Abstract
We have investigated the influences of gamma interferon (IFN-γ) and interleukin-12 (IL-12) on the efficacy of posaconazole (POS) treatment of acute experimental infections with Trypanosoma cruzi; the standard drug, benznidazole (BZ), was used as a positive control. Wild-type (WT) mice infected with T. cruzi and treated with POS or BZ had no parasitemia, 100% survival, and cure rates of 86 to 89%. IFN-γ-knockout (KO) mice infected with T. cruzi and treated with BZ controlled the infection during treatment but relapsed after the drug pressure ceased and had 0% survival, while those receiving POS better controlled the infection after the end of treatment and had 70% survival (P < 0.0001 compared to the results for both untreated and BZ-treated animals). IL-12-KO mice infected and treated with POS or BZ had intermediate results, displaying enhanced parasitemia, decreased survival (77 to 83%), and reduced cure rates (35 to 39%) compared with those of the WT animals. Our results demonstrate that either IFN-γ or IL-12 deficiency reduces the efficacy of POS or BZ in this experimental model but also indicate that the anti-T. cruzi activity of POS is much less dependent on the activity of IFN-γ than that of BZ is.
Chagas' disease, which is caused by the protozoan Trypanosoma cruzi, affects approximately 18 million inhabitants in Latin America (40). Substantial advances in measures that can be used to control disease have been made, e.g., by combating the triatomine vector with insecticides. Nevertheless, Chagas' disease still remains a public health problem. Only two drugs are clinically used for treatment of the disease, the nitroimidazole benznidazole (BZ) and the nitrofuran nifurtimox (NFX). Both drugs possess limited efficacy during the acute phase (76%) and the chronic phase (8%) (8). The presence of strains naturally resistant to BZ and NFX (12) may be an important factor that explains the low cure rates shown by some of the treated patients with Chagas' disease. However, little is known about the influences of the host parameters related to therapeutic failure. One of the major factors that may interfere with the efficacy of treatment of Chagas' disease is the host immunological response through cooperative action with the drug (4, 24, 31).
The host immunological response has been implicated in resistance to infection as a result of inhibition of parasite replication and modulation of the onset of T. cruzi infection (25, 27, 32). Interleukin-12 (IL-12) is an important cytokine of the innate protective mechanism that stimulates gamma interferon (IFN-γ) production by natural killer cells. IFN-γ is a cytokine that activates macrophages, enhancing their trypanocidal activities by increasing the levels of phagocytosis and nitric oxide production (14, 34). Moreover, these cytokines play important roles in immunological response differentiation (26). Mice treated with IL-12- and IFN-γ-neutralizing antibodies, as well as those with genetic deficiencies in such cytokines, are more susceptible to T. cruzi infection, showing high parasitemia and mortality levels, compared to those of wild-type (WT) mice (1, 18, 24). Importantly, chagasic patients infected with human immunodeficiency virus, patients with autoimmune diseases, or patients receiving immunosuppressive therapy posttransplantation present episodes of reactivation of the T. cruzi infection (10, 16, 21, 22). Indeed, the occurrence of meningoencephalitis, a rare though severe form of Chagas' disease, was observed in those patients (13).
A cooperative effect between drugs and the host immune system has been reported in the literature for parasitic diseases such as avian malaria (30), murine schistosomiasis (11), and canine visceral leishmaniasis (23). For Chagas' disease, immune system activation with recombinant IL-12 has been shown to enhance drug efficacy during BZ chemotherapy in experimental models (17). Likewise, the efficacy of BZ treatment in IFN-γ- and IL-12-knockout (KO) mice was shown to be reduced compared to that in other mice of similar genetic backgrounds but with intact immune systems (24). Furthermore, patients who were treated with BZ and NFX and cured have been shown to have IFN-γ levels higher than those in patients who were treated but not cured (3).
Novel antifungal triazole derivatives, developed for the treatment of invasive fungal infections, have arisen as alternative treatments for Chagas' disease. They inhibit T. cruzi ergosterol synthesis, which is fundamental for parasite growth and survival, and have pharmacokinetic properties suitable for the treatment of this disseminated intracellular infection. Experimentally, several triazole derivatives have been tested, including D0870 (19), posaconazole (POS) (20, 35), ravuconazole (39), albaconazole (15, 36), and TAK-187 (9, 38). In particular, POS (Schering-Plough Research Institute) has previously been shown to have potent in vitro and in vivo activities, inducing parasitological cure in mice with acute and chronic infections, including those caused by T. cruzi BZ-resistant strains (20). Therefore, POS has recently been proposed as a rational candidate for clinical trials with Chagas' disease patients (37). The present work was aimed at investigating the influences of the proinflammatory cytokines IL-12 and IFN-γ on the effectiveness of POS in providing a cure for acute T. cruzi infection in a murine model.
