Abstract
The objective of this study was to evaluate the pharmacokinetics of atazanavir (ATV), saquinavir (SQV), and ritonavir (RTV) in a boosted double-protease inhibitor (PI) therapy regimen without reverse transcriptase inhibitors (RTIs). The study design was as follows. Patients with limited RTI options received a PI combination of 300/100 mg ATV/RTV once daily and 1,000 mg SQV twice daily (group 1; n = 49) without RTI comedication. The results were compared to the plasma concentrations of PIs of patients taking either 300 mg ATV/100 mg RTV once daily plus RTIs (group 2; n = 72) or patients taking 1,000 mg SQV/100 mg RTV plus RTIs (group 3; n = 90). The study methods were as follows. Patients were given a 12/24-h pharmacokinetic assessment at steady state. Drug concentrations were measured by liquid chromatography-tandem mass spectrometry. The minimum and maximum concentrations (Cmin and Cmax), area under the concentration-time curve under steady-state conditions (AUCss), elimination half-life, time of maximum concentration and lag time were subject to statistical analysis. The results show that patients treated with ATV/SQV/RTV exhibited significantly high SQV concentrations and moderate enhancement of the AUCss of ATV in comparison to those of patients of the control groups: for SQV in groups 1 and 3, the geometric mean (GM) of the AUCss was 22,794 versus 15,759 ng·h/ml (GM ratio [GMR] = 1.45; P < 0.05), the GM of the Cmax was 3,257 versus 2,331 ng/ml (GMR = 1.40; P < 0.05), and the GM of the Cmin was 438 versus 437 ng/ml (GMR = 1.00); for ATV in groups 1 and 2, the GM of the AUCss was 39,154 versus 33,626 ng·h/ml (GMR = 1.16), the GM of the Cmax was 3,488 versus 2,924 ng/ml (GMR = 1.20), and the GM of the Cmin was 515 versus 428 ng/ml (GMR = 1.21). RTV levels were comparable for all groups. A subgroup analysis detected only marginal differences in ATV plasma exposure if combined with tenofovir-disoproxilfumarate and without it. We conclude that our pharmacokinetic results support the use of a boosted double-PI regimen of ATV/SQV/RTV as a treatment option for patients who need antiretroviral therapy without RTIs.
Therapy options for patients who no longer have treatment alternatives with reverse transcriptase inhibitors (RTIs) due to toxicity or resistance are limited. Boosted double-protease inhibitor (PI) regimens have been described and are currently used in clinics as one therapy option for these patients.
However, the use of boosted dual-PI therapy regimens can be limited by various factors, such as resistance, toxicity, or unwanted pharmacokinetic interactions. Most human immunodeficiency virus (HIV) PIs are metabolized by the same hepatic cytochrome P450 (CYP) isoenzymes. PIs are not only substrates, but induce or inhibit cytochromes to a certain degree. The unwanted pharmacokinetic interactions between tipranavir, inducing CYP3A, and either saquinavir (SQV), lopinavir, or amprenavir led to the recommendation not to use these combinations in patients (4). Also, fosamprenavir and lopinavir-ritonavir (RTV) should not be used together, as the plasma concentrations of both PIs were significantly reduced when measured at steady state (23). Other combinations, such as SQV and lopinavir-RTV, are not limited by pharmacokinetic interactions and show a sufficient immunological and virological response even in heavily pretreated patients (45, 46, 47). As CYP3A4/5 is responsible for at least 90% of the metabolism of SQV and atazanavir (ATV), pharmacological enhancement with low-dose RTV, which is a potent inhibitor of CYP3A4/5, has been extensively described (9, 10); the autoinduction of cytochromes leads to changing plasma concentrations over the first 2 weeks of treatment (10, 17, 18), whereas in the following phase, RTV constantly inhibits CYP3A4/5, with a Ki of 0.019 to 0.03 μM (10, 15). In addition, RTV inhibits and induces a number of cytochromes, such as CYP1A2, CYP2C9, CYP2D6, CYP2E1, and CYP3A7, to various degrees (9, 10, 15). Since it has been shown, at least for SQV, that its metabolism does not consistently rely on CYP1A2, CYP2C9, or CYP2E1 (11), these interactions should play a minor role in the investigated combination. However, if more than two PIs are coadministered, it is difficult to predict drug-drug interactions in such combinations. SQV, for example, is also a substrate of P-glycoprotein, which is inhibited by RTV, and therefore shows markedly enhanced plasma concentrations in animal models, as discussed previously (19-21, 33, 49), although the impact on bioavailability and metabolism in humans may be restricted (7). SQV mesylate is an established PI with well-described pharmacokinetics, safety profile, and efficacy, currently available as a 500-mg tablet formulation (2). RTV-boosted SQV has been approved for the twice-daily dosage of 1,000/100 mg SQV/RTV (27; Fortovase product information, 27898325-702, Roche Laboratories Inc., NJ, 2002; Invirase product information, 27898310-702, Roche Laboratories Inc., NJ).
ATV is an azapeptide PI which shows good bioavailability and plasma protein binding of about 86%. ATV is a substrate and an inhibitor of CYP3A4/5 (38) and demonstrates nonlinear pharmacokinetics with greater than dose-proportional increases in the area under the concentration-time curve (AUC) and the maximum concentration (Cmax) within the dosing interval over the dose range of 200 to 800 mg. When coadministered, low-dose RTV significantly enhances ATV plasma levels (29; Reyataz product information, F1-B001-06-03, 2003, Bristol-Myers Squibb Company, Princeton, NJ).
ATV is the least likely HIV PI to contribute to dyslipidemia (6). Also, ATV shows a nonoverlapping resistance profile with SQV for HIV type 1 (HIV-1) protease (14). The unique I50L primary mutation for ATV in previously PI-naïve patients does not change or may even enhance the susceptibility of the virus to SQV and other PIs (8). The ATV dosage in different therapy regimens has been either 400 mg unboosted (35) or 300 mg ATV boosted with 100 mg RTV once daily, plus nucleoside or nucleotide reverse transcriptase inhibitors (NRTIs) (Reyataz product information, F1-B001-06-03, 2003, Bristol-Myers Squibb Company, Princeton, NJ).
In addition to the pharmacological interactions apparent in PI combinations, resistance or toxicity can limit the therapeutic options of boosted dual-PI regimens (13, 32). Thus, the goal of this observational study was to evaluate the steady-state pharmacokinetic interactions and safety during the first 12 weeks of therapy with a regimen of 300 mg ATV/100 mg RTV once daily in combination with 1,000 mg SQV twice daily in the absence of RTIs in multiply pretreated HIV-1-infected adults.
MATERIALS AND METHODS
Patients.
Forty-nine patients with limited options for therapy with RTIs were treated with ATV/RTV (300/100 mg once daily) and SQV (1,000 mg twice daily) without the addition of RTIs (group 1). These patients had a long treatment history (Table 1) and had experienced either toxicities or viral resistance, especially following the intake of RTIs. Two groups of patients who were predominantly antiretroviral treatment-naïve served as controls: group 2 consisted of 72 patients taking 300 mg ATV/100 mg RTV plus two or three NRTIs, and the 90 patients in group 3 received 1,000 mg SQV/100 mg RTV twice daily, also in combination with two or three NRTIs. All patients taking SQV mesylate in this study randomly received either the soft-gelatin capsule (as Fortovase; Hoffman-La Roche, Basel, Switzerland; n = 61) or the hard-gelatin capsule (Invirase; Hoffman-La Roche, Basel, Switzerland; n = 78). The choice of soft- or hard-gelatin capsules was not part of the study design, but given by the assignment of an individual patient to one of the presently available formulations during the transition of commercial availability from Fortovase to Invirase during the study period.
TABLE 1.
