Abstract
In vitro drug susceptibility testing with the malaria parasite has been used to assess the antimalarial activities of new compounds and to monitor drug resistance in field isolates. We investigated the validity of a SYBR green I fluorescent-based assay under various culture conditions and compared the assay results to those of previously published histidine-rich protein II (HRPII) enzyme-linked immunosorbent assay (ELISA) methods. Reference strains of Plasmodium falciparum were cultured in vitro by using standard conditions in complete medium with and without phenol red before they were dispensed into 96-well plates predosed with chloroquine, mefloquine, or quinine. Following incubation, the culture supernatants were divided and the 50% inhibitory concentrations (IC50s) were determined by using a SYBR green I-based method and the HRPII capture ELISA method. There were no significant differences in IC50 values when phenol red was included in the medium. The IC50s and the IC90s of the antimalarials tested by both methods were similar or identical for each of the reference strains. Fresh clinical isolates of P. falciparum collected from imported cases of malaria in Lyon, France, were tested for in vitro resistance to chloroquine and mefloquine by using the validated SYBR green I and HRPII ELISA methods. The SYBR green I-based method was able to calculate IC50 and IC90 values similar or identical to those calculated by the HRPII assay with fresh clinical samples without removal of white blood cells. The SYBR green I-based method for determination of drug sensitivity levels produced results comparable to those produced by other methods, showing that this method can be used routinely to conduct surveillance for drug resistance in P. falciparum with fresh or cultured parasites.
Currently, in vivo drug efficacy studies are the “gold standard” for assessment of drug resistance in malaria parasites (20). While such studies are routinely conducted on a small scale, the cost associated with in vivo drug studies with the malaria parasite prohibits sustained surveillance at the required level of vigilance. Molecular marker determination has been used to identify the single-nucleotide polymorphisms commonly associated with drug resistance in malaria parasites; however, the presence of molecular markers does not always correlate with in vivo drug resistance (25). Other molecular biology methodologies have been developed to assess the flow of drug resistance markers by using known microsatellite markers, but this technology requires specialized equipment and can be quite costly (10, 26). Although microsatellite differentiation is quite powerful and informative, it would be difficult to conduct microsatellite differentiation in the field in real time.
A quick and very cost-effective alternative means to determination of the level of resistance is in vitro drug susceptibility testing (4, 17). Unlike the in vivo test for drug resistance, the in vitro assay is designed to assess the parasites' responses to drugs without interference from host factors, such as acquired immunity and pharmacokinetic profiles (poor absorption, biotransformation, concentration in certain tissues, rapid clearance) (3). The in vitro drug susceptibility assay can be used with cultivated parasites to assess new antimalarials (16, 18, 27) or can be used with fresh clinical isolates to conduct epidemiological studies (3, 5, 21). An inherent problem associated with the use of cultured parasites for in vitro testing is the potential for the selection of a dominant clone during the culturing phase, which could alter the 50% inhibitory concentrations (IC50s) (5). The use of fresh isolates overcomes this laboratory-induced artifact and gives true values for the parasite populations obtained from infected patients. Procedures for in vitro drug sensitivity assays that use cultured parasites and an array of different means for determination of the IC50 are well established (5, 16, 23).
The most widely published and accepted method for the determination of IC50 values is the isotopic assay (9). However, this method is time-consuming and the need for the disposal of radioactive materials has limited its widespread use, especially in countries that cannot afford the prohibitive cost of a liquid scintillation counter and where reagents are difficult, if not impossible, to obtain. Another method commonly used to determined susceptibility levels is the WHO microtest, which uses microscopic evaluation to enumerate the quantity of parasites following growth in the presence of antimalarials (8, 17). Although this method is inexpensive, it is highly labor-intensive and subjective due to variations in the expertise of the microscopists responsible for reading the slides. Two enzyme-linked immunosorbent assay (ELISA)-based methods that use monoclonal antibodies to Plasmodium spp. or plasmodial lactate dehydrogenase (pLDH) (13) and histidine-rich protein II (HRPII) (16, 18, 19) have begun to be widely used in recent years due to their ease of use. However, the test kits can be difficult to obtain, and the performance of these multiple-step assays is time-consuming. Both ELISA-based assays have been compared in a side-by-side comparison with the isotopic assay, which is considered the gold standard method for determination of inhibitory concentrations (13, 18). Currently, the pLDH monoclonal antibodies are not available for purchase, while the HRPII capture and detection antibodies can be purchased separately and used in an in-house ELISA, as described previously (16). The in-house 96-well plate method is less expensive than the commercial kits, but the antibodies are not widely available from multiple sources. In areas where genetic diversity is high, the use of monoclonal antibodies can be hindered by a decrease in binding affinities due to genetic variation within the protein (2, 11), leading to a decrease in sensitivity and low IC50 values.
Recently, two fluorescent-based techniques that use SYBR green I to determine parasite growth following the in vitro drug susceptibility assay have been described (6, 23). While each method used SYBR green I, there were differences between the published reports. For instance, Smilkstein et al. reported the use of a lysis buffer containing SYBR green I prior to determination of the fluorescent values (23), while Bennett et al. did not lyse the red blood cells (RBCs) but instead added the dye directly to each well (6). SYBR green I has been used extensively to stain DNA following agarose gel electrophoresis and as a reporter molecule in real-time quantitative PCR. The fluorescence-based method is a simple, one-step procedure that is cost-effective, and the dye is readily available from multiple sources worldwide. We report here the inhibitory concentration values for control isolates and fresh clinical isolates from travelers returning from regions where malaria is endemic cultured in vitro. These values were obtained from studies conducted in parallel by using a slightly modified SYBR green I method and an HRPII ELISA method.