MATERIALS AND METHODS
Animals.
C57BL/6 mice with KO for IL-12p40 (IL-12 KO) or IFN-γ (IFN-γ KO), as well as C57BL/6 (WT) mice, obtained from the Departamento de Imunologia, Faculdade de Medicina de Ribeirão Preto (FMRP/USP, São Paulo, Brazil), were used in the present investigation. WT and KO male mice (age, 8 to 10 weeks) were infected with T. cruzi and kept under standard conditions with experimental isolation barriers at the animal house of the Centro de Pesquisa René Rachou in Belo Horizonte, state of Minas Gerais, Brazil. All the procedures that involved the use of animals in this study followed the ethical principles on the use of laboratory animals supplied by the Brazilian College of Animal Experimentation-Cobea and the Manual on the Care and Use of Laboratory Animals of the National Research Council (20a). Moreover, the study was committed to the principle of replacement, reduction, and refinement, the basic objective of which is the use of alternative methods with the use of animals, as well as the use of a technician whose aim is to diminish whenever possible the suffering and the number of animals necessary to perform the project.
Mice infection and parasitemia curve.
WT and KO mice were infected by intraperitoneal injection of 5,000 blood trypomastigote forms of the Y strain of T. cruzi (29), which had been kept in our laboratory by serial passage in Swiss-Webster mice. The presence of infection was confirmed 4 days postinfection (dpi) and was monitored to 60 dpi by examination of fresh blood collected from the tails of the mice. Parasite numbers were estimated daily from 4 to 15 dpi and thereafter every other day up to 60 dpi (5).
Treatment of infected mice.
At 4 dpi, WT and KO mice were treated orally with POS (20 mg/kg of body weight/day, divided into two daily doses) or BZ (100 mg/kg/day, given once a day) for 20 consecutive days. BZ was dissolved in water containing arabic gum, and POS was dissolved in 2% methylcellulose and 0.5% Tween 80. Nontreated, infected WT and KO mice were used as controls.
Hemoculture.
Hemoculture was used as a parasitological cure indicator. At 60 dpi, mice with no parasitemia, as determined by the observation of blood under an optical microscopy, were aseptically bled from the venous orbital sinus, and 0.5 ml of blood was collected from each mouse. Blood samples were distributed into two tubes containing 5 ml of liver infusion tryptose medium (7). The tubes were incubated for 30 to 60 days at 28°C and then microscopically examined for parasite detection.
Statistical analysis.
The means and standard deviations of the parasitemia levels were calculated with Microsoft Excel software (for Windows). Comparisons between parasitemia graphs regarding either the POS-treated or the BZ-treated mice were carried out by the Mann-Whitney test, a nonparametric method, in that the data were asymmetric. Comparison of the cure rates for the infected, treated mice was done by using the chi-square test and the Bonferroni method with the software package Minitab (Minitab Inc., State College, PA). Survival analysis was carried out by the nonparametric Kaplan-Meier method and by the log rank and Peto-Peto-Wilcoxon tests, implemented with the StatView 4.5 software package (Abacus Concepts, Berkeley, CA). Differences were considered significant when P was <0.05.
RESULTS
C57BL/6 mice infected with T. cruzi are highly responsive to posaconazole and benznidazole treatment.