Patients' baseline characteristics
| Characteristic | Group and regimen (no. of patients)
|
|||||
|---|---|---|---|---|---|---|
| 1, ATV/SQV/RTV (49)
|
2, ATV/RTV+NRTI (72)
|
3, SQV/RTV+NRTI (90)
|
||||
| Mean or no. | % or range | Mean or no. | % or range | Mean or no. | % or range | |
| Male/female (no.) | 43/6 | 87.8/12.2 | 47/25 | 65.3/34.7 | 63/27 | 70.0/30.0 |
| Caucasian/other (no.) | 43/6 | 87.8/12.2 | 64/8 | 88.9/11.1 | 74/16 | 82.2/17.8 |
| Age (yr) | 45.1 | 28.0-71.0 | 41.5 | 23.0-72.0 | 41.0 | 19.0-60.0 |
| Body mass index (kg/m2) | 24.9 | 18.5-33.0 | 24.9 | 16.2-38.6 | 23.2 | 18.8-36.0 |
| CDC status C3/B3 (no.) | 39 | 79.6 | 46 | 63.9 | 55 | 61.1 |
| Hepatitis B virus s antigen (HBsAg)/HBeAg, HCV PCR positive (no.) | 6 | 12.2 | 18 | 25.0 | 11 | 15.3 |
| CD4 cell count/μl | 297 | 10-779 | 290 | 4-1,082 | 138 | 1-624 |
| HIV viral load, log10 | 3.02 | 1.28-5.88 | 3.71 | 1.28-6.00 | 4.72 | 1.28-6.00 |
| Previous treatments (no.) | 7.1 | 0-24 | 4.6 | 0-30 | 3.5 | 0-10 |
| Time to pharmacokinetic assessment (wks) | 5.3 | 2-24 | 16.0 | 2-140 | 8.0 | 2-48 |
| Treatment naive (no.) | 1 | 2.0 | 7 | 9.7 | 68 | 75.6 |
| Saquinavir formulation (no.) | ||||||
| SQV in soft-gelatin capsule | 10 | 20.4 | 43 | 47.8 | ||
| SQV in hard-gelatin capsule | 39 | 79.6 | 47 | 52.2 | ||
| NRTI comedication (no. receiving) | ||||||
| Abacavir | 7 | 9.7 | 16 | 17.8 | ||
| Didanosine | 25 | 34.7 | 9 | 10.0 | ||
| Emtricitabine | 19 | 26.4 | ||||
| Lamivudine | 24 | 33.3 | 81 | 90.0 | ||
| Stavudine | 3 | 4.2 | 4 | 4.4 | ||
| Tenofovir-DF | 53 | 73.6 | 20 | 22.2 | ||
| Zidovudine | 8 | 11.1 | 64 | 71.1 | ||
There were no CD4 cell count or viral load restrictions included in this study in any of the three treatment groups. HIV-1 RNA was measured using a COBAS AmpliPrep/COBAS TaqMan HIV-1 test (Roche Diagnostics, Mannheim, Germany) with a quantification range of 50 to 10,000,000 copies/ml. PI therapy-experienced patients were made eligible for the ATV/SQV/RTV therapy regimen after the interpretation of genotypic-resistance testing (according to the International AIDS Society—USA Drug Resistance Mutations Group algorithm) (22) showed sufficient susceptibility of the patient's HIV-1 virus to the therapy compounds.
Patients completed a 12/24-h pharmacokinetic measurement following a standardized pharmacokinetic protocol which is used for all outpatients (47). Pharmacokinetic data were assessed for samples from all patients who started with a new therapy regimen between March 2002 (group 3) and March 2003 (groups 1 and 2) and participated in the therapeutic-drug monitoring (TDM) procedure until June 2005 (all groups).
Routine liver function tests during the study assured that patients with hepatic impairment defined by Child-Pugh classification B or C and patients receiving comedication with CYP3A4/5 enzyme inhibitors or inducers as well as antacids were not included in the study; this exclusion also included patients treated with antibiotics or antifungals, such as azoles, that are frequently used in therapy for opportunistic infections of HIV patients. An exception was cotrimoxazole, 960 mg taken thrice weekly as a Pneumocystis carinii pneumonia prophylaxis, which is known to have no pharmacological-interaction impact on PI metabolism (31). Data were obtained as part of the TDM that is regularly performed in the HIV Treatment and Clinical Research Unit at the J. W. Goethe University Hospital, Frankfurt, Germany. Verbal consent for the TDM procedure was obtained from patients and documented in the patients' records. This pharmacokinetic study design is observational; no additional intervention was performed, and ethics approval was not obtained, according to the National Medical Act and the advice of the responsible ethics committee. Nevertheless, patients were individually informed about the TDM procedure and agreed with the documentation of their comedication and compliance.
Study protocol.
After at least 2 weeks on the regimen (median of 4 weeks for all groups; mean values are shown in Table 1), patients underwent a pharmacokinetic assessment following a standardized protocol at steady-state conditions. The schedule of drug intake was documented by the patients for 3 days prior to the pharmacokinetic assessment. In addition, all concomitant drug intake had to be documented by the patient and physician, including daily intake of herbal agents or nutritive supplements. On the day of the pharmacokinetic assessment, fasting trough levels (Ctrough) were obtained immediately before drug intake, followed by a standardized breakfast of about 2,500 kJ (25% from fat; 20 g butter, 50 g bread, 40 g cornflakes, 20 g jam, 150 ml yogurt [3.5% fat], 200 ml apple juice, and 400 ml tea). Plasma samples were then collected at 1, 2, 4, 6, 9, 12, and 24 h after the drug intake; the second daily dose of SQV for patients in group 1 was taken after 12 h.
Pharmacokinetic assay.
Plasma concentrations of ATV, SQV, and RTV were determined by validated high-pressure liquid chromatography-tandem mass spectrometry methods (equipment from Merck-Hitachi, Germany, and Applied Biosystems/Canada at Therapia GmbH, Berlin, Germany) that are described elsewhere (28). The reliable lower limit of quantification was 20 ng/ml, and the linearity of the calibration curve for all tested compounds was proved up to 20,000 ng/ml (3, 28).
Pharmacokinetic evaluation.
Pharmacokinetic calculations were based on plasma concentrations which exceeded the lower limit of quantification. The pharmacokinetic parameters were calculated according to a noncompartmental approach from the 0-to-24-h data and after fitting the data to an open two-compartment model. This model was chosen instead of a one-compartment model after comparison of the quality of the fit according to the Akaike criterion. The minimum concentration (Cmin) and Cmax values of the noncompartmental analysis were read directly from the plasma concentration-time curves of ATV, SQV, and RTV within the standard dosing interval (τ, specifically 12 or 24 h).
The AUCss(τ) is obtained with the logarithmic trapezoidal rule. The elimination half-life (t1/2) is calculated from the elimination constant (λz) with the equation t1/2 = ln2/λz (time). The lag time (Tlag) equals the time until resorption of the dose begins.
The Tlag and the time of the maximum concentration (Tmax) of either substance in blood were obtained by using a two-compartment analysis model. The appropriateness of the pharmacokinetic assessment (8 samples over 24 h) under open conditions in an outpatient center was demonstrated by comparing the robust noncompartmental analysis with the fit to a predefined pharmacokinetic model in which accuracy depends on the closeness of sampling. The accordance of the primary pharmacokinetic variables, as assessed by the noncompartmental analysis, with the results of the two-compartment model after curve fitting was proved by linear regression analysis. The two-compartment model is necessary to evaluate Tlag and Tmax data providing additional information about the resorption and distribution of the compounds in patients if blood assessment during dissolution and absorption of the compounds (0 to 4 h) is limited due to the TDM setting.
All pharmacokinetic analyses were performed with TOPFIT2.0 (16).
Pharmacodynamic measurements.
The study focused on the pharmacokinetic interaction between the combination partners. Nevertheless, toxicity parameters and adverse events (grade two or more according to the common toxicity criteria [CTC] v3.0; http://www.fda.gov/cder/cancer/toxicityframe.htm, accessed November 3, 2006), infections, and AIDS-related diagnoses (category C according to the guidelines of the Centers for Disease Control and Prevention [CDC]; http://wonder.cdc.gov/wonder/help/AIDS/MMWR-12-18-1992.html, accessed November 3, 2006) were simultaneously recorded over the first 12 weeks of therapy.
