Abstract
Coagulase-negative staphylococci (CoNS) and Candida are among the most common causes of single infections and coinfections in neonates after 72 h of age. In neonates, coinfection increases the rate of mortality threefold and results in significantly greater morbidity compared to those that result from single infections. In an effort to better understand this phenomenon, we developed the first neonatal animal model of coinfection (with CoNS and Candida) and evaluated its effects on mortality and morbidity and the impact of antifungal prophylaxis with fluconazole. Neonatal Wistar rats were infected with Candida albicans and/or Staphylococcus epidermidis with doses of 2 × 108 and 2 × 106 CFU subcutaneously in different combinations and were monitored for mortality, weight gain, and bacteremia. The in vitro sensitivity of C. albicans to fluconazole was evaluated and the MIC was determined. A subset of rats in these experiments received fluconazole at 10 mg/kg of body weight/dose intraperitoneally starting 24 h before infection for 4 days, and the serum trough levels of fluconazole were measured. Coinfection in the suckling rat significantly increased the rate of mortality compared to that after infection with a single species (P < 0.001) and resulted in deaths even at sublethal doses. Coinfection also impaired weight gain significantly in severely infected pups compared to that achieved after infection with a single species (P < 0.001). Fluconazole prophylaxis significantly reduced mortality by 30% in the Candida group and 36% in the coinfection group and improved weight gain in this neonatal model of coinfection (P < 0.001). We developed a neonatal model of coinfection with Candida and CoNS, observed significantly greater mortality and morbidity with coinfection, and found that fluconazole prophylaxis significantly reduced the rates of both mortality and morbidity. Further research on neonatal coinfection is urgently needed to improve clinical outcomes.
Sepsis is a major cause of neonatal mortality and morbidity (21). “All-cause mortality” is approximately 18% in very-low-birth-weight infants with late-onset (after 72 h of age) sepsis. In addition, neonatal late-onset sepsis is associated with an increased incidence of cerebral palsy in premature infants, even after adjustment for gestational age (32); an increased length of hospital stay; and a significant risk of chronic lung disease (34). In a large cohort study of 6,093 extremely-low-birth-weight infants, infants who were infected had significantly adverse neurodevelopmental outcomes at follow-up, characterized by cerebral palsy, low Bayley scores, and vision impairment compared with the outcomes for infants who were not infected (35).
The most common causative organisms of late-onset sepsis in very-low-birth-weight infants are coagulase-negative staphylococci (CoNS) (∼48%), Staphylococcus aureus (∼7%), and Candida albicans (∼6%) (34). The clinical risk factors for neonatal Candida or CoNS infection appear to be similar (9, 30; M. P. Venkatesh, M. Fein, A. Weyand, L. Kong, and L. E. Weisman, Pediatr. Acad. Soc. Annu. Meet., abstr. 625, 2005), and frequently these organisms coexist. Concurrent infection with two or more organisms (coinfection or polymicrobial sepsis) is found in 4 to 24% of all neonatal infections (12, 34). Candida infection with CoNS or enterococci infection is the most common coinfection reported in neonates (18). Thirty percent of infants with Candida sepsis also have a concurrent bacteremia (17, 18). Such coinfections appear to significantly impair the clinical outcome. Several authors have reported that infants with concurrent fungemia and bacteremia have a higher incidence of endocarditis than infants with fungemia alone (10, 29). In addition, mortality due to infections with multiple organisms is significantly greater than that due to infections with a single species of organism (70% versus 23%) (11).
To date, research on coinfection in the neonate has been entirely observational. Thus, there is an urgent need for research to better understand coinfection and develop effective strategies for treatment and prevention. Coinfection with S. aureus and Candida in an adult mouse model has been studied and reported (6-8), but we are not aware of any studies of coinfection with CoNS and Candida in a neonatal animal model. Therefore, we designed a model of coinfection with CoNS and Candida in neonatal suckling rats and evaluated their mortality and morbidity. In addition, we investigated the impact of antifungal prophylaxis with fluconazole in this coinfection model.
MATERIALS AND METHODS
Organisms.
Candida albicans (strain ATCC 32354) was plated on Sabouraud dextrose agar for 48 to 72 h and then inoculated in 62.5 ml of glucose yeast extract peptone (GYEP) broth and incubated at 37°C for 24 h. The optical density at 540 nm was adjusted to 1.8 by adding more broth, and the strain was further incubated at 37°C for 6 h. The suspension was washed twice at 3,000 rpm for 10 min with normal saline, and the final solution was adjusted to an optical density at 540 nm of 1.9. This resulted in a concentration of approximately 108 CFU/ml (range, 0.5 × 107 to 0.5× 108 CFU/ml), as determined by previous growth curves. The suspension was then concentrated or diluted appropriately to give the desired concentrations. Serial quantitative cultures confirmed the final concentration.