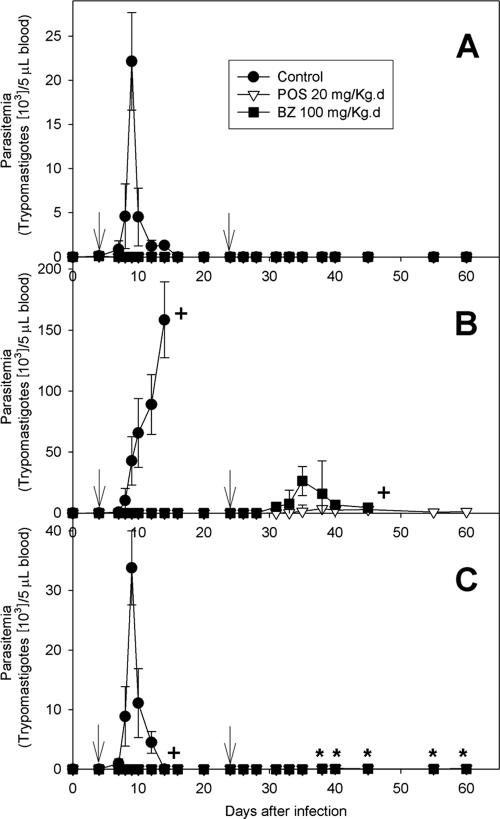
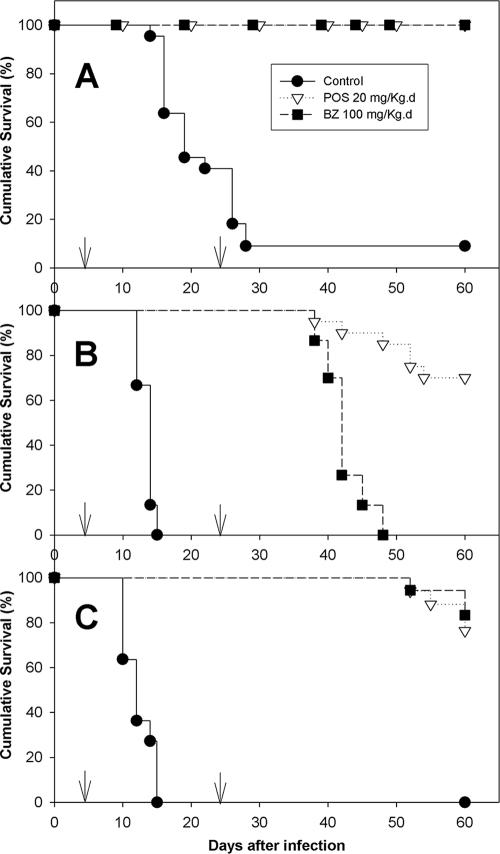
Figure 1A shows the mean parasitemia levels for nontreated (control) WT mice infected with the T. cruzi Y strain and WT mice infected with the T. cruzi Y strain and treated with BZ and POS. The control mice had patent parasitemia starting at 4 dpi; the peak was reached at 9 dpi and the parasitemia became undetectable by 16 dpi. Thereafter, rare circulating parasites appeared intermittently up to 60 dpi. POS- and BZ-treated mice showed subpatent parasitemia throughout the whole observation period. They were then submitted to hemoculture in order to estimate cure rates. Ninety-one percent of the infected nontreated WT mice were dead by 60 dpi (mean survival time, 21.2 ± 1.1 days), while all treated animals survived the observation period (Table 1 and Fig. 2A). Parasitological cure rates, determined by examination of fresh blood and hemoculture, were 0% for WT nontreated mice and 86 to 89% for WT mice treated with BZ or POS. There were significant differences in the parasitemia, mortality, and cure rates between the POS- and BZ-treated mice and nontreated mice (P < 0.05). No significant differences were observed between the POS- and BZ-treated mice.
FIG. 1.
Parasitemia levels of WT (A), IFN-γ-KO (B), and IL-12-KO (C) mice infected with the Y strain of Trypanosoma cruzi. Arrows indicate treatment period. +, death of the whole group mice; *, rare parasites were found in blood samples from treated mice. The results correspond to the averages of two independent experiments. Note the change in the vertical scale for panel B. For more details, see Material and Methods.
TABLE 1.
Parasitological cure and mortality rates and mean survival times of WT, IFN-γ-KO, and IL-12-KO mice infected with T. cruzi strain Y and treated with posaconazole and benznidazole
| Mice | Untreated
|
Benznidazole
|
Posaconazole
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mortality (no. of mice that died/total no. tested [%]) | Mean survival time (days) | Curea (no. of mice cured/total no. tested [%]) | Mortality (no. of mice that died/total no. tested [%]) | Mean survival time (days) | Cure (no. of mice cured/total no. tested [%]) | Mortality (no. of mice that died/total no. tested [%]) | Mean survival time (days) | Cure (no. of mice cured/total no. tested [%]) | |
| WT | 20/22 (91)f | 21.2 ± 1.1 | 0/22 (0) | 0/29 (0) | (>60) | 25/29 (86) | 0/18 (0) | >60 | 16/18 (89) |
| IFN-γ KO | 15/15 (100) | 13.5 ± 0.3b | 0/15 (0) | 15/15 (100) | (42.1 ± 0.8)c | 0/15 (0) | 6/20 (30) | 52.1 ± 1.1c,d,f | 3/20 (15) |
| IL-12 KO | 11/11 (100) | 12.3 ± 0.6b | 0/11 (0) | 3/18 (17) | (59.6 ± 0.6)e,f | 7/18 (39) | 4/17 (23) | 59.2 ± 0.6e,f | 6/17 (35) |
Proportion of the number of mice with negative parasitemia to the number of mice examined by fresh blood examination and hemoculture after chemotherapy.
