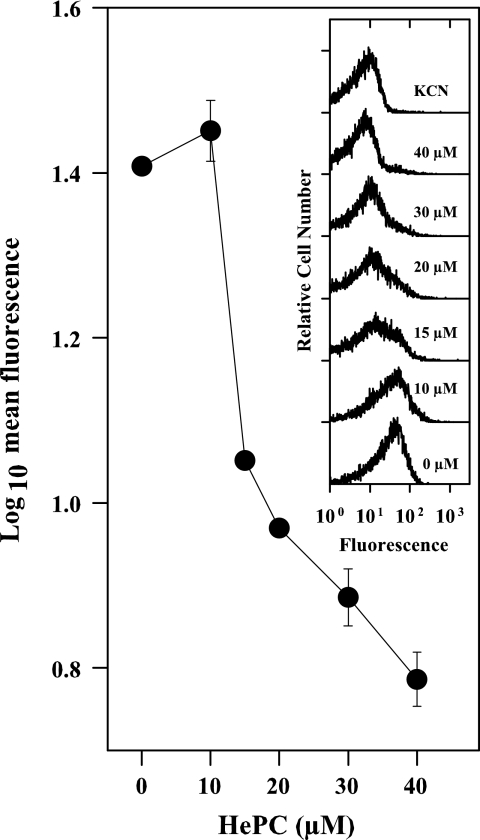
The incubation of promastigotes with HePC for 14 h induced a dose-dependent decrease in rhodamine 123 accumulation and, thus, a gradual depolarization of ΔΨm (Fig. 3), which at 40 μM HePC was comparable to that obtained with 10 mM KCN. After 14 h of incubation with HePC, a decrease in the oxygen consumption rate was observed (Table 1); the inhibition at 40 μM reached ca. 55% of that for the nontreated parasites. Even after this long incubation with HePC, the integrity of the mitochondrial inner membrane was preserved, as state 3 was restored in digitonized parasites by ADP addition (data not shown). Altogether, HePC significantly affects the mitochondrial functionality, in agreement with the findings of other authors (38).
FIG. 3.
Variation of ΔΨm of L. donovani promastigotes, as monitored by rhodamine 123 accumulation. The geometric mean from the fluorescence histograms was plotted against the HePC concentration. Parasites preincubated for 14 h with HePC were loaded with 0.3 μg/ml rhodamine 123, and the fluorescence level was measured by cytofluorometry. (Inset) Fluorescence histograms for the respective experiments. The drug concentration is shown at the side of each trace. Fully depolarized parasites were obtained by incubation with 10 mM KCN.
TABLE 1.
Variation of oxygen consumption rates of L. donovani promastigotes after incubation with HePC for 14 h
| HePC concn (μM) | O2 consumption rate (nmol O2 × min−1 × 10−8 cells) | % Consumption relative to that for control |
|---|---|---|
| 0 | 19.6 | 100.0 |
| 10 | 18.7 | 95.4 |
| 20 | 14.7 | 75.0 |
| 40 | 8.7 | 44.4 |
Identification of HePC target in the respiratory chain.
Next, HePC inhibition of the respiratory chain was characterized by using succinate as the substrate. To this end, HePC was added to digitonized parasites; and the recovery of their oxygen consumption rate, achieved after the chain was fed with specific substrates, was monitored. Similar to the previous experiment with parasites incubated for a long period with HePC, the integrity of the inner membrane was evidenced by the increase in respiration after the addition of ADP (state 3) (48). The detergent effect of HePC was neutralized by the high concentration of fatty acid-free bovine serum albumin present in the respiration buffer.
