Abstract
Disk diffusion was a reliable, easy, and inexpensive method for testing the susceptibility of Campylobacter jejuni to erythromycin (215 susceptible and 45 resistant isolates) and to ciprofloxacin (154 susceptible, two intermediate, and 124 resistant isolates) using, respectively, an erythromycin disk and ciprofloxacin and nalidixic acid disks.
Campylobacter jejuni subsp. jejuni (C. jejuni) is a major human pathogen responsible of 85 to 95% of enterocolitis caused by Campylobacter spp. These infections need to be treated with an antimicrobial agent in less than 50% of cases (1). Macrolides and fluoroquinolones are the first- and second-choice antimicrobial agents for that purpose (1, 4, 8, 9, 11, 13). With the development of resistance of C. jejuni strains to both erythromycin and ciprofloxacin, routine susceptibility testing has become a very important tool for appropriate antimicrobial treatment when needed (4, 6-9, 11, 13). The main erythromycin resistance mechanism of C. jejuni strains (mutation of 23S ribosomal rRNA and proteins) confers a high-level resistance with MICs of ≥128 μg/ml (9). The main quinolone resistance mechanism (mutation[s] of DNA gyrase A) can confer a low, intermediate, or high resistance level to ciprofloxacin and confers a high resistance level to nalidixic acid (4, 14).
Antimicrobial susceptibility testing of Campylobacter spp. with an agar dilution method has been standardized by the CLSI (2), and the erythromycin, ciprofloxacin, tetracycline, and doxycycline MIC interpretive criteria for C. jejuni and Campylobacter coli have been reported previously (3). The agar dilution method, however, is not convenient for testing a few isolates at a time, and the disk diffusion method has not been standardized yet.
The aim of this study was to compare the results obtained by the disk diffusion method with those obtained with the reference agar dilution method to test the susceptibility of C. jejuni isolates to erythromycin and ciprofloxacin.
A total of 280 C. jejuni organisms isolated at Hôpital Saint-Luc (HSL) from 2002 to 2006 were used in this study. All the Campylobacter spp. isolates included in this study were microaerobic spiral gram-negative rods that were hippurate positive, resistant to cephalothin, and grew well at 42°C, confirming their identification as C. jejuni subsp. jejuni. The identification tests were done by using the recommended methods (12). The identification at the genus and species levels of 92 of these 280 isolates was confirmed by the Laboratoire de santé publique du Québec (LSPQ). The erythromycin (Sigma Chemical Co., St. Louis, MO) and ciprofloxacin (Bayer Leverkusen, Germany) MICs of the C. jejuni isolates were determined at HSL by using the agar dilution method at 37°C, as recommended by CLSI (2). C. jejuni ATCC 33560 was used as the control strain (2). For the erythromycin and ciprofloxacin disk diffusion testing carried out at HSL, inocula, prepared in Mueller-Hinton (MH) broth at a density adjusted to a 0.5 McFarland turbidity standard, were delivered onto 5% sheep blood MH agar plates (Difco, Becton Dickinson, Sparks, MD). Plates were incubated at 37°C for 48 h, and zone diameters were measured with slipping calipers. A study carried out to determine the presence or absence of an inhibition zone using erythromycin and nalidixic acid disks was also done, at 37°C and 42°C, at HSL (143 isolates tested) and at Hôpital Sacré-Coeur (HSC) (100 isolates tested). The study on the presence or absence of an inhibition zone using nalidixic acid disks was also done at 37°C, at LSPQ (92 isolates). For these studies, inocula were prepared in MH broth at a density adjusted to a 0.5 McFarland turbidity standard at HSL and HSC and in BHI broth at a density adjusted to a 1.0 McFarland turbidity standard, as recommended for Campylobacter identification (10), at LSPQ. Inocula were delivered onto 5% sheep blood (MH), Trypticase soy (TS) (Quélab, Montréal, Québec, Canada), or brain heart infusion (Difco, Becton Dickinson, Sparks, MD) agar plates. Incubation was carried out under a microaerobic atmosphere obtained with a gas generator envelope or in a microaerobic incubator, either at 37°C for 48 h or at 42°C for 24 h. C. jejuni ATCC 33291 and Campylobacter fetus subsp. fetus ATCC 27374 were used as the control strains at LSPQ (10). Erythromycin (15 μg), ciprofloxacin (5 μg), or nalidixic acid (30 μg) disks (BBL, Becton Dickinson, Sparks, MD) were used.