P < 0.0001 compared with the results for the WT untreated control. All P values were determined by the log rank and Peto-Peto-Wilcoxon tests.
P < 0.0001 compared with the results for the IFN-KO untreated control.
P < 0.0001 compared with the results for the IFN-KO BZ-treated animals.
P < 0.0001 compared with the results for the IL-12-KO untreated control.
Biased results because the mean survival times were calculated assuming that the death (failure) time of the survivors was exactly 60 days. They provide a subestimation of the true mean survival times when the observation period is limited.
FIG. 2.
Survival levels of WT (A), IFN-γ KO (B), and IL-12 KO (C) mice infected with the Y strain of Trypanosoma cruzi. Arrows indicate treatment period. For more details, see Material and Methods.
IFN-γ-KO mice have increased susceptibility to T. cruzi infection and are significantly less responsive to benznidazole than to posaconazole.
T. cruzi-infected IFN-γ-KO mice were found to be highly susceptible to T. cruzi infection, with peak parasitemia levels for the untreated animals being eightfold higher than those for the WT animals (Fig. 1B), and had a shortened mean survival time (13.5 ± 0.3 days [P < 0.0001 compared with the results for the WT mice]). Treatment with BZ and POS still kept parasitemia levels subpatent during the treatment period (from 4 to 24 dpi). However, 2 days after the end of BZ treatment there was a reactivation of the infection and parasites appeared in the bloodstream, reaching a peak on day 11 after the end of treatment (Fig. 1B). Such reactivation was intense and was associated with 100% mortality of those animals by 48 dpi and a mean survival time of 42.1 ± 0.8 days, which was nevertheless significantly higher than that for the untreated controls (P < 0.0001) (Table 1). In contrast, infected IFN-γ-KO mice treated with POS had only scarce circulating parasites after the end of the treatment, and the parasitemia was significantly lower than that found in BZ-treated animals (P < 0.05). The first circulating parasites were observed in this group on day 7 after the end of treatment and persisted intermittently until day 36, but in 30% of these animals no circulating parasites were detected through the whole observation period of 60 days. Consistently, the cumulative mortality rate at 60 dpi was just 30% for POS-treated animals (Fig. 2B), with a mean survival time of 52.1 ± 1.1 days (P < 0.0001 compared with the results for both untreated control and BZ-treated animals) (Table 1). The parasitological cure rates were 0% for BZ-treated IFN-γ-KO mice and 15% for POS-treated mice, which were significantly lower than those observed for the WT animals.
IL-12-KO mice have increased susceptibility to T. cruzi infection but are responsive to both POS and BZ treatment.
Figure 1C shows that T. cruzi-infected and nontreated IL-12-KO mice exhibited a peak parasitemia at 9 dpi. The peak parasitemia was only slightly higher than that for the WT mice (Fig. 1A) and was significantly lower than that observed for IFN-γ KO mice (Fig. 1B). The levels of parasitemia for T. cruzi-infected IL-12-KO mice treated with either POS or BZ were subpatent during the whole treatment period (Fig. 1C), but low levels of circulating parasites appeared 14 days after the end of treatment with both drugs and persisted intermittently throughout the rest of the observation period. The cumulative rates of mortality at 60 dpi were 100% for IL-12-KO nontreated mice, 17% for BZ-treated mice, and 23% for POS-treated mice (Fig. 2C), with mean survival times of 12.3 ± 0.6, 59.6 ± 0.6, and 59.2 ± 0.6 days, respectively (Table 1). No significant differences in survival were observed among the IL-12-KO mice treated with either drug (P > 0.5), but both treated groups had significantly higher rates of survival than the untreated animals (P < 0.001). The cure rates for the IL-12-KO infected mice were 0% for the nontreated mice, 39% for the BZ-treated mice, and 35% for the POS-treated mice (Table 1); no significant differences in cure rates were observed between the BZ- and POS-treated groups.