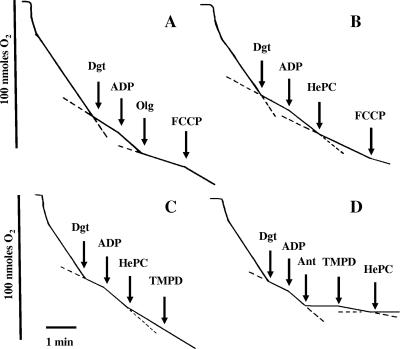
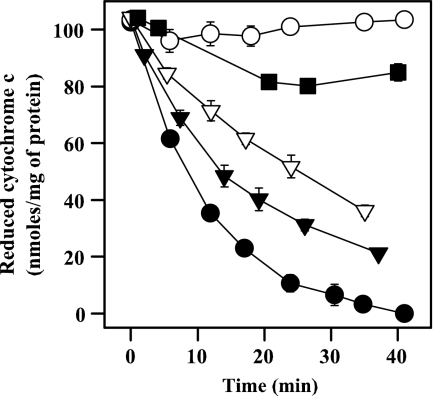
Figure 4 shows the variation in oxygen consumption rates under different conditions. Figure 4A illustrates the respiration pattern in untreated parasites. The initial rate was partially inhibited by digitonin, and state 3 was obtained after ADP addition. The inhibition by oligomycin, a typical ATPase inhibitor, and the increase in the O2 consumption rate produced by FCCP as an uncoupling agent ensured that the synthesis of ATP and the respiratory chain were coupled. In Fig. 4B, HePC decreased the rate of state 3 by 40%, and this was not reversed by FCCP, ruling out the inhibition of ATPase. The inhibition of the oxygen consumption rate after the addition of HePC (Fig. 4C) was not reversed by feeding complex IV directly with TMPD-ascorbate, pointing toward CcO as a likely target. Nevertheless, an unspecific impairment of mitochondrial functionality may account for this effect as well. To rule out this possibility, the succinate-dependent respiration was inhibited with antimycin A at the level of complex III, and oxygen consumption was allowed to proceed with TMPD-ascorbate as the only available substrates. This allows monitoring of CcO activity, regardless of the integrity of the electron transport chain upstream of complex IV. As shown in Fig. 4D, 25μM HePC fully inhibited TMPD-ascorbate-dependent oxygen consumption, which provides evidence of the specific inhibition of CcO. To corroborate this assumption, the inhibition of this enzyme by increasing HePC concentrations was checked in mitochondrial fractions. Figure 5 shows the direct and dose-dependent inhibition of CcO activity by HePC. Total inhibition of this enzyme was achieved with 10 mM KCN, which was used as a reference. The specificity of this effect was confirmed by the fact that the activity of cytochrome c reductase (complex III) in the mitochondrial fraction was barely inhibited (5%) with 40 μM HePC, the highest concentration tested (data not shown).
FIG. 4.
Oxygen consumption rates of digitonin-permeabilized L. donovani promastigotes. The oxygen consumption rates before HePC addition were 13.1 nmol × min−1 × 10−8 cells when 5 mM succinate was used as the substrate. Other substrates and inhibitors were added at their respective final concentrations, as follows: 60 μM digitonin (Dgt), 100 μM ADP, 12.6 μM oligomycin (Olg), 10 μM FCCP, 25 μM HePC, 1 μM antimycin A (Ant), and 0.1 mM TMPD plus 1.7 mM ascorbate (TMPD).
FIG. 5.
CcO activity with HePC. The mean CcO activity ± standard deviation was monitored by determination of the decrease in the absorbance at 550 nm that a reduced cytochrome c solution (32 μM) underwent when it was oxidized by CcO at 37°C. The spontaneous oxidation rate was determined in samples previously incubated with 10 mM KCN. The following HePC concentrations (μM) were tested: 40 (▪), 25 (▿), 15 (▾), and 0 (no treatment) (•). ○, 10 mM KCN.
DISCUSSION
A substantial number of leishmanicidal drugs have the mitochondrion as at least one of their targets. These include pentamidine, sitamaquine, and paromomycin, all of which are in clinical use or preclinical trials (1), as well as others tested successfully as antileishmanial agents in vitro or in animal models, such as licochalcones (51), phenylphenalenones (23), and aurones (20), among others. The study of the mitochondrion as a likely target for miltefosine on Leishmania is relevant because (i) the leishmanicidal mechanisms of HePC involve apoptosis, with the mitochondrion being a key organelle in the execution of apoptosis by the intrinsic pathway (8); (ii) functional and morphological mitochondrial alterations have been described for different trypanosomatids after LPA treatment (31, 50); and (iii) a low level of activity of HePC has been reported against the bloodstream form of African trypanosomes (6), a stage that lacks ATP production by oxidative phosphorylation due to the absence of classical cytochrome-containing respiratory chain complexes III and IV (15).
