Abstract
Dalbavancin, a semisynthetic lipoglycopeptide being developed for the treatment of skin and skin structure infections (SSSIs), has a half-life of 5 to 7 days in humans and offers promise for a convenient weekly dosing regimen. We studied the in vitro bactericidal activity of dalbavancin against target organisms, using the concentrations that are maintained in human blood with the proposed dosage regimen. Dalbavancin minimal bactericidal concentrations (MBCs) were ≤0.5 μg/ml for eight staphylococcal isolates; and for six of these strains, including one vancomycin-intermediate Staphylococcus aureus (VISA) isolate, the MBCs were equal to or within 1 doubling dilution of the MIC. Dalbavancin MICs for all three Streptococcus pyogenes strains were 0.008 μg/ml, as were the MBCs for two of the isolates. In time-kill studies conducted with a different set of seven strains (two methicillin-susceptible S. aureus isolates, three methicillin-resistant S. aureus isolates, one VISA isolate, and one S. pyogenes isolate), all strains exhibited a ≥3-log10 decrease in their viable counts when they were exposed to ≥1 μg/ml of dalbavancin for 24 h. Resistance development studies by both direct selection (resistance frequency, <10−10) and serial passage failed to produce stable mutants with decreased susceptibility to dalbavancin. These observations suggest that dalbavancin will be an effective choice for the management of patients with SSSIs.
Dalbavancin is a novel semisynthetic lipoglycopeptide that is administered intravenously and that is currently under regulatory review for the treatment of complicated skin and skin structure infections (SSSIs) caused by gram-positive bacteria. Taking advantage of the dalbavancin half-life of 5 to 7 days, the proposed dosing regimen in humans, which consists of 1 g on day 1 and 0.5 g on day 8, was designed to maintain bactericidal concentrations of dalbavancin in blood over the entire 2-week treatment period. The maintenance of therapeutic levels throughout therapy should contribute to improved outcomes and should also reduce the likelihood of the emergence of resistance. The convenience of once-weekly intravenous infusion was another consideration in developing this regimen. While a number of studies have been conducted to establish dalbavancin's MIC profile, studies of its bactericidal activity, particularly at pharmacokinetically relevant concentrations, have been limited (9, 10, 12, 13, 18, 19). Using information on its pharmacokinetics and estimates of protein binding, we studied the bactericidal activity of dalbavancin against the main SSSI target organisms, Staphylococcus aureus and Streptococcus pyogenes (5, 7, 18), over a range of concentrations that included approximations of the levels of free drug present in human blood after infusion of a dose and 1 week later.
At the time that this study was initiated, the pharmacokinetic profile of dalbavancin in humans had been characterized. The concentrations of total drug of 20 to 40 μg/ml were present 7 days after a therapeutic dose, and peak concentrations at the end of infusion were >200 and >150 μg/ml for the first and second doses, respectively. In addition, dalbavancin is approximately 93% protein bound (2, 16).
MATERIALS AND METHODS
The staphylococcal and S. pyogenes isolates used in this study were selected as clinical strains of known resistance phenotypes (e.g., methicillin-resistant S. aureus [MRSA] and vancomycin-intermediate S. aureus [VISA]) from various hospitals in the United States. MICs, minimal bactericidal concentrations (MBCs), and time-kill profiles were determined according to Clinical and Laboratory Standards Institute (CLSI; formerly NCCLS) guidelines (14, 15). MICs and MBCs were determined with validated dry-form microtiter panels provided by TREK Diagnostics (8). Time-kill studies were performed by using plastic tubes and cation-adjusted Mueller-Hinton broth with 0.02% polysorbate-80 (P-80). Addition of P-80 prevents dalbavancin from sticking to plastic; and in broth microdilution MIC determinations by the CLSI methodology, P-80 has been shown to provide the same results obtained with dry-form panels, which do not require P-80 (1, 8). Broth microdilution determination of the MIC for dalbavancin by the CLSI methodology has now been standardized with a 0.002% final concentration of P-80 (4). For S. pyogenes, the medium was supplemented with 2 to 5% lysed horse blood. The starting inoculum was approximately 107 CFU/ml, and the concentrations of dalbavancin tested were chosen to reflect the calculated free levels of dalbavancin in plasma over a 1-week dosing interval (1, 4, and 16 μg/ml) as well as a lower concentration (0.25 μg/ml) which more closely reflected the MBCs for some strains. Estimates of free dalbavancin levels in blood were based on the extent of protein binding (93%) in human plasma and total human blood levels (≥200 and ca. 150 μg/ml after infusion of the first and second doses, respectively; >20 μg/ml at the end of each 1-week dosing interval) (2).