MATERIALS AND METHODS
Antimalarials.
Chloroquine diphosphate (molecular weight [MW], 515.9; Sterling-Winthrop Research Institute, Rensselaer, NY), mefloquine hydrochloride (MW, 414.8; Ash Stevens Inc., Detroit, MI), and quinine hydrochloride (MW, 782.9; Mallinckrodt/Baker, Phillipsburg, NJ) were used for the in vitro test and were kindly provided by WRAIR and the WRAIR Chemical Repository. Solutions of each drug were prepared by adding 5 mg of drug to 5 ml of water (chloroquine), 70% ethanol (quinine), or methanol (mefloquine), followed by sonication until the drugs were dissolved. Stock solutions were diluted with complete RPMI medium with and without phenol red supplemented with 0.5% Albumax I (0.005 g/ml) and HEPES (5.94 g/liter) to the desired concentrations. The antimalarial drugs were diluted twofold, and 20 μl of each dilution (chloroquine, 3.8 nM to 242 nM; quinine, 10.8 nM to 346 nM; and mefloquine, 1.1 nM to 37.7 nM) was added in duplicate to a 96-well plate (Costar; Corning Inc., Corning, NY). The plates were stored frozen at −80°C for up to 1 month.
In vitro parasite cultivation.
Asexual Plasmodium falciparum cultures of reference clones D6/Sierra Leone (D6; chloroquine sensitive and mefloquine resistant), W2/Indochina (W2; chloroquine resistant and mefloquine sensitive; kindly provided by Dennis Kyle at Division of Experimental Therapeutics, WRAIR, Silver Spring, MD), and P. falciparum clone 3D7 (chloroquine and mefloquine sensitive; the clone was from isolate ATCC/MR4, collected at the Amsterdam, The Netherlands, airport) were initiated from stabilates preserved in liquid nitrogen and maintained in culture by using the method of Trager and Jenson (24). After the parasites were thawed, they were resuspended in RPMI 1640 (Invitrogen, Carlsbad, CA) medium supplemented with 20% human serum, HEPES (5.94 g/liter), and NaHCO3 (2.4 g/liter); placed in a 25-cm2 tissue culture flask in a total volume of 5 ml containing a 6% RBC suspension; and incubated at 37°C under a gas mixture of 5% O2, 5% CO2, and 90% N2. The cultures were incubated until 5% parasitemias were reached, followed by 1:1 dilution in fresh medium containing 10% human serum. The diluted cultures were grown until a 7 to 8% parasitemia with a ring-stage parasite concentration of at least 70% was achieved.
In vitro drug sensitivity assay with cultured parasites.
After they were taken out of liquid nitrogen, the cultured parasites were grown for 10 to 12 days until a predominance of ring-stage parasites of no less than 70% was reached. These parasites were used for the drug sensitivity assays. Once the parasitemia levels of the in vitro cultures reached an optimum density of 5 to 8%, the infected red blood cells were centrifuged at 350 × g for 5 min; the supernatant was aspirated; and the cells were suspended in RPMI with and without phenol red (Invitrogen) supplemented with 0.5% Albumax I (0.005 g/ml), HEPES (5.94 g/liter), and NaHCO3 (2.4 g/l) or 10% human serum and HEPES and NaHCO3. An aliquot of the culture was diluted to reduce the parasitemia to 0.5%, and the hematocrit was adjusted to 2% with fresh RBCs. A total of 180 μl/well of 0.5% parasitized RBCs at 2% hematocrit was added, and the plates were incubated in a humidified modular incubator chamber (Flow Laboratories) at 37°C under a gas mixture of 5% O2, 5% CO2, and 90% N2 for 72 h. The chamber was flushed daily for 1 to 2 min with a mixture of gas (5% CO2, 5% O2, and 90% N2).
IC50 determination by the SYBR green I assay.
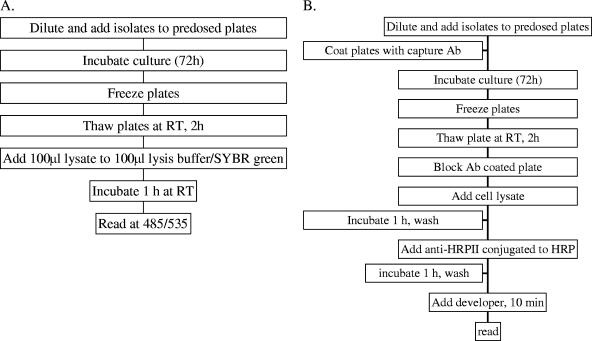
Following incubation, the plates were frozen and stored at −80°C until the SYBR green I assay was performed. The plates were thawed for 2 h at room temperature and each sample was mixed by pipetting. A diagram depicting the steps involved in the SYBR green I method are shown in Fig. 1A. Briefly, a total of 100 μl of the culture was transferred to a new 96-well plate, followed by the addition of 100 μl of SYBR green I (Molecular Probes, Invitrogen, Carlsbad, CA) in lysis buffer (0.2 μl of SYBR green I/ml of 2× lysis buffer, which consisted of Tris 20 mM [pH 7.5], EDTA [5 mM], saponin [0.008%; wt/vol], and Triton X-100 [0.08%; wt/vol]) (23). The remaining lysate was used for the HRPII assay described below. The plates were covered and incubated at room temperature for 1 h. The fluorescence intensity was measured from below with a GENius Plus plate reader (Tecan USA, Research Triangle, NC) with excitation and emission wavelengths of 485 nm and 535 nm, respectively, and with the gain set at 60.