Our initial observation was a quantitative and significant decrease in the overall bioenergetic metabolism of the living promastigotes induced after incubation with HePC; this was evidenced by the decrease in the cytoplasmic levels of ATP relative to those in the nontreated parasites, as monitored by the in vivo luminescence of strain 3-Luc promastigotes. The loss of ATP due to plasma membrane permeabilization (25) was discarded as a cause, given the low level of SYTOX green uptake induced in promastigotes. Therefore, the most likely origin for this effect was impaired ATP synthesis resulting either from an overall decrease in parasite homeostasis or, as demonstrated in this work, from inhibition of oxidative phosphorylation, the main source of ATP production in Leishmania (47). After HePC incubation, the parasites showed a significant decrease in the oxygen consumption rate that paralleled the drop in ΔΨm, as monitored by the decrease in rhodamine 123 accumulation; this finding has also previously been reported by other authors for L. amazonensis promastigotes (38).
To get a deeper insight into the inhibition of ATP synthesis, we carried out a systematic dissection of the process using digitonized parasites endowed with a selective plasma membrane permeation (48). The inhibition caused by HePC was not reverted by FCCP, a typical uncoupling agent. This pointed toward defective electron transport by the respiratory chain; furthermore, when complex IV, the last complex involved in electron transport, was externally fed with its selective substrates, TMPD-ascorbate, the inhibition persisted. The specificity of the process is evidenced by the fact that HePC inhibits the oxygen consumption rate resulting from the addition of TMPD-ascorbate to a respiratory chain blocked upstream of complex IV by the prior addition of antimycin A. This clearly hints that CcO is a likely target of HePC. In fact, this complex played a key role in the control of ΔΨm and, hence, in the progression of apoptosis (19).
How this inhibition is achieved is unknown at present. A likely mechanism may be the alteration of the phospholipid environment of CcO, as its activity is highly dependent on cardiolipin molecules physically associated with the complex in yeast and mammalian cells (14, 40). HePC at a high concentration may displace, at least partially, some of these molecules, with an ensuing effect on their activities. Aside from this environmental regulation, inhibition of the internal proton transfer in CcO by some specific detergents has been described for CcO from beef mitochondria (3, 46); testing of the feasibility of this hypothesis in Leishmania will require further experimental work.
Altogether, mitochondria and, more specifically, CcO appear to be important targets for HePC, as their inhibition by this drug runs parallel to the alteration of processes such as O2 consumption and ΔΨm, as well as the drop in ATP levels. Nevertheless, we cannot single out CcO as the unique target for HePC. The existence of a plurality of HePC targets in Leishmania is supported by an extensive body of knowledge; the only traits reported nowadays for HePC resistance in Leishmania are strongly associated either with a faulty uptake or its regulation or with efflux pumps (35); in other words, resistance is associated with a scarce HePC intracellular concentration but not a specific and single mutation of a putative intracellular target. Very similar conclusions have been obtained for edelfosine in tumor cells (12). Furthermore, in L. donovani promastigotes, the intracellular HePC concentration based on previously reported data (5 nmol/mg of protein [32] and an internal volume of 4.3 μl/mg of protein [52]) is close to 1 mM, and it could be even higher if a privileged accumulation in a specific organelle were to take place. This means that the affinity of HePC would not be required to be excessively high, favoring a multiplicity of targets. Finally, the genes encoding for different CcO subunits showed a strong polymorphism, having been extensively used in evolutionary studies of Leishmania (17). It is tempting to speculate whether specific haplotypes of the CcO subunits in Leishmania might have a correlation with the outcome of HePC treatment. In fact, recently, a link between a specific haplotype of subunit II of CcO and an unusual mucosal form of L. donovani infection in Ethiopia has been described (27).
