Abstract
Emerging resistance threatens the usefulness of linezolid for the treatment of severe infections caused by multidrug-resistant gram-positive bacteria. Optimal pharmacokinetic (PK)/pharmacodynamic (PD) indices have been described for the antimicrobial efficacy of linezolid (area under the concentration-time curve over 24 h at steady state divided by the MIC, >100; the cumulative percentage of a 24-h period that the drug concentration exceeds the MIC under steady-state PK conditions, >85). The aim of this study was to investigate the influence of these PK/PD indices on the development of resistance to linezolid by using an in vitro PK/PD model. Four dosage regimens were simulated over 72 h (two intermittent bolus regimens of 600 mg every 12 h [q12h] and 120 mg q12h and two continuous-infusion regimens of 120 mg/24 h and 30 mg/24 h) against four reference strains: methicillin-resistant Staphylococcus aureus (MRSA), heteroresistant vancomycin-intermediate S. aureus (hVISA), vancomycin-intermediate S. aureus (VISA), and vancomycin-resistant Enterococcus faecium (VRE). Linezolid concentrations were measured by high-performance liquid chromatography. Changes in susceptibility were characterized by pre- and posttreatment MIC measurements and population analysis profiles (PAPs). The linezolid concentrations that were achieved closely matched those that were targeted. The simulation with 600 mg q12h provided a >3-log10 reduction in the number of CFU/ml for all four strains, as did the 120-mg-q12h regimen for hVISA and VISA and the 30-mg/24-h continuous infusion for VRE and VISA. After 72 h of exposure to the 120-mg/24-h continuous-infusion simulation, the area under the PAP curve for all strains increased substantially (40 to 178%); increases in the MICs for the MRSA and hVISA strains were observed. The results demonstrate that PK/PD considerations are important in optimizing both antibacterial activity and the development of resistance to linezolid. The potential for resistance development appears to be higher when a constant concentration is maintained in the vicinity of the MIC of the bacteria.
The widespread and increasing prevalence of antimicrobial resistance threatens the management of serious bacterial infections worldwide (14). The oxazolidinone linezolid is one of only a few new agents approved in recent times for the treatment of infections caused by multidrug-resistant gram-positive bacteria (3a, 26, 29).
Linezolid has demonstrated bacteriostatic (1, 4, 12, 15, 17) and bactericidal (1, 5, 6, 21) activities against target pathogens and minimal concentration-dependent killing (2). The key pharmacokinetic (PK) characteristics of linezolid include approximately 100% bioavailability and a relatively low level of plasma protein binding (approximately 30%) (7). The relative influence of integrated PK/pharmacodynamic (PD) indices (including the area under the concentration-time curve over 24 h at steady state divided by the MIC [AUC/MIC], the cumulative percentage of a 24-h period that the drug concentration exceeds the MIC under steady-state PK conditions [%TMIC], and the peak concentration [Cmax] divided by the MIC [Cmax/MIC]) on the activity of linezolid has been investigated. The antimicrobial activity of linezolid against Staphylococcus aureus has been linked to the AUC/MIC in mouse thigh infection studies (2, 19a) and to %TMIC in rabbit endocarditis studies (9, 15, 21). The PK/PD analysis of a large compassionate-use study of linezolid correlated AUC/MIC and %TMIC values of >100 and >85, respectively, with clinical cure and bacterial eradication end points (23).
Despite the brief existence of linezolid, the rate of resistance to the drug is growing (11, 16, 30). To date, risk factors for resistance, including the use of inadequate linezolid doses, long durations of therapy, and the nature of the infection, have been implicated (3, 22, 24). Patients in the compassionate-use study who developed decreased susceptibility to linezolid (fourfold or greater increases in the MIC) during treatment also exhibited AUC/MIC and %TMIC values <100, suggesting an influence of PK/PD in linezolid resistance development (23a).
While relationships between linezolid PK/PD indices and efficacy have been examined, no systematic investigation into their influence on the development of resistance to linezolid has been reported. Accordingly, the objective of this study was to use an in vitro model to examine the PK/PD influences of the emergence of resistance to linezolid in four gram-positive multidrug-resistant strains (methicillin-resistant S. aureus [MRSA], vancomycin- heteroresistant S. aureus and intermediate-resistant S. aureus [hVISA and VISA, respectively], and vancomycin-resistant Enterococcus faecium [VRE]). Dosage regimens were chosen to provide maximal differentiation between the two relevant PK/PD indices, AUC/MIC and %TMIC.