DISCUSSION
Previous investigations with humans and experimental models have demonstrated the importance of the host immune system in the efficacy of chemotherapeutic treatments against parasitic diseases (31, 4, 24). In the present work, we investigated the effects of IFN-γ and IL-12 deprivation on the antiparasitic activities of POS and BZ in a murine model of acute Chagas' disease. Our results demonstrate the substantial participation of an intact immune system in the antiparasitic activities of both drugs.
Nowadays, BZ is the only available drug used for the chemotherapy of Chagas' disease in Brazil. It provides up to 76% cure rates in acute-phase infections but only an 8% cure rate during the chronic phase of the disease (8). Previous work has shown that the efficacy of BZ is markedly reduced in immunosuppressed mice (33, 24). Although the mechanism of action of BZ has not been clearly established yet, it apparently involves reductive stress (37); however, the reduction of its activity in immunosuppressed mice also points toward a direct role of the host immune system in clearing the infection. The possibility that POS will become an alternative for the treatment of immunosuppressed individuals has been suggested (20, 37). POS treatment of cyclophosphamide-immunosuppressed mice infected with BZ-susceptible and BZ-resistant T. cruzi strains showed trypanocidal activity similar to that observed in mice with intact immunological systems (20). Furthermore, the cure rates achieved with POS were higher than those obtained with BZ in the same experimental model.
The results of the present work confirm that both POS and BZ are very effective in increasing the rate of survival and inducing parasitological cure in WT mice with acute T. cruzi infections, but it also showed that the antiparasitic activities of both drugs are reduced in IFN-γ-KO or IL-12-KO mice. However, the activity of POS was found to be significantly less dependent on IFN-γ than that of BZ was (Fig. 1B and 2B and Table 1). Several investigations have emphasized the importance of IFN-γ and IL-12 in natural resistance to T. cruzi infection. They have also been implicated in the response to BZ treatment in murine models (17, 24) and in humans (3). IFN-γ plays a major role in natural resistance to T. cruzi infection by activating macrophages and driving T-helper-cell differentiation during T. cruzi infection (6, 14, 26, 34). In this work we found that infected nontreated IFN-γ-KO mice are much more susceptible to T. cruzi infection than WT mice, confirming previous results (18). Although we observed that in IFN-γ-deficient animals both drugs were able to suppress parasite proliferation during the treatment interval, it reactivated after the drug pressure ceased, indicating that the drugs had trypanostatic rather than trypanocidal activities under these conditions. Nevertheless, the observed reactivation of the infection was more severe in BZ-treated animals than in those that received POS, leading to a higher rate of survival of the latter group. This could have been due to a more effective reduction of the parasite load by POS or to different outcomes of the combined effects of the drug's action with the macrophage's killing mechanisms. The reactivation of subpatent infection in immunosuppressed Chagas' disease patients has also been reported to be a consequence of secondary diseases or other clinical conditions that lead to immunosuppression, such as human immunodeficiency virus infection, the immunosuppressive treatment administered to transplant patients, and autoimmune diseases (13, 16).
IL-12-KO T. cruzi-infected mice were more resistant to T. cruzi infection than IFN-γ-KO ones: untreated IL-12-KO mice had peak parasitemia levels comparable to those of WT animals, and the parasitemia reactivation after BZ or POS treatment was much milder in IL-12-KO mice than in the IFN-γ-KO animals (Fig. 1C). Previous studies have reported that IL-12 deficiency increases parasitemia and mortality rates in acutely infected mice (2, 28). According to Silva et al. (28), T. cruzi-infected IL-12p40-KO mice present an intense tissue parasitism, the absence of inflammatory infiltrates, and the defective production of IFN-γ by splenocytes. In the present investigation, it was found that IL-12-KO infected mice, in contrast to the IFN-γ-KO animals, were responsive to both POS and BZ treatments, which led to high rates of survival in both treated groups (Fig. 2C and Table 1); however, the cure rates achieved in those animals were much lower than those observed in WT animals, showing that IL-12 is probably essential for clearance of the infection.
In conclusion, the present results suggest that the effectiveness of POS and BZ for the treatment of T. cruzi infection relies not only on the direct trypanocidal effect but also on a cooperative effect with the host immune system, hereby emphasized by the essential roles of IL-12 and IFN-γ. Importantly, we found that POS was more efficient, although not totally independently, than BZ for the treatment of T. cruzi infection in IFN-γ-deficient mice.