Acknowledgments
This work was supported by grant QLK2-CT-2001-01404 (EU), the Fondo de Investigaciones Sanitarias (grants PI 061125 and RD 06/0021/0006), and CYCyT grant BIO2003-09056-CO2-02.
The technical assistance with cytofluorimetry provided by Javier Moreno and Jose M. Saugar (CIB-CSIC) is greatly appreciated. We thank Eduardo Rial (CIB-CSIC) for his helpful experimental suggestions and David Andreu (Universitat Pompeu Fabra, Barcelona, Spain) for his criticisms and careful editing of the manuscript.
Footnotes
Published ahead of print on 5 February 2007.
REFERENCES
- 1.Alvar, J., S. Croft, and P. Olliaro. 2006. Chemotherapy in the treatment and control of leishmaniasis. Adv. Parasitol. 61:223-274. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Fortes, E., L. M. Ruiz-Perez, F. Bouillaud, E. Rial, and L. Rivas. 1998. Expression and regulation of mitochondrial uncoupling protein 1 from brown adipose tissue in Leishmania major promastigotes. Mol. Biochem. Parasitol. 93:191-202. [DOI] [PubMed] [Google Scholar]
- 3.Antalik, M., D. Jancura, G. Palmer, and M. Fabian. 2005. A role for the protein in internal electron transfer to the catalytic center of cytochrome c oxidase. Biochemistry 44:14881-14889. [DOI] [PubMed] [Google Scholar]
- 4.Chen, M., L. Zhai, S. B. Christensen, T. G. Theander, and A. Kharazmi. 2001. Inhibition of fumarate reductase in Leishmania major and L. donovani by chalcones. Antimicrob. Agents Chemother. 45:2023-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chicharro, C., C. Granata, R. Lozano, D. Andreu, and L. Rivas. 2001. N-terminal fatty acid substitution increases the leishmanicidal activity of CA1-7M2-9, a cecropin-melittin hybrid peptide. Antimicrob. Agents Chemother. 45:2441-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Croft, S. L., K. Seifert, and M. Duchene. 2003. Antiprotozoal activities of phospholipid analogues. Mol. Biochem. Parasitol. 126:165-172. [DOI] [PubMed] [Google Scholar]
- 7.Croft, S. L., S. Sundar, and A. H. Fairlamb. 2006. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 19:111-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debrabant, A., N. Lee, S. Bertholet, R. Duncan, and H. L. Nakhasi. 2003. Programmed cell death in trypanosomatids and other unicellular organisms. Int. J. Parasitol. 33:257-267. [DOI] [PubMed] [Google Scholar]
- 9.de Castro, S. L., R. M. Santa-Rita, J. A. Urbina, and S. L. Croft. 2004. Antiprotozoal lysophospholipid analogues: a comparison of their activity against trypanosomatid parasites and tumor cells. Mini Rev. Med. Chem. 4:141-151. [DOI] [PubMed] [Google Scholar]
- 10.Desjeux, P., and J. Alvar. 2003. Leishmania/HIV co-infections: epidemiology in Europe. Ann. Trop. Med. Parasitol. 97(Suppl. 1):3-15. [DOI] [PubMed] [Google Scholar]
- 11.Diaz-Achirica, P., J. Ubach, A. Guinea, D. Andreu, and L. Rivas. 1998. The plasma membrane of Leishmania donovani promastigotes is the main target for CA1-8M1-18, a synthetic cecropin A-melittin hybrid peptide. Biochem. J. 330:453-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gajate, C., R. I. Fonteriz, C. Cabaner, G. Alvarez-Noves, Y. Alvarez- Rodriguez, M. Modolell, and F. Mollinedo. 2000. Intracellular triggering of Fas, independently of FasL, as a new mechanism of antitumor ether lipid-induced apoptosis. Int. J. Cancer 85:674-682. [DOI] [PubMed] [Google Scholar]
- 13.Gajate, C., and F. Mollinedo. 2002. Biological activities, mechanisms of action and biomedical prospect of the antitumor ether phospholipid ET-18-OCH3 (edelfosine), a proapoptotic agent in tumor cells. Curr. Drug Metab. 3:491-525. [DOI] [PubMed] [Google Scholar]