Statistical methods.
Primary target variables were the AUCss(τ), Cmax, Cmin, total clearance (CLtot), t1/2, Tmax, and Tlag of ATV, SQV, and RTV. The statistical analysis used the t test to compare the means of the primary target variables. Absence of a significant difference in the levels of ATV, SQV, and RTV exposure between groups was suggested when no significant difference in the geometric mean ratio (GMR), together with a 90% confidence interval, was determined. This statistical procedure follows the current Food and Drug Administration guidelines for in vivo pharmacokinetic evaluation (12). The sample-size calculation for the comparison between groups was based on the detection of a difference of ±20% in the AUCss(0-τ) of ATV/SQV for either control group. A minimum sample size of 45 subjects per group was calculated for a power of 80% and a two-sided significance level of α, 0.05, for the analysis of variance (ANOVA) (H. Ackermann, BIAS for Windows, version 7.06, 2004).
The descriptive statistical analyses used SPSS version 11.5 for Windows (2004).
RESULTS
Disposition of patients.
The demographics and characteristics of patients at the therapy baseline assessment were comparable in all groups, except for parameters influenced by the pretreatment history (Table 1). The mean ages were 45.1 (range, 28.0 to 71.0), 41.5 (range, 23.0 to 72.0), and 41.0 (range, 19.0 to 60.0) years for groups 1, 2, and 3, respectively. The majority of patients were male and Caucasian, with comparable body mass indexes and HIV status according to CDC guidelines for diagnosis. The baseline viral loads and CD4 cells counts differed between the groups according to the variability of length and history of pretreatment. The baseline CD4 cell counts exhibited means of 297 (range, 10 to 779) μl−1, 290 (range, 4 to 1,082) μl−1, and 138 (range, 1 to 624) μl−1 for groups 1, 2, and 3, respectively (Kruskal-Wallis, P > 0.001). The baseline viral loads showed a reciprocal relationship, with means of 3.02 (range, 1.28 to 5.88), 3.71 (range, 1.28 to 6.00), and 4.72 (range, 1.28 to 6.00) log10 copies/ml for groups 1, 2, and 3, respectively (Kruskal-Wallis, P > 0.001). A higher number of hepatitis B antigen-positive or hepatitis C virus PCR-positive patients was found in group 2 (25%) at the baseline assessment, but clinical and laboratory anamneses detected neither markedly elevated liver enzymes (>twofold the upper limit of normality [ULN]) nor signs of cirrhosis (Child-Pugh B or C) in these patients (Table 1). In contrast to the control groups, most of the patients in the ATV/SQV/RTV group (group 1) were multiply pretreated patients who had experienced prior treatment failure with RTIs. In groups 1, 2, and 3, 89.8, 62.5, and 14.4%, respectively, of the patients were PI experienced. The mean numbers of previously taken PIs were 2.3, 1.8, and 1.4 for groups 1, 2, and 3, respectively. A considerable number of patients experienced a structured-treatment inhibition prior to the boosted double-PI therapy (28.6% and 12.5% of groups 1 and 2), whereas 75.6% of patients in group 3 were treatment naïve at the baseline assessment. The assignment of patients to either formulation of SQV mesylate was slighly uneqally distributed in groups 1 and 3, as 36.7% of patients received the soft-gelatin capsule formulation (Fortovase) and 63.3% of patients received the hard-gelatin formulation (Invirase) in group 1, and 47.8% of patients received the soft-gelatin capsule formulation and 52.2% of patients received the hard-gelatin formulation in group 3. This represents no significant difference between the groups (Fisher's exact test of Fortovase/Invirase versus groups 1/3, P = 0.283). Thirty-nine out of 216 patients received 960 mg cotrimoxazole thrice weekly as a Pneumocystis carinii pneumonia prophylaxis (group 1, n = 7 [14.3%], versus group 2, n = 15 [16.7%], versus group 3, n = 17 [34.7%]; Pearson χ2, P = 0.298).
Pharmacokinetics.
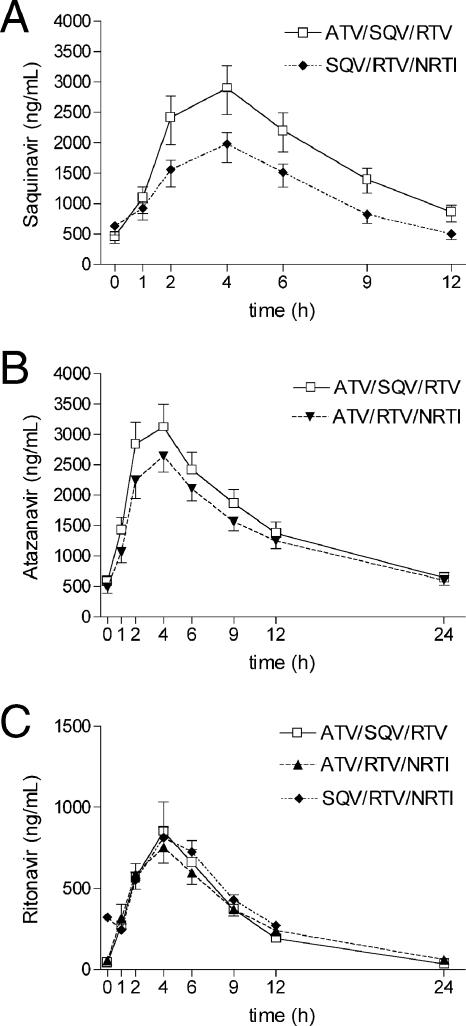
The plasma concentrations of SQV for group 1, when coadministered with ATV, and group 3, without ATV, were significantly different. Table 2 shows all pharmacokinetic results given as GMs (90% confidence interval [CI]) and GMRs (including P values for the ANOVA). The measured plasma concentrations of SQV showed a considerable difference over the entire 12-h dosing interval (Fig. 1A): the GM (90% CI) of the AUCss of SQV was 22,794 (range, 19,959 to 26,032) ng·h/ml in group 1 versus 15,759 (range, 13,880 to 17,892) ng·h/ml in group 3 (GMR = 1.45; P = 0.012) and the GM of the Cmax was 3,257 ng/ml for group 1 versus 2,331 ng/ml for group 3 (GMR = 1.40; P = 0.014). By contrast, the GM of the Cmin did not vary between group 1 (438 ng/ml) and group 3 (437 ng/ml), although SQV was taken without RTV the evening before the pharmacokinetic assessment. Furthermore, the GM of the t1/2 of SQV was significantly prolonged when ATV was coadministered (4.53 h for group 1 versus 3.86 h for group 3; GMR = 1.17; P = 0.023).
TABLE 2.
