Abstract
The echinocandin susceptibilities of bloodstream Candida isolates growing in a biofilm was investigated. Within the therapeutic range of concentrations of each drug, caspofungin and micafungin were active against biofilms formed by Candida albicans or C. glabrata but not those formed by C. tropicalis or C. parapsilosis.
Biofilm-mediated antifungal resistance is a well-documented phenomenon for Candida species and probably contributes to Candida pathogenicity in catheter-related bloodstream infections (BSIs) (6, 10, 16). Although fungal biofilm-associated infections are frequently refractory to conventional antifungal therapy, the echinocandins, which constitute a new class of antifungals that inhibit 1,3-β-d-glucan synthase, have recently been demonstrated to be active against Candida albicans biofilms (3, 14). While C. albicans is the most commonly isolated Candida species, other non-C. albicans species have been increasingly recognized as catheter-related BSI pathogens (4, 16). However, there have been few comparisons of the activities of echinocandins against biofilms formed by different Candida species. We compared the in vitro activities of caspofungin, micafungin, fluconazole, and amphotericin B against biofilms formed by BSI isolates of four different Candida species.
We examined 43 Candida species isolates, including 12 C. albicans, 12 Candida parapsilosis, 10 Candida tropicalis, and 9 Candida glabrata isolates. All of the isolates were from blood cultures acquired at Chonnam National University Hospital, Gwangju, South Korea, between January 1999 and December 2003. The MICs for planktonic cells were determined by the standard CLSI (Clinical and Laboratory Standards Institute) M27-A2 broth microdilution method (5). The MICs of the two echinocandins for planktonic cells were defined as the lowest concentrations resulting in the prominent inhibition of growth as determined after 24 h of incubation (11).
The MICs for sessile cells (biofilms) were determined using a microtiter-based assay (3, 15). In this system, mature biofilms were allowed to form in 96-well microtiter plates for 48 h and the cell densities of the biofilms were estimated using the 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrozolium-5-carboxanilide (XTT) absorbance assay. The drugs were prepared in a series of twofold dilutions as follows: fluconazole, 1,024 to 2 μg/ml; amphotericin B, 32 to 0.06 μg/ml; and the two echinocandins, 16 to 0.03 μg/ml. The inhibitory effects of the antifungals were measured as the optical densities (ODs) of the antifungal-treated wells relative to those of the control (antifungal-free) wells (considered to be 100%) as determined in the XTT assays and are expressed as percentages of the values for control wells. The MIC50 and MIC80 of each drug for sessile cells were determined and compared to the controls (15). All isolates were tested at least twice.
The ODs of the different Candida species were compared by the Mann-Whitney U test by using the SPSS Win 10.0 program. Differences between the species were considered to be significant for P of <0.05. Correlations between the MICs for planktonic cells and those for sessile cells and between the MICs of caspofungin and micafungin for sessile cells were examined by the least-squares method (13). Alpha was set at 0.05, and all the P values were two tailed.
The distributions of antifungal MICs for planktonic and sessile cells of the different Candida species are shown in Tables 1, 2, and 3. The median MIC50s and MIC80s of fluconazole for sessile cells of all Candida species were >1,024 μg/ml. The median MIC50 of amphotericin B for sessile cells of each of the four Candida species ranged from 0.5 to 1 μg/ml, which was similar to the median MIC for planktonic cells (0.5 μg/ml), while the median MIC80 for sessile cells ranged from 2 to >32 μg/ml. These data show that amphotericin B is moderately effective against the biofilms of all four species, whereas fluconazole is ineffective.
TABLE 1.
Distributions of fluconazole MICs for different Candida species strains under planktonic or biofilm (sessile-cell) growth conditions
| Species | No. of isolates tested | Type of MICa | No. of isolates for which indicated MIC (μg/ml) was:
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | >1,024 | |||
| C. albicans | 12 | MIC for planktonic cells | 3 | 4 | 1 | 3 | 1 | ||||||||
| MIC50 for sessile cells | 1 | 1 | 2 | 1 | 7 | ||||||||||
| MIC80 for sessile cells | 12 | ||||||||||||||
| C. parapsilosis | 12 | MIC for planktonic cells | 1 | 4 | 4 | 3 | |||||||||
| MIC50 for sessile cells | 2 | 10 | |||||||||||||
| MIC80 for sessile cells | 12 | ||||||||||||||
| C. tropicalis | 10 | MIC for planktonic cells | 1 | 6 | 1 | 2 | |||||||||
| MIC50 for sessile cells | 2 | 8 | |||||||||||||
| MIC80 for sessile cells | 10 | ||||||||||||||
| C. glabrata | 9 | MIC for planktonic cells | 1 | 2 | 5 | 1 | |||||||||
| MIC50 for sessile cells | 1 | 8 | |||||||||||||
| MIC80 for sessile cells | 9 | ||||||||||||||
MIC50s and MIC80s were determined by measuring XTT activity reduction.