FIG. 1.
Diagram of the steps involved in performing the SYBR green I (A) and HRPII ELISA (B) methods. Ab, antibody; RT, room temperature; HRP, horseradish peroxidase; 485/535, excitation and emission wavelengths of 485 and 535 nm, respectively.
IC50 determination by the HRPII assay.
The capture and detector antibodies were purchased from Immunology Consultants Laboratories Inc. (Newberg, OR). The HRPII ELISA was performed as described previously (16), and a diagram depicting the steps involved in the method are shown in Fig. 1B. An immunoglobulin M (IgM) capture antibody (MPFM-45A) specific for P. falciparum HRPII was diluted to 1 μg/ml in phosphate-buffered saline (PBS), and 100 μl was transferred to each well of a 96-well plate. The plates were covered and placed at 4°C overnight. Following incubation, the contents of the wells were removed, the plates were washed three times with PBS, and nonspecific sites were blocked by using 200 μl/well of 2% bovine serum albumin diluted in PBS. Following the blocking step, the microtiter plates were washed as described above, 100 μl of the remaining lysate from parasites grown for 72 h in the presence of antimalarial drugs was added per well, and the plates were incubated for 1 h at room temperature. The plates were washed, 100 μl of the detector antibody (MPFG-45P) conjugated to horseradish peroxidase diluted to 0.05 μg/ml in PBS was added to each well, and the plates were incubated at 37°C. Following a subsequent wash step, 100 μl of nitroblue tetrazolium chromogen was added to each well for 10 min, followed by the addition of 50 μl of 1 M sulfuric acid. The absorbance was determined by using the Tecan GENius Plus plate reader at a wavelength of 450 nm.
In vitro susceptibility assay with fresh clinical isolates.
Between September and December 2005, fresh isolates were collected before treatment from patients hospitalized in the Lyon University Hospital for imported malaria. A diagnosis of malaria was established by microscopic examination of Giemsa-stained blood smears, and the diagnosis was confirmed by using the real-time PCR previously described by De Monbrison et al. (8). Blood samples collected from the patients with confirmed cases of P. falciparum malaria were maintained at room temperature for, on average, less than 12 h until they were diluted and added to 96-well plates precoated with chloroquine and mefloquine. The contents of the plates were tested by the SYBR green I assay after incubation times of 48 and 72 h and by the HRPII assay after an incubation time of 72 h. The assays were performed as follows. Blood samples were washed three times in complete RPMI medium and were then adjusted to an optimum parasite density of 0.05 to 0.1% and a hematocrit of 1.5% with freshly washed uninfected erythrocytes. Two hundred microliters of this preparation was distributed in plates precoated with chloroquine or mefloquine. The plates were incubated at 37°C in 5% CO2, 5% O2, and 90% N2 and were frozen at −20°C after 48 and 72 h. The plates were thawed just before the contents were tested. For the SYBR green I assay, 100 μl of lysate from each well of the plates incubated for 48 and 72 h was transferred to a new 96-well plate and mixed with 100 μl of the lysis buffer-SYBR green I mixture (final concentration, 1/10,000). After 1 h of incubation at room temperature in the dark, the fluorescence values were read on a Twinkle fluorometer (Berthold, Thoiry, France) at excitation and emission wavelengths of 485 and 535 nm, respectively. For the HRPII assay, 100 μl of lysate was removed following 72 h of incubation and processed as described above. The absorbance at 450 nm was then determined.
Data analysis.
The IC50s and IC90s obtained after incubation times of 48 and 72 h were calculated by using HN-nonlin V1.O5 beta program (16). Statistical analysis was performed by nonparametric correlation analysis (Mann-Whitney U test) with Prism 4.0 software for Windows (Graphpad Software, San Diego, CA).
RESULTS
Effects of culture conditions on IC50 values.
The initial experimentation was designed to replicate the work published previously (6, 23). We sought to determine if various components in the culture medium had adverse effects on the IC50 values using cultured parasites and the SYBR green I in vitro assay. Our results showed that there were no statistically significant differences (P > 0.05) in the IC50 values for clones D6, 3D7, and W2 when the parasites were grown in complete medium with or without phenol red, providing evidence that the phenol red does not interfere with the assay or complex with the SYBR green I, which would have produced erroneous fluorescent readings (data not shown). We did observe that parasites cultured in complete medium with phenol red grew better, as determined from the higher parasite counts determined by microscopy and the overall higher fluorescent values, an indication of a higher DNA content, following addition of SYBR green I at the conclusion of the incubation process (data not shown). There was no other difference in the medium composition except for the presence or absence of phenol red. The fluorescent values recorded for parasites grown in medium with phenol red were 0.2- to 1.4-fold greater in the drug-free control wells than in the wells with the highest concentration of drug (data not shown). In order to determine if sufficient parasite growth had occurred to produce valid IC50 results, we investigated the fold increase in fluorescent units between the control well without drugs and the wells containing the highest drug concentration. Even with only a 0.5-fold difference in the fluorescent values for the control wells containing parasites and no antimalarials and the wells containing parasites and the highest concentration of antimalarials, the sigmoidal curves were similar to those reported previously (18, 23), with a squared correlation coefficient of 0.95. On average, the fold increase in fluorescent intensity was one- to threefold higher between the drug-free wells and the wells containing the highest drug concentration. Each plate contained a negative control well with uninfected RBCs that was used to determine background fluorescent intensities. Compared to the fluorescence of the wells that contained parasites and no antimalarial drugs, the negative control wells that only contained uninfected RBCs produced a background fluorescence that was 5- to 20-fold lower (data not shown). In addition, the IC50 values for culture medium supplemented with human serum and those for culture medium supplemented with Albumax I did not differ (data not shown). It should be noted that there was more background fluorescence with medium supplemented with human serum than with medium supplemented with Albumax I (data not shown), which could have been due to the exogenous DNA in the human serum or proteins that react with the SYBR green I, which would cause an increase in fluorescence. Another change to the original procedure was the addition of the freezing step following incubation but before the addition of lysis buffer-SYBR green I. Freezing of the plates produced results that were more consistent and allowed the plates to be read within 5 min after addition of the SYBR green I dye (data not shown). The incubation period can be critical, depending on which in vitro method is used (3, 16). Two incubation times (48 h and 72 h) were tested in triplicate for the SYBR green I assay with the four different clones (data not shown). There were no significant differences between the values obtained after 48 h or 72 h of incubation.