Direct selection and serial passage studies for the detection of resistance development were performed with one methicillin-susceptible S. aureus (MSSA) isolate, three MRSA isolates, one VISA isolate, and one methicillin-resistant Staphylococcus epidermidis isolate. The procedures described by Silverman et al. (17) were used as a guideline for these studies. To detect spontaneous mutants, an inoculum of approximately 1 × 109 to 1 × 1010 CFU was plated onto the surfaces of Mueller-Hinton agar plates containing dalbavancin at concentrations of 0.5×, 1×, 2×, 4×, and 8× the initial broth microdilution MIC. The dalbavancin MICs of any potential mutants growing on these selective plates were determined by broth microdilution.
The same staphylococcal strains were subjected to serial passage in the presence of sub-MICs of dalbavancin over 20 consecutive days by a broth microdilution method with dry-form panels. For each passage day the entire content of the dalbavancin microtiter well (100 μl) with the highest concentration of drug that allowed growth was used to generate the inoculum for a fresh microtiter plate. After 20 passages, the dalbavancin MICs of each strain were determined and compared with those obtained prior to the initiation of serial passage.
RESULTS AND DISCUSSION
The MICs and MBCs of dalbavancin, vancomycin, and teicoplanin for five S. aureus isolates, five coagulase-negative staphylococci, three S. pyogenes isolates, and two isolates each of Enterococcus faecalis and Enterococcus faecium are presented in Table 1. In every case, dalbavancin had lower MICs and MBCs than both vancomycin and teicoplanin, including those for a VISA strain and coagulase-negative staphylococcal isolates with reduced susceptibilities to vancomycin and/or teicoplanin. The dalbavancin MICs ranged from 0.03 to 0.12 μg/ml for 9 of 10 staphylococcal strains, and the dalbavancin MIC was 1 μg/ml for the VISA strain. Dalbavancin MBCs were ≤0.5 μg/ml for eight of the staphylococcal isolates; and for six strains, including the VISA strain, the dalbavancin MBCs were equal to or within 1 doubling dilution of the MIC. The dalbavancin MICs for all three S. pyogenes strains were 0.008 μg/ml, as were the MBCs for two of the isolates. The one S. pyogenes isolate that had the high dalbavancin MBC/MIC ratio also had high ratios with teicoplanin and vancomycin, which indicates that this particular strain may have some underlying physiology that allows it to be relatively refractory to this class of agents. Nonetheless, the dalbavancin MBC for this strain was well below the therapeutic levels of this drug. The MICs and MBCs of dalbavancin for this species were at least an order of magnitude lower than those of vancomycin. Against both the E. faecalis and the E. faecium strains, the MBC-to-MIC ratios for dalbavancin, teicoplanin, and vancomycin were all high. Although this group of agents is not considered effectively bactericidal against enterococci, the MBCs obtained with dalbavancin were lower than those obtained with either vancomycin or teicoplanin.
TABLE 1.
Comparison of minimal bactericidal concentrations of dalbavancin, vancomycin, and teicoplanin
| Isolate | Culture identifier | Phenotype | Dalbavancin
|
Teicoplanin
|
Vancomycin
|
|||
|---|---|---|---|---|---|---|---|---|
| MIC (μg/ml) | MBC (μg/ml) | MIC (μg/ml) | MBC (μg/ml) | MIC (μg/ml) | MBC (μg/ml) | |||