TABLE 2.
Distributions of amphotericin B MICs for different Candida species strains under planktonic or biofilm growth conditions
| Species | No. of isolates tested | Type of MICa | No. of isolates for which indicated MIC (μg/ml) was:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | >32 | |||
| C. albicans | 12 | MIC for planktonic cells | 3 | 9 | ||||||||||
| MIC50 for sessile cells | 3 | 3 | 6 | |||||||||||
| MIC80 for sessile cells | 4 | 4 | 4 | |||||||||||
| C. parapsilosis | 12 | MIC for planktonic cells | 2 | 2 | 8 | |||||||||
| MIC50 for sessile cells | 2 | 5 | 5 | |||||||||||
| MIC80 for sessile cells | 2 | 2 | 1 | 7 | ||||||||||
| C. tropicalis | 10 | MIC for planktonic cells | 1 | 9 | ||||||||||
| MIC50 for sessile cells | 4 | 2 | 4 | |||||||||||
| MIC80 for sessile cells | 1 | 1 | 2 | 1 | 1 | 4 | ||||||||
| C. glabrata | 9 | MIC for planktonic cells | 1 | 1 | 6 | 1 | ||||||||
| MIC50 for sessile cells | 1 | 2 | 4 | 1 | 1 | |||||||||
| MIC80 for sessile cells | 1 | 1 | 2 | 1 | 1 | 1 | 2 | |||||||
MIC50s and MIC80s were determined by measuring XTT activity reduction.
TABLE 3.
Distribution of caspofungin and micafungin MICs for different Candida species strains under planktonic or biofilm growth conditions
| Drug | Species | No. of isolates tested | Type of MICa | No. of isolates for which indicated MIC (μg/ml) was:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | >16 | ||||
| Caspofungin | C. albicans | 12 | MIC for planktonic cells | 6 | 4 | 2 | ||||||||
| MIC50 for sessile cells | 1 | 1 | 1 | 9 | ||||||||||
| MIC80 for sessile cells | 9 | 3 | ||||||||||||
| C. parapsilosis | 12 | MIC for planktonic cells | 6 | 5 | 1 | |||||||||
| MIC50 for sessile cells | 1 | 2 | 2 | 2 | 5 | |||||||||
| MIC80 for sessile cells | 12 | |||||||||||||
| C. tropicalis | 10 | MIC for planktonic cells | 4 | 5 | 1 | |||||||||
| MIC50 for sessile cells | 6 | 2 | 2 | |||||||||||
| MIC80 for sessile cells | 10 | |||||||||||||
| C. glabrata | 9 | MIC for planktonic cells | 2 | 5 | 2 | |||||||||
| MIC50 for sessile cells | 1 | 1 | 6 | 1 | ||||||||||
| MIC80 for sessile cells | 3 | 6 | ||||||||||||
| Micafungin | C. albicans | 12 | MIC for planktonic cells | 12 | ||||||||||
| MIC50 for sessile cells | 2 | 6 | 3 | 1 | ||||||||||
| MIC80 for sessile cells | 1 | 2 | 6 | 3 | ||||||||||
| C. parapsilosis | 12 | MIC for planktonic cells | 3 | 7 | 2 | |||||||||
| MIC50 for sessile cells | 2 | 5 | 1 | 4 | ||||||||||
| MIC80 for sessile cells | 1 | 11 | ||||||||||||
| C. tropicalis | 10 | MIC for planktonic cells | 10 | |||||||||||
| MIC50 for sessile cells | 1 | 1 | 1 | 1 | 1 | 1 | 4 | |||||||
| MIC80 for sessile cells | 1 | 9 | ||||||||||||
| C. glabrata | 9 | MIC for planktonic cells | 9 | |||||||||||
| MIC50 for sessile cells | 2 | 6 | 1 | |||||||||||
| MIC80 for sessile cells | 3 | 2 | 2 | 1 | 1 | |||||||||
MIC50s and MIC80s were determined by measuring XTT activity reduction.