Comparison of SYBR green I and HRPII ELISA in vitro drug susceptibility methods.
We were interested in comparing the SYBR green I in vitro method described by Smilkstein et al. (23) and the HRPII ELISA method (16, 18). The HRPII assay has been used extensively in various geographical locations worldwide and is considered an acceptable alternative to the isotopic assay so was therefore considered the gold standard to which the SYBR green I method should be compared. Cultured D6, 3D7, and W2 parasites were used for the side-by-side comparison of the two methods. Cultures were grown for 72 h in the presence of the antimalarial drugs, followed by a freeze-thaw step. A total of 100 μl of the lysate from each well was used for each of the tests, as described previously (16, 23). As shown in Table 1, there were no significant differences (P > 0.05) in the IC50s and IC90s calculated by both the SYBR green I method and the HRPII capture ELISA for each reference strain and antimalarial drug. An excellent and typical fit of the data to the polynomial regression model was observed and resulted in squared correlation coefficients between 0.88 and 1.0.
TABLE 1.
Comparison of IC50 and IC90 values for three reference clones of P. falciparum determined by the SYBR green I method and HRPII capture ELISA after 72 h of incubationa
| Drug and strain | IC50 (nM)
|
IC90 (nM)
|
||||||
|---|---|---|---|---|---|---|---|---|
| SYBR green I assayb
|
HRPII ELISAc
|
SYBR green I assay
|
HRPII ELISA
|
|||||
| Mean | 95% CId | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Chloroquine | ||||||||
| 3D7 | 8.1 | 6.6-10.9 | 7.5 | 4.9-8.6 | 26.4 | 12.5-55.6 | 33.2 | 16.8-55.6 |
| D6 | 6.1 | 4.7-7.9 | 5.9 | 4.5-7.2 | 16.1 | 12.4-20.9 | 15.8 | 16.5-19.7 |
| W2 | 35.1 | 26.2-46.9 | 33.2 | 21.2-47.2 | 125 | 61.9-252.7 | 128.7 | 104.5-162 |
| Mefloquine | ||||||||
| 3D7 | 9.4 | 6.2-14.2 | 8.4 | 5.8-13.9 | 29.8 | 23-38.6 | 25.6 | 14.2-37.6 |
| D6 | 6.4 | 3.2-12.8 | 4.7 | 3.8-5.7 | 23.3 | 14.7-36.8 | 18.7 | 14.9-32.3 |
| W2 | 3.1 | 0.7-13.2 | 1.9 | 1.3-2.8 | 12.2 | 4.4-33.8 | 8.0 | 4.3-10.6 |
| Quinine | ||||||||
| 3D7 | 6.9 | 4.5-10.5 | 5.9 | 5.5-6.2 | 63.7 | 20.5-195.5 | 42.6 | 23-0.62 |
| D6 | 6.6 | 3.8-11.3 | 6.7 | 4.8-9.2 | 12.4 | 6.7-22.8 | 13.2 | 7.1-23.5 |
| W2 | 22.2 | 7.4-66.7 | 15.1 | 4.5-24 | 116 | 98.2-136.7 | 128 | 93.5-182.9 |
None of the differences was significant by the Mann-Whitney U test.
Values are averages of n = 12.
Values are averages of n = 12.
CI, confidence interval.
In vitro testing of fresh clinical isolates.
Malaria parasite-infected blood was collected from nonimmune travelers with a mean level of parasitemia of 0.75% returning from regions of West Africa where malaria is endemic following 2- to 24-week stays (median stay, 5 weeks). Three of the patients had been in Cameroon, while three had been in Burkina Faso, three had been in Mali, two had been in Ivory Coast, one had been in Gabon, and one had been in the Central African Republic. All but one of the patients reported the use of prophylaxis, and each patient was treated with quinine following diagnosis, with no reported treatment failures. A total of 10 of 25 (45%) blood samples collected from the patients with malaria were successfully analyzed after both incubation times by the SYBR green I and HRPII assay methods. The reason for failure could have been a lack of growth of the parasites, confirmed by the observation of Giemsa-stained smears from control wells, which could be attributed to a prolonged time between the times when the samples were collected and when they were tested, or the starting parasitemias may have been too low. No IC50 could be calculated by the SYBR green I assay for 3 of the 10 samples at 48 h, whereas at 72 h, all 10 samples gave typical IC50 curves (Table 2). This supports our earlier observation that the results were more accurate at 72 h than at 48 h. Some discrepancies between the inhibitory concentrations obtained after 48 h and 72 h could be observed, with the highest correlation coefficients obtained with chloroquine. A comparison of the IC50 and IC90 values from the SYBR green I assay and the HRPII assay are shown in Table 2. The results for the calculated IC50 and IC90 values were similar or identical, with no significant differences.