Erythromycin susceptibility testing.
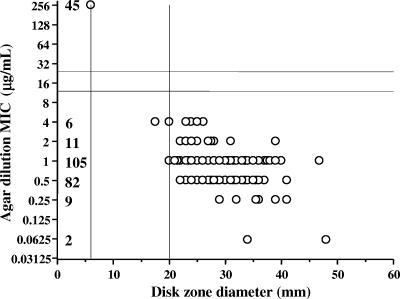
In Fig. 1, the results of disk diffusion testing carried out at 37°C with 260 C. jejuni isolates were compared to those of agar dilution for erythromycin, using a scattergram. The 215 susceptible isolates had MICs of 0.06 to 4 μg/ml and zone diameters of 17.5 to 48 mm around the erythromycin disk; 87% of the susceptible isolates had MICs of 0.5 or 1 μg/ml and 97% had zone diameters of 20 to 39 mm; only one isolate had a diameter less than 20 mm, at 17.5 mm. The 45 resistant isolates had MICs of ≥256 μg/ml and zone diameters of 6 mm (i.e., no zone around the disk). No isolates had MICs of 8 to 128 μg/ml. Zone diameters of 6 mm and ≥20 mm around the erythromycin disk are respectively suggested as resistant and susceptible breakpoints of C. jejuni isolates. Using the disk diffusion method at 42°C, the zone diameters obtained for erythromycin (57 susceptible isolates, including those with zone diameters nearest to the tentative breakpoint, and the 45 resistant isolates) were within the zone diameter breakpoints suggested for the disk diffusion method at 37°C (data not shown). When the susceptibility of 146 C. jejuni isolates to erythromycin was determined at HSL and HSC on MH or TS blood agar plates, at 37°C or 42°C, using as a criterion for susceptibility or resistance the presence or absence of an inhibition zone, all 101 isolates with a zone and all 45 isolates without a zone around the erythromycin disk were respectively susceptible (MICs of 0.06 to 4 μg/ml) and resistant (MICs of ≥256 μg/ml) to erythromycin. Of the 45 C. jejuni isolates resistant to erythromycin, 27 were from a cluster (8; unpublished data) and the 18 others were not related to one another or to the epidemic strain. The reported rate of erythromycin resistance is low (<5%) for these bacteria in most studies (1, 4, 6, 7, 9) and was also relatively low (2.7%) at HSL from 2002 to 2006.
FIG. 1.
Correlation between disk diffusion and MICs of erythromycin for 260 C. jejuni isolates. The number of isolates is indicated for each MIC.
Ciprofloxacin susceptibility testing.