Staphylococcus epidermidis (strain Hay, ATCC 55133) was plated on blood agar plates for 24 h and inoculated in 25 ml of Trypticase soy broth and incubated at 37°C for 2 h. The suspension was washed twice at 3,000 rpm for 10 min with normal saline. The optical density at 650 nm of the suspension was adjusted to 0.8, which results in a concentration of approximately 108 CFU/ml (range, 0.5 × 107 to 0.5× 108 CFU/ml), as determined by previous growth curves. This suspension was then diluted or concentrated to give the desired concentrations. Serial quantitative cultures confirmed the final concentration.
Animals.
Wistar rats that were timed to have been pregnant for 14 days were obtained from Charles River Laboratories Inc. and received antibiotic-free water and food ad libitum. Each dam delivered approximately 10 pups at 21 to 22 days of gestation. The pups were kept with their dams throughout the experiment. One- to 2-day-old pups (day 1 of study) received 0.2 ml of 20% intralipid solution intraperitoneally as two doses on the first day of the study 4 h apart and as one dose each on day 2 and day 3 of the study. The intralipid suspension was administered to obtain an immunosuppressant effect, probably effected through reticuloendothelial cell system blockade. Littermate pups were randomly assigned to different groups and were infected subcutaneously with 0.2 ml of 109 CFU/ml (dose of 2 × 108 CFU, which is the 50% lethal dose) or 107 CFU/ml (dose of 2 × 106 CFU, which is a sublethal dose) of S. epidermidis or C. albicans, or both, in different combinations on day 2 and day 3 of the study. The doses of infection were given at different subcutaneous sites cephalad to the tail. Control animals received the same intralipid doses as other animals and 0.2 ml of normal saline subcutaneously instead of organisms.
In subsequent experiments, fluconazole or placebo was given intraperitoneally (i.p.) at 10 mg/kg of body weight/day for 4 days, beginning 24 h before infection. Littermate pups were randomly assigned to different treatment groups and were infected subcutaneously with 0.2 ml of 109 CFU/ml (dose of 2 × 108 CFU, which is the 50% lethal dose) of C. albicans alone or with 107 CFU/ml of S. epidermidis (dose of 2 × 106 CFU) on day 2 and day 3 of the study. The doses of infection were given at different subcutaneous sites cephalad to the tail. Control animals received the same intralipid doses as other animals and 0.2 ml of normal saline subcutaneously instead of organisms. The Animal Use Committee at the Baylor College of Medicine reviewed and approved this project.
Survival, weight gain, and bacteremia.
Animal survival and nonedematous weight were monitored daily for 9 days, which was based on our previous observations of no mortality after 9 days. Quantitative blood samples for culture were obtained from the tail vein of random animals on day 4 of the study (24 h after the second dose of the infection). Ten microliters of blood was obtained from the tail vein of each pup of randomly selected litters, serially diluted, and plated on blood agar plates. After incubation at 37°C for 18 h, the colonies on each plate were counted and the cultures were expressed as the number of CFU/ml.
Serum fluconazole assay.
Serum trough levels of fluconazole were determined at 24, 48, 72, 96, and 144 h of the experiment. This correlates to the times just prior to the second, third, and fourth doses and 24 and 48 h after the fourth dose of fluconazole, respectively. For each time point, 12 to 15 rats were euthanized and blood from 3 rats was pooled as one sample, giving us four to five datum points for each time point. The blood samples were obtained by cardiac puncture, whole blood was centrifuged at 3,000 rpm for 10 min, and serum samples were stored at −70°C and assayed in one batch. The fluconazole assay was performed at the Fungus Testing Laboratory at the University of Texas Health Sciences at San Antonio by gas-liquid chromatography (16). Serum standards and controls were analyzed along with the study samples. The extraction recovery rate is approximately 86 to 93% for fluconazole. The assay is linear from 0.2 to 200 μg/ml, with the lowest limit of detection being 0.2 μg/ml by nonweighted linear regression analysis. For the controls, with doses of 2, 12.5, and 30 μg/ml, the interday coefficients of variation were 8.73%, 4.19%, and 5.91%, respectively, and the intraday coefficients of variation were 8.04%, 3.42%, and 1.87%, respectively.
Fluconazole MIC for C. albicans (ATCC 32354).