Summary of the geometric mean (90% CI) steady-state pharmacokinetic parameters for ATV, SQV, and RTV, including GMR and P values (two-sided ANOVA) for the differences between groups
| Drug | Group and regimen (no. of patients) | Parameter [GM (90% CI)]
|
|||||
|---|---|---|---|---|---|---|---|
| Cmin (ng/ml) | Cmax (ng/ml) | AUCss (ng·h/ml) | t1/2 (h) | Tmax (h) | Tlag (h) | ||
| ATV | 1, SQV/RTV (49) | 515 (440, 604) | 3,488 (3,081, 3,949) | 39,154 (34,255, 44,752) | 7.64 (7.12, 8.19) | 2.43 (1.52, 2.90) | 0.47 (0.29, 0.76) |
| 2, RTV/NRTI (72) | 428 (390, 503) | 2,924 (2,869, 3,366) | 33,626 (31,143, 38,431) | 8.06 (7.73, 8.63) | 2.67 (1.92, 2.83) | 0.51 (0.42, 0.72) | |
| 1 vs 2 | 1.21 (1.14, 1.55) | 1.20 (1.09, 1.38) | 1.16 (1.12, 1.44) | 0.94 (0.82, 0.95) | 0.91 (0.53, 1.51) | 0.92 (0.40, 1.81) | |
| SQV | 1, ATV/RTV (49) | 438 (357, 537) | 3,257 (2,869, 3,697) | 22,794 (19,959, 26,032) | 4.52 (4.28, 4.76) | 2.94 (2.70, 3.21) | 1.02 (0.84, 1.24) |
| 3, RTV/NRTI (90) | 437 (374, 512) | 2,331 (2,069, 2,627) | 15,759 (13,880, 17,892) | 3.86 (3.67, 4.06) | 2.95 (1.97, 3.22) | 0.78 (0.63, 0.97) | |
| 1 vs 3 | 1.00 (0.70, 1.53) | 1.40 (1.09, 1.79)a | 1.45 (1.12, 1.88)a | 1.17 (1.05, 1.30)a | 1.00 (0.84, 1.63) | 1.31 (0.87, 1.97) | |
| RTV | 1, ATV/SQV (49) | 29 (24, 35) | 1,050 (933, 1,183) | 8,100 (7,076, 9,270) | 3.76 (3.52, 4.01) | 2.67 (1.65, 3.22) | 0.30 (0.17, 0.54) |
| 2, ATV/NRTI (72) | 50 (47, 58) | 943 (931, 1,092) | 8,450 (8,239, 9,586) | 4.82 (4.63, 5.08) | 2.70 (1.66, 2.61) | 0.16 (0.13, 0.28) | |
| 3, SQV/RTV (90) | 231 (208, 251) | 970 (878, 1,071) | 6,899 (6,286, 7,573) | 3.85 (3.65, 4.05) | 3.34 (1.97, 3.57) | 0.55 (0.36, 0.85) | |
| 1 vs 2 | 0.58 (0.41, 0.74)c | 1.11 (0.87, 1.24) | 0.96 (0.74, 1.13) | 0.78 (0.69, 0.87)b | 0.99 (0.63, 1.94) | 1.88 (0.61, 4.15)c | |
| 1 vs 3 | 0.13 (0.10, 0.17)c | 1.08 (0.87, 1.24) | 1.17 (0.93, 1.47) | 0.98 (0.87, 1.10) | 0.80 (0.46, 1.63)b | 0.55 (0.20, 1.50)c | |
Statistically significant; P < 0.05.
P < 0.01.
P < 0.001 (two-sided ANOVA).
FIG. 1.
Noncompartmental GM (90% CI) plasma concentration-time profiles for SQV in the presence of either ATV/RTV or RTV/NRTI (A); noncompartmental GM (90% CI) plasma concentration-time profiles for ATV in the presence of either SQV/RTV or RTV/NRTI (B); and noncompartmental GM (90% CI) plasma concentration-time profiles for RTV in the presence of ATV/SQV, SQV/NRTI, or ATV/NRTI (C).
The ATV plasma levels in patients differed moderately between groups 1 and 2, without reaching a level of statistical significance. The GM (90% CI) of the AUCss of ATV was 39,154 ng·h/ml (range, 34,255 to 44,752) for group 1 versus 33,626 ng·h/ml for group 2 (range, 31,143 to 38,431) with a GMR of 1.16 (Table 2 and Fig. 1B). The GM of the Cmax of ATV was 3,488 ng/ml for group 1 versus 2,924 ng/ml for group 2 (GMR = 1.20). The CLtot (range, 132 versus 149 ml/min; GMR = 0.89) and the Cmin (515 versus 428 ng/ml; GMR = 1.21) did not present significant differences.
Low-dose RTV in both groups resulted in only marginally higher RTV plasma concentrations in the presence of ATV, without reaching statistical significance. The GMs (90% CI) of the AUCss of RTV were 8,100 ng·h/ml for group 1 (range, 7,076 to 9,270) and 8,450 ng·h/ml for group 2 (range, 8,239 to 9,586), compared to 6,899 (range, 6,286 to 7,573) ng·h/ml for group 3 (Table 2, Fig. 1C). The significant difference in the Cmin values of RTV was due to the different dosing intervals in all groups. When combined with ATV/SQV or ATV/NRTI, RTV was dosed once daily, and together with SQV/NRTI, twice daily. Therefore, the GMs (90% CI) of the Cmin of RTV were 29 (range, 24 to 35) ng/ml for group 1 and 50 (range, 47 to 58) ng/ml for group 2, versus 231 (range, 208 to 251) ng/ml for group 3 (GMR = 0.58 for group 1 versus 2 and GMR = 0.13 for group 1 versus 3; P < 0.001).
Statistically significant differences were detected by the two-compartment model for the Tmax of RTV, which exhibited a considerable reduction in the presence of ATV. The Tmax was reached at 2.67 and 2.70 h for groups 1 and 2 versus 3.34 h for group 3 (GMR = 0.80 and 0.81, respectively; P = 0.007).
We found a strong correlation between the values for the AUCss, which is the primary parameter, for all PIs derived from the noncompartmental analysis and those estimated by fitting to the curve in a two-compartment model. The linear regression analysis of the AUCss values produced a mean r2 of 0.97 (standard deviation [SD] = 0.032) for ATV, a mean r2 of 0.98 (SD = 0.019) for SQV, and a mean r2 of 0.95 (SD = 0.041) for RTV.
No impact of the formulation taken as Fortovase/Invirase, dichotomized as 0 or 1, on minimum concentrations in plasma (ANOVA, P = 0.32) or the AUCss (P = 0.85) of SQV was seen. Chronic thrice-weekly intake of 960 mg cotrimoxazole, dichotomized as 0 (no intake) or 1 (intake), also had no significant impact on the Cmin (ANOVA, P = 0.57) or AUCss (ANOVA, P = 0.32) of SQV.
Sex and weight tested in a Spearman regression analysis were correlated with the Cmin (r2 = 0.23 and P = 0.001 for weight/Cmin), Cmax (r2 = 0.19 and P = 0.006 for weight/Cmax; r2 = 0.19 and P = 0.007 for sex/Cmax), and AUC (r2 = 0.16 and P = 0.020 for weight/AUC; r2 = 15 and P = 0.033 for sex/AUC) of RTV but not with the plasma concentrations of SQV or ATV. However, taken as regressors in a multilinear regression analysis, these parameters had no significant influence on the pharmacokinetics of either PI. The CD4 cell count, which exhibited marked differences between the groups, was not correlated to the Cmin, Cmax, or AUC of either PI in the Spearman regression analysis.
Subgroup analysis of ATV/tenofovir comedication.
A subgroup analysis of group 2 tested for the differences in ATV plasma exposure between patients who received ATV with and those who received it without tenofovir as part of the NRTI comedication. As contradictory data have been published concerning the supposed decreasing influence of tenofovir coadministration on ATV plasma levels (15-17), we compared patients (n = 53) who took tenofovir with patients (n = 19) who did not receive tenofovir. The differences detected were marginal: the GM (90% CI) of the AUCss of ATV was 32,487 (range, 26,850 to 39,308) ng·h/ml when tenofovir was coadministered versus 34,076 (range, 30,022 to 38,677) ng·h/ml without tenofovir (GMR = 0.95; P = 0.421). This was also reflected by the following: the GM (90% CI) of the Cmax was 2,747 (range, 2,283 to 3,301) ng·h/ml versus 2,954 (range, 2,595 to 3,362) ng/ml (GMR = 0.93; P = 0.461) and the GM of the Cmin was 452 (range, 331 to 619) versus 399 (range, 333 to 478) ng/ml (GMR = 1.13; P = 0.661).
Safety results from the first 3 months of therapy.