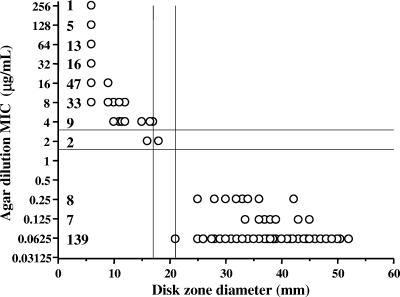
In Fig. 2, the results of disk diffusion testing carried out at 37°C with 280 C. jejuni isolates were compared to those of agar dilution for ciprofloxacin, using a scattergram. The 154 susceptible isolates had MICs of 0.06 to 0.25 μg/ml and zone diameters of 21 to 52 mm around the ciprofloxacin disk; 90% of the susceptible isolates had an MIC of 0.06 μg/ml and 88% had zone diameters of 30 to 49 mm. The 124 resistant isolates had MICs of 4 to ≥256 μg/ml and zone diameters of 6 to 17 mm; 93% of these isolates had MICs of 8 to ≥256 μg/ml and 89% had zone diameters of 6 mm. The two isolates intermediate to ciprofloxacin had an MIC of 2 μg/ml and zone diameters of 16 and 18 mm. When the susceptibility of 146 C. jejuni isolates to ciprofloxacin was determined at HSL, HSC, and LSPQ on MH, TS, or brain heart infusion blood agar plates, at 37°C or 42°C, using as a criterion for susceptibility or resistance the presence or absence of an inhibition zone around a nalidixic acid disk, all 76 isolates with a zone and 68 of the 70 isolates without a zone around the nalidixic acid disk were respectively susceptible (MICs of 0.06 to 0.25 μg/ml) and resistant (MICs of 4 to 128 μg/ml) to ciprofloxacin. The two remaining isolates without a zone around the nalidixic acid disk were intermediate (MICs of 2 μg/ml) to ciprofloxacin. The other 134 C. jejuni isolates (56 resistant and 78 susceptible) were tested at HSL at 37°C using the agar dilution method, which showed a complete correlation between the nalidixic acid disk (presence or absence of an inhibition zone) and the ciprofloxacin MICs (data not shown). Based on these results, for ciprofloxacin susceptibility testing of C. jejuni isolates, zone diameters of ≤17 mm and ≥21 mm around the ciprofloxacin disk and the absence or the presence of an inhibition zone around the nalidixic acid disk are suggested as breakpoints for resistance and susceptibility, respectively. Using the disk diffusion method at 42°C, the zone diameters obtained for ciprofloxacin (47 susceptible isolates and 37 resistant isolates, including those with zone diameters nearest to the tentative breakpoints) were within the zone diameter breakpoints suggested for the disk diffusion method at 37°C (data not shown).
FIG. 2.
Correlation between disk diffusion and MICs of ciprofloxacin for 280 C. jejuni isolates. The number of isolates is indicated for each MIC.
Using the above criteria, in comparison to the agar dilution method, one isolate intermediate to ciprofloxacin was found resistant using the ciprofloxacin disk, giving one minor error (0.4%), and the two isolates intermediate to ciprofloxacin were found resistant using the nalidixic acid disk, giving two minor errors (1.4%). These two isolates are probably not susceptible to ciprofloxacin, but molecular studies will be needed to find the true answer. The nalidixic acid disk is also recommended for the identification of Campylobacter at the species level (10, 12) and is reported as a marker of ciprofloxacin susceptibility (4-8, 11-14). Other studies (5, 11) have shown a correlation between the disk diffusion and agar dilution methods for the susceptibility of C. jejuni to erythromycin and ciprofloxacin, but the MIC interpretive criteria were reported only in 2006 (3).
Concluding remarks.
Present results suggest that zone diameters of 6 mm and ≥20 mm around the erythromycin disk could be considered as breakpoints for resistance and susceptibility, respectively, for C. jejuni isolates. MIC determination using the agar dilution method for isolates with a zone diameter of more than 6 mm and of less than 20 mm is recommended. Of the 260 C. jejuni isolates tested in this study, only one isolate would have needed an erythromycin MIC test.
For ciprofloxacin susceptibility of C. jejuni isolates, zone diameters of ≤17 mm and ≥21 mm around the ciprofloxacin disk and the absence or the presence of an inhibition zone around the nalidixic acid disk are suggested as breakpoints for resistance and susceptibility, respectively. If the results obtained with both disks are consistent, the isolate could be reported accordingly. If the two disks give discordant results and reidentification confirms a C. jejuni isolate, we suggest determining a ciprofloxacin MIC using the agar dilution method. In our experience, of the 280 C. jejuni isolates tested, only one isolate would have needed a ciprofloxacin MIC.
Since the collection of C. jejuni studied in this work included only two isolates intermediate to ciprofloxacin and no isolate intermediate to erythromycin, it would be important to further evaluate the performance of the disk diffusion test with a larger number of intermediate isolates.
In this study, disk diffusion was a reliable, easy, and inexpensive method for testing the susceptibility of C. jejuni to erythromycin and to ciprofloxacin. In 2006, CLSI recommended that the susceptibility of C. jejuni to erythromycin and to ciprofloxacin should be considered for testing. Until it becomes standardized, the disk diffusion method for erythromycin (determined with the erythromycin disk) and ciprofloxacin (determined with the ciprofloxacin and nalidixic acid disks) susceptibility of C. jejuni should be considered a screening method. To implement this method in a laboratory, we suggest using C. jejuni ATCC 33560 and other strains for which the MICs are known as quality controls.