(These results were presented at the Australian Society for Antimicrobials 7th Annual Scientific Meeting, Sydney, Australia, 23 to 25 February 2006.)
MATERIALS AND METHODS
Bacterial strains and media.
Four relevant reference strains were used in the current study and studied in duplicate: three strains of S. aureus (MRSA ATCC 43300 [American Type Culture Collection; Manassas, VA], hVISA ATCC 700698 [Mu3], and VISA ATCC 700699 [Mu50]) and one strain of E. faecium (VRE strain ATCC 700221). The isolates were stored in tryptone soy broth (Oxoid Australia, West Heidelberg, Victoria, Australia) with 20% glycerol (Ajax Finechem, Seven Hills, NSW, Australia) at −80°C in cryovials (Simport Plastics, Boloeil, Quebec, Canada). The isolates were subcultured onto horse blood agar for 24 h at 35°C before each experiment (Media Preparation Unit, Melbourne, Victoria, Australia).
Antimicrobials.
Linezolid was kindly supplied by Pharmacia & Upjohn Company (Kalamazoo, MI). Immediately prior to each experiment, linezolid was weighed and dissolved in Milli-Q water (Millipore Australia, North Ryde, NSW, Australia) and sterilized by passage through a 0.2-μm-pore-size syringe filter (Sartorius, Geoffingen, Germany).
In vitro PK/PD model.
The one-compartmental PK/PD model used in this study was based upon a previous design (27). Briefly, the system consisted of three sealed central chambers (compartments), each of which contained 290 ml of brain heart infusion (BHI) broth (Oxoid Australia) and a magnetic stir bar to ensure adequate mixing; the chambers were placed in a paraffin bath at 37°C. A flowthrough system was created, whereby BHI broth was added to each chamber continuously by using a peristaltic pump (Cole Parmer Instrument Company); an equal volume of medium was displaced. The flow rate set on the peristaltic pump and the volume of the central chamber were chosen to simulate human drug clearance and a 5-h linezolid half-life (28).
The model was used to simulate four different dosage regimens (two intermittent bolus regimens and two continuous-infusion regimens) to provide maximal differentiation between the indices AUC/MIC and %TMIC (Table 1). The baseline linezolid MIC for all strains was determined by the standard microdilution method (see below). The 24-h AUC (μg·h/ml) was calculated by using the linear trapezoidal method.
TABLE 1.
Simulated linezolid dosage regimens
| Regimen no. | Simulated human regimen | Targeted concn | In vitro regimena | Targeted PK/PD valuesb
|
|
|---|---|---|---|---|---|
| AUC/MIC | %TMIC | ||||
| 1 | 600 mg q12h | Cmax, 20 μg/mlc; Cmin, 3.8 μg/ml | 5.8-mg loading dose + 4.7-mg maintenance dose q12h | 120 | 100 |
| 2 | 120 mg/24 h | Continuous maintenance of 2.0 μg/ml | 2.0-μg/ml continuous infusion | 24 | 100 |
| 3 | 120 mg q12h | Cmax, 4.0 μg/ml; Cmin, 0.76 μg/ml | 1.2-mg loading dose + 0.94-mg maintenance dose q12h | 24 | 42 |
| 4 | 30 mg/24 h | Continuous maintenance of 0.50 μg/ml | 0.50-μg/ml continuous infusion | 6 | 0 |
Volume of distribution, 290 ml; half-life, 5 h.
MIC of 2 μg/ml for all strains.
Approximating Cmax (for the total plasma concentration) (Zyvox product information, 21 February 2002).
At the beginning of each experiment, exponentially growing bacteria were inoculated into two experimental central chambers of the model at 106 CFU/ml; appropriate growth controls were used. Administration of linezolid was performed to generate the concentrations and PK/PD indices described in Table 1. Samples (1 ml) were collected aseptically from each central chamber via a rubber septum-sealed port over 72 h (0, 1, 2, 4, 8, 24, 48, and 72 h) for determination of viable counts and measurement of linezolid concentrations. Samples for counting of viable bacteria were diluted with 0.9% saline, and 20-μl aliquots were manually plated onto nutrient agar plates (Media Preparation Unit), which were then incubated at 37°C for 24 h. The minimum, accurately quantifiable number was 50 CFU/ml. S. aureus identification was performed by using chromatographic medium (SAID; BioMérieux, Baulkham Hills, NSW, Australia) at the end of each experiment.