TABLE 2.
IC50s and IC90s obtained with fresh clinical isolates after 48 and 72 h of incubation by the SYBR green I and HRPII assays
| Patient no. | Chloroquine
|
Mefloquine
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SYBR green I
|
HRPII ELISA, 72 h
|
SYBR green I
|
HRPII ELISA, 72 h
|
|||||||||
| 48 h
|
72 h
|
48 h
|
72 h
|
|||||||||
| IC50 (nM) | IC90 (nM) | IC50 (nM) | IC90 (nM) | IC50 (nM) | IC90 (nM) | IC50 (nM) | IC90 (nM) | IC50 (nM) | IC90 (nM) | IC50 (nM) | IC90 (nM) | |
| 1 | 58 ± 39 | 482 ± 191 | 12.5 ± 0 | 24 ± 14 | 13 ± 0 | 14 ± 2 | 33 ± 21 | 146 ± 20 | 14 ± 6 | 131 ± 23 | 5 ± 0.5 | 11 ± 1 |
| 2 | 24 ± 6 | 377 ± 4 | 20 ± 5 | 43 ± 12.5 | 27 ± 6 | 77 ± 7 | 42 ± 15 | 101 ± 32 | 23 ± 3 | 69 ± 11 | 14 ± 2 | 68 ± 1 |
| 3 | 38 ± 4 | 77 ± 12 | 35 ± 1 | 80 ± 15.5 | 43 ± 4 | 95 ± 10 | 37 ± 8 | 108 ± 16 | 41 ± 10 | 110 ± 15 | 36 ± 11 | 88 ± 6 |
| 4 | 36 ± 1 | 97 ± 1 | 37 ± 1 | 53 ± 13 | NDa | ND | 34 ± 6 | 94 ± 14 | 43 ± 8 | 109 ± 13 | ND | ND |
| 5 | 67 ± 3 | 160 ± 18 | 53 ± 13 | 165 ± 25.5 | 67 ± 37 | 516 ± 37 | 28 ± 4 | 86 ± 19 | 64 ± 7 | 167 ± 18 | 16 ± 10 | 53 ± 39 |
| 6 | ND | ND | 56 ± 5 | 140 ± 14 | 15 ± 1 | 39 ± 3 | ND | ND | ND | ND | 27 ± 3 | 64 ± 4 |
| 7 | ND | ND | 243 ± 39 | 374 ± 37 | 258 ± 20 | 354 ± 31 | ND | ND | 42 ± 14 | 114 ± 38 | 16 ± 11 | 49 ± 16 |
| 8 | 164 ± 1 | 478 ± 190 | 437 ± 90 | 679 ± 120 | ND | 626 ± 2 | 18 ± 3 | 60 ± 13 | 20 ± 13 | 49 ± 16 | 9 ± 0.5 | 22 ± 2 |
| 9 | 390 ± 55 | 732 ± 19 | 370 ± 35 | 742 ± 11 | 256 ± 73 | 538 ± 109 | 43 ± 15 | 106 ± 21 | 43 ± 10 | 115 ± 15 | 8 ± 0.5 | 56 ± 3 |
| 10 | ND | ND | 410 ± 73 | ND | 366 ± 21 | 707 ± 27 | ND | ND | 21 ± 4 | 59 ± 14 | 18 ± 1 | 34 ± 0.5 |
ND, the inhibitory concentration was not determined due to insufficient growth of the parasites or a low correlation coefficient.
DISCUSSION
The use of in vitro drug susceptibility testing has become a common practice as a means to screen new antimalarial compounds or antimalarial combination therapies with known reference strains (28) and to monitor temporal changes in resistance in field isolates (1, 15). Since the advent of the technology (9), the [3H]hypoxanthine method has been the most widely used and has been considered the gold standard method for the determination of IC50 and IC90 values. Due to the cost of specialized equipment and issues associated with the disposal of radioactive waste, this method is not practical in many places of the world today. Other non-radioactivity-based methods, such as the morphological WHO microtest, the SYBR green I assay, the HRPII capture ELISA, and the pLDH ELISA can be used routinely to conduct in vitro drug susceptibility testing. Each assay has been studied and reported on extensively in the literature, with each assay providing similar results; but most of these assays are cumbersome and may require either specialized equipment or extensive time to complete, making them unsuitable for the performance of high-throughput drug screening assays for new antimalarial chemotherapies.