The caspofungin MIC50s for C. albicans sessile cells ranged from 0.06 to 0.5 μg/ml, similar to the values reported previously (3). The median caspofungin MIC80s for sessile cells of C. albicans, C. tropicalis, C. parapsilosis, and C. glabrata were 0.5, >16, >16, and 1 μg/ml, respectively. The median micafungin MIC80s for sessile cells were 0.5 μg/ml and 0.25 μg/ml for C. albicans and C. glabrata, respectively, and >16 μg/ml for both C. parapsilosis and C. tropicalis (P < 0.01). There was no correlation between the MICs for planktonic cells and those for sessile cells of a given strain, but a positive correlation between the caspofungin and micafungin MICs for sessile cells was noted (R, 0.4 for MIC50 for sessile cells; P = 0.014; R, 0.9 for MIC80 for sessile cells; P < 0.01).
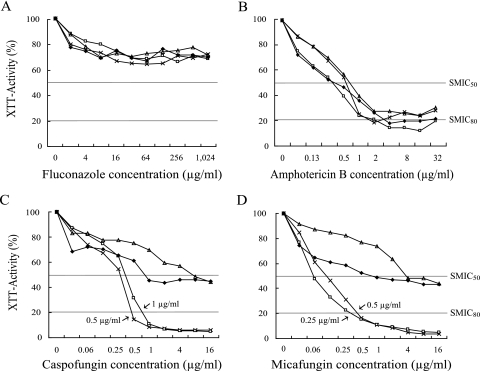
Figure 1 shows the inhibition curves for biofilm-grown Candida species in the presence of different concentrations of the four antifungal agents. The inhibitory effects of fluconazole and amphotericin B showed no significant differences among the Candida species. However, there were significant species-specific differences in the echinocandin activities against the Candida biofilms (P < 0.05). In contrast to that in the C. parapsilosis and C. tropicalis biofilms, a reduction in XTT activity of up to 80% in the C. albicans and C. glabrata biofilms was observed following exposure to relatively low concentrations (0.25 to 1 μg/ml) of caspofungin and micafungin.
FIG. 1.
In vitro activities of different concentrations of drugs against biofilms formed by four individual bloodstream isolates representing four different Candida species. Symbols: ×, C. albicans; ▵, C. parapsilosis; ⧫, C. tropicalis; □, C. glabrata. The inhibitory effect of each concentration of antifungal was measured as the average OD of all antifungal-treated wells and expressed as a percentage of the OD of control (antifungal-free) wells (considered to be 100%) as determined in XTT reduction assays. Species-specific differences are evident for the activities of caspofungin and micafungin against Candida biofilms (P < 0.05), in contrast to the activities of fluconazole and amphotericin B. SMIC50, MIC50 for sessile cells; SMIC80, MIC80 for sessile cells.
Kuhn et al. (9) have shown that both echinocandins inhibit one of two C. parapsilosis strains while exhibiting high MICs for the other strain. In the present study, the biofilms formed by C. parapsilosis (12 strains) were less susceptible to both echinocandins in vitro. These interstudy differences may be due to the differences in the Candida biofilm models used or to the biofilm-forming abilities of the Candida isolates tested (8, 16, 17).
We selected 43 out of the 95 isolates from preliminary experiments in which the biofilms had high turbidities at 48 h (OD > 0.3), since the biofilms with lower turbidities gave nonreproducible MIC results for sessile cells, mainly due to the low ODs of the control wells. Therefore, the criterion that we used for isolate selection may have introduced a bias. Further studies are needed on the potential associations between the densities of biofilms (particularly those formed by C. parapsilosis and C. tropicalis isolates) and echinocandin susceptibilities.
The mechanisms of echinocandin activity against biofilms formed by different Candida species are unknown (7). However, differences related to the composition of the Candida biofilm matrix (1, 8), metabolic activity (12), and the rate of drug diffusion through the biofilm (2) have been reported for different Candida species. These data suggest different drug resistance mechanisms for different biofilm-forming Candida species. Clinical significance remains to be verified, given the variations in testing methods and isolate selection. The observed differences in echinocandin susceptibilities among different Candida species suggest the involvement of novel biochemical and genetic mechanisms in biofilm formation.
Acknowledgments
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2005-E00082).
Footnotes
Published ahead of print on 5 February 2007.