Determination of linezolid concentrations in broth.
Samples collected in the in vitro PK/PD experiments were stored at −20°C until analysis. Total linezolid concentrations were measured by a previously validated high-performance liquid chromatography assay (18); quality control samples with nominal concentrations of 0.05, 1.0, and 12.0 μg/ml had measured concentrations (mean ± standard deviation) of 0.052 ± 0.007, 0.976 ± 0.061, and 12.3 ± 0.59 μg/ml, respectively.
Monitoring emergence of resistance.
Changes in susceptibility were monitored by serial measurement of MIC and by use of population analysis profiles (PAPs). The MICs were determined for the reference strains at the baseline and for the bacteria isolated from the in vitro model after 24, 48, and 72 h of exposure to the different dosage regimens; cation-adjusted Mueller-Hinton broth (Oxoid Australia) was used with a final inoculum of 106 CFU/ml, according to the standard microdilution method (8). PAPs were also measured for the reference strains at the baseline and for the bacteria isolated from the in vitro model after 72 h of exposure; 50 μl of a 0.5 McFarland bacterial suspension and/or appropriate serial dilutions were spiral plated onto BHI agar plates (Media Preparation Unit) containing linezolid (0, 0.5, 1.0, 1.5, 2, 3, 4, and 5 μg/ml) (13). The choice of the linezolid concentration range was based on the susceptibility breakpoint for linezolid being 4 μg/ml (19). After incubation at 35°C for 48 h, the colonies were counted by an automated colony counter (ProtoCOL; Synbiosis, Cambridge, United Kingdom), with a limit of counting of 20 CFU/ml. Changes in the PAPs were characterized by plotting the log10 CFU/ml count against the BHI agar plate linezolid concentration (0 to 5 μg/ml) and calculating the area under the PAP curve (AUPAP) at time zero and 72 h by using the linear trapezoidal rule. The stability of any changes in susceptibility observed after 72 h of treatment was assessed by serial passaging in drug-free BHI broth (16 daily passages), with daily PAP determination followed by final MIC measurement.
RESULTS
The baseline MICs for all strains in four replicates were 2 μg/ml. Excellent agreement was observed between the targeted and the achieved linezolid concentrations. The observed half-life (mean ± standard deviation) for the simulated intermittent-dosage regimens (600 mg every 12 h [q12h] and 120 mg q12h) was 5.32 ± 0.30 h, which compares well with the targeted 5-h half-life; the AUC and Cmax values achieved were within 13% and 16% of the targeted values, respectively.
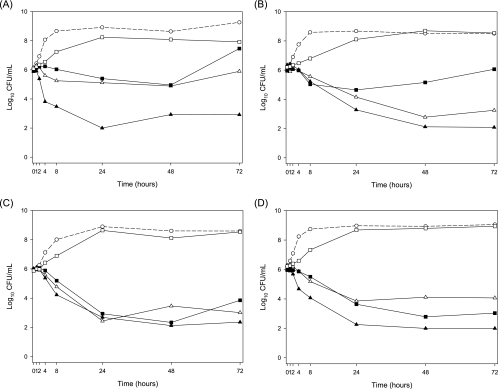
The killing of MRSA, hVISA, VISA, and VRE by the four different simulated dosage regimens are presented in Fig. 1. The growth inhibition observed for the 600-mg-q12h, 120-mg-q12h, and 120-mg/24-h continuous-infusion regimens was delayed for at least the first 2 h of linezolid treatment. The regimen simulating the human linezolid dosage regimen of 600 mg q12h provided bactericidal activity (a >3-log10 reduction in the numbers of CFU/ml) against all four strains at 24 h, with mean log10 CFU/ml decreases of 3.9 for MRSA, 3.1 for hVISA, 3.3 for VISA, and 3.8 for VRE. For the 120-mg-q12h simulated regimen, a greater than 3-log10 reduction in the numbers of CFU/ml was observed at 24 h for VISA and at 48 h for hVISA. The same magnitude of reduction occurred at 48 h for the 120-mg/24-h continuous-infusion simulated regimen for VISA and VRE. Minor regrowth of hVISA after 24 h of exposure and of MRSA and VISA after 48 h of exposure to the 120-mg/24-h continuous-infusion simulation was observed. Over the duration of the treatment period for all strains, the 30-mg/24-h infusion simulation produced growth similar to that of the control.