An in vitro assay that uses the incorporation of SYBR green I into the genomic DNA of the malaria parasites present in the wells of the reaction plates was originally reported by two independent groups in 2004 (6, 23). SYBR green I is used extensively for the visualization of DNA and RNA in agarose gels and as a reporter molecule in real-time quantitative PCR (14). The SYBR green I molecule intercalates in the genomic DNA of the parasite and fluoresces once it is in position in the DNA. The method yields results similar to those of the radioactive method at a fraction of the cost and without the production of harmful waste material (23). A variable that we investigated was what effects the phenol red in the complete medium had on the SYBR green I-based in vitro assay. It had been postulated that the phenol red dye could interfere with the binding of the SYBR green I dye or cause an increase in the background fluorescence when it is included in the culture medium used for cultivation of the parasites during the SYBR green I in vitro method. We performed a side-by-side comparison of complete media with and without phenol red and found no statistically significant difference in the IC50 values calculated for the cultured parasite. However, we did observe that parasites that were grown in complete medium with phenol red grew better than those cultured in complete medium without phenol red, as indicated by a greater ratio of fluorescence units between the drug-free control wells and the test wells with the highest concentration of antimalarial. Other fluorescent dyes have been used for in vitro drug susceptibility assays (7, 22). PicoGreen has been used and was found to produce results comparable to those of the standard method based on the uptake of [3H]hypoxanthine (7). The SYBR green I method has been reported to produce results similar to those of the method based on the uptake of [3H]hypoxanthine (23). A recently published study compared the PicoGreen method to isotopic and microscopic assays for the measurement of the chloroquine sensitivities of fresh and cryopreserved isolates of P. vivax (12). The authors reported that there was no significant difference in the IC50 values, regardless of the method used. They also reported similar results with D6 (sensitive) and Ki (resistant) control isolates of P. falciparum (12). They also reported that the WHO microtest assay was more likely than the PicoGreen and isotopic methods to produce valid results. We were successful in determining IC50 values for 45% of the fresh clinical isolates of P. falciparum at 72 h. Further studies will be needed to compare the sensitivities of PicoGreen and SYBR green I.
It has been shown that one of the most crucial steps in the in vitro assay is the culturing of parasites (3). Storage of fresh clinical samples infected with P. falciparum parasites more than 24 h after collection was shown to drastically affect IC50 values, and there is evidence that the in vitro cultivation of fresh isolates can select for a subset of the population collected at the time of infection (5). For the current study, we used fresh clinical isolates adjusted to 1.5% hematocrit in complete medium with Albumax I. The hematocrit is a variable that needs to be addressed since IC50 values have been found to increase dramatically as the hematocrit increases (5). The length of time that the cultures are permitted to grow greatly affects the choice of assay used for in vitro testing. While the SYBR green I assay, hypoxanthine, and pLDH can be used at either 48 or 72 h, the HRPII assay requires a 72-h incubation (18). The production of HRPII occurs between 48 and 72 h in the in vitro drug susceptibility test, making this method unsuitable for the shorter in vitro assays. Another benefit of the use of SYBR green I or other DNA-staining molecules (or dyes), such as PicoGreen, is that the results will not be biased on the basis of the growth stage of the parasites, as is the case with HRPII. Analysis of the DNA content versus the drug concentration correlates directly with the drug-induced inhibition of replication (22). Additionally, the use of monoclonal antibodies for the detection of the HRPII from P. falciparum can be hampered by genetic variation within the circulating population of malaria parasites (2, 11). It was reported that a false-negative result rate of 20% was observed with rapid dipstick tests based on the HRPII from confirmed P. falciparum cases originating in Nigeria (11). Similar genetic diversity in HRPII is seen from isolates originating in the Asia-Pacific region, with only 84% of the isolates reacting and with parasite densities less than 250 asexual parasites/μl (2).
We found that freezing of the plates produced results that were more reliable. Smilkstein et al. (23) reported little to no difference between the results obtained by the use of lysis buffer with SYBR green I on plates that recently finished the incubation step and the results obtained when a freeze-thaw step was performed prior to the addition of SYBR green I. We found that freezing the plates provided the convenience of not having to conduct the experiment at the time that incubation was complete and also led to better lysis of the infected RBCs and parasites, thus releasing the DNA, which could then freely react with the SYBR green I. The regression plots became more reproducible and there was less variation between replicate wells, which decreased the standard error. The addition of a freezing step permits long-term storage and shipping of assay plates. This has proved to be quite beneficial and has allowed the assay to be conducted in remote regions of the Amazon, where access to a fluorometer is limited. Addition of a lysis step also permitted an incubation of less than 5 min following the addition of the SYBR green I due to the presence of free DNA from the lysed parasites. We found that the IC50 values were similar after 5 min and 1 h of incubation with SYBR green I. The choice of method used to perform the assay is not crucial in the in vitro process, as shown in this study. There was little to no significant difference between the calculated IC50 and IC90 values and the published values when the results of the SYBR green I, HRPII, and PCR methods were compared. When there was a difference in the mean, the 95% confidence intervals were within the published confidence intervals. As is the case with the selection of the most suitable assay for any method, the cost, the ease of use, and the time required to conduct the study are factors that need to be considered when one is choosing the most appropriate method. Until now, the fluorescent-based techniques with SYBR green I have been reported in the literature only for parasites grown in vitro without host cell white blood cells (WBCs) (6, 23). The HRPII assay has been used previously with fresh clinical isolates without removal of the WBCs before culture (16). The HRPII assay uses two monoclonal antibodies that specifically recognize the malaria parasite protein without appreciable cross-reactivity. Originally, we were conducting a laborious method of removing the WBCs prior to the in vitro assay with slow-spin centrifugation and size-exclusion columns but found that this step was unnecessary. We found that while the WBCs did contribute to the background fluorescence, the background could be removed or left in the final calculation of the IC50. If adequate growth, as determined by the ratio of the fluorescence of wells with no drugs and that of wells with the highest drug concentration, is achieved, then an accurate IC50 value could be calculated from an excellent and typical fit of the data to the polynomial regression model, with a resultant squared correlation coefficient greater than 0.9.