Acknowledgments
This work received financial support from the Conselho Nacional de Pesquisas (CNPq, Brazil), Fundação Oswaldo Cruz (Brazil), and the Howard Hughes Medical Institute (Chevy Chase, Maryland; grant 55000620 to J.A.U.).
We thank João Santana Silva for providing the IL-12- and IFN-γ-KO mice.
Footnotes
Published ahead of print on 12 January 2007.
REFERENCES
- 1.Abrahamsohn, I. A., and R. Coffman. 1995. Cytokine and nitric oxide regulation of the immunosuppresion in Trypanosoma cruzi infection. J. Immunol. 155:3955-3963. [PubMed] [Google Scholar]
- 2.Aliberti, J. C. S., M. A. G. Cardoso, G. A. Martins, R. T. Gazzinelli, L. Q. Vieira, and J. S. Silva. 1996. Interleukin-12 mediates resistance to Trypanosoma cruzi in mice and is produced by murine macrophages in response to live trypomastigotes. Infect. Immun. 64:1961-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahia-Oliveira, L. M. G., J. A. S. Gomes, J. R. Cançado, T. C. Ferrari, E. M. Lemos, Z. M. P. Luz, M. C. Moreira, G. Gazzinelli, and R. Corrêa-Oliveira. 2000. Immunological and clinical evaluation of chagasic patients subjected to chemotherapy during the acute phase of Trypanosoma cruzi infection 14-30 years ago. J. Infect. Dis. 182:634-638. [DOI] [PubMed] [Google Scholar]
- 4.Berger, B. J., and A. H. Fairlamb. 1992. Interactions between immunity and chemotherapy in the treatment of trypanosomiases and leishmaniases. Parasitology 105:S71-S78. [DOI] [PubMed] [Google Scholar]
- 5.Brener, Z. 1962. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev. Inst. Med. Trop. Sao Paulo 4:389-396. [PubMed] [Google Scholar]
- 6.Brener, Z., and R. T. Gazzinelli. 1997. Immunological control of Trypanosoma cruzi infection and pathogenesis of Chagas' disease. Int. Arch. Allergy Immunol. 114:103-110. [DOI] [PubMed] [Google Scholar]
- 7.Camargo, E. P. 1964. Growth and differentiation in Trypanosoma cruzi. I. Origin of metacyclic trypanosome in liquid media. Rev. Inst. Med. Trop. Sao Paulo 6:93-100. [PubMed] [Google Scholar]
- 8.Cançado, J. R. 2002. Long term evaluation of etiological treatment of Chagas disease with Benznidazole. Rev. Inst. Med. Trop. Sao Paulo 44:29-37. [PubMed] [Google Scholar]
- 9.Corrales, M., R. Cardozo, M. A. Segura, J. A. Urbina, and M. A. Basombrio. 2005. Comparative efficacies of TAK-187, a long-lasting ergosterol biosynthesis inhibitor, and benznidazole in preventing cardiac damage in a murine model of Chagas' disease. Antimicrob. Agents Chemother. 49:1556-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Ávila, S. C., A. M. D'Ávila, C. Pagliari, V. M. Gonçalves, and M. I. Duarte. 2005. Erythema nodoso in reactivation of Chagas' disease after cardiac transplantation. Rev. Soc. Bras. Med. Trop. 38:61-63. [DOI] [PubMed] [Google Scholar]
- 11.Doenhoff, M. J., A. A. A. Sabah, C. Fletcher, G. Webbe, and J. Bain. 1987. Evidence for an immune-dependent action of praziquantel on Schistosoma mansoni in mice. Trans. R. Trop. Med. Hyg. 81:947-951. [DOI] [PubMed] [Google Scholar]
- 12.Filardi, L. S., and Z. Brener. 1987. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans. R. Soc. Trop. Med. Hyg. 81:755-759. [DOI] [PubMed] [Google Scholar]
- 13.Galhardo, M. C. G., I. A. Martins, A. H. Moreno, S. S. Xavier, J. M. C. Coelho, A. C. V. Vasconcelos, and R. S. Ribeiro. 1999. Reativação da infecção por Trypanosoma cruzi em paciente com síndrome da imunodeficiência adquirida. Rev. Soc. Bras. Med. Trop. 32:291-294. [DOI] [PubMed] [Google Scholar]
- 14.Gazzinelli, R. T., I. P. Oswald, S. Hieny, S. L. James, and A. Sher. 1992. The microbicidal activity of interferon-gamma-treated macrophages against Trypanosoma cruzi involves an l-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-beta. Eur. J. Immunol. 22:2501-2506. [DOI] [PubMed] [Google Scholar]