All grade 2, 3, and 4 adverse events or laboratory abnormalities occurring during the first 12 weeks of therapy, recorded simultaneously with the pharmacokinetic assessment, are listed in Table 3. Two cases of bilirubin elevation of >2.5 ULN and jaundice were reported in group 1 (ATV/SQV/RTV) during the first 12 weeks of the study, causing early termination of treatment in one case. The comparison of the control groups showed a significant increase in the incidence of grade 3 or 4 bilirubin elevation and jaundice in group 2, taking ATV/RTV plus nucleosides (n = 5), while no such case occurred in group 3, taking SQV/RTV plus nucleosides (Pearson χ2, P = 0.047). Group 3, taking SQV/RTV, exhibited higher numbers of severe constitutional symptoms (n = 7), such as chronic asthenia and insomnia, which occurred in only one patient in each of the other groups (Pearson χ2, P = 0.028). As patients in group 3 also exhibited a much lower CD4 cell count and a higher viral load at the baseline assessment, the constitutional symptoms were most likely due to the immunological status of these patients.
TABLE 3.
Grade 2 to 4 adverse events or laboratory abnormalities through week 12a
| Event or abnormality | No. of cases
|
Pearson χ2 test, P value | ||
|---|---|---|---|---|
| ATV/SQV/RTV (n = 49) | ATV/RTV/NRTI (n = 72) | SQV/RTV/NRTI (n = 90) | ||
| Hematology | ||||
| Hemoglobin < 10 mg/dl | 7 | 0.008 | ||
| Thrombocytes < 25,000 | 1 | NS | ||
| Lymphopenia 1.0-1.4/nl | 1 | NS | ||
| Cytokine kinase increase > 2.0 × ULN | 1 | NS | ||
| Metabolic abnormalities | ||||
| Cholesterol > 250 mg/dl | 1 | NS | ||
| Amylase/lipase > 2.1 × ULN | 1 | NS | ||
| Pathological oral glucose tolerance test | 1 | NS | ||
| Liver | ||||
| Bilirubin > 3.0 × ULN | 2 | 5 | 0.047 | |
| Aspartate-aminotransferase, alanine-aminotransferase > 2.6 × ULN | 2 | NS | ||
| Alkaline phosphatase > 2.6 × ULN | 1 | NS | ||
| Gastrointestinal | ||||
| Diarrhea (grade 3) | 4 | 4 | 1 | NS |
| Vomiting (grade 3) | 4 | NS | ||
| Ulcus ventriculi | 1 | NS | ||
| Constitutional symptoms | ||||
| Asthenia, sleepiness | 1 | 1 | 7 | 0.028 |
| Skin | ||||
| Exanthema | 3 | NS | ||
| Eczema | 2 | NS | ||
| Other | 2 | 3 | 1 | NS |
| Peripheral nervous system | ||||
| Polyneuropathy | 1 | NS | ||
| Other | ||||
| Pancreatitis | 1 | NS | ||
| Psychiatric disorders | 1 | 2 | NS | |
| Orthostatic dizziness | 1 | NS | ||
Adverse events are reported according to the common terminology criteria for adverse events (CTCAE) v3.0, http://safetyprofiler-ctep.nci.nih.gov/CTC/CTC.aspx. NS, not statistically significant.
Regarding AIDS-defining diagnoses, two new cases of Kaposi's sarcoma were diagnosed in both control groups and one case of Pneumocystis carinii and one case of cytomegaly virus pneumonia, which were already suspected at the time of the baseline evaluation, occurred in group 3 shortly after the start of therapy.
Regarding non-AIDS-related infections, eight patients in group 3 had an oral and/or pharyngeal Candida albicans infection, compared to only one patient in each of groups 1 and 2 (Pearson χ2, P = 0.050), at the start of therapy. All cases were treated and resolved within 4 weeks. The pharmacokinetic assessments in these patients took place at least 2 weeks after the cessation of fluconazole therapy. Three cases of herpes zoster infection (Pearson χ2, P = 0.054) and two cases of syphilis infection (Pearson χ2, P = 0.144) occurred in group 2, but none were recorded in the other two groups.
DISCUSSION
Our study focused on the pharmacokinetic interactions and possibly related adverse events of a three-drug combination of 300 mg ATV/100 mg RTV once daily plus 1,000 mg SQV twice daily in the absence of RTIs.
Boffito et al. (3) recently published pharmacokinetic data from a once-daily regimen containing boosted SQV/ATV/RTV (1,600/300/100 mg). SQV and RTV levels were enhanced significantly in this regimen, while differences in the ATV plasma exposure were not tested. One result of this pharmacokinetic study was the relatively low plasma levels of SQV (the GM of the Ctrough was 184 ng/ml and the GM of the AUC was 29,445 ng·h/ml) over the dosing interval of 24 h compared to levels with the twice-daily dosing with 1,000 mg SQV (the GM of the Cmin was 438 ng/ml and the GM of the AUCss was 22,794 ng·h/ml for 12 h) described above. Another previously conducted study with a combination of unboosted 1,200 mg SQV/400 mg ATV plus NRTIs once daily resulted in the plasma concentrations of SQV dropping below the proposed minimum therapeutic concentration and insufficient viral suppression in highly treatment-experienced patients (42; E. Seminari et al., presented at the Second International AIDS Society Conference on HIV Pathogenesis and Treatment, Paris, France, 2003). These patients also received tenofovir-disoproxilfumarate (DF) as part of the NRTI backbone, which is known to impair the plasma concentrations of unboosted ATV, as previously described (24; G. H. Kruse et al, presented at the Fifth International Workshop on Clinical Pharmacology of HIV Therapy, Rome, Italy, 2004). Therefore, we investigated the three-way pharmacokinetic interactions between ATV, SQV, and low-dose RTV in a twice-daily dosage regimen without the coadministration of NRTIs in therapy-experienced patients in an observational HIV cohort.
Pharmacokinetics of ATV/RTV plus SQV.
The key findings of the pharmacokinetic analysis were the significant enhancement of plasma concentrations of SQV and a moderate enhancement of the AUC of ATV when coadministered in this regimen. The overall levels plasma exposure of the booster, RTV, were comparable for all groups, although it was taken once daily when combined with ATV.
Although Kilby et al. (25) showed that RTV doses between 100 mg and 400 mg had equally distinct enhancing effects on SQV plasma concentrations, suggesting a saturation of the boosting effect, we detected significantly increased SQV concentrations in the presence of ATV in our study.
Two findings in this study are most likely to explain these results. First, the SQV elimination half-life was significantly prolonged in the presence of ATV and correlated with ATV plasma concentrations (Spearman's ρ for the t1/2 of SQV correlated to ATV, P > 0.001 and r2 = 0.54 for the Cmin, P = 0.002 and r2 = 0.43 for the Cmax, and P = 0.001 and r2 = 0.45 for the AUC), suggesting an enhanced hepatic CYP3A4/5 inhibition by ATV.
Secondly, a shorter Tlag and an earlier Tmax of RTV in the presence of ATV are markers of a faster absorption, leading to an earlier beginning and therefore a prolonged pharmacokinetic-enhancing effect of RTV on SQV over almost the entire dosing interval (Spearman's ρ for the t1/2 of SQV correlated with RTV Tlag, P = 0.050 and r2 = 0.17, and Tmax, P = 0.047 and r2 = 0.17).
The assumption of an enhanced absorption of SQV is supported by the work of Sinko et al., who demonstrated that the inhibition of intestinal but not hepatic CYP3A4/5 contributed significantly to the bioavailability of SQV in rabbits (44). As SQV is a fast-extracted drug with a comparably low and variable bioavailability, influencing the net absorption in the intestine considerably increases plasma drug concentrations (43, 50). In addition, recent works by Lucia et al. and Perloff et al. (30, 38) showed an in vitro inhibitory effect of ATV on P-glycoprotein, which may also play a role in altering the bioavailability of SQV, which is a known substrate of this transporter of drugs across different cell barriers.
A further result of our study was that the Ctrough of SQV at steady state exhibited values comparable to those of a twice-daily SQV/RTV intake. The once-daily intake of RTV together with ATV sufficiently enhanced the SQV plasma concentrations over both daily dosing intervals, although the Cmin of SQV showed none of the differences detected in the Cmax and AUC.
As a result, the dosage of RTV can be reduced to 100 mg once daily in this combination of drugs, lowering the pill burden and possibly RTV-induced side effects, especially in the intestine, without jeopardizing the efficacy of this therapy regimen.