The cost of the SYBR green I assay is approximately $0.88 per 96-well plate, while the cost per HRPII assay by use of an in-house ELISA method (16) was approximately $10 per 96-well plate (Table 3). The HRPII ELISA kit is available from a commercial company but exceeds $50 per 96-well plate, making it less cost-effective, especially as a tool for high-throughput screening and for use in regions of the world where available funding is limited. The SYBR green I assay can be conducted in 5 min, while the HRPII assay takes more than 3 to 4 h. When the time required to perform the HRPII assay is taken into consideration, the cost of the HRPII assay is much higher, especially when multiple tests are conducted simultaneously.
TABLE 3.
Cost comparison of the SYBR green I-based and HRPII ELISA methods used to determine inhibitory concentrations
| Method | Cost ($)/96-well platea
|
Time (h) per assay | ||||
|---|---|---|---|---|---|---|
| Antibodiesb | ELISA reagentsd | SYBR green Ie | SYBR green lysis bufferf | Total | ||
| SYBR green I | NAc | NA | 0.20 | 0.68 | 0.88 | 1 |
| HRPII ELISA | 2.55 | 7.33 | NA | NA | 9.88 | 3 |
The final costs are estimates based on the amounts of the reagents required to perform assays with one full 96-well plate and are calculated from the actual cost of ordering the reagent from a U.S.-based manufacturer. Shipping costs were not taken into consideration.
The price includes those for both the IgM capture and the IgG detection antibodies.
NA, not applicable.
The price is for blocking reagents, wash buffer reagents, and tetramethylbenzidine.
Cost of the required amount of SYBG green I used per assay plate.
Reagents required to make lysis buffer used in the SYBR green method.
In conclusion, the choice of method to be conducted for in vitro drug susceptibility testing is less crucial than control of the culture conditions. With several of the most common methods producing similar IC50 and IC90 values, the choice of method can be made on the basis of the cost per plate required to conduct the experiment, the availability of reagents, the ease of use, and the comfort of the laboratory personnel conducting the work.
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily reflect the official policy of the U.S. Department of the Navy, the U.S. Department of Defense, or the U.S. government.
This work was supported by funds provided by the U.S. Department of Defense Global Emerging Infectious System under unit number 847705.82000.256B,B0016.
We express our appreciation to Andreas Lescano for his assistance with statistical analysis.
Footnotes
Published ahead of print on 12 January 2007.
REFERENCES
- 1.Babiker, H. A., G. Satti, H. Ferguson, R. Bayoumi, and D. Walliker. 2005. Drug resistant Plasmodium falciparum in an area of seasonal transmission. Acta Trop. 94:260-268. [DOI] [PubMed] [Google Scholar]
- 2.Baker, J., J. McCarthy, M. Gatton, D. E. Kyle, V. Belizario, J. Luchavez, D. Bell, and Q. Cheng. 2005. Genetic diversity of Plasmodium falciparum histidine-rich protein 2 (PfHRP2) and its effect on the performance of PfHRP2-based rapid diagnostic tests. J. Infect. Dis. 192:870-877. [DOI] [PubMed] [Google Scholar]
- 3.Basco, L. K. 2003. Molecular epidemiology of malaria in Cameroon. XV. Experimental studies on serum substitutes and supplements and alternative culture media for in vitro drug sensitivity assays using fresh isolates of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 69:168-173. [PubMed] [Google Scholar]
- 4.Basco, L. K. 2003. Molecular epidemiology of malaria in Cameroon. XVII. Baseline monitoring of atovaquone-resistant Plasmodium falciparum by in vitro drug assays and cytochrome b gene sequence analysis. Am. J. Trop. Med. Hyg. 69:179-183. [PubMed] [Google Scholar]
- 5.Basco, L. K. 2004. Molecular epidemiology of malaria in Cameroon. XX. Experimental studies on various factors of in vitro drug sensitivity assays using fresh isolates of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 70:474-480. [PubMed] [Google Scholar]
- 6.Bennett, T. N., M. Paguio, B. Gligorijevic, C. Seudieu, A. D. Kosar, E. Davidson, and P. D. Roepe. 2004. Novel, rapid, and inexpensive cell-based quantification of antimalarial drug efficacy. Antimicrob. Agents Chemother. 48:1807-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corbett, Y., L. Herrera, J. Gonzalez, L. Cubilla, T. L. Capson, P. D. Coley, T. A. Kursar, L. I. Romero, and E. Ortega-Barria. 2004. A novel DNA-based microfluorimetric method to evaluate antimalarial drug activity. Am. J. Trop. Med. Hyg. 70:119-124. [PubMed] [Google Scholar]
- 8.de Monbrison, F., D. Raynaud, C. Latour-Fondanaiche, A. Staal, S. Favre, K. Kaiser, F. Peyron, and S. Picot. 2003. Real-time PCR for chloroquine sensitivity assay and for pfmdr1-pfcrt single nucleotide polymorphisms in Plasmodium falciparum.J. Microbiol. Methods 54:391-401. [DOI] [PubMed] [Google Scholar]