| S. aureus | FB892379 | Methicillin susceptible | 0.06 | 0.06 | 0.5 | 0.5 | 1 | 1 |
| S. aureus | FB1050986 | Methicillin susceptible | 0.06 | 1 | 1 | 4 | 1 | 2 |
| S. aureus | FB1051634 | Methicillin resistant | 0.06 | 0.06 | 0.5 | 0.5 | 1 | 1 |
| S. aureus | FB1065524 | Methicillin resistant | 0.06 | 0.5 | 1 | 1 | 1 | 1 |
| S. aureus | FB988644 | Vancomycin intermediate | 1 | 2 | 4 | 8 | 8 | 8 |
| S. epidermidis | FB758988 | Methicillin resistant | 0.06 | 0.06 | 2 | 4 | 2 | 2 |
| S. epidermidis | FB750345 | Methicillin resistant | 0.06 | 0.06 | 4 | 8 | 2 | 2 |
| S. epidermidis | FB896283 | Methicillin resistant | 0.03 | 0.03 | 8 | 32 | 2 | 2 |
| S. haemolyticus | FB953975 | 0.12 | 0.25 | 32 | >32 | 4 | 4 | |
| S. haemolyticus | FB954177 | 0.03 | 0.12 | 0.5 | 0.5 | 1 | >32 | |
| S. pyogenes | FB764371 | Erythromycin resistant | 0.008 | 0.008 | 0.03 | 0.03 | 0.5 | 0.5 |
| S. pyogenes | FB1041775 | Erythromycin susceptible | 0.008 | 0.25 | 0.06 | 0.5 | 0.5 | >2 |
| S. pyogenes | FB1041626 | Erythromycin susceptible | 0.008 | 0.008 | 0.06 | 0.06 | 0.5 | 0.5 |
| E. faecalis | FB766323 | Vancomycin susceptible | 0.03 | 8 | 0.25 | 16 | 1 | >32 |
| E. faecalis | FB766782 | Vancomycin susceptible | 0.06 | 4 | 0.25 | 16 | 1 | >32 |
| E. faecium | FB769334 | Vancomycin susceptible | 0.015 | 2 | ≤0.12 | 8 | 0.5 | 16 |
| E. faecium | FB767191 | Vancomycin susceptible | 0.03 | 2 | 0.25 | 16 | 2 | >32 |
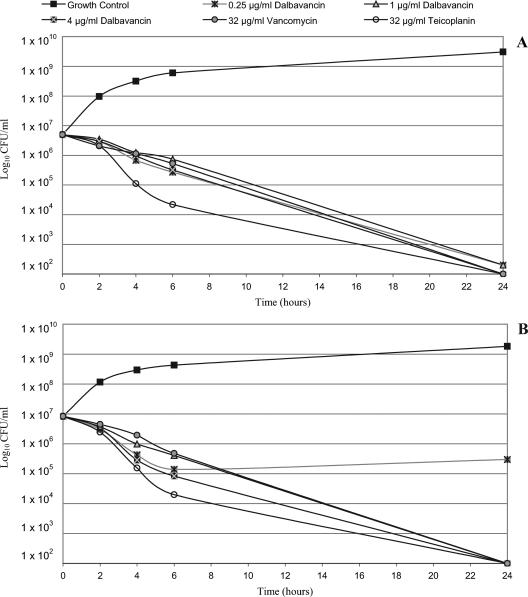
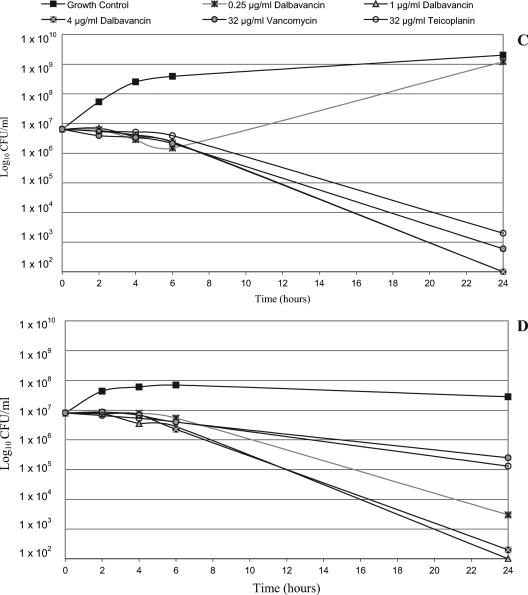
Time-kill studies were conducted with a different set of six strains of S. aureus (two MSSA strains, three MRSA strains, and one VISA strain) and one S. pyogenes strain. Regardless of phenotype, all strains exhibited a ≥3-log10 decrease in viable counts and, in most cases, exhibited about a 5-log10 decrease when they were exposed to ≥1 μg/ml of dalbavancin for 24 h. Two strains (one MSSA strain and one MRSA strain) were also killed to that extent by the lowest dalbavancin concentration tested, 0.25 μg/ml. The data for three of the S. aureus isolates (one isolate each of MSSA, MRSA, and VISA) are shown in Fig. 1A to C, respectively. No further killing was obtained by increasing the dalbavancin concentration to 4 μg/ml (Fig. 1) or even to 16 μg/ml (data not shown). At higher concentrations (8 and 32 μg/ml), a ≥3-log10 killing of all of the S. aureus isolates was also seen with vancomycin and teicoplanin, but in some cases the extent of killing was less than that achieved with 0.25 or 1 μg/ml of dalbavancin (data not shown). Dalbavancin also produced >3-log10 killing of S. pyogenes at 0.25 μg/ml and about a 5-log10 reduction at ≥1 μg/ml (Fig. 1D). Vancomycin and teicoplanin had notably poorer bactericidal activities against this organism, achieving <2-log10 killing. Thus, even when it was tested at very low concentrations, dalbavancin had more potent bactericidal activity than vancomycin and teicoplanin. These observations correlate with the findings of studies that demonstrated that lower and less frequent doses of dalbavancin were effective in animal infection models (3, 6). The strong bactericidal activity of dalbavancin at concentrations of free drug that are sustained in humans throughout the therapeutic interval (approximately 15 to 20 μg/ml after infusion and 1.5 to 3 μg/ml after 7 days) suggests a low potential for the selection of resistance in patients.