FIG. 1.
Antibacterial effects of linezolid (means for two replicates per strain). (A) MRSA; (B) hVISA; (C) VISA; (D) VRE. ○, growth control; ▴, 600 mg q12h; ▵, 120 mg q12h; ▪, 120-mg/24-h continuous infusion; □, 30-mg/24-h continuous infusion.
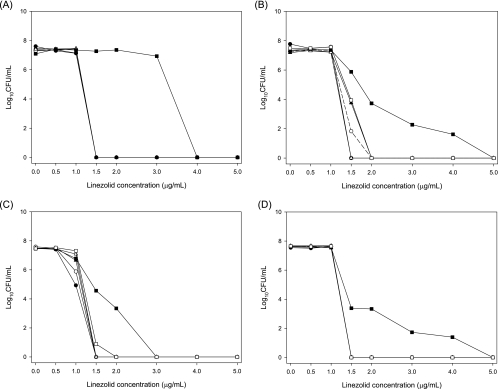
Increases in MICs to values ≥8 μg/ml were observed only for both MRSA replicates and one hVISA replicate after exposure to the 120-mg/24-h continuous-infusion simulation. The PAPs for each strain before and after 72 h of exposure to the four linezolid regimens are presented in Fig. 2; the means of two replicates are shown except for hVISA, VISA, and VRE exposed to the 600-mg-q12h regimen due to a lack of quantifiable colonies for one of the two replicates. For the baseline (time zero) and growth control (at 72 h), the growth of all four strains was observed on PAP plates containing 0, 0.5 and 1.0 μg/ml of linezolid; while mild growth was observed on the plate containing 1.5 μg/ml, colonies were not countable due to their small size. For VISA, the extent of growth at 1.0 μg/ml was lower than that for the other strains (Fig. 2). After treatment with linezolid for 72 h, the PAPs for each strain were generally similar for three of the four simulated regimens; the one exception was the continuous-infusion regimen of 120 mg/24 h. For this regimen, the AUPAPs were 178%, 70.2%, 90.2%, and 40.0% greater than that of the 72-h growth control for MRSA, hVISA, VISA, and VRE, respectively. No changes in PAPs were observed after each of the 16 passages in drug-free broth, and no changes in the MIC measurements were observed after the final passage.
FIG. 2.
PAPs before (baseline) and after 72 h of exposure to linezolid or the growth control. •, baseline; ○, growth control; ▴, 600 mg q12h; ▵, 120 mg q12h; ▪, 120-mg/24-h continuous infusion; □, 30-mg/24-h continuous infusion.
DISCUSSION
In light of emerging resistance to antimicrobials like the oxazolidinone linezolid, the optimization of antimicrobial use through the integration of PK and PD data is important. Based on one large compassionate-use study, both an AUC/MIC of >100 and a %TMIC of >85 have been described as optimal PK/PD targets for linezolid clinical efficacy (23). A previous study reported that patients who developed decreased susceptibility to linezolid during therapy were exposed to the drug with AUC/MIC and %TMIC values <100 (23a). Accordingly, the present study systematically investigated in an in vitro model the importance of PK/PD indices on the potential emergence of linezolid resistance.
Bactericidal activity was observed against all reference strains after 24 h of exposure to the 600-mg-q12h regimen, against VISA and VRE after 48 h exposure to the 120-mg/24-h continuous-infusion regimen, and against VISA and hVISA after 24 h and 48 h of exposure to the 120-mg-q12h regimen, respectively. Of these regimens, the 600-mg-q12h regimen is the only one for which previous in vitro model data are available; both bacteriostatic activity (against MRSA [1, 12, 17] and VRE [1]) and bactericidal activity (against MRSA [5] and VRSA [1, 5]) have been reported. The differences in antibacterial activity (i.e., bactericidal versus bacteriostatic activity) seen among the present and previous studies may relate to variations in the particular in vitro models and bacterial strains used. Within the current study, minor strain-to-strain differences in the responses to the four different regimens were observed, which warrants further investigation in future studies.