The fluconazole MICs were determined by the broth microdilution method, as described by the Clinical and Laboratory Standards Institute (formerly NCCLS) guidelines in the M27-A2 standard (28). A fluconazole stock solution (2 mg/ml; manufactured by Ben Venue Labs Inc., Bedford, OH, for Bedford Labs, Bedford, OH) was obtained and appropriately diluted to give twofold dilutions starting from 125-μg/ml to 0.25-μg/ml concentrations. C. albicans was grown from stored vials on Sabouraud dextrose agar plates for 24 h. Five colonies of approximately 1 mm in diameter were picked up and incubated in GYEP broth for 4 to 6 h. This mixture was centrifuged; the sediment was constituted in normal saline; and the optical density at 540 nm was adjusted to 0.6, which corresponds to 107 CFU/ml. Concentrations of 1 to 5 × 103 CFU/ml of the organisms were obtained by appropriate dilutions with RPMI 1640 medium without bicarbonate buffered with 3-(N-morpholino)propanesulfonic acid to a pH of 7 at 25°C. One hundred microliters of this suspension was added to 100 μl of serial dilutions of fluconazole in 96-well microtiter plate. The microtiter plates were incubated at 35°C for 48 h. The MIC endpoint was defined as the lowest concentration of fluconazole at which a prominent decrease (defined as ≥50%) in turbidity was noted (28). The plates were read visually at 24 and 48 h, and any trailing growth at 48 h was assumed to be fluconazole susceptible. Drug-free medium with organisms (positive control) and organism-free medium with fluconazole (negative control) were used for comparison.
Statistical analyses.
The survival data were summarized and tabulated. Survival data were assessed for differences between the groups by the chi-square test and between two individual groups by the two-sided Fisher exact test. Nonedematous weight curves were constructed for individual suckling rats, and their slopes were calculated. The means of the slopes of the weight curves for the different groups were compared by one-way analysis of variance. Statistical significance was assumed at a P value of ≤0.05. The statistical packages SPSS (version 12) and Graph Pad Prism (version 4) were used for statistical analyses.
RESULTS
Survival in coinfection model.
The rate of survival after coinfection with both organisms at a dose of 2 × 108 CFU was significantly lower than that achieved after infection with either of the species singly at a dose of 2 × 108 CFU (P < 0.001) (Table 1). The rate of survival after coinfection with both organisms at a dose of 2 × 106 CFU was significantly lower than that achieved after infection with a single species at the same dose (P < 0.001). The survival rates achieved after coinfection with a combination of S. epidermidis at 2 × 106 and C. albicans at 2 × 108 CFU were lower than those achieved after single infections with S. epidermidis or C. albicans at 2 × 108 CFU, but the differences did not achieve statistical significance (P = 0.79 and 0.13, respectively). Coinfection with a combination of S. epidermidis at 2 × 108 and C. albicans 2 × 106 CFU resulted in a statistically significant reduction in survival compared with that achieved with C. albicans alone at 2 × 108 CFU (P = 0.003), and although the survival rate after coinfection was lower than that achieved after infection with S. epidermidis alone at 2 × 108 CFU/ml, the difference did not reach statistical significance (P = 0.213). Blood cultures confirmed infection in all infection and coinfection groups.
TABLE 1.
Survival in the neonatal rat after coinfectiona
| Organism (dose [CFU]) in infection no.:
|
Total no. of animals | % Survival | |
|---|---|---|---|
| 1 | 2 | ||
| S. epidermidis (2 × 108) | None | 230 | 48.6 |
| C. albicans (2 × 108) | None | 219 | 57.5 |
| S. epidermidis (2 × 108) | C. albicans (2 × 108) | 158 | 6.96 |
| S. epidermidis (2 × 106) | None | 84 | 98.8 |
| C. albicans (2 × 106) | None | 131 | 100 |
| S. epidermidis (2 × 106) | C. albicans (2 × 106) | 195 | 91.3 |
| S. epidermidis (2 × 108) | C. albicans (2 × 106) | 158 | 41.8 |
| S. epidermidis (2 × 106) | C. albicans (2 × 108) | 69 | 46.4 |
| None | None | 126 | 100 |
The rate of survival after coinfection at 2 × 108 CFU was significantly lower than that after infection with either organism singly at a dose of 2 × 108 CFU (P < 0.001). The rate of survival after coinfection at 2 ×106 CFU was significantly lower than that after infection with either organism singly at the same dose (P < 0.001). Coinfection with a combination of S. epidermidis at 2 × 108 and C. albicans at 2 × 106 CFU resulted in a statistically significant reduction in the rate of survival compared with that after infection with C. albicans at 2 × 108 CFU (P = 0.003) but not compared with that after infection with S. epidermidis at 2 × 108 CFU/ml (P = 0.213). Coinfection with a combination of S. epidermidis at 2 × 106 CFU and C. albicans at 2 × 108 CFU resulted in lower survival rates compared to those after infections with S. epidermidis or C. albicans alone at 2 × 108 CFU, but the difference did not achieve statistical significance (P = 0.79 and 0.13, respectively).