FIG. 1.
Time kill curves of dalbavancin versus S. aureus and S. pyogenes isolates. (A) Methicillin-susceptible S. aureus (strain FB1109397; dalbavancin MIC, 0.06 μg/ml; teicoplanin MIC, 1 μg/ml; vancomycin MIC, 1 μg/ml); (B) methicillin-resistant S. aureus (strain FB1109400; dalbavancin MIC, 0.06 μg/ml; teicoplanin MIC, 1 μg/ml; vancomycin MIC, 1 μg/ml); (C) vancomycin-intermediate S. aureus (strain Mu50 Japan; dalbavancin MIC, 0.5 μg/ml; teicoplanin MIC, 4 μg/ml; vancomycin MIC, 4 μg/ml); (D) S. pyogenes (strain FB1110016; dalbavancin MIC, 0.008 μg/ml; teicoplanin MIC, 0.03 μg/ml; vancomycin MIC, 0.5 μg/ml).
The potential for staphylococci to develop resistance to dalbavancin was studied by means of direct selection and by serial passage at subtherapeutic concentrations. In direct selection experiments with the six strains tested (one MSSA strain, three MRSA strains, one VISA strain, and one methicillin-resistant Staphylococcus epidermidis strain), no colonies with increased MICs were obtained on dalbavancin-containing plates (frequency, <10−10). The same six strains were passaged in the presence of subinhibitory concentrations of dalbavancin. After 20 passages, four strains (including the VISA strain) had a dalbavancin MIC that was within 1 doubling dilution of the MIC obtained prior to the 20 passages. For two MRSA strains, increases of 2 or 3 dilutions were seen (to 0.25 and 0.5 μg/ml). When these isolates were grown on drug-free medium for three consecutive days and retested, the MICs were equivalent or within 1 doubling dilution of the starting MICs.
This study was conducted to evaluate the bactericidal activity of dalbavancin over a range of concentrations of free drug that are present in human blood, as well as a lower concentration, and to assess the propensities of target organisms to develop resistance on exposure to this agent. Due to the bactericidal levels present throughout the dosing interval, the selection of secondary resistance in patients undergoing therapy with dalbavancin would seem unlikely. Also, it is thought that VISA strains may be selected as a result of the twice-daily occurrence of subtherapeutic trough levels of vancomycin in some patients; the effects of low but prolonged posttherapy levels could affect the susceptibilities of colonizing strains. In the present study, the results of both direct selection and serial passage experiments in vitro suggest a low potential for the selection of dalbavancin-resistant variants. Notably, increased MICs were not seen with a VISA strain. Similar results were previously reported from a more limited study with single strains of S. aureus and Staphylococcus haemolyticus (11, 13).
In summary, dalbavancin has potent in vitro bactericidal activity against staphylococci and S. pyogenes at concentrations that will be maintained in human blood over the entire dosing interval. Concentrations of free dalbavancin considerably higher than the MBCs are present throughout most of the dosing interval. The potent bactericidal activity of dalbavancin for target organisms and the low propensity for the selection of resistant variants in vitro, even with subtherapeutic concentrations, are desirable attributes for a new glycopeptide therapeutic agent.
Acknowledgments
This study was funded by Pfizer Inc, Pfizer Global Pharmaceuticals, New York, NY.
Footnotes
Published ahead of print on 12 January 2007.