Acknowledgments
We thank Huguette Gilbert, France Boucher, Suzanne Hallée, Lise Bourbonnais, Denis Boudreau, and Sophie Grenier for technical assistance. We also thank Robert Boileau for assistance with the scattergrams.
Footnotes
Published ahead of print on 29 January 2007.
REFERENCES
- 1.Blaser, M. J. 2005. Campylobacter jejuni and related species, p. 2548-2557. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 6th ed. Elsevier Churchill Livingston, Philadelphia, PA.
- 2.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing; fifteenth informational supplement; no. M100-S15 (M7-A6), vol. 25, no. 1. Clinical and Laboratory Standards Institute, Wayne, PA.
- 3.Clinical and Laboratory Standards Institute. 2006. Methods for antimicrobial dilution and disk susceptibility testing for infrequently-isolated or fastidious bacteria; approved guideline. Publication no. M45-A. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Engberg, J., F. M. Aarestrup, D. E. Taylor, P. Gerner-Smidt, and I. Nachamkin. 2001. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates. Emerg. Infect. Dis. 7:24-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaudreau, C., and H. Gilbert. 1997. Comparison of disc diffusion and agar dilution methods for antibiotic susceptibility testing of Campylobacter jejuni subsp. jejuni and Campylobacter coli. J. Antimicrob. Chemother. 39:707-712. [DOI] [PubMed] [Google Scholar]
- 6.Gaudreau, C., and H. Gilbert. 1998. Antimicrobial resistance of clinical strains of Campylobacter jejuni subsp. jejuni isolated from 1985 to 1997 in Quebec, Canada. Antimicrob. Agents Chemother. 42:2106-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaudreau, C., and H. Gilbert. 2003. Antimicrobial resistance of Campylobacter jejuni subsp. jejuni strains isolated from humans in 1998 to 2001 in Montréal, Canada. Antimicrob. Agents Chemother. 47:2027-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaudreau, C., and S. Michaud. 2003. Cluster of erythromycin- and ciprofloxacin-resistant Campylobacter jejuni subsp. jejuni from 1999 to 2001 in men who have sex with men, Québec, Canada. Clin. Infect. Dis. 37:131-136. [DOI] [PubMed] [Google Scholar]
- 9.Gibreel, A., V. N. Kos, M. Keelan, C. A. Trieber, S. Levesque, S. Michaud, and D. E. Taylor. 2005. Macrolide resistance in Campylobacter jejuni and Campylobacter coli: molecular mechanism and stability of the resistance phenotype. Antimicrob. Agents Chemother. 49:2753-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isenberg, H. D. 2004. Fecal culture for Campylobacter and related organisms, p. 3.8.2. In H. D. Isenberg (ed.), Clinical microbiology procedure handbook, 2nd ed. American Society for Microbiology, Washington, DC.
- 11.Nachamkin, I., J. Engberg, and F. M. Aarestrup. 2000. Diagnosis and antimicrobial susceptibility of Campylobacter species, p. 45-66. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. American Society for Microbiology, Washington, DC.
- 12.Nachamkin, I. 2003. Campylobacter and Arcobacter, p. 902-914. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. American Society for Microbiology Washington, DC.
- 13.Smith, K. E., J. M. Besser, C. W. Hedberg, F. T. Leano, J. B. Bender, J. H. Wicklund, B. P. Johnson, K. A. Moore, M. T. Osterholm, and the Investigation Team. 1999. Quinolone-resistant Campylobacter jejuni infections in Minnesota, 1992-1998. N. Engl. J. Med. 340:1525-1532. [DOI] [PubMed] [Google Scholar]
- 14.Trieber, C. A., and D. E. Taylor. 2000. Mechanisms of antibiotic resistance in Campylobacter, p. 441-454. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. American Society for Microbiology, Washington, DC.