At the same time, we measured the GMRs of the Cmin (GMR = 1.21), Cmax (GMR = 1.20), and AUC (GMR = 1.16) of ATV when combined with SQV compared to the GMRs in the control group. This result is not statistically significant and is of limited clinical relevance for the first 3 months of therapy, since higher ATV plasma concentrations were not correlated with more contemporary side effects (for the comparison between groups 1 and 2, see Table 3). In general there are various examples for drug-drug interactions in PI combinations that were described but not sufficiently explained. This is especially the case for RTV, which autoinduces its own metabolism and inhibits the metabolism of other participating PIs (18, 25, 26; Norvir product information, Ref. 03-2337-R17-Rev., 2001, Abbott Laboratories, Chicago, IL) if it is mutually boosted by the comedication in a multiple-antiretroviral therapy which contains more than two PIs.
This is not only due to the fact that all PIs are substrates of CYP450. Furthermore, still-unknown mechanisms of interaction of the cytochromes or drug transporters such as P-glycoprotein in the gastrointestinal mucosa or the target tissues may play a role, possibly influencing the resorption and distribution of SQV and ATV to different human compartments. The results of several studies have shown that, at least, the plasma concentrations of SQV as a substrate of P-glycoprotein are influenced by genotypes encoding P-glycoprotein expression in human cells or P-glycoprotein inhibition by comedication (19, 20, 33, 36, 37, 39, 44, 51), and not much is known about such interactions for ATV so far. As MDR-1 or CYP genotyping was not done for our patients, we cannot exclude a bias concerning P-glycoprotein or CYP3A4/5 expression in the gastrointestinal mucosa or hepatocytes.
ATV levels lower than those from previously published data.
Another result of this investigation was the measurement of the plasma concentrations following 300 mg of boosted ATV once daily, which showed levels generally lower than those previously reported (3, 5; Reyataz product information, F1-B001-06-03, 2003, Bristol-Myers Squibb Company, Princeton, NJ). A reason for this could be the different setting of the pharmacokinetic assessments, which in our study took place after a median of 4 weeks on therapy. The period from baseline to pharmacokinetic assessment in other studies was shorter (3), and a decline of the Ctrough of ATV over time was detected, if repeatedly assessed (G. Kruse et al., presented at the Fifth International Workshop on Clinical Pharmacology of HIV Therapy, Rome, Italy, 2004). As a result, our patients may have exhibited lower ATV levels due to the extended autoinduction of CYP3A4 in liver and gastrointestinal mucosa.
The concomitant intake of tenofovir-DF also has to be discussed as a reason for lower ATV plasma concentrations. Previous analyses investigating potential interactions of 300 mg tenofovir-DF and boosted ATV showed only slightly decreased ATV plasma levels in the presence of tenofovir-DF, not reaching statistical significance (24, 48; G. Kruse et al., presented at the Fifth International Workshop on Clinical Pharmacology of HIV Therapy, Rome, Italy, 2004). These results correspond with the data of our subgroup analysis showing almost no difference in ATV plasma concentrations when combined with tenofovir-DF or without it. As these data were generated as part of an observational study not addressing this question, it is not suitable to draw any further conclusions. However, it is demonstrated that, at least in this study, there is no bias caused by the concomitant intake of tenofovir with boosted ATV.
Safety of ATV/RTV plus SQV.
The higher ATV plasma concentrations in the double-PI regimen were not correlated with a higher rate of acute gastrointestinal or hematological side effects. However, as Barrios et al. detected a significant correlation of transient hyperbilirubinemia to the Cmin of ATV at week 12 of therapy (1) and Zhang et al. described the inhibitory potency of ATV for UDP-glucuronyltransferase (52), patients should be monitored for hyperbilirubinemia. A correlation with high ATV plasma levels was not detected, and genotypic screening for the homozygous UGT1A1*28 allele or the MDR-1 3435 C→T polymorphism, which were shown to be the most likely reasons for episodes of hyperbilirubinemia or jaundice in patients (40, 41), was not done in this study.
The markedly higher plasma exposure of SQV was not related to a considerable hyperlipidemic effect until week 12 of therapy, which was in accordance with other study results (E. Seminari et al., presented at the Second International AIDS Society Conference on HIV Pathogenesis and Treatment, Paris, France, 2003). However, higher SQV levels may also cause an increased rate of gastrointestinal side effects, as RTV does, although the AUCss of RTV, for instance, is still much lower than that with the therapeutic dosage (26). Four patients in each group taking ATV/RTV plus either SQV or RTIs (Pearson χ2, P = 0.113) suffered from severe but reversible episodes of diarrhea, and none of the patients stopped therapy because of these side effects as of week 12. The markedly higher numbers of constitutional symptoms in group 3 (SQV/RTV) are most likely due to a multifactorial genesis and the immunological status of a comparably low CD4 cell count and a high viral load of patients of group 3. Again, no correlation was detected between these symptoms and the plasma levels of the PIs. The clinical investigation is still ongoing and definitive conclusions about the clinical impacts of this therapy regimen cannot yet be drawn.
Summary.
Although observational outpatient studies are not commonly used to investigate drug-drug interactions, this sampling strategy is appropriate to pharmacokinetic evaluation and keeps as close as possible to real-life conditions. The sizes of 968 ATV, 973 SQV, and 1,598 RTV samples should limit the influence of intraindividual variability on the results, which has been reported recently for the repeated sampling of PI levels at single time points in a small number of patients (34). This was reflected by the normal distribution of values for all time points and the AUCss in the tested groups. The appropriateness of the data fit to a pharmacokinetic two-compartment model and the concordance of the results with other studies corroborate the scientific approach to clinical pharmacokinetics under ambulatory conditions.
If plasma drug levels are seen as an important reason for therapeutic drug monitoring and a predictive marker for therapy outcome, then these results point towards the potential benefit of therapeutic drug monitoring for the individual patient.
Finally, we can state that 300 mg ATV/100 mg RTV once daily plus 1,000 mg SQV twice daily is a boosted double-PI combination of virologically potent compounds with preferable pharmacokinetics, considerable short-term safety, and a divergent resistance profile. This new therapy regimen should be clinically evaluated for its long-term safety and efficacy and compared to other boosted double-PI regimens, such as SQV/lopinavir/RTV.
Footnotes
Published ahead of print on 12 February 2007.
REFERENCES
- 1.Barrios, A., A. Rendon, O. Rios, L. Martin-Carbonero, D. Gonzalez de Requena, O. Gallego, L. Valer, I. Maida, I. Jimenez-Nacher, J. Gonzalez-Lahoz, and V. Soriano. 2004. Atazanavir plasma levels associated with efficacy and safety in protease inhibitor-experienced HIV-infected patients, abstr. 606. Eleventh Conf. Retrovir. Oppor. Infect., San Francisco, CA.
- 2.Bittner, B., M. Riek, B. Holmes, and S. Grange. 2005. Saquinavir 500 mg film-coated tablets demonstrate bioequivalence to saquinavir 200 mg hard capsules when boosted with twice-daily ritonavir in healthy volunteers. Antivir. Ther. 10:803-810. [PubMed] [Google Scholar]
- 3.Boffito, M., M. Kurowski, G. Kruse, A. Hill, A. A. Benzie, M. Nelson, G. Moyle, B. Gazzard, and A. Pozniak. 2004. Atazanavir enhances saquinavir hard-gel concentrations in a ritonavir boosted once-daily regimen. AIDS 18:1291-1297. [DOI] [PubMed] [Google Scholar]
- 4.Boffito, M., D. Maitland, and A. Pozniak. 2006. Practical perspectives on the use of tipranavir in combination with other medications: lessons learned from pharmacokinetic studies. J. Clin. Pharmacol. 46:130-139. [DOI] [PubMed] [Google Scholar]
- 5.Boffito, M., D. Maitland, Y. Samarasinghe, and A. Pozniak. 2005. The pharmacokinetics of HIV protease inhibitor combinations. Curr. Opin. Infect. Dis. 18:1-7. [DOI] [PubMed] [Google Scholar]
- 6.Cahn, P., N. Percival, P. Phanuphak, I. Sanne, T. Kelleher, and M. Giordano. 2001. Phase II 24-week data from study AI424-008: comparative results of BMS-232632, stavudine, lamivudine as HAART for treatment of naïve HIV-infected patients, abstr. 5. First Int. AIDS Soc. Conf. HIV Pathog. Treat., Buenos Aires, Argentina.