Following 72 h of exposure to the 120-mg/24-h continuous-infusion regimen, changes in AUPAP provided evidence of decreased susceptibility for all four strains, possibly due to the low AUC/MIC of 24 (23). While increases in AUPAPs occurred for all four strains with this regimen, twofold increases in postexposure MICs (to the resistance breakpoint of 8 μg/ml [19]) were observed only for both MRSA replicates and one hVISA replicate. This may relate to the higher sensitivity of the PAP method at detecting changes in susceptibility within subpopulations through the use of multiple sub- and supra-MICs of linezolid. The lack of resistance following exposure to the 600-mg-q12h regimen is consistent with the findings of previous in vitro studies that used the same regimen for 72 h (5, 17) or for shorter durations (1, 12). The methods of resistance detection used in the previous studies have included pre- and postexperimental MIC determination and plating on agar containing linezolid concentrations of four- and eightfold the MIC (1, 5, 12, 17). The lack of emergence of linezolid resistance following exposure to both the 120-mg-q12h and 30-mg/24-h continuous-infusion simulations is interesting, given that both PK/PD indices were low in both regimens (23).
In addition to the impact of the different PK/PD targets attained, the differences in effects on susceptibility observed across the four different regimens may have been influenced by the various PK profiles generated (peak and trough linezolid concentrations fluctuating above and around the MIC versus maintenance of constant linezolid concentrations either at or below the MIC), which may relate to the concept of the mutant selection window (MSW). Fluoroquinolone resistance occurs in a stepwise fashion; it has been demonstrated that an MSW exists, ranging from approximately the MIC to the concentration required to inhibit the growth of first-step mutants (10). There is very limited information on the MSW for linezolid against any bacteria (25) and none against the strains used in this study. Linezolid resistance is most often conferred through the G2576T point mutation in domain V of the 23S rRNA of the 50S ribosomal subunit (16, 20, 30). Multiple copies of the 23S rRNA gene exist in clinically relevant species, and the level of linezolid resistance has been correlated with allelic frequencies (20). The accumulation of mutations in these alleles may lead to a stepwise lowering of susceptibility to linezolid. It is possible that the resistance obtained with the 120-mg/24-h continuous-infusion simulation occurred because the linezolid concentration (2 μg/ml) was maintained within an MSW for the duration of the simulation. Conversely, the constant concentration (0.5 μg/ml) provided by the 30-mg/24-h continuous-infusion simulation may have been lower than the MSW. The peak and trough linezolid concentrations generated by the intermittent regimens may not have been within the MSW for a period of time sufficient to select for resistant mutants.
The lack of resistance emergence following exposure to three of the four regimens may also be related to the relatively short treatment period used (72 h), given that long durations of therapy have been implicated as a risk factor for clinical resistance development (22). It is also possible that minor alterations in mutation frequency may have gone undetected without the use of higher inocula when the emergence of resistance is investigated. Future studies should consider these potential limitations and look to the use of a larger number of regimens and bacterial strains, which may allow determination of specific PK/PD breakpoints for linezolid resistance. In any attempts to relate PK/PD indices determined in the in vitro model to the clinical situation, recognition would need to be made of the fact that linezolid is approximately 30% protein bound in human plasma (7). In addition, care is needed in extrapolating the results from in vitro models to the in vivo situation, where host immune defense systems exist.
In summary, this study has found that antibacterial activity is dependent upon the attainment of optimal PK/PD indices. Through systematic investigation of the influence of PK/PD indices on the emergence of linezolid resistance, the AUC/MIC in particular has been shown to play an important role. It is also apparent that the potential for resistance development may be substantial when constant concentrations of linezolid are maintained around the MIC.
Footnotes
Published ahead of print on 22 January 2007.