- 14.Gohil, V. M., P. Hayes, S. Matsuyama, H. Schagger, M. Schlame, and M. L. Greenberg. 2004. Cardiolipin biosynthesis and mitochondrial respiratory chain function are interdependent. J. Biol. Chem. 279:42612-42618. [DOI] [PubMed] [Google Scholar]
- 15.Hellemond, J. J., B. M. Bakker, and A. G. Tielens. 2005. Energy metabolism and its compartmentation in Trypanosoma brucei. Adv. Microb. Physiol. 50:199-226. [DOI] [PubMed] [Google Scholar]
- 16.Holzmuller, P., D. Sereno, and J. L. Lemesre. 2005. Lower nitric oxide susceptibility of trivalent antimony-resistant amastigotes of Leishmania infantum. Antimicrob. Agents Chemother. 49:4406-4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibrahim, M. E., and D. C. Barker. 2001. The origin and evolution of the Leishmania donovani complex as inferred from a mitochondrial cytochrome oxidase II gene sequence. Infect. Genet. Evol. 1:61-68. [DOI] [PubMed] [Google Scholar]
- 18.Jendrossek, V., and R. Handrick. 2003. Membrane targeted anticancer drugs: potent inducers of apoptosis and putative radiosensitisers. Curr. Med. Chem. Anticancer Agents 3:343-353. [DOI] [PubMed] [Google Scholar]
- 19.Kadenbach, B., S. Arnold, I. Lee, and M. Huttemann. 2004. The possible role of cytochrome c oxidase in stress-induced apoptosis and degenerative diseases. Biochim. Biophys. Acta 1655:400-408. [DOI] [PubMed] [Google Scholar]
- 20.Kayser, O., M. Chen, A. Kharazmi, and A. F. Kiderlen. 2002. Aurones interfere with Leishmania major mitochondrial fumarate reductase. Z. Naturforsch. Sect. C 57:717-720. [DOI] [PubMed] [Google Scholar]
- 21.Kiderlen, A. F., and P. M. Kaye. 1990. A modified colorimetric assay of macrophage activation for intracellular cytotoxicity against Leishmania parasites. J. Immunol. Methods 127:11-18. [DOI] [PubMed] [Google Scholar]
- 22.Lira, R., L. M. Contreras, R. M. Rita, and J. A. Urbina. 2001. Mechanism of action of anti-proliferative lysophospholipid analogues against the protozoan parasite Trypanosoma cruzi: potentiation of in vitro activity by the sterol biosynthesis inhibitor ketoconazole. J. Antimicrob. Chemother. 47:537-546. [DOI] [PubMed] [Google Scholar]
- 23.Luque-Ortega, J. R., S. Martinez, J. M. Saugar, L. R. Izquierdo, T. Abad, J. G. Luis, J. Pinero, B. Valladares, and L. Rivas. 2004. Fungus-elicited metabolites from plants as an enriched source for new leishmanicidal agents: antifungal phenyl-phenalenone phytoalexins from the banana plant (Musa acuminata) target mitochondria of Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 48:1534-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luque-Ortega, J. R., O. M. Rivero-Lezcano, S. L. Croft, and L. Rivas. 2001. In vivo monitoring of intracellular ATP levels in Leishmania donovani promastigotes as a rapid method to screen drugs targeting bioenergetic metabolism. Antimicrob. Agents Chemother. 45:1121-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luque-Ortega, J. R., J. M. Saugar, C. Chiva, D. Andreu, and L. Rivas. 2003. Identification of new leishmanicidal peptide lead structures by automated real-time monitoring of changes in intracellular ATP. Biochem. J. 375:221-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lux, H., N. Heise, T. Klenner, D. Hart, and F. R. Opperdoes. 2000. Ether-lipid (alkyl-phospholipid) metabolism and the mechanism of action of ether-lipid analogues in Leishmania. Mol. Biochem. Parasitol. 111:1-14. [DOI] [PubMed] [Google Scholar]