Weight gain as a measure of morbidity in the coinfection model.
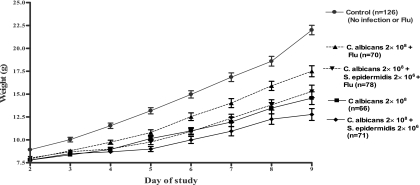
There was a significant difference among the slopes of the weight curves for the different groups when they were compared by one-way analysis of variance (P < 0.05) (Fig. 1). Coinfection at a dose of 2 × 108 CFU/ml tended to impair growth significantly more than either single infection at a dose of 2 × 108 CFU/ml (P < 0.001). Coinfection at a dose of 2 × 106 CFU did not show a statistically significant impairment of weight gain compared with that achieved for the control or animals infected with either S. epidermidis or C. albicans alone at a dose of 2 × 106 CFU. Coinfection with a combination of both S. epidermidis at dose of 2 × 108 CFU and C. albicans at a dose of 2 × 106 CFU also did not result in a statistically significant difference in weight gain compared to that for animals infected with S. epidermidis alone at a dose of 2 × 108 CFU (P = 1.0). The weight gain achieved after coinfection with a combination of S. epidermidis at dose of 2 × 106 CFU and C. albicans at dose of 2 × 108 CFU was significantly different from that after infection with C. albicans alone at a dose of 2 × 108 CFU (P < 0.001).
FIG. 1.
Weight gain of the neonatal rat after infection and coinfection. The values 2 × 108 and 2 × 106 indicate the dose (in CFU) of each infection. Coinfection or infection with a single species at 2 × 108 CFU significantly reduced the weight gain compared to that for the controls (P < 0.05). Infection with any organism at 2 × 106 CFU did not affect weight gain. Coinfection at 2 × 108 CFU significantly reduced weight gain compared to that after infection with a single species at 2 × 108 CFU. Coinfection with one organism at 2 × 108 CFU and the other organism at 2 × 106 CFU decreased weight gain but not significantly (P > 0.05) compared to that achieved with infection with a single species at 2 × 108 CFU.
Fluconazole MIC.
Fluconazole at a concentration of 1 μg/ml (MIC) inhibited C. albicans, but it did not inhibit S. epidermidis at any concentration, as assayed by the broth microdilution method.
Serum fluconazole levels.
Serum trough levels of fluconazole were 1.55 ± 0.007 μg/ml (mean ± standard deviation) at 24 h, 2.5 ± 0.007 μg/ml at 48 h, 1.51 ± 0.13 μg/ml at 72 h, 1.03 ± 0.35 μg/ml at 96 h, and 0 μg/ml at 144 h (Fig. 2). The mean 24-h trough levels were all above the MIC during the period of prophylaxis, suggesting the adequacy of our 10-mg/kg/dose schedule in achieving effective concentrations of fluconazole for prophylaxis.
FIG. 2.
Serum assay of fluconazole by enzyme-linked immunosorbent assay displayed as raw data with the means and standard deviations. There were five datum points at 24 and 72 h each and four datum points at 48, 96, and 144 h. Each datum point is for pooled serum from three rat pups. The trough levels of fluconazole were assayed at 24, 48, 72, 96, and 144 after the start of fluconazole prophylaxis. Mean 24-h trough levels were above 1 μg/ml, the MIC.
Survival in the coinfection model after fluconazole treatment.
Fluconazole prophylaxis significantly enhanced the rate of survival against infection with Candida at a dose of 2 × 108 CFU from 56.1% to 85.7% (P = 0.0003) and enhanced the rate of survival against coinfection with doses of 2 × 108 CFU of C. albicans and 2 × 106 CFU of S. epidermidis from 39.4% to 75.6% (P < 0.001) (Table 2).
TABLE 2.
Survival in the neonatal rat after fluconazole prophylaxis and coinfectiona
| Organism (dose [CFU]) in infection no.:
|
Fluconazole treatment | No. of animals that survived | Total no. of animals | % Survival | |
|---|---|---|---|---|---|
| 1 | 2 | ||||
| C. albicans (2 × 108) | None | No | 37 | 66 | 56.06 |
| C. albicans (2 × 108) | None | Yes | 60 | 70 | 85.71 |
| S. epidermidis (2 × 106) | C. albicans (2 × 108) | No | 28 | 71 | 39.44 |
| S. epidermidis (2 × 106) | C. albicans (2 × 108) | Yes | 59 | 78 | 75.64 |
Fluconazole prophylaxis for coinfection significantly improved the rates of survival both against severe C. albicans infection (2 × 108 CFU) from 56.1% to 85.7% (P = 0.0003) and against coinfection with C. albicans at 2 × 108 CFU with S. epidermidis at 2 × 106 CFU from 39.4% to 75.6% (P < 0.001).