- 7.Chiou, W. L., S. M. Chung, T. C. Wu, and C. Ma. 2001. A comprehensive account on the role of efflux transporters in the gastrointestinal absorption of 13 commonly used substrate drugs in humans. Int. J. Clin. Pharmacol. Ther. 39:93-101. [DOI] [PubMed] [Google Scholar]
- 8.Colonno, R., R. Rose, C. Ciani, G. Aldovani, N. Parkin, and J. Frigborn. 2003. Emergence of atazanavir resistance and maintenance of susceptibility to other PIs is associated with the I50L substitution in HIV-protease, abstr. 597. Tenth Conf. Retrovir. Oppor. Infect., Boston, MA.
- 9.Cooper, C. L., R. P. van Heeswijk, K. Gallicano, and D. W. Cameron. 2003. A review of low-dose ritonavir in protease inhibitor combination therapy. Clin. Infect. Dis. 36:1585-1592. [DOI] [PubMed] [Google Scholar]
- 10.Eagling, V. A., D. J. Back, and M. G. Barry. 1997. Differential inhibition of cytochrome P450 isoforms by the protease inhibitors ritonavir, saquinavir and indinavir. Br. J. Clin. Pharmacol. 44:190-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eagling, V. A., H. Wiltshire, I. W. Whitcombe, and D. J. Back. 2002. CYP3A4-mediated hepatic metabolism of the HIV-1 protease inhibitor saquinavir in vitro. Xenobiotica 32:1-17. [DOI] [PubMed] [Google Scholar]
- 12.Food and Drug Administration. 2002. Guidance for industry. Bioavailability and bioequivalence studies for orally administered drug products: general considerations. www.fda.gov/cder/guidance/4964dft.pdf.
- 13.Gallant, J. E. 2000. Strategies for long-term success in the treatment of HIV infection. JAMA 283:1329-1334. [DOI] [PubMed] [Google Scholar]
- 14.Gong, Y.-F., B. S. Robinson, R. E. Rose, C. Deminie, T. P. Spicer, D. Stock, R. J. Colonno, and P. F. Lin. 2000. In vitro resistance profile of the human immunodeficiency virus type 1 protease inhibitor BMS-232632. Antimicrob. Agents Chemother. 44:2319-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granfors, M. T., J. S. Wang, L. I. Kajosaari, J. Laitila, P. J. Neuvonen, and J. T. Backman. 2006. Differential inhibition of cytochrome P450 3A4, 3A5 and 3A7 by five human immunodeficiency virus (HIV) protease inhibitors in vitro. Basic Clin. Pharmacol. Toxicol. 98:79-85. [DOI] [PubMed] [Google Scholar]
- 16.Heinzel, G. 1993. TOPFIT 2.0 pharmacokinetic and pharmacodynamic data analysis system for the PC. Gustav Fischer Verlag, Stuttgart, Germany.
- 17.Hsu, A., G. R. Granneman, and R. J. Bertz. 1998. Ritonavir. Clinical pharmacokinetics and interactions with other anti-HIV agents. Clin. Pharmacokinet. 35:275-291. [DOI] [PubMed] [Google Scholar]
- 18.Hsu, A., G. R. Granneman, G. Witt, C. Locke, J. Denissen, A. Molla, J. Valdes, J. Smith, K. Erdman, N. Lyons, P. Niu, J.-P. Decourt, J.-B. Fourtillan, J. Girault, and J. M. Leonard. 1997. Multiple-dose pharmacokinetics of ritonavir in human immunodeficiency virus-infected subjects. Antimicrob. Agents Chemother. 41:898-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huisman, M. T., J. W. Smit, K. M. Crommentuyn, N. Zelcer, H. R. Wiltshire, J. H. Beijnen, and A. H. Schinkel. 2002. Multidrug resistance protein 2 (MRP2) transports HIV protease inhibitors, and transport can be enhanced by other drugs. AIDS 16:2295-2301. [DOI] [PubMed] [Google Scholar]
- 20.Huisman, M. T., J. W. Smit, H. R. Wiltshire, J. H. Beijnen, and A. H. Schinkel. 2003. Assessing safety and efficacy of directed P-glycoprotein inhibition to improve the pharmacokinetic properties of saquinavir coadministered with ritonavir. J. Pharmacol. Exp. Ther. 304:596-602. [DOI] [PubMed] [Google Scholar]
- 21.Huisman, M. T., J. W. Smit, H. R. Wiltshire, R. M. Hoetelmans, J. H. Beijnen, and A. H. Schinkel. 2001. P-glycoprotein limits oral availability, brain, and fetal penetration of saquinavir even with high doses of ritonavir. Mol. Pharmacol. 59:806-813. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, V., F. Brun-Vezinet, B. Clotet, B. Conway, D. Kuritzkes, D. Pillay, J. Schapiro, A. Telenti, and D. Richman. 2005. Update of the drug resistance mutations in HIV-1: fall 2005. Top. HIV Med. 13:125-131. [PubMed] [Google Scholar]
- 23.Kashuba, A., C. Tierney, G. Downey, E. Acosta, E. Vergis, and K. Klingman. 2005. Combining fosamprenavir with lopinavir/ritonavir substantially reduces amprenavir and lopinavir exposure: ACTG protocol A 5143 results. AIDS 19:145-152. [DOI] [PubMed] [Google Scholar]
- 24.Kaul, S., K. Bassi, B. Damle, et al. 2003. Pharmacokinetic evaluation of the combination of atazanavir (ATV), enteric coated didanosine (ddI-EC), and tenofovir disoproxil fumarate (TDF) for a once-daily antiretroviral regimen, abstr. A-1616. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL.
- 25.Kilby, J. M., A. Hill, and N. Buss. 2002. The effect of ritonavir on saquinavir plasma concentration is independent of ritonavir dosage: combined analysis of pharmacokinetic data from 97 subjects. HIV Med. 3:97-104. [DOI] [PubMed] [Google Scholar]
- 26.Kilby, J. M., G. Sfakianos, N. Gizzi, P. Siemon-Hryczyk, E. Ehrensing, C. Oo, N. Buss, and M. S. Saag. 2000. Safety and pharmacokinetics of once-daily regimens of soft-gel capsule saquinavir plus minidose ritonavir in human immunodeficiency virus-negative adults. Antimicrob. Agents Chemother. 44:2672-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitchen, V. S., C. Skinner, K. Ariyoshi, E. A. Lane, I. B. Duncan, J. Burckhardt, H. U. Burger, K. Bragman, A. J. Pinching, and J. N. Weber. 1995. Safety and activity of saquinavir in HIV infection. Lancet 345:952-955. [DOI] [PubMed] [Google Scholar]
- 28.Kurowski, M., T. Sternfeld, A. Sawyer, A. Hill, and C. Mocklinghoff. 2003. Pharmacokinetic and tolerability profile of twice-daily saquinavir hard gelatin capsules and saquinavir soft gelatin capsules boosted with ritonavir in healthy volunteers. HIV Med. 4:94-100. [DOI] [PubMed] [Google Scholar]
- 29.Le Tiec, C., A. Barrail, C. Goujard, and A. M. Taburet. 2005. Clinical pharmacokinetics and summary of efficacy and tolerability of atazanavir. Clin. Pharmacokinet. 44:1035-1050. [DOI] [PubMed] [Google Scholar]
- 30.Lucia, M. B., C. Golotta, S. Rutella, E. Rastrelli, A. Savarino, and R. Cauda. 2005. Atazanavir inhibits P-glycoprotein and multidrug resistance-associated protein efflux activity. J. Acquir. Immune Defic. Syndr. 39:635-637. [PubMed] [Google Scholar]
- 31.Maserati, R., P. Villani, L. Cocchi, and M. Regazzi. 1998. Co-trimoxazole administered for Pneumocystis carinii pneumonia prophylaxis does not interfere with saquinavir pharmacokinetics. AIDS 12:815-816. [PubMed] [Google Scholar]
- 32.Montaner, J. S., P. R. Harrigan, N. Jahnke, J. Raboud, E. Castillo, R. S. Hogg, B. Yip, M. Harris, V. Montessori, and M. V. O'Shaughnessy. 2001. Multiple drug rescue therapy for HIV-infected individuals with prior virologic failure to multiple regimens. AIDS 15:61-69. [DOI] [PubMed] [Google Scholar]
- 33.Mouly, S. J., M. F. Paine, and P. B. Watkins. 2004. Contributions of CYP3A4, P-glycoprotein, and serum protein binding to the intestinal first-pass extraction of saquinavir. J. Pharmacol. Exp. Ther. 308:941-948. [DOI] [PubMed] [Google Scholar]
- 34.Nettles, R., T. Kieffer, T. Parsons, J. Johnson, T. Quinn, B. Jackson, J. Cofranesco, J. Gallant, K. Carson, R. Siliciano, and C. Flexner. 2005. Frequent sampling in virologically suppressed patients taking HIV protease inhibitors of non-nucleoside reverse transcriptase inhibitors defines intraindividual pharmacokinetic variability, abstr. 642. Twelfth Conf. Retrovir. Oppor. Infect., Boston, MA.