REFERENCES
- 1.Allen, G. P., R. Cha, and M. J. Rybak. 2002. In vitro activities of quinupristin-dalfopristin and cefepime, alone and in combination with various antimicrobials, against multidrug-resistant staphylococci and enterococci in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 46:2606-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andes, D., M. L. van Ogtrop, J. Peng, and W. A. Craig. 2002. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob. Agents Chemother. 46:3484-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birmingham, M. C., C. R. Rayner, A. K. Meagher, S. M. Flavin, D. H. Batts, and J. J. Schentag. 2003. Linezolid for the treatment of multidrug-resistant, gram-positive infections: experience from a compassionate-use program. Clin. Infect Dis. 36:159-168. [DOI] [PubMed] [Google Scholar]
- 3a.Cammarata, S. K., G. S San Pedro, J. A. Timm, K. A. Hempsall, W. M. Todd, and T. H. Oliphant. 2000. Comparison of linezolid versus ceftriaxone/cefpodoxime in the treatment of hospitalized patients with community-acquired pneumonia. Clin. Microbiol. Infect. 6(Suppl. l):136. [Google Scholar]
- 4.Cha, R., R. L. Akins, and M. J. Rybak. 2003. Linezolid, levofloxacin, and vancomycin against vancomycin-tolerant and fluoroquinolone-resistant Streptococcus pneumoniae in an in vitro pharmacodynamic model. Pharmacotherapy 23:1531-1537. [DOI] [PubMed] [Google Scholar]
- 5.Cha, R., W. J. Brown, and M. J. Rybak. 2003. Bactericidal activities of daptomycin, quinupristin-dalfopristin, and linezolid against vancomycin-resistant Staphylococcus aureus in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 47:3960-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cha, R., and M. J. Rybak. 2003. Linezolid and vancomycin, alone and in combination with rifampin, compared with moxifloxacin against a multidrug-resistant and a vancomycin-tolerant Streptococcus pneumoniae strain in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 47:1984-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clemett, D., and A. Markham. 2000. Linezolid. Drugs 59:815-827. [DOI] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 6th ed. Approved standard M7-A6. Clinical and Laboratory Standards Institute, Wayne, PA.
- 9.Dailey, C. F., C. L. Dileto-Fang, L. V. Buchanan, M. P. Oramas-Shirey, D. H. Batts, C. W. Ford, and J. K. Gibson. 2001. Efficacy of linezolid in treatment of experimental endocarditis caused by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:2304-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drlica, K. 2003. The mutant selection window and antimicrobial resistance. J. Antimicrob. Chemother. 52:11-17. [DOI] [PubMed] [Google Scholar]
- 11.Gonzales, R. D., P. C. Schreckenberger, M. B. Graham, S. Kelkar, K. DenBesten, and J. P. Quinn. 2001. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 357:1179. [DOI] [PubMed] [Google Scholar]
- 12.Gunderson, B. W., K. H. Ibrahim, C. A. Peloquin, L. B. Hovde, and J. C. Rotschafer. 2003. Comparison of linezolid activities under aerobic and anaerobic conditions against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium. Antimicrob. Agents Chemother. 47:398-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiramatsu, K., N. Aritaka, H. Hanaki, S. Kawasaki, Y. Hosoda, S. Hori, Y. Fukuchi, and I. Kobayashi. 1997. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 350:1670-1673. [DOI] [PubMed] [Google Scholar]
- 14.Infectious Diseases Society of America. 2004. Bad bugs, no drugs. http://www.idsociety.org/pa/IDSA_Paper4_final_web.pdf. Infectious Diseases Society of America, Alexandria, VA.
- 15.Jacqueline, C., E. Batard, L. Perez, D. Boutoille, A. Hamel, J. Caillon, M. F. Kergueris, G. Potel, and D. Bugnon. 2002. In vivo efficacy of continuous infusion versus intermittent dosing of linezolid compared to vancomycin in a methicillin-resistant Staphylococcus aureus rabbit endocarditis model. Antimicrob. Agents Chemother. 46:3706-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones, R. N., P. Della-Latta, L. V. Lee, and D. J. Biedenbach. 2002. Linezolid-resistant Enterococcus faecium isolated from a patient without prior exposure to an oxazolidinone: report from the SENTRY Antimicrobial Surveillance Program. Diagn. Microbiol. Infect. Dis. 42:137-139. [DOI] [PubMed] [Google Scholar]
- 17.LaPlante, K. L., and M. J. Rybak. 2004. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 48:4665-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, J., C. R. Rayner, S. Dixson, and R. L. Nation. 2004. Simple method for the assay of linezolid in brain heart infusion broth by high-performance liquid chromatography. Biomed. Chromatogr. 18:1-5. [DOI] [PubMed] [Google Scholar]
- 19.Livermore, D. M., S. Mushtaq, and M. Warner. 2001. Susceptibility testing with linezolid by different methods, in relation to published ‘general breakpoints’. J. Antimicrob. Chemother. 48:452-454. [DOI] [PubMed] [Google Scholar]
- 19a.Louie, A., W. Lui, M. Deziel, M. Drusano, and G. Drusano. 2004. Pharmacodynamics of linezolid in a neutropenic mouse thigh model of Staphylococcus aureus infection. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-1865.