- 27.Mahdi, M., E. M. Elamin, S. E. Melville, A. M. Musa, J. M. Blackwell, M. M. Mukhtar, A. M. Elhassan, and M. E. Ibrahim. 2005. Sudanese mucosal leishmaniasis: isolation of a parasite within the Leishmania donovani complex that differs genotypically from L. donovani causing classical visceral leishmaniasis. Infect. Genet. Evol. 5:29-33. [DOI] [PubMed] [Google Scholar]
- 28.Mukherjee, S. B., M. Das, G. Sudhandiran, and C. Shaha. 2002. Increase in cytosolic Ca2+ levels through the activation of non-selective cation channels induced by oxidative stress causes mitochondrial depolarization leading to apoptosis-like death in Leishmania donovani promastigotes. J. Biol. Chem. 277:24717-24727. [DOI] [PubMed] [Google Scholar]
- 29.Olliaro, P. L., P. J. Guerin, S. Gerstl, A. A. Haaskjold, J. A. Rottingen, and S. Sundar. 2005. Treatment options for visceral leishmaniasis: a systematic review of clinical studies done in India, 1980-2004. Lancet Infect. Dis. 5:763-774. [DOI] [PubMed] [Google Scholar]
- 30.Ouellette, M., J. Drummelsmith, and B. Papadopoulou. 2004. Leishmaniasis: drugs in the clinic, resistance and new developments. Drug Resist. Update 7:257-266. [DOI] [PubMed] [Google Scholar]
- 31.Paris, C., P. M. Loiseau, C. Bories, and J. Breard. 2004. Miltefosine induces apoptosis-like death in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 48:852-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Victoria, F. J., S. Castanys, and F. Gamarro. 2003. Leishmania donovani resistance to miltefosine involves a defective inward translocation of the drug. Antimicrob. Agents Chemother. 47:2397-2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez-Victoria, F. J., F. Gamarro, M. Ouellette, and S. Castanys. 2003. Functional cloning of the miltefosine transporter. A novel P-type phospholipid translocase from Leishmania involved in drug resistance. J. Biol. Chem. 278:49965-49971. [DOI] [PubMed] [Google Scholar]
- 34.Perez-Victoria, F. J., M. P. Sanchez-Canete, S. Castanys, and F. Gamarro. 2006. Phospholipid translocation and miltefosine potency require both L. donovani miltefosine transporter and the new protein LdRos3 in Leishmania parasites. J. Biol. Chem. 281:23766-23775. [DOI] [PubMed] [Google Scholar]
- 35.Perez-Victoria, F. J., M. P. Sanchez-Canete, K. Seifert, S. L. Croft, S. Sundar, S. Castanys, and F. Gamarro. 2006. Mechanisms of experimental resistance of Leishmania to miltefosine: implications for clinical use. Drug Resist. Update 9:26-39. [DOI] [PubMed] [Google Scholar]
- 36.Rakotomanga, M., M. Saint-Pierre-Chazalet, and P. M. Loiseau. 2005. Alteration of fatty acid and sterol metabolism in miltefosine-resistant Leishmania donovani promastigotes and consequences for drug-membrane interactions. Antimicrob. Agents Chemother. 49:2677-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santa-Rita, R. M., H. S. Barbosa, and S. L. de Castro. 2006. Ultrastructural analysis of edelfosine-treated trypomastigotes and amastigotes of Trypanosoma cruzi. Parasitol. Res. 100:187-190. [DOI] [PubMed] [Google Scholar]
- 38.Santa-Rita, R. M., A. Henriques-Pons, H. S. Barbosa, and S. L. de Castro. 2004. Effect of the lysophospholipid analogues edelfosine, ilmofosine and miltefosine against Leishmania amazonensis. J. Antimicrob. Chemother. 54:704-710. [DOI] [PubMed] [Google Scholar]