- 35.Nieto-Cisneros, L., C. Zala, W. Fessel, J. Gonzalez-Garcia, C. Cohen, and R. McGovern. 2003. Atazanavir (ATV) versus lopinavir/ritonavir (LPV/RTV) in patients with virologic failure to a protease inhibitor (PI): 24-week results from BMS AI424-043, abstr. 117. Second Int. AIDS Soc. Conf. HIV Pathog. Treat., Paris, France.
- 36.Owen, A., B. Chandler, P. G. Bray, S. A. Ward, C. A. Hart, D. J. Back, and S. H. Khoo. 2004. Functional correlation of P-glycoprotein expression and genotype with expression of the human immunodeficiency virus type 1 coreceptor CXCR4. J. Virol. 78:12022-12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park, S., and P. J. Sinko. 2005. P-Glycoprotein and multidrug resistance-associated proteins limit the brain uptake of saquinavir in mice. J. Pharmacol. Exp. Ther. 312:1249-1256. [DOI] [PubMed] [Google Scholar]
- 38.Perloff, E. S., S. X. Duan, P. R. Skolnik, D. J. Greenblatt, and L. L. von Moltke. 2005. Atazanavir: effects on P-glycoprotein transport and CYP3A metabolism in vitro. Drug Metab. Dispos. 33:764-770. [DOI] [PubMed] [Google Scholar]
- 39.Profit, L., V. A. Eagling, and D. J. Back. 1999. Modulation of P-glycoprotein function in human lymphocytes and Caco-2 cell monolayers by HIV-1 protease inhibitors. AIDS 13:1623-1627. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez Novoa, S., P. Barreiro, A. Rendon, A. Barrios, A. Corral, I. Jimenez-Nacher, J. Gonzalez-Lahoz, and V. Soriano. 2006. Plasma levels of atazanavir and the risk of hyperbilirubinemia are predicted by the 3435C→T polymorphism at the multidrug resistance gene 1. Clin. Infect. Dis. 42:291-295. [DOI] [PubMed] [Google Scholar]
- 41.Rotger, M., P. Taffe, G. Bleiber, H. F. Gunthard, H. Furrer, P. Vernazza, H. Drechsler, E. Bernasconi, M. Rickenbach, A. Telenti, et al. 2005. Gilbert syndrome and the development of antiretroviral therapy-associated hyperbilirubinemia. J. Infect. Dis. 192:1381-1386. [DOI] [PubMed] [Google Scholar]
- 42.Seminari, E., M. Guffanti, P. Villani, N. Gianotti, M. Cusato, G. Fusetti, A. Galli, A. Castagna, M. Regazzi, and A. Lazzarin. 2005. Steady-state pharmacokinetics of atazanavir given alone or in combination with saquinavir hard-gel capsules or amprenavir in HIV-1-infected patients. Eur. J. Clin. Pharmacol. 61:545-549. [DOI] [PubMed] [Google Scholar]
- 43.Shen, D. D., K. L. Kunze, and K. E. Thummel. 1997. Enzyme-catalyzed processes of first-pass hepatic and intestinal drug extraction. Adv. Drug Deliv. Rev. 27:99-127. [DOI] [PubMed] [Google Scholar]
- 44.Sinko, P. J., J. R. Kunta, H. H. Usansky, and B. A. Perry. 2004. Differentiation of gut and hepatic first pass metabolism and secretion of saquinavir in ported rabbits. J. Pharmacol. Exp. Ther. 310:359-366. [DOI] [PubMed] [Google Scholar]
- 45.Staszewski, S., E. Babacan, C. Stephan, A. Haberl, A. Carlebach, P. Gute, S. Klauke, Y. Hermschulte, M. Stuermer, B. Dauer, et al. 2006. The LOPSAQ study: 48 week analysis of a boosted double protease inhibitor regimen containing lopinavir/ritonavir plus saquinavir without additional antiretroviral therapy. J. Antimicrob. Chemother. 58:1024-1030. [DOI] [PubMed] [Google Scholar]
- 46.Staszewski, S., B. Dauer, C. Stephan, M. Kurowski, M. Stürmer, and P. Gute. 2002. A new strategy: boosted double protease inhibitor (PI) regimen lopinavir/r plus saquinavir (LPVr/SQV) without reverse transcriptase inhibitors (RTI), abstr. H-176. Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., San Diego, CA.
- 47.Stephan, C., N. von Hentig, I. Kourbeti, B. Dauer, M. Mösch, T. Lutz, S. Klauke, S. Harder, and S. Staszewski. 2004. Saquinavir drug exposure is not impaired by the boosted double protease inhibitor combination of lopinavir/ritonavir. AIDS 18:503-508. [DOI] [PubMed] [Google Scholar]
- 48.von Hentig, N., A. Mueller, A. Haberl, T. Lutz, G. Knecht, M. Kurowski, S. Harder, and S. Staszewski. 2004. Pharmacokinetic interactions of atazanavir (AZV) and saquinavir (SQV) in a ritonavir (RTV) boosted protease inhibitor therapy regimen, abstr. WeOrB1235. Fifteenth World AIDS Conf., Bangkok, Thailand.
- 49.Washington, C. B., H. R. Wiltshire, M. Man, T. Moy, S. R. Harris, E. Worth, P. Weigl, Z. Liang, D. Hall, L. Marriott, and T. F. Blaschke. 2000. The disposition of saquinavir in normal and P-glycoprotein deficient mice, rats, and in cultured cells. Drug Metab. Dispos. 28:1058-1062. [PubMed] [Google Scholar]
- 50.Williams, P. E. O., G. J. Muirhead, M. J. Madigan, A. M. Mitchell, and T. Shaw. 1992. Disposition and bioavailability of the HIV-protease inhibitor Ro 31-8959 after single doses in healthy volunteers. Br. J. Clin. Pharmacol. 34:155P-156P. [Google Scholar]
- 51.Woodahl, E. L., Z. Yang, T. Bui, D. D. Shen, and R. J. Ho. 2005. MDR1 G1199A polymorphism alters permeability of HIV protease inhibitors across P-glycoprotein-expressing epithelial cells. AIDS 19:1617-1625. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, D., T. J. Chando, D. W. Everett, C. J. Patten, S. S. Dehal, and W. G. Humphreys. 2005. In vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationship of this property to in vivo bilirubin glucuronidation. Drug Metab. Dispos. 33:1729-1739. [DOI] [PubMed] [Google Scholar]