- 20.Marshall, S. H., C. J. Donskey, R. Hutton-Thomas, R. A. Salata, and L. B. Rice. 2002. Gene dosage and linezolid resistance in Enterococcus faecium and Enterococcus faecalis. Antimicrob. Agents Chemother. 46:3334-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oramas-Shirey, M. P., L. V. Buchanan, C. L. Dileto-Fang, C. F. Dailey, C. W. Ford, D. H. Batts, and J. K. Gibson. 2001. Efficacy of linezolid in a staphylococcal endocarditis rabbit model. J. Antimicrob. Chemother. 47:349-352. [DOI] [PubMed] [Google Scholar]
- 22.Pai, M. P., K. A. Rodvold, P. C. Schreckenberger, R. D. Gonzales, J. M. Petrolatti, and J. P. Quinn. 2002. Risk factors associated with the development of infection with linezolid- and vancomycin-resistant Enterococcus faecium. Clin. Infect. Dis. 35:1269-1272. [DOI] [PubMed] [Google Scholar]
- 23.Rayner, C. R., A. Forrest, A. K. Meagher, M. C. Birmingham, and J. J. Schentag. 2003. Clinical pharmacodynamics of linezolid in seriously ill patients treated in a compassionate use programme. Clin. Pharmacokinet. 42:1411-1423. [DOI] [PubMed] [Google Scholar]
- 23a.Rayner, C. R., A. Forrest, A. K. Meagher, M. C. Birmingham, and J. J. Schentag. 2000. Population pharmacodynamics of linezolid in seriously-ill adult patients from a compassionate-use protocol. Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1390.
- 24.Roberts, S. M., A. F. Freeman, S. M. Harrington, S. M. Holland, P. R. Murray, and A. M. Zelazny. 2006. Linezolid-resistant Staphylococcus aureus in two pediatric patients receiving low-dose linezolid therapy. Pediatr. Infect. Dis. J. 25:562-564. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez, J. C., L. Cebrian, M. Lopez, M. Ruiz, I. Jimenez, and G. Royo. 2004. Mutant prevention concentration: comparison of fluoroquinolones and linezolid with Mycobacterium tuberculosis. J. Antimicrob. Chemother. 53:441-444. [DOI] [PubMed] [Google Scholar]
- 26.Rubinstein, E., S. Cammarata, T. Oliphant, and R. Wunderink. 2001. Linezolid (PNU-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multicenter study. Clin. Infect. Dis. 32:402-412. [DOI] [PubMed] [Google Scholar]
- 27.Rybak, M., G. Allen, and E. Hershberger. 2002. In vitro antibiotic pharmacodynamic models, p. 41-45. In C. H. Nightingale, T. Murukawa, and P. G. Ambrose (ed.), Antimicrobial pharmacodynamics in theory and clinical Practice. Marcel Dekker, Inc., New York, NY.
- 28.Stalker, D. J., G. L. Jungbluth, N. K. Hopkins, and D. H. Batts. 2003. Pharmacokinetics and tolerance of single- and multiple-dose oral or intravenous linezolid, an oxazolidinone antibiotic, in healthy volunteers. J. Antimicrob. Chemother. 51:1239-1246. [DOI] [PubMed] [Google Scholar]
- 29.Stevens, D. L., L. G. Smith, J. B. Bruss, M. A. McConnell-Martin, S. E. Duvall, W. M. Todd, and B. Hafkin. 2000. Randomized comparison of linezolid (PNU-100766) versus oxacillin-dicloxacillin for treatment of complicated skin and soft tissue infections. Antimicrob. Agents Chemother. 44:3408-3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsiodras, S., H. S. Gold, G. Sakoulas, G. M. Eliopoulos, C. Wennersten, L. Venkataraman, R. C. Moellering, and M. J. Ferraro. 2001. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 358:207-208. [DOI] [PubMed] [Google Scholar]