- 15.Guedes, P. M., J. A. Urbina, M. de Lana, L. C. Afonso, V. M. Velosos, W. L. Tafuri, G. L. Machado-Coelho, E. Chiari, and M. T. Bahia. 2004. Activity of new triazole derivate albaconazole against Trypanosoma (Schizotrypanum) cruzi in dog host. Antimicrob. Agents Chemother. 48:4286-4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madalosso, G., A. C. Pellini, M. J. Vasconcelos, A. F. Ribeiro, L. Weissmann, G. S. Oliveira Filho, A. C. Penalva de Oliveira, and J. E. Vidal. 2004. Chagasic meningoencephalitis: case reported of a recently included AIDS-defining illness in Brazil. Rev. Inst. Med. Trop. Sao Paulo 46:199-202. [DOI] [PubMed] [Google Scholar]
- 17.Michailowsky, V., S. M. F. Murta, L. Carvalho-Oliveira, M. E. S. Pereira, L. R. P. Ferreira, Z. Brener, A. J. Romanha, and R. T. Gazzinelli. 1998. Interleukin-12 enhances in vivo parasiticidal effect of benznidazole during acute experimental infection with a naturally drug-resistant strain of Trypanosoma cruzi. Antimicrob. Agents Chemother. 42:2549-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michailowsky, V., N. M. Silva, C. D. Rocha, L. Q. Vieira, J. Lannes-Vieira, and R. T. Gazzinelli. 2001. Pivotal role of interleukin-12 and interferon-gamma axis in controlling tissue parasitism and inflammation in the heart and central system during Trypanosoma cruzi infection. Am. J. Pathol. 159:1723-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molina, J., Z. Brener, A. J. Romanha, and J. A. Urbina. 2000. In vivo activity of the bis-triazole D0870 against drug-susceptible and drug-resistant strains of the protozoan parasite Trypanosoma cruzi. J. Antimicrob. Chemother. 46:137-140. [DOI] [PubMed] [Google Scholar]
- 20.Molina, J., O. Martins-Filho, Z. Brener, A. J. Romanha, D. Loebenberg, and J. A. Urbina. 2000. Activities of the triazole derivate SCH 56592 (posaconazole) against drug-resistant strains of the protozoan parasite Trypanosoma (Schizotrypanum) cruzi in immunocompetent and immunosuppressed murine hosts. Antimicrob. Agents Chemother. 44:150-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.National Research Council. 2003. Manual on the care and use of laboratory animals. National Academy Press, Washington, DC.
- 21.Rassi, A., V. Amato Neto, A. F. Siqueira, F. F. Filho, V. S. Amato, G. G. Rassi, and A. Rassi, Jr. 2002. Tratamento da fase crônica da doença de Chagas com nifurtimox associado a corticóide. Rev. Soc. Bras. Med. Trop. 35:547-550. [DOI] [PubMed] [Google Scholar]
- 22.Rassi, A., V. Amato Neto, A. F. Siqueira, F. F. Filho, V. S. Amato, and A. Rassi Jr. 1999. Efeito protetor do benznidazol contra a reativação parasitária em pacientes cronicamente infectados pelo Trypanosoma cruzi e tratados com corticóides em virtude de afecções secundárias. Rev. Soc. Bras. Med. Trop. 32:475-482. [DOI] [PubMed] [Google Scholar]
- 23.Rhalem, A., H. Sahibi, S. Lasri, and C. L. Jaffe. 1999. Analysis of immune responses in dogs with canine visceral leishmaniosis before, and after, drug treatment. Vet. Immunol. Immunopathol. 71:69-76. [DOI] [PubMed] [Google Scholar]
- 24.Romanha, A. J., R. O. Alves, S. M. F. Murta, J. S. Silva, C. Ropert, and R. T. Gazzinelli. 2002. Experimental chemotherapy against Trypanosoma cruzi infection: role of endogenous interferon-γ in mediating parasitologic cure. J. Infect. Dis. 186:823-828. [DOI] [PubMed] [Google Scholar]
- 25.Rottenberg, M. E., A. Riarte, L. Sporrong, J. Altcheh, P. Petray, A. M. Ruiz, H. Wigzell, and A. Orn. 1995. Outcome of infection with differents strains of Trypanosoma cruzi in mice lacking CD4 and/or CD8. Immunol. Lett. 45:53-60. [DOI] [PubMed] [Google Scholar]