Weight gain in coinfection model after fluconazole treatment.
Fluconazole prophylaxis improved weight gain after infection with Candida at a dose of 2 × 108 CFU (P < 0.001) and coinfection with C. albicans at a dose of 2 × 108 CFU and S. epidermidis at a dose of 2 × 106 CFU (P < 0.001) (Fig. 3).
FIG. 3.
Weight gain of neonatal rats in fluconazole (Flu) prophylaxis experiments. The values 2 × 108 and 2 × 106 indicate the dose (in CFU) of each infection. Fluconazole prophylaxis enhances growth after infection with Candida albicans at 2 × 108 CFU and coinfection with C. albicans at 2 × 108 CFU plus S. epidermidis at 2 ×106 CFU (P < 0.05).
DISCUSSION
Since coinfection in the neonate is a very significant event, we designed and developed a neonatal rat model that provides a platform with which to investigate the mechanisms of disease and effective prevention and treatment strategies. Neonatal animals are different from adult animals in their responses to infection, hence the need for a neonatal model. To our knowledge the effects of coinfection have not been evaluated in such a neonatal animal model. Similar to clinical observations, in neonatal animals coinfection significantly reduced the rate of survival compared to that after infection with a single species. A decrease in survival was noted on addition of even a sublethal dose of a second organism.
The most commonly seen coinfection in premature neonates is that with CoNS and Candida, which is why these organisms were selected for use in our model. Synergistic effects on mortality in coinfections or polymicrobial infections in general may be due to but not limited to the following: (i) physical interactions between the organisms that give them a survival advantage; (ii) impairment or overwhelming of the host's immune system, thereby enhancing proliferation of organisms; and (iii) host factors that predispose the individual to multiple infections, e.g., a breach of the mucosal (e.g., gastrointestinal tract) or skin integrity, immunodeficiency, or the presence of a foreign body (catheters) that facilitates the formation of polymicrobial biofilms, which can then disseminate, causing infection and increased mortality.
A physical interaction between Candida and staphylococci may be responsible for the synergistic effects of infection with these two organisms on mortality, and this has been suggested by in vivo and in vitro observations. The synergistic effects of coinfection on mortality have also been observed in adult animal models. C. albicans and S. aureus have been studied in an adult mouse model of coinfection (6-8) in which doses of 107 CFU/ml of S. aureus or 108 CFU/ml of C. albicans given intraperitoneally and/or subcutaneously were studied in different combinations. A synergistic effect of dual infections on mouse mortality was noted. The rate of mortality was the highest (81.2%) when both organisms were given intraperitoneally. There were no deaths when C. albicans was given subcutaneously. Heat-inactivated C. albicans had an effect similar to that of viable C. albicans. When both viable C. albicans and S. aureus were injected at different sites, a mixed infection was established at the site of the C. albicans infection but not at the site of the S. aureus infection. On pathological examination, bacteria were observed in the center of the fungal growth. The interaction between these two organisms in some way appears to be beneficial to each other, resulting in increased mortality. In mixed-species biofilms of S. epidermidis and C. albicans in vitro, an extracellullar polymer produced by S. epidermidis inhibited fluconazole penetration and the presence of C. albicans appeared to protect S. epidermidis against vancomycin (1). Mixed-species biofilms are more resistant to antimicrobial agents and may give them a survival advantage. Medical prosthetic devices, namely, central venous catheters, endotracheal tubes, biliary stents, and acrylic dentures, are often the sites of mixed-biofilm formation (15); and central venous catheter infections have been associated with polymicrobial septicemia (5, 18) and high rates of mortality (11, 26).
Coinfection or polymicrobial infection is known to impair or alter the host immune mechanisms (2), which may contribute to the observed synergistic effect on mortality. Polymicrobial sepsis has been shown to impair leukocyte migration, suppress polymorphonuclear leukocyte function (27, 33), alter cytokine and chemokine expression (24), alter dendritic cell function (13), and induce organ-specific apoptosis (14), as well as increase the level of inducible apoptosis of CD4+ lymphocytes (3).
In our neonatal model of coinfection, the mechanisms contributing to synergistic effects on mortality were very likely the physical interaction between C. albicans and S. epidermidis, even though they were administered at different subcutaneous sites, and the immune dysfunction induced by the coinfection. Our future experiments would focus on evaluating the inflammatory response in the rats after infection with a single species and coinfections and the effects of inflammation after coinfection in the brain, lungs, and the liver and studying the physical interactions between the organisms in vitro.