- 9.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semi-automated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escalante, A. A., O. E. Cornejo, A. Rojas, V. Udhayakumar, and A. A. Lal. 2004. Assessing the effect of natural selection in malaria parasites. Trends Parasitol. 20:388-395. [DOI] [PubMed] [Google Scholar]
- 11.Happi, C. T., G. O. Gbotosho, A. Sowunmi, C. O. Falade, D. O. Akinboye, O. Oladepo, and A. M. Oduola. 2004. Malaria diagnosis: false negative Parasight-F tests in falciparum malaria patients in Nigeria. Afr. J. Med. Med. Sci. 33:15-18. [PubMed] [Google Scholar]
- 12.Kosaisavee, V., R. Suwanarusk, F. Nosten, D. E. Kyle, M. Barrends, J. Jones, R. Price, B. Russell, and U. Lek-Uthai. 2006. Plasmodium vivax: isotopic, PicoGreen, and microscopic assays for measuring chloroquine sensitivity in fresh and cryopreserved isolates. Exp. Parasitol. 114:34-39. [DOI] [PubMed] [Google Scholar]
- 13.Makler, M. T., J. M. Ries, J. A. Williams, J. E. Bancroft, R. C. Piper, B. L. Gibbins, and D. J. Hinrichs. 1993. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am. J. Trop. Med. Hyg. 48:739-741. [DOI] [PubMed] [Google Scholar]
- 14.Mangold, K. A., R. U. Manson, E. S. C. Koay, L. Stephens, M. Regner, R. B. Thomson, Jr., L. R. Peterson, and K. L. Kaul. 2005. Real-time PCR for detection and identification of Plasmodium spp. J. Clin. Microbiol. 43:2435-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mbaisi, A., P. Liyala, F. Eyase, R. Achilla, H. Akala, J. Wangui, J. Mwangi, F. Osuna, U. Alam, B. L. Smoak, J. M. Davis, D. E. Kyle, R. L. Coldren, C. Mason, and N. C. Waters. 2004. Drug susceptibility and genetic evaluation of Plasmodium falciparum isolates obtained in four distinct geographical regions of Kenya. Antimicrob. Agents Chemother. 48:3598-3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noedl, H., J. Bronnert, K. Yingyuen, B. Attlmayr, H. Kollaritsch, and M. Fukuda. 2005. Simple histidine-rich protein 2 double-site sandwich enzyme-linked immunosorbent assay for use in malaria drug sensitivity testing. Antimicrob. Agents Chemother. 49:3575-3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noedl, H., W. H. Wernsdorfer, S. Krudsood, P. Wilairatana, P. Viriyavejakul, H. Kollaritsch, G. Wiedermann, and S. Looareesuwan. 2001. In vivo-in vitro model for the assessment of clinically relevant antimalarial cross-resistance. Am. J. Trop. Med. Hyg. 65:696-699. [DOI] [PubMed] [Google Scholar]
- 18.Noedl, H., W. H. Wernsdorfer, R. S. Miller, and C. Wongsrichanalai. 2002. Histidine-rich protein II: a novel approach to malaria drug sensitivity testing. Antimicrob. Agents Chemother. 46:1658-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noedl, H., C. Wongsrichanalai, and W. H. Wernsdorfer. 2003. Malaria drug-sensitivity testing: new assays, new perspectives. Trends Parasitol. 19:175-181. [DOI] [PubMed] [Google Scholar]
- 20.Ringwald, P. 2003. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. World Health Organization, Geneva, Switzerland.
- 21.Russell, B. M., R. Udomsangpetch, K. H. Rieckmann, B. M. Kotecka, R. E. Coleman, and J. Sattabongkot. 2003. Simple in vitro assay for determining the sensitivity of Plasmodium vivax isolates from fresh human blood to antimalarials in areas where P. vivax is endemic. Antimicrob. Agents Chemother. 47:170-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smeijsters, L. J., N. M. Zijlstra, F. F. Franssen, and J. P. Overdulve. 1996. Simple, fast, and accurate fluorometric method to determine drug susceptibility of Plasmodium falciparum in 24-well suspension cultures. Antimicrob. Agents Chemother. 40:835-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smilkstein, M., N. Sriwilaijaroen, J. X. Kelly, P. Wilairat, and M. Riscoe. 2004. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 48:1803-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trager, W., and J. B. Jenson. 1978. Cultivation of malarial parasites. Nature 273:621-622. [DOI] [PubMed] [Google Scholar]
- 25.Vinayak, S., S. Biswas, V. Dev, A. Kumar, M. A. Ansari, and Y. D. Sharma. 2003. Prevalence of the K76T mutation in the pfcrt gene of Plasmodium falciparum among chloroquine responders in India. Acta Trop. 87:287-293. [DOI] [PubMed] [Google Scholar]
- 26.Wilson, P. E., A. P. Alker, and S. R. Meshnick. 2005. Real-time PCR methods for monitoring antimalarial drug resistance. Trends Parasitol. 21:278-283. [DOI] [PubMed] [Google Scholar]
- 27.Wongsrichanalai, C., K. Lin, L. W. Pang, M. A. Faiz, H. Noedl, T. Wimonwattrawatee, A. Laoboonchai, and F. Kawamoto. 2001. In vitro susceptibility of Plasmodium falciparum isolates from Myanmar to antimalarial drugs. Am. J. Trop. Med. Hyg. 65:450-455. [DOI] [PubMed] [Google Scholar]
- 28.Zhu, S., T. H. Hudson, D. E. Kyle, and A. J. Lin. 2002. Synthesis and in vitro studies of novel pyrimidinyl peptidomimetics as potential antimalarial therapeutic agents. J. Med. Chem. 45:3491-3496. [DOI] [PubMed] [Google Scholar]