- 26.Seder, R. A., R. Gazzinelli, A. Sher, and W. E. Paul. 1993. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc. Natl. Acad. Sci. USA 90:10188-10192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva, A. P. G., J. F. Jacysyn, and I. A. Abrahamsohn. 2003. Resistant mice lacking interleukin-12 become susceptible to Trypanosoma cruzi infection but fail to mount a T helper type 2 response. Immunity 108:230-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silva, J. S., J. C. S. Aliberti, G. A. Martins, M. A. Souza, J. T. Souto, and M. A. Pádua. 1998. The role of IL-12 in experimental Trypanosoma cruzi infection. Braz. J. Med. Biol. Res. 31:111-115. [DOI] [PubMed] [Google Scholar]
- 29.Silva, L. H. P., and V. Nussenzweigh. 1953. Sobre uma cepa de Trypanosoma cruzi altamente virulenta para o camundongo branco. Folia Clin. Biol. 20:191-208. [Google Scholar]
- 30.Taliaferro, W. H. 1948. The role of the spleen and lymphoid macrophage system in the quinine treatment of Gallinaceum malaria. I. Acquired immunity and phagocytosis. J. Infect. Dis. 83:164-180. [DOI] [PubMed] [Google Scholar]
- 31.Targett, G. A. 1985. Chemotherapy and the immune response in parasitic infections. Parasitology 90:661-673. [DOI] [PubMed] [Google Scholar]
- 32.Tarleton, R. L., B. H. Koller, A. Latour, and M. Postan. 1992. Susceptibility of beta 2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature 356:338-340. [DOI] [PubMed] [Google Scholar]
- 33.Toledo, M. J. O., G. B. N. Machado, M. E. S. Pereira, and Z. Brener. 1991. Results of treatment in mice immunossupressed inoculated with different Trypanosoma cruzi strains. Mem. Inst. Oswaldo Cru z 836:237. [Google Scholar]
- 34.Torrico, F., H. Heremans, M. T. Rivera, E. van Marck, A. Billiau, and Y. Carlier. 1991. Endogenous IFN-γ is required for resistance to acute Trypanosoma cruzi infection in mice. J. Immunol. 146:3626-3632. [PubMed] [Google Scholar]
- 35.Urbina, J. A., G. Payares, C. Sanoja, J. Molina, M. M. Piras, R. Piras, N. Perez, P. Wincker, and D. Loebenberg. 1998. Antiproliferative effects and mechanism of action of SCH 56592 against Trypanosoma (Schizotrypanum) cruzi: in vitro and in vivo studies. Antimicrob. Agents Chemother. 42:1771-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urbina, J. A., R. Lira, G. Visbal, and J. Bartrolí. 2000. In vitro antiproliferative effects and mechanism of action of the new triazole derivative UR-9825 against the protozoan parasite Trypanosoma (Schizotrypanum) cruzi. Antimicrob. Agents Chemother. 44:2498-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urbina, J. A., and R. Docampo. 2003. Specific chemotherapy of Chagas disease: controversies and advances. Trends Parasitol. 19:495-500. [DOI] [PubMed] [Google Scholar]
- 38.Urbina, J. A., R. Lira, Z. Brener, and A. J. Romanha. 2003. Parasitological cure of acute and chronic experimental Chagas disease using the long-acting experimental triazole TAK-187. Activity against drug-resistant Trypanosoma cruzi strains. Int. J. Antimicrob. Agents 21:39-48. [DOI] [PubMed] [Google Scholar]
- 39.Urbina, J. A., G. Payares, C. Sanoja, R. Lira, and A. J. Romanha. 2003. In vitro and in vivo activities of ravuconazole on Trypanosoma cruzi, the causative agent of Chagas disease. Int. J. Antimicrob. Agents 21:27-38. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization. 2002. Chagas disease. Tropical disease research, p. 112-123. In Eighteenth programme report of UDNPD/World Bank/WHO Special Programme for Research and Training in Tropical Disease Research: progress 1995-1996. World Health Organization, Geneva, Switzerland.