We evaluated impairment of nonedematous weight gain as a measure of short-term morbidity, which has been described in other animal models (36). Compared to the weight gain of the controls, weight gain in neonatal suckling rats was impaired after a severe infection with either organism and in the coinfection groups when the infection with at least one organism was severe. Weight gain was maximally impaired in the coinfection group when the infections with both organisms were severe, which was also associated with the highest rate of mortality. It may be difficult to assess weight curves in a group which has a high mortality rate. We attempted to overcome this problem by computing the slopes of the growth curves for individual animals, which could be calculated with only two measurements. Most of the deaths in all groups occurred on day 3 or day 4 of the study. Therefore, at least two weight measurements were available for all animals, even those in the group with the highest mortality rate. The reasons for weight gain impairment in the infected animals may be multifactorial, most plausibly due to a catabolic state induced by infection or the inability of the infected pups to compete with the other pups during suckling.
In our experiments with fluconazole prophylaxis, we used fluconazole at 10 mg/kg/dose for four doses given 24 h apart i.p. We chose the i.p. route because intravenous cannulation of neonatal rats is difficult and the peritoneal surface offers a wide area for absorption. The intraperitoneal dose of 10 mg/kg every 24 h was based on and modified from a previous study by Marchetti et al. which evaluated serum levels after intraperitoneal administration of fluconazole in adult Wistar rats (25) and whose findings were supported by our pharmacokinetic observations. A dose that varies from 3 to 6 mg/kg/dose intravenously or orally every 72 to 48 h has been used effectively for prophylaxis of Candida infections in very-low-birth-weight infants (4, 20, 22).
The in vitro assay of susceptibility of the strain of C. albicans used in this study (strain ATCC 32354) to fluconazole was performed by the microdilution method, as recommended by the Clinical and Laboratory Standards Institute (28). There is an excellent correlation of the results of antifungal susceptibility testing between the broth macrodilution and the broth microdilution techniques (28). The advantage of the microdilution technique lies in its simplicity and convenience. The strain of C. albicans was susceptible to fluconazole with an MIC of 1 μg/ml, similar to that for most but not all strains.
A potential concern with fluconazole prophylaxis is the development of resistance over time. C. albicans is the most common colonizing and invading species of Candida in neonatal units and is susceptible to fluconazole, whereas the non-C. albicans species, i.e., C. parapsilosis and C. glabrata, have MICs of fluconazole greater than those for C. albicans (23). There is also a concern that resistant non-C. albicans species may replace fluconazole-susceptible C. albicans strains in neonatal intensive care units after years of fluconazole prophylaxis. Recently, fluconazole-resistant C. parapsilosis strains have been reported in animals and neonates after years of prophylaxis (31, 37). However, fluconazole prophylaxis currently remains an effective option for reduction of Candida colonization and infection if it is given in an optimal dosage regimen.
Fluconazole prophylaxis in our neonatal rat model of coinfection significantly enhanced survival and weight gain after Candida infection and coinfection with C. albicans and S. epidermidis. The improvement in survival in the coinfection group with fluconazole prophylaxis is significantly more than what one would expect with just the eradication of C. albicans. As speculated earlier in our discussion, changing the pathology from coinfection or a polymicrobial infection to an infection with a single species eliminates the synergistic effect of infections with two species. We also speculate that fluconazole prophylaxis, by converting coinfection to an infection with a single species, may also reduce the immune dysfunction and improve survival and weight gain. However, these speculations must be researched further by focusing on the host's inflammatory response and immune status, as well as the tissue histopathology for evidence of inflammatory injury.
The neonatal rat coinfection described here is analogous to the situation in premature infants who are at risk for multiple infections and coinfection. Our data reaffirm the beneficial effects of fluconazole prophylaxis in high-risk infants, in terms of a reduction in mortality and short-term morbidity and with no overt adverse effects attributable to fluconazole. Antifungal prophylaxis may have a role in preventing fungal infections in extremely premature infants (19). The prevention of coinfections with Candida in high-risk infants who are prone to multiple infections assumes potential clinical significance in terms of preventing mortality and possibly mitigating the common morbidities associated with the premature neonate (e.g., chronic lung disease, intraventricular hemorrhage, and neurodevelopment).
Footnotes
Published ahead of print on 29 January 2007.
REFERENCES
- 1.Adam, B., G. S. Baillie, and L. J. Douglas. 2002. Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J. Med. Microbiol. 51:344-349. [DOI] [PubMed] [Google Scholar]
- 2.Ayala, A., and I. H. Chaudry. 1996. Immune dysfunction in murine polymicrobial sepsis: mediators, macrophages, lymphocytes and apoptosis. Shock 6(Suppl. 1):S27-S38. [PubMed] [Google Scholar]
- 3.Ayala, A., C. S. Chung, Y. X. Xu, T. A. Evans, K. M. Redmond, and I. H. Chaudry. 1999. Increased inducible apoptosis in CD4+ T lymphocytes during polymicrobial sepsis is mediated by Fas ligand and not endotoxin. Immunology 97:45-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertini, G., S. Perugi, C. Dani, L. Filippi, S. Pratesi, and F. F. Rubaltelli. 2005. Fluconazole prophylaxis prevents invasive fungal infection in high-risk, very low birth weight infants. J. Pediatr. 147:162-165. [DOI] [PubMed] [Google Scholar]
- 5.Bonadio, W. A. 1988. Polymicrobial bacteremia in children. An 11-year experience. Am. J. Dis. Child. 142:1158-1160. [DOI] [PubMed] [Google Scholar]
- 6.Carlson, E. 1983. Effect of strain of Staphylococcus aureus on synergism with Candida albicans resulting in mouse mortality and morbidity. Infect. Immun. 42:285-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson, E. 1982. Synergistic effect of Candida albicans and Staphylococcus aureus on mouse mortality. Infect. Immun. 38:921-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson, E., and G. Johnson. 1985. Protection by Candida albicans of Staphylococcus aureus in the establishment of dual infection in mice. Infect. Immun. 50:655-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou, C. Y., M. T. Kao, H. L. Kuo, J. S. Liu, Y. L. Liu, and C. C. Huang. 2006. Gram-negative and polymicrobial peritonitis are associated with subsequent fungal peritonitis in CAPD patients. Perit. Dial. Int. 26:607-608. [PubMed] [Google Scholar]
- 10.Daher, A. H., and F. E. Berkowitz. 1995. Infective endocarditis in neonates. Clin. Pediatr. (Philadelphia) 34:198-206. [DOI] [PubMed] [Google Scholar]
- 11.Faix, R. G., and S. M. Kovarik. 1989. Polymicrobial sepsis among intensive care nursery infants. J. Perinatol. 9:131-136. [PubMed] [Google Scholar]
- 12.Fanaroff, A. A., S. B. Korones, L. L. Wright, E. C. Wright, R. L. Poland, C. B. Bauer, J. E. Tyson, J. B. Philips III, W. Edwards, J. F. Lucey, et al. 1994. A controlled trial of intravenous immune globulin to reduce nosocomial infections in very-low-birth-weight infants. N. Engl. J. Med. 330:1107-1113. [DOI] [PubMed] [Google Scholar]
- 13.Flohe, S. B., H. Agrawal, D. Schmitz, M. Gertz, S. Flohe, and F. U. Schade. 2006. Dendritic cells during polymicrobial sepsis rapidly mature but fail to initiate a protective Th1-type immune response. J. Leukoc. Biol. 79:473-481. [DOI] [PubMed] [Google Scholar]
- 14.Franklin, G. A., M. Turina, J. F. Kuhn, R. Turpen, J. C. Peyton, and W. G. Cheadle. 2006. Organ specific apoptosis following polymicrobial intraperitoneal infection. Inflamm. Res. 55:136-143. [DOI] [PubMed] [Google Scholar]
- 15.Fux, C. A., J. W. Costerton, P. S. Stewart, and P. Stoodley. 2005. Survival strategies of infectious biofilms. Trends Microbiol. 13:34-40. [DOI] [PubMed] [Google Scholar]
- 16.Harris, S. C., J. E. Wallace, G. Foulds, and M. G. Rinaldi. 1989. Assay of fluconazole by megabore capillary gas-liquid chromatography with nitrogen-selective detection. Antimicrob. Agents Chemother. 33:714-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlowicz, M. G., P. J. Giannone, J. Pestian, A. L. Morrow, and J. Shults. 2000. Does candidemia predict threshold retinopathy of prematurity in extremely low birth weight (</=1000 g) neonates? Pediatrics 105:1036-1040. [DOI] [PubMed] [Google Scholar]