REFERENCES
- 1.Anderegg, T. R., D. J. Biedenbach, and R. N. Jones. 2003. Initial quality control evaluations for susceptibility testing of dalbavancin (BI397), an investigational glycopeptide with potent gram-positive activity. J. Clin. Microbiol. 41:2795-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckwalter, M., and J. A. Dowell. 2005. Population pharmacokinetic analysis of dalbavancin, a novel lipoglycopeptide. J. Clin. Pharmacol. 45:1279-1287. [DOI] [PubMed] [Google Scholar]
- 3.Candiani, G., M. Abbondi, M. Borgonovi, G. Romano, and F. Parenti. 1999. In-vitro and in-vivo antibacterial activity of BI 397, a new semi-synthetic glycopeptide antibiotic. J. Antimicrob. Chemother. 44:179-192. [DOI] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing; sixteenth informational supplement, vol. 26, no. 3. M100-S16. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Dorr, M. B., D. Jabes, M. Cavaleri, J. Dowell, G. Mosconi, A. Malabarba, R. J. White, and T. J. Henkel. 2005. Human pharmacokinetics and rationale for once-weekly dosing of dalbavancin, a semi-synthetic glycopeptide. J. Antimicrob. Chemother. 55(Suppl. S2):ii25-ii30. [DOI] [PubMed] [Google Scholar]
- 6.Jabes, D., G. Candiani, G. Romano, C. Brunati, S. Riva, and M. Cavaleri. 2004. Efficacy of dalbavancin against methicillin-resistant Staphylococcus aureus in the rat granuloma pouch infection model. Antimicrob. Agents Chemother. 48:1118-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jauregui, L. E., S. Babazadeh, E. Seltzer, L. Goldberg, D. Krievins, M. Frederick, D. Krause, I. Satilovs, Z. Endzinas, J. Breaux, and W. O'Riordan. 2005. Randomized, double-blind comparison of once-weekly dalbavancin versus twice-daily linezolid therapy for the treatment of complicated skin and skin structure infections. Clin. Infect. Dis. 41:1407-1415. [DOI] [PubMed] [Google Scholar]
- 8.Jones, R. N., J. M. Streit, and T. R. Fritsche. 2004. Validation of commercial dry-form broth microdilution panels and test reproducibility for susceptibility testing of dalbavancin, a new very long-acting glycopeptide. Int. J. Antimicrob. Agents 23:197-199. [DOI] [PubMed] [Google Scholar]
- 9.Jones, R. N., M. G. Stilwell, H. S. Sader, T. R. Fritsche, and B. P. Goldstein. 2006. Spectrum and potency of dalbavancin tested against 3322 gram-positive cocci isolated in the United States Surveillance Program (2004). Diagn. Microbiol. Infect. Dis. 54:149-153. [DOI] [PubMed] [Google Scholar]
- 10.Jones, R. N., T. R. Fritsche, H. S. Sader, and B. P. Goldstein. 2005. Antimicrobial spectrum and potency of dalbavancin tested against clinical isolates from Europe and North America (2003): initial results from an international surveillance protocol. J. Chemother. 17:593-600. [DOI] [PubMed] [Google Scholar]
- 11.Lefort, A., J. Pavie, L. Garry, F. Chau, and B. Fantin. 2004. Activities of dalbavancin in vitro and in a rabbit model of experimental endocarditis due to Staphylococcus aureus with or without reduced susceptibility to vancomycin and teicoplanin. Antimicrob. Agents Chemother. 48:1061-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin, G., K. Credito, L. M. Ednie, and P. C. Appelbaum. 2005. Antistaphylococcal activity of dalbavancin, an experimental glycopeptide. Antimicrob. Agents Chemother. 49:770-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez, S., C. Hackbarth, G. Romano, J. Trias, D. Jabes, and B. P. Goldstein. 2005. In vitro antistaphylococcal activity of dalbavancin, a novel glycopeptide. J. Antimicrob. Chemother. 55(Suppl. 2):ii21-iii24. [DOI] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 6th ed., vol. 23, no. 2. Approved standard M7-A6. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 15.National Committee for Clinical Laboratory Standards. 1998. Methods for determining bactericidal activity of antimicrobial agents. Approved guideline M26-A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 16.Seltzer, E., M. B. Dorr, B. P. Goldstein, M. Perry, J. A. Dowell, T. Henkel, and the Dalbavancin Skin and Soft-Tissue Infection Study Group. 2003. Once-weekly dalbavancin versus standard-of-care antimicrobial regimens for treatment of skin and soft-tissue infections. Clin. Infect. Dis. 37:1298-1303. [DOI] [PubMed] [Google Scholar]
- 17.Silverman, J. A., N. Oliver, T. Andrew, and T. Li. 2001. Resistance studies with daptomycin. Antimicrob. Agents Chemother. 45:1799-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Streit, J. M., H. S. Sader, T. R. Fritsche, and R. N. Jones. 2005. Dalbavancin activity against selected populations of antimicrobial-resistant gram-positive pathogens. Diagn. Microbiol. Infect. Dis. 53:307-310. [DOI] [PubMed] [Google Scholar]
- 19.Streit, J. M., T. R. Fritsche, H. S. Sader, and R. N. Jones. 2004. Worldwide assessment of dalbavancin activity and spectrum against over 6,000 clinical isolates. Diagn. Microbiol. Infect. Dis. 48:137-143. [DOI] [PubMed] [Google Scholar]