REFERENCES
- 1.Al-Fattani, M. A., and L. J. Douglas. 2006. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J. Med. Microbiol. 55:999-1008. [DOI] [PubMed] [Google Scholar]
- 2.Al-Fattani, M. A., and L. J. Douglas. 2004. Penetration of Candida biofilms by antifungal agents. Antimicrob. Agents Chemother. 48:3291-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmann, S. P., K. VandeWalle, G. Ramage, T. F. Patterson, B. L. Wickes, J. R. Graybill, and J. L. Lopez-Ribot. 2002. In vitro activity of caspofungin against Candida albicans biofilms. Antimicrob. Agents Chemother. 46:3591-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blumberg, H. M., W. R. Jarvis, J. M. Soucie, J. E. Edwards, J. E. Patterson, M. A. Pfaller, M. S. Rangel-Frausto, M. G. Rinaldi, L. Saiman, R. T. Wiblin, and R. P. Wenzel. 2001. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. The National Epidemiology of Mycosis Survey. Clin. Infect. Dis. 33:177-186. [DOI] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A2, 2nd ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Kojic, E. M., and R. O. Darouiche. 2004. Candida infections of medical devices. Clin. Microbiol. Rev. 17:255-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuhn, D. M., and M. A. Ghannoum. 2004. Candida biofilms: antifungal resistance and emerging therapeutic options. Curr. Opin. Investig. Drugs 5:186-197. [PubMed] [Google Scholar]
- 8.Kuhn, D. M., J. Chandra, P. K. Mukherjee, and M. A. Ghannoum. 2002. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect. Immun. 70:878-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhn, D. M., T. George, J. Chandra, P. K. Mukherjee, and M. A. Ghannoum. 2002. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 46:1773-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis, R. E., D. P. Kontoyiannis, R. O. Darouiche, I. I. Raad, and R. A. Prince. 2002. Antifungal activity of amphotericin B, fluconazole, and voriconazole in an in vitro model of Candida catheter-related bloodstream infection. Antimicrob. Agents Chemother. 46:3499-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odds, F. C., M. Motyl, R. Andrade, J. Bille, E. Canton, M. Cuenca-Estrella, A. Davison, C. Durussel, D. Ellis, E. Foraker, A. W. Fothergill, M. A. Ghannoum, R. A. Giacobbe, M. Gobernado, R. Handke, M. Lavediere, W. Lee-Yang, W. G. Merz, L. Ostrosky-Zeichner, J. Peman, S. Perea, J. R. Perfect, M. A. Pfaller, L. Prioa, J. H. Rex, M. G. Rinaldi, J. L. Rodriguez-Tudela, W. A. Schell, C. Shields, D. A. Sutton, P. E. Verweij, and D. W. Warnock. 2004. Interlaboratory comparison of results of susceptibility testing with caspofungin against Candida and Aspergillus species. J. Clin. Microbiol. 42:3475-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parahitiyawa, N. B., Y. H. Samaranayake, L. P. Samaranayake, J. Ye, P. W. Tsang, B. P. Cheung, J. Y. Yau, and S. K. Yeung. 2006. Interspecies variation in Candida biofilm formation studied using the Calgary biofilm device. APMIS 114:298-306. [DOI] [PubMed] [Google Scholar]
- 13.Pfaller, M. A., S. A. Messer, L. Boyken, R. J. Hollis, C. Rice, S. Tendolkar, and D. J. Diekema. 2004. In vitro activities of voriconazole, posaconazole, and fluconazole against 4,169 clinical isolates of Candida spp. and Cryptococcus neoformans collected during 2001 and 2002 in the ARTEMIS global antifungal surveillance program. Diagn. Microbiol. Infect. Dis. 48:201-205. [DOI] [PubMed] [Google Scholar]
- 14.Ramage, G., K. VandeWalle, S. P. Bachmann, B. L. Wickes, and J. L. Lopez-Ribot. 2002. In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob. Agents Chemother. 46:3634-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramage, G., K. Vande Walle, B. L. Wickes, and J. L. Lopez-Ribot. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin, J. H., S. J. Kee, M. G. Shin, S. H. Kim, D. H. Shin, S. K. Lee, S. P. Suh, and D. W. Ryang. 2002. Biofilm production by isolates of Candida species recovered from nonneutropenic patients: comparison of bloodstream isolates with isolates from other sources. J. Clin. Microbiol. 40:1244-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song, J. W., J. H. Shin, D. H. Shin, S. I. Jung, D. Cho, S. J. Kee, M. G. Shin, S. P. Suh, and D. W. Ryang. 2005. Differences in biofilm production by three genotypes of Candida parapsilosis from clinical sources. Med. Mycol. 43:657-661. [DOI] [PubMed] [Google Scholar]