- 18.Karlowicz, M. G., L. N. Hashimoto, R. E. Kelly, Jr., and E. S. Buescher. 2000. Should central venous catheters be removed as soon as candidemia is detected in neonates? Pediatrics 106:E63. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman, D. 2003. Strategies for prevention of neonatal invasive candidiasis. Semin. Perinatol. 27:414-424. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman, D., R. Boyle, K. C. Hazen, J. T. Patrie, M. Robinson, and L. G. Donowitz. 2001. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N. Engl. J. Med. 345:1660-1666. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman, D., and K. D. Fairchild. 2004. Clinical microbiology of bacterial and fungal sepsis in very-low-birth-weight infants. Clin. Microbiol. Rev. 17:638-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kicklighter, S. D., S. C. Springer, T. Cox, T. C. Hulsey, and R. B. Turner. 2001. Fluconazole for prophylaxis against candidal rectal colonization in the very low birth weight infant. Pediatrics 107:293-298. [DOI] [PubMed] [Google Scholar]
- 23.Long, S. S., and D. K. Stevenson. 2005. Reducing Candida infections during neonatal intensive care: management choices, infection control, and fluconazole prophylaxis. J. Pediatr. 147:135-141. [DOI] [PubMed] [Google Scholar]
- 24.Maier, S., K. Emmanuilidis, M. Entleutner, N. Zantl, M. Werner, K. Pfeffer, and C. D. Heidecke. 2000. Massive chemokine transcription in acute renal failure due to polymicrobial sepsis. Shock 14:187-192. [DOI] [PubMed] [Google Scholar]
- 25.Marchetti, O., J. M. Entenza, D. Sanglard, J. Bille, M. P. Glauser, and P. Moreillon. 2000. Fluconazole plus cyclosporine: a fungicidal combination effective against experimental endocarditis due to Candida albicans. Antimicrob. Agents Chemother. 44:2932-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKenzie, F. E. 2006. Case mortality in polymicrobial bloodstream infections. J. Clin. Epidemiol. 59:760-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreno, S. E., J. C. Alves-Filho, F. Rios-Santos, J. S. Silva, S. H. Ferreira, F. Q. Cunha, and M. M. Teixeira. 2006. Signaling via platelet-activating factor receptors accounts for the impairment of neutrophil migration in polymicrobial sepsis. J. Immunol. 177:1264-1271. [DOI] [PubMed] [Google Scholar]
- 28.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 2nd ed. NCCLS document M27-A2. NCCLS, Wayne, PA.
- 29.Noyola, D. E., M. Fernandez, E. H. Moylett, and C. J. Baker. 2001. Ophthalmologic, visceral, and cardiac involvement in neonates with candidemia. Clin. Infect. Dis. 32:1018-1023. [DOI] [PubMed] [Google Scholar]
- 30.Saiman, L., E. Ludington, M. Pfaller, S. Rangel-Frausto, R. T. Wiblin, J. Dawson, H. M. Blumberg, J. E. Patterson, M. Rinaldi, J. E. Edwards, R. P. Wenzel, W. Jarvis, et al. 2000. Risk factors for candidemia in neonatal intensive care unit patients. Pediatr. Infect. Dis. J. 19:319-324. [DOI] [PubMed] [Google Scholar]
- 31.Sarvikivi, E., O. Lyytikainen, D. R. Soll, C. Pujol, M. A. Pfaller, M. Richardson, P. Koukila-Kahkola, P. Luukkainen, and H. Saxen. 2005. Emergence of fluconazole resistance in a Candida parapsilosis strain that caused infections in a neonatal intensive care unit. J. Clin. Microbiol. 43:2729-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shelley, O., T. Murphy, H. Paterson, J. A. Mannick, and J. A. Lederer. 2003. Interaction between the innate and adaptive immune systems is required to survive sepsis and control inflammation after injury. Shock 20:123-129. [DOI] [PubMed] [Google Scholar]
- 33.Simms, H. H., and R. D'Amico. 1992. Polymicrobial sepsis disrupts normal neutrophil extracellular matrix protein interactions. Circ. Shock 38:1-8. [PubMed] [Google Scholar]
- 34.Stoll, B. J., N. Hansen, and A. A. Fanaroff. 2002. Late onset sepsis in very low-birth weight neonates:the experience of the National Institute of Child-Health and Human Development Neonatal Research Network. Pediatrics 110:285-291. [DOI] [PubMed] [Google Scholar]
- 35.Stoll, B. J., N. I. Hansen, I. Adams-Chapman, A. A. Fanaroff, S. R. Hintz, B. Vohr, and R. D. Higgins. 2004. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA 292:2357-2365. [DOI] [PubMed] [Google Scholar]
- 36.Tran, T. V., and L. E. Weisman. 2004. Dexamethasone effects on group B streptococcal infection in newborn rats. Pediatr. Infect. Dis. J. 23:47-52. [DOI] [PubMed] [Google Scholar]
- 37.Yoder, B. A., D. A. Sutton, V. Winter, and J. J. Coalson. 2004. Resistant Candida parapsilosis associated with long term fluconazole prophylaxis in an animal model. Pediatr. Infect. Dis. J. 23:687-688. [DOI] [PubMed] [Google Scholar]