- 39.Santa-Rita, R. M., R. Lira, H. S. Barbosa, J. A. Urbina, and S. L. de Castro. 2005. Anti-proliferative synergy of lysophospholipid analogues and ketoconazole against Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae): cellular and ultrastructural analysis. J. Antimicrob. Chemother. 55:780-784. [DOI] [PubMed] [Google Scholar]
- 40.Sedlak, E., M. Panda, M. P. Dale, S. T. Weintraub, and N. C. Robinson. 2006. Photolabeling of cardiolipin binding subunits within bovine heart cytochrome c oxidase. Biochemistry 45:746-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seifert, K., S. Matu, F. Javier Perez-Victoria, S. Castanys, F. Gamarro, and S. L. Croft. 2003. Characterisation of Leishmania donovani promastigotes resistant to hexadecylphosphocholine (miltefosine). Int. J. Antimicrob. Agents 22:380-387. [DOI] [PubMed] [Google Scholar]
- 42.Sereno, D., P. Holzmuller, I. Mangot, G. Cuny, A. Ouaissi, and J. L. Lemesre. 2001. Antimonial-mediated DNA fragmentation in Leishmania infantum amastigotes. Antimicrob. Agents Chemother. 45:2064-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sindermann, H., S. L. Croft, K. R. Engel, W. Bommer, H. J. Eibl, C. Unger, and J. Engel. 2004. Miltefosine (Impavido): the first oral treatment against leishmaniasis. Med. Microbiol. Immunol. (Berlin) 193:173-180. [DOI] [PubMed] [Google Scholar]
- 44.Soto, J., B. A. Arana, J. Toledo, N. Rizzo, J. C. Vega, A. Diaz, M. Luz, P. Gutierrez, M. Arboleda, J. D. Berman, K. Junge, J. Engel, and H. Sindermann. 2004. Miltefosine for New World cutaneous leishmaniasis. Clin. Infect. Dis. 38:1266-1272. [DOI] [PubMed] [Google Scholar]
- 45.Sottocasa, G. L., B. Kuylenstierna, L. Ernster, and A. Bergstrand. 1967. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J. Cell Biol. 32:415-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tarasev, M., and B. C. Hill. 2002. Detergent modulation of electron and proton transfer reactions in bovine cytochrome c oxidase. Arch. Biochem. Biophys. 400:162-170. [DOI] [PubMed] [Google Scholar]
- 47.Van Hellemond, J. J., and A. G. Tielens. 1997. Inhibition of the respiratory chain results in a reversible metabolic arrest in Leishmania promastigotes. Mol. Biochem. Parasitol. 85:135-138. [DOI] [PubMed] [Google Scholar]
- 48.Vercesi, A. E., C. F. Bernardes, M. E. Hoffmann, F. R. Gadelha, and R. Docampo. 1991. Digitonin permeabilization does not affect mitochondrial function and allows the determination of the mitochondrial membrane potential of Trypanosoma cruzi in situ. J. Biol. Chem. 266:14431-14434. [PubMed] [Google Scholar]
- 49.Vergnes, B., B. Gourbal, I. Girard, S. Sundar, J. Drummelsmith, and M. Ouellette. 2007. A proteomic screen implicates HSP83 and a small kinetoplastid calpain-related protein in drug resistance in Leishmania donovani clinical field isolates by modulating drug-induced programmed cell death. Mol. Cell. Proteomics 6:88-101. [DOI] [PubMed] [Google Scholar]
- 50.Verma, N. K., and C. S. Dey. 2004. Possible mechanism of miltefosine-mediated death of Leishmania donovani. Antimicrob. Agents Chemother. 48:3010-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhai, L., J. Blom, M. Chen, S. B. Christensen, and A. Kharazmi. 1995. The antileishmanial agent licochalcone A interferes with the function of parasite mitochondria. Antimicrob. Agents Chemother. 39:2742-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zilberstein, D., and D. M. Dwyer. 1985. Proton motive force-driven active transport of d-glucose and l-proline in the protozoan parasite Leishmania donovani. Proc. Natl. Acad. Sci. USA